Abstract
The realization that medications used to treat movement disorders and psychiatric conditions of basal ganglia origin have significant shortcomings, as well as advances in the understanding of the functional organization of the brain, has led to a renaissance in functional neurosurgery, and particularly the use of deep brain stimulation (DBS). Movement disorders are now routinely being treated with DBS of ‘motor’ portions of the basal ganglia output nuclei, specifically the subthalamic nucleus and the internal pallidal segment. These procedures are highly effective and generally safe. Use of DBS is also being explored in the treatment of neuropsychiatric disorders, with targeting of the ‘limbic’ basal ganglia-thalamocortical circuitry. The results of these procedures are also encouraging, but many unanswered questions remain in this emerging field. This review summarizes the scientific rationale and practical aspects of using DBS for neurologic and neuropsychiatric disorders.
Keywords: Parkinson’s disease, Dystonia, Obsessive compulsive disorder, Depression, Tourette syndrome, Circuit disorder
1. Introduction
Over the last two decades deep brain stimulation (DBS) has emerged as a major new therapeutic modality for disorders involving the basal ganglia, specifically Parkinson’s disease and dystonia, as well as Tourette syndrome (TS), obsessive compulsive disorder (OCD), and treatment-resistant depression (TRD). In the United States and other developed countries, DBS has now largely replaced traditional ablative neurosurgical treatments for these disorders because of its less invasive nature, reversibility and adjustability. In this review we will briefly survey the functional organization of the basal ganglia and related structures, followed by a discussion of DBS therapy.
2. Basal ganglia-thalamocortical circuits
The basal ganglia are components of massive parallel and largely closed cortical-subcortical circuits, in which information is sent from different cortical areas to spatially separate domains of the basal ganglia, processed, and then returned to the frontal cortical area of origin via the thalamus [1–5]. Based on the presumed function(s) of the cortical region involved, the initial description of the circuits included “motor,” “oculomotor,” “prefrontal,” (or “associative”) and “limbic” circuits [1–3, 6, 7]. Each of these broadly indentified circuits is now thought to be comprised of multiple functionally and anatomically distinct subcircuits [8, 9]. This so-called segregated-circuit hypothesis is of fundamental importance for our understanding of the use and effects of ablative procedures and DBS in movement disorders and other conditions, because it explains how focal interventions in small areas of the basal ganglia will have functionally specific effects.
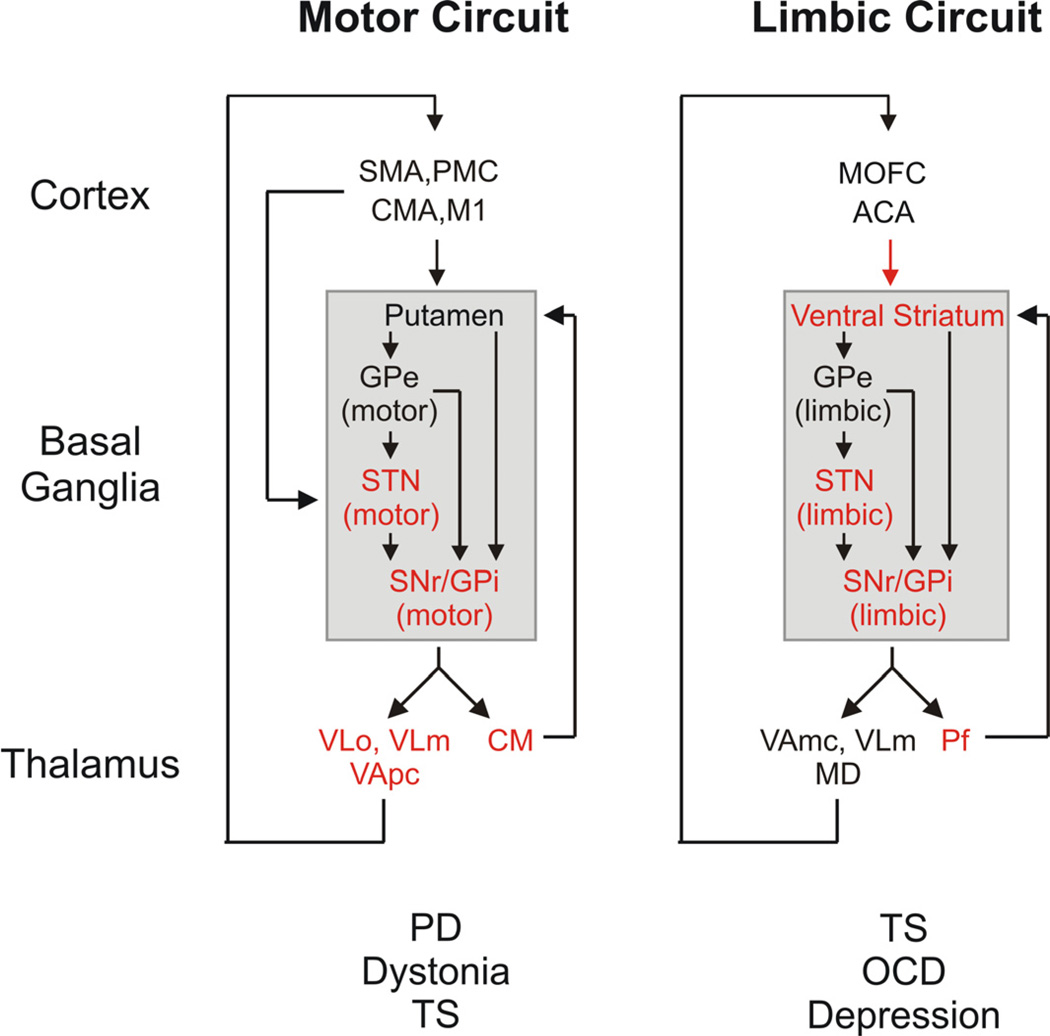
