Abstract
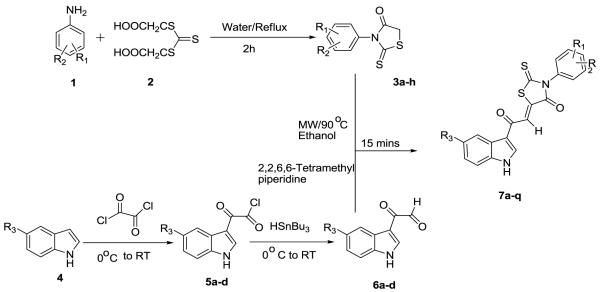
(Z)-5-(2-(1H-Indol-3-yl)-2-oxoethylidene)-3-phenyl-2-thioxothiazolidin-4-one (7a-q) derivatives have been synthesized by the condensation reaction of 3-phenyl-2-thioxothiazolidin-4-ones (3a-h) with suitably substituted 2-(1H-indol-3-yl)-2-oxoacetaldehyde (6a-d) under microwave condition. The thioxothiazolidine-4-ones were prepared from corresponding aromatic amines (1a-e) and di-(carboxymethyl)-trithiocarbonyl (2). The aldehydes (6a-h) were synthesized from the corresponding acidchlorides (5a-d) using HSnBu3.
Keywords: 3-phenyl-2-thioxothiazolidin-4-one, 2-(1H-indol-3-yl)-2-oxoacetaldehyde, acid chloride, HSnBu3, MW irradiation
Introduction
United Nations Program On HIVAIDS (UNAIDS) estimates approximately 35.2 million people worldwide are living with HIV and more than 25 million people have died of AIDS. Thus so far, 28 anti-HIV drugs have been licensed by the United States Food and Drug Administration (FDA). Most of these drugs belong to two categories: reverse transcriptase inhibitors (RTI) and protease inhibitors (PI). Combined application of these antiretroviral drugs has shown significant synergistic effects.1 However, an increasing number of patients with HIV infection/AIDS can no longer use such drugs as a result of drug resistance and serious adverse effects.2 Therefore it is essential to develop novel anti-HIV drugs targeting HIV entry. A recent report3 identified 2-aryl-5-(4-oxo-3- phenethyl-2-thioxothiazolidinylidenemethyl)-furans (A, Fig. 1) with Rhodanine as a core molecule exhibited anti-HIV-1 activity. On the other hand, Rhodanine4 and its derivatives possessing hydrogen attached to the nitrogen atom have been patented as fungicides while the compounds containing nitrogen atom5 were patented as pesticides, with mention being made of their usefulness as fungicides. 5-Benzylidine-3-pheny-2-thioxo-thiazolidin-4-one core (B, Fig. 1) was shown to inhibit the Jun NH2-terminal kinase (Jnk) stimulatory phosphatase-1 (JSP-1).6 In addition Rhodanine-based molecules are popular as small molecule inhibitors of numerous targets such as HCV NS3 protease,7a aldose reductase,7b,c β-lactamase,7d UDP-N-acetylmuramate/L-alanine ligase,7e antidiabetic agents,7f cathepsin D,7g and histidine decarboxylase.7h For the past few years our group has been preparing and evaluating biologically important compounds.8 Herein we report the synthesis of a novel class of rhodanine-based small molecule with the aim of investigating their inhibitory properties against JSP-1 and HIV-1 in the low micro molar range.
Fig. 1.
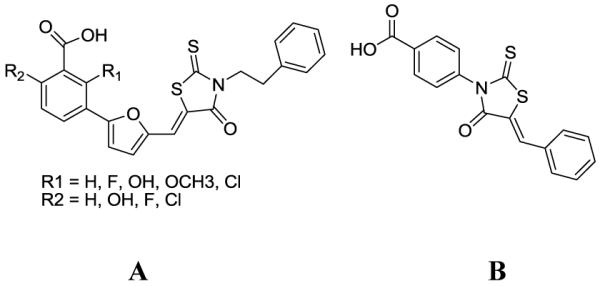
Competitive inhibitor of HIV-1 and JSP-1
5-(2-(1H-Indol-3-yl)-2-oxoethylidene)-3-phenyl-2-thiox-othiaz- olidin-4-ones (7a-q) were synthesized by a Knoevenagel condensation of 3-phenyl-2-thioxothiazolidin-4-ones (3a-h) derivatives with suitably substituted 2-(1H-indol-3-yl)-2-oxoacetaldehydes (6a-d) using MW irradiation and catalytic amount of 2,2,6,6-tetramethyl piperidine (TMP) in ethanol (Scheme 1). Although other bases (Table 1) can be used as catalyst {e.g,; piperidine, pyridine, N-methyl piperidine (NMP), DBU}, TMP works best. The same reaction under conventional reflux condition using ethanol as solvent gave lower yields (11 to 69%),3 longer time (5 h) and/or compounds required rigorous purification. However the MW reaction provides cleaner reaction, 15 min time and the products only required to be washed with cold ethanol. The yields are good to excellent (Table 3). The optimum temperature and condition for this MW assisted reaction was determined by a series of reaction of 3-phenyl-2-thioxothiazolidin-4-one (3b) and 2-(1H-indol-3-yl)-2-oxoacetaldehyde (6a). The results are summarized in Table 1. From this table it is clear that MW irradiation at 90 °C for 15 min in ethanol is the optimum condition for the synthesis of these molecules. All the compounds 7a-q were isolated as single (Z ) isomer and was confirmed by comparing the vinylic proton shift in 1H NMR with previously reported3,6 data which appears around δ 8.00 ppm.
Scheme 1.
Schematic representation for the synthesis of 7a-q
Table 1.
Screening of solvents, reaction time and temperature for the synthesis of 7b
| Base | Conditiona | Temp (°C) |
Time (mins) |
Yield b (%) |
|---|---|---|---|---|
| - | No solvent | 90 | 15 | Trace |
| - | Ethanol | 90 | 15 | Trace |
| Piper idene |
Ethanol | 90 | 15 | 80 |
| TMP | Ethanol | 90 | 15 | 96 |
| TMP | No solvent | 90 | 15 | Trace |
| DBU / pyrid ine |
Ethanol | 90 | 20 | 20 |
| TMP | Acetonitrile | 90 | 15 | 76 |
| TMP | Acetonitrile | 130 | 15 | 15 |
| DBU | Acetonitrile | 90 | 30 | Trace |
| TMP | DMF | 90 | 15 | 45 |
| DMF | 90 | 30 | 10 | |
| NMP | ||||
| DBU | DMF | 120- 140 |
15 | Trace |
| TMP | Water | 90 | 15 | Trace |
| TMP | Water | 130 | 15 | Trace |
| - | Water | 130 | 30 | Trace |
| TMP | Toluene | 90 | 15 | Trace |
| TMP | Isopropanol | 90 | 15 | 45 |
| TMP | THF | 90 | 15 | 38 |
| TMP | n-Butanol | 90 | 15 | 33 |
All the reaction was carried out in equimolar amount of each compound in 2 ml of solvent at 150 psi pressure.
Isolated yield
Table 3.
Reaction of various indole based aldehydes with Rhodanine derivatives
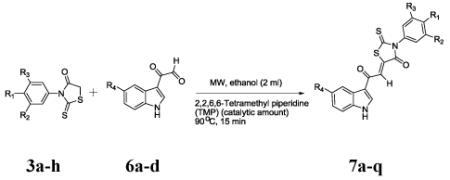
| Entry (%) |
R1 | R2 | R3 | R4 | product | Yielda,b |
|---|---|---|---|---|---|---|
| 1 | H | H | H | H | 7a | 95 |
| 2 | CH3 | H | H | H | 7b | 96 |
| 3 | CH3 | CH3 | H | H | 7c | 90 |
| 4 | CH3 | H | H | OCH3 | 7d | 93 |
| 5 | OCH3 | H | H | H | 7e | 95 |
| 6 | OCH3 | H | H | OCH3 | 7f | 92 |
| 7 | H | H | H | OCH3 | 7g | 94 |
| 8 | CN | H | H | OCH3 | 7h | 90 |
| 9 | CN | H | H | H | 7i | 91 |
| 10 | H | H | H | Cl | 7j | 89 |
|
H | H | 7k | 95 | ||
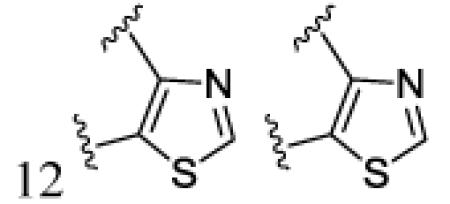
|
H | OCH3 | 7l | 93 | ||
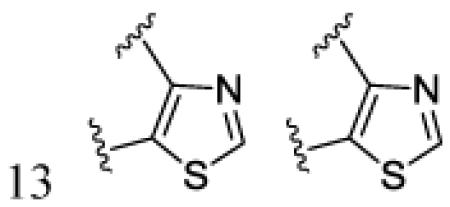
|
H | CN | 7m | 91 | ||
| 14 | OH | Br | Br | H | 7n | 90 |
| 15 | OH | Cl | Cl | H | 7o | 89 |
| 16 | OH | Br | Br | OCH3 | 7p | 96 |
| 17 | OH | Cl | Cl | OCH3 | 7q | 90 |
Isolated yield;
All the compounds were characterized by 1H NMR, 13C NMR, DEPT-135, IR and HRMS analysis.