Basal ganglia disorders are thought to arise from abnormalities in one or more of these segregated circuits (see figure 1). Thus, most movement disorders are believed to result from disturbances in the basal ganglia thalamo-cortical ‘motor circuit’. This circuit originates from pre- and post-central sensorimotor areas, including the primary motor cortex (M1), the supplementary motor area (SMA), the premotor cortex (PMC), the cingulate motor area (CMA), and related post-central sensory cortical areas. The circuit then engages motor portions of the basal ganglia, including the motor area of the striatum, the post-commissural putamen, the dorsolateral subthalamic nucleus (STN), and the ventral posterolateral internal pallidal segment of the globus pallidus (GPi). The ‘motor’ projections from GPi reach the ventrolateral nucleus in the thalamus (VL), which then projects back to M1 and the premotor areas [8, 10, 11]. By contrast, neuropsychiatric disorders, such as OCD or TS, are thought to result from disturbances within the ‘limbic’ circuit, which takes origin from the anterior cingulate and medial orbitofrontal cortices, passes through the ventral striatum, the medial and caudal STN, the ventral and rostromedial GPi and rostrodorsal SNr, the paramedian portion of the mediodorsal nucleus (MD) and the magnocellular ventral anterior nucleus of the thalamus (VA), and then projects back to the anterior cingulate cortex.
Figure 1.
‘Motor’ and ‘limbic’ cortex-basal ganglia-thalamocortical circuits. The targets of current DBS treatments are labeled with asterisks (*), and movement disorders and neuropsychiatric disorders caused by dysfunction of these circuits are listed below the circuit diagrams. Abbreviations: ACA, anterior cingulate area; CMA, cingulate motor area; M1, primary motor cortex; MD, mediodorsal nucleus of thalamus; MOFC, medial orbitofrontal cortex; PMC, premotor cortex; SMA, supplementary motor area; VApc, ventral anterior nucleus of thalamus, pars parvocellularis; VAmc, ventral anterior nucleus of thalamus, pars magnocellularis; VLm, ventrolateral nucleus of thalamus, pars medialis; VLo, ventrolateral nucleus of thalamus, pars oralis. See text for other abbreviations. Modified from a figure in ref. [251], used with permission.
The circuit elements that link cortex, basal ganglia and thalamus appear to be relatively similar between the different functional circuits. In each circuit, the striatum and STN are the main entry points for cortical and thalamic inputs to the basal ganglia, and the GPi and the substantia nigra pars reticulata (SNr) provide basal ganglia output to the thalamus and brainstem. The striatum and GPi/SNr are linked via two anatomically distinct pathways. The ‘direct’ pathway arises from striatal medium spiny neurons that project monosynaptically to neurons in GPi and SNr. The ‘indirect’ pathway arises from other striatal neurons that project to the external segment of the globus pallidus [GPe, ref. 12, 13]. The GPe then projects to GPi and SNr either directly or via the STN.