Compounds 7a-q were obtained in 89-96% yields with high melting point (>300 °C). They were insoluble in usual organic solvent, water or hexane. The IR (KBr) spectra of compounds 7a-q exhibit absorption bands due to the stretching vibrations of NH group of indole cycles (3200 cm−1 range). The spectra of compounds 7a-q display characteristic bands and C=S group (intense bands at 1628-1610 cm−1). The 3-phenyl due to stretching vibrations of two C=O groups (1720 cm−1 range -2-thioxothiazolidin-4-ones (3a-h) derivatives were prepared by the literature9 procedure by refluxing equimolar amounts of suitably substituted aromatic amines (1a-e) and di-(carboxymethyl)-trithiocarbonyl (2). The aldehydes 6a-d were synthesized by treating corresponding acid chlorides with HSnBu3.10 The acid chlorides were prepared by acylation of indole (or substituted indole) with oxalylchloride.11 All compounds were characterized by 1H NMR, 13C NMR, DEPT-135, IR and HRMS studies.
In conclusion we have successfully developed an easy access to a novel series of (Z)-5-(2-(1H-indol-3-yl)-2-oxoethylidene)-3-phenyl-2-thioxothiazolidin-4-one (7a-q) derivatives. The mild reaction conditions, easy workup, good to excellent yields, and easily available substrate make this reaction an attractive method for the preparation of 3-phenyl-2-thioxothiazolidin-4-ones. Efforts towards the synthesis of other important drug molecules with a Rhodanine moiety by MW irradiation are ongoing in our laboratory. Also work is in progress to obtain biological activity (antibacterial, antifungal, anticancer and neuroprotective kinase inhibitor activity) of these important compounds. Results in these areas will be presented in due course.
Supplementary Material
Table 2.
Reaction of various aromatic amines with di-(carboxymethyl)-trithiocarbonyl
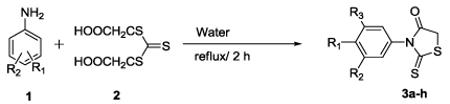
| Entry | R1 | R2 | R3 | Time (h) | Product | Yielda (%) |
|---|---|---|---|---|---|---|
| 1 | H | H | H | 3.0 | 3a | 75 |
| 2 | CH3 | H | H | 2.5 | 3b | 73 |
| 3 | CH3 | CH3 | H | 2.5 | 3c | 76 |
| 4 | OCH3 | H | H | 2.0 | 3d | 75 |
| 5 | CN | H | H | 4.0 | 3e | 71 |
| 6 | OH | Br | Br | 3.0 | 3f | 78 |
| 7 | OH | Cl | Cl | 3.0 | 3g | 76 |
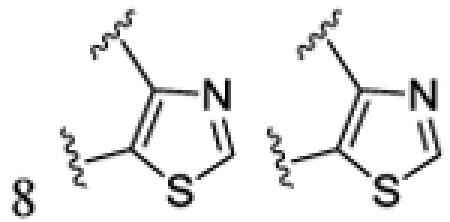
|
H | 4.0 | 3h | 75 | ||
Isolated yield.
Acknowledgments
The authors are grateful to NIH (1RC2NS064950) for generous financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.De Clercq E. J. Clin. Virol. 2004;30:115–133. doi: 10.1016/j.jcv.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 2(a).Johnson VA, Brun-Vezinet F, Clotet B, Conway B, D’Aquila RT, Demeter LM, Kuritzkes DR, Pillay D, Schapiro JM, Telenti A, Richman DD. Top. HIV Med. 2003;11:215–221. [PubMed] [Google Scholar]; (b) Richman DD, Morton SC, Wrin T, Hellmann N, Berry S, Shapiro MF, Bozette SA. AIDS. 2004;18:1393–1401. doi: 10.1097/01.aids.0000131310.52526.c7. [DOI] [PubMed] [Google Scholar]; (c) Carr A, Cooper DA. Lancet. 2000;356:1423–1430. doi: 10.1016/S0140-6736(00)02854-3. [DOI] [PubMed] [Google Scholar]
- 3.Jiang S, Tala SR, Lu H, Abo-Dya NE, Avan I, Gyanda K, Lu L, Katritzky AR, Debnath A. J. Med. Chem. 2011;54:572–579. doi: 10.1021/jm101014v. [DOI] [PubMed] [Google Scholar]
- 4.Alvord E. 1962109 U.S. Patent. 1934 June 5;
- 5.CIBA AG. 242300 Swiss Patent. 1946 Oct 1;
- 6.Cutshall NS, O’Day C, Prezhdo M. Biorg. Med. Chem. Lett. 2005;15:3374–3379. doi: 10.1016/j.bmcl.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 7(a).Sing WT, Lee CL, Yeo SL, Lim SP, Sim M. Biorg. Med. Chem. Lett. 2001;11:91–94. doi: 10.1016/s0960-894x(00)00610-7. [DOI] [PubMed] [Google Scholar]; (b) Bruno G, Costantino L, Curinga C, Maccari R, Monforte F, Nicolo F, Ottana R, Vigorita MG. Biorg. Med. Chem. Lett. 2002;10:1077–1084. doi: 10.1016/s0968-0896(01)00366-2. [DOI] [PubMed] [Google Scholar]; (c) Fujishima H, Tsuboto K. Br. J. Ophthalmol. 2002;86:860. doi: 10.1136/bjo.86.8.860. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Grant EB, Guiadeen D, Baum EZ, Foleno BD, Jin H, Montenegro DA, Nelson EA, Bush K, Hlasta DJ. Biorg. Med. Chem. Lett. 2000;10:2179–2182. doi: 10.1016/s0960-894x(00)00444-3. [DOI] [PubMed] [Google Scholar]; (e) Sim MM, Ng SB, Buss AD, Crasta SC, Goh KL, Lee SK. Biorg. Med. Chem. Lett. 2002;12:697–699. doi: 10.1016/s0960-894x(01)00832-0. [DOI] [PubMed] [Google Scholar]; (f) Momose Y, Meguro K, Ikeda H, Hatanaka C, Oi S, Sohda T. Chem. Pharm. Bull. 1991;39:1440–1445. doi: 10.1248/cpb.39.1440. [DOI] [PubMed] [Google Scholar]; (g) Whitesitt CA, Simon RL, Jon K. Reel, Sigmund SK, Phillips ML, Shadle JK, Heinz LJ, Koppel GA, Hundel DC, Lifer SL, Berry D, Ray J, Little SP, Liu X, Marshall WS, Panetta JA. Biorg. Med. Chem. Lett. 1996;6:2157–2162. [Google Scholar]; (h) Free CA, Majchrowicz E, Hess SM. Biochem. Pharm. 1971;20:1421–1428. doi: 10.1016/0006-2952(71)90269-3. [DOI] [PubMed] [Google Scholar]
- 8(a).Ankati H, Akubathini SK, D’Mello SR, Biehl ER. Synthetic Communications. 2010;40(16):2364–2376. [Google Scholar]; (b) Wang L, Ankati H, Akubathini SK, Balderamos M, Storey CA, Patel AV, Kretzschmar D, Biehl ER, D’Mello SR. Journal of Neuroscience Research. 2010;88:1970–1984. doi: 10.1002/jnr.22352. [DOI] [PubMed] [Google Scholar]; (c) Balderamos M, Ankati H, Akubathini SK, Patel AV, Kamila S, Mukherjee C, Wang L, Biehl ER, D’Mello SR. Experimental Biology and Medicine (Maywood, N. J, United States) 2008;233(11):1395–1402U. doi: 10.3181/0805-RM-153. [DOI] [PubMed] [Google Scholar]