The glutamatergic STN, an important DBS target and a key component of the indirect pathway, also receives segregated projections from cortical motor and non-motor areas and from the thalamic caudal intralaminar nuclei (i.e., the centromedian [CM] and parafascicular nuclei [Pf]). The cortical projection to the STN and the subsequent STN projections to the GPi/SNr have been referred to as the “hyperdirect” pathway, emphasizing the possibility that this pathway may provide a faster route for cortical input to modulate the activity of the output nuclei of the basal ganglia than the direct/indirect striatal pathway systems [14–16].
Collaterals from the pallido- and nigrothalamic projections also reach the CM/Pf nuclei. These projections are not parts of the trans-thalamic cortico-cortical re-entrant loops mentioned above, but are primarily components of a thalamostriatal feedback system [17–19]. Like the thalamocortical system, the primate CM/Pf-striatal projections are strictly topographically arranged. The CM receives motor input from the basal ganglia and projects to the motor portions of putamen and STN, whereas Pf inputs and output are related to associative and limbic territories of the basal ganglia [17, 20, 21]. In addition to this feedback function, the intralaminar nuclei provide saliency information to the striatum which may be important for procedural learning [22, 23].
In addition to their ascending projections, the basal ganglia output nuclei also send collateral projections to brainstem areas, such as the superior colliculus and the pedunculopontine nucleus [PPN, see 24, 25, 26]. The PPN plays a role in the regulation of gait and balance. Ascending projections from the PPN are returned to the basal ganglia as well as to the thalamus and basal forebrain. A basal ganglia projection to the superior colliculus arises from the SNr. This projection is involved with the control of saccadic and head/neck movements [27–30].
For decades, clinicians and researchers have believed that the trans-thalamic loops involving the basal ganglia and cerebellum are separate. However, there is now evidence that these structures are, in fact, interconnected [31, 32]. It was shown recently in rodents and primates that the cerebellum influences the basal ganglia via a bisynaptic pathway involving the thalamus and terminating in the striatum [31, 33], while the basal ganglia appear to influence the cerebellum via connections from the STN to the pontine nuclei, which then project to the cerebellar cortex [32]. As discussed below, these recently recognized subcortical connections are potentially very important for our understanding of the pathophysiologic basis of and surgical approaches to what have been traditionally considered as exclusive ‘basal ganglia disorders. Of course, interactions between basal ganglia and cerebellar output have long been known to also exist at the cortical level [see, for instance, ref. 34].
3. DBS for disorders involving the basal ganglia
Dysfunction within the basal ganglia circuits has been found to lead to a wide spectrum of motor and non-motor behavioral abnormalities. The abnormalities in a given disorder are thought to arise because of the presence of a signature pattern of neuronal dysfunction within one or more of the basal ganglia-thalamocortical circuits. Focal functional neurosurgical approaches, such as ablation or DBS aim to selectively modulate the activity in the diseased circuits without affecting non-involved circuits or unrelated networks, thus avoiding the widespread side effects associated with pharmacologic and other less targeted therapeutic approaches.
DBS therapy consists of chronic local electrical stimulation of discrete brain targets via an implanted wire bundle electrode with multiple contacts and a small implanted subcutaneous, externally programmable pulse generator. The primary targets for DBS are specific regions of the STN, GPi or ventral striatum. Other areas outside of the basal ganglia-thalamocortical circuits, typically receiving input from or projecting to the basal ganglia, are also targeted for specific applications, including the PPN, the zona incerta and the CM/Pf. During DBS surgery, electrodes are stereotactically placed into the brain, guided by neuroimaging and/or electrophysiological recording. These electrodes contain four separate contacts, spaced 0.5–1.5 mm apart along their distal end. The proximal (extra-cranial) portion of the electrodes is connected to a pulse generator which is placed in the sub-clavicular region. Many patients require bilateral DBS leads; in these cases, two independent single-channel stimulators or a single dual-channel stimulator can be used. Multi-site DBS lead implantations can be done during single surgical sessions or as staged procedures.
Many aspects of the stimulation can be controlled via telemetric adjustments of the pulse generator, including the choice of electrode contacts, the stimulation voltage or current, the width of the stimulation pulses, and the frequency of stimulation. The stimulation parameters vary between patients, stimulation targets and specific disorders, but typically, pulses are delivered at 60–185 Hz, with an amplitude of less than 4 V, and a pulse width between 60 and 200 µs.
4. Movement Disorders
4.1. Parkinsonism
After tremor, Parkinson’s disease is the second most common movement disorder, involving approximately 1–1.5 million patients in North America. The cardinal motor features of Parkinson’s disease, i.e., the triad of akinesia/bradykinesia, tremor at rest and muscular rigidity, result from decreased dopaminergic transmission in the motor portions of the basal ganglia, due to progressive loss of dopaminergic neurons in the substantia nigra pars compacta (SNc). Other features of the disease such as depression, autonomic dysfunction, sleep disorders, cognitive impairment, and gait/balance problems are now ascribed to widespread progressive pathologic changes outside of the dopaminergic system [35, 36].
Studies in animals with experimental dopamine depletion and in humans with Parkinson’s disease have provided evidence for significant abnormalities of activity within the direct and indirect pathways, suggesting that neuronal discharge may be reduced in GPe, and increased in STN, GPi and SNr [37–45]. These abnormalities are postulated to result from the lack of dopaminergic transmission in the striatum which is thought to lead to increased activity in striatal neurons of the indirect pathway, resulting in inhibition of GPe, and subsequent disinhibition of STN and GPi/SNr, and to reduced activity along the direct pathway, resulting in disinhibition of GPi and SNr. Abnormalities of neuronal firing patterns, such as an increased tendency of basal ganglia neurons to discharge in bursts [40, 41, 46–48], oscillatory firing patterns [42, 47–55] or increased synchrony [41, 47] are now thought to be at least as important for the development of parkinsonism as changes in tonic discharge rate. Of these, the presence of synchronized neuronal oscillations in the beta-range of frequencies, and the failure to generate gamma-band oscillations before and during movement have been specifically emphasized, based on finding these oscillatory pattern abnormalities in parkinsonian patients off medications with macroelectrodes placed in the basal ganglia or with cortical EEG [47, 49, 50, 54].
After its introduction in the 1990s, DBS rapidly became the preferred treatment for patients with advanced Parkinson’s disease. The procedure is now commonly used in patients with intractable tremor, or with disabling drug-induced complications, especially motor fluctuations and/or dyskinesias. A favorable clinical outcome of DBS surgery is generally seen in patients who strongly respond to levodopa and who do not show evidence for atypical PD, dementia or psychiatric diseases. While generally highly effective, DBS in its current form is a symptomatic treatment that does not alter the progression of the disease, and does not affect the non-levodopa responsive motor and non-motor aspects of the disorders such as levodopa-refractory freezing of gait and balance problems.
To specifically treat the motor signs of Parkinson’s disease, neurosurgeons target most commonly the motor portions of GPi or STN [56–60]. Although unilateral DBS is highly effective for asymmetrical symptoms, and can have significant bilateral effects, most patients with advanced Parkinson’s disease undergo bilateral DBS. Most of the recent trials comparing DBS at the STN and GPi have not found significant overall differences in terms of the antiparkinsonian benefits of the procedure, measured with scores from the United Parkinson’s disease rating scale (UPDRS) and scales to quantify activities of daily living or the quality of life. DBS at either target was found to alleviate parkinsonian motor signs, particularly during ‘off’ periods, and to reduce drug-induced side effects such as dyskinesias, dystonia or motor fluctuations. In patients with advanced Parkinson’s disease, DBS in GPi or DBS strongly improves the patient’s quality of life and is more effective than medical management [e.g., 58, 61]. In contrast to patients with GPi-DBS, those with STN-DBS are often able to substantially reduce their medications, which may, in part, account for the anti-dyskinetic effects of the procedure [62].
The risk of significant direct surgical complications such as intracerebral hemorrhage is small (1–2%), although infection is sometimes as high as 10% [61]. Stimulation, particularly of the STN, may also produce significant functional side effects, including the induction of paresthesias, involuntary movements, worsening of gait or speech, gaze deviation or paralysis, as well as cognitive and mood side effects. Many of these side effects can be eliminated by adjusting the stimulation parameters. Cognitive side effects, specifically reduced verbal fluency and declines in executive functions [63, 64], and postoperative depression (even resulting in suicide), mania, anxiety, and apathy have been reported [65–67], perhaps caused by inadvertent stimulation of limbic circuit elements [e.g., 68, 69]. Suicide in post-operative patients with PD has been correlated with unrecognized and untreated depression. Such inadvertent effects appear to be less likely with GPi-DBS than with STN-DBS, likely because of the wider separation of motor and non-motor functions in this nucleus. The apparent better benefit/side-effect ratio of GPi-DBS may in the future result in increased use of the GPi target for the treatment of parkinsonian signs [70].
Other DBS targets may be useful in patients with specific clinical