- 9.Yarovenko VN, Nikitina AS, Zavarzin IV, Krayuskin MM, Kovalenko LV. Synthesis. 2006:1246–1248. [Google Scholar]
- 10.Kuivila HG. J. Org. Chem. 1960;25:284–290. [Google Scholar]
- 11(a).Speeter NE, Anthony WC. J. Am. Chem. Soc. 1954;76:6208–6209. [Google Scholar]; (b) Kharasch MS, Kane SS, Brown HC. J. Am. Chem. Soc. 1940;62:2242–2243. [Google Scholar]; (c) Brutcher FV, Vanderwerff WD. J. Org.Chem. 1958;23:146. [Google Scholar]; (d) Millich F, Becker E. J. Org.Chem. 1958;23:1096–1099. [Google Scholar]; (e) Aubry C, Wilson AJ, Emmerson D, Murphy E, Chan YY, Dickens MP, Garcia MD, Jenkins PR, Mahale S, Chaudhuri B. Bioorg. Med. Chem. 2009;17:6073–6084. doi: 10.1016/j.bmc.2009.06.070. [DOI] [PubMed] [Google Scholar]; (f) Vermeulen ES, van Smeden M, Schmidt AW, Sprouse JS, Wikstrom HV, Grol CJ. J. Med. Chem. 2004;47:5451–5466. doi: 10.1021/jm049743b. [DOI] [PubMed] [Google Scholar]
- 12(a).Lebeuf R, Ferezou I, Rossier J, Arseniyadis S, Cossy J. Organic Letters. 2009;11(21):4822–4825. doi: 10.1021/ol901846g. [DOI] [PubMed] [Google Scholar]; (b) Garraway JL. J. Chem. Soc. 1961:3733–3735. [Google Scholar]
- 13.Brown FC, Bradsher CK, Morgan EC, Tetenbaum M, Wilder P., Jr. J. Am. Chem. Soc. 1956;78:384–388. [Google Scholar]
- 14.All compounds gave satisfactory spectroscopic and analytical data. Compounds 3a,13 3b,13 3c,13 3d 12 were prepared according to the method described in the literature. The other related compounds, 3e, 3f, 3g and 3h were prepared by the same method. Foe details, see supplementary data.
- 15.MW assisted general synthesis of compound 7a-q: Equimolar amount of 3a and 6a were mixed properly in a mortar pestle and was taken in specially designed MW test tube. Ethanol (2 mL) was added to the mixture followed by 1-2 drops of TMP. The test tube after being sealed was irradiated for 15 min at 90 °C and 150 psi pressure. After cooling, the solid mass was crushed into 20 ml of 95% ethanol and filtered. The solid mass collected was washed with ethanol (20 ml) and dried under vacuum to get the desired (Z)-5-(2-(1H-Indol-3-yl)-2-oxoethylidene)-3-phenyl-2-thioxothiazolidin-4-one (7a) as orange solid. Mp 324-326 °C. IR (KBr-disk) 3375, 1726, 1626, 1555, 1516, 1496, 1439, 1219, 1168 cm−1. 1H NMR (500 Mhz, dmso- d6): δ 12.44 (brs, 1H, NH), 8.96 (s, 1H, Ar-CH), 8.28 (dd, J = 1.7 Hz, 8 Hz, 1H, Ar-CH), 8.02 (s, 1H, vinylic proton), 7.55-7.39 (m, 6H, Ar-CH), 7.27-7.25 (m, 2H, Ar-CH). 13C NMR (500 Mhz, dmso- d6) 201.1 (C=S), 182.3 (C=O), 167.3 (C=O), 138.2 (C), 137.8 (CH), 137.6 (C), 129.9 (CH), 129.8 (CH), 129.3 (CH), 125.9 (C), 124.5 (CH), 123.3 (CH), 122.1 (CH), 113.1 (CH). HRMS (ESI+) m/z 365.0430, calcd for C19H12N2O2S2 : 365.0420. The related compounds (7b-q) were prepared in the same way.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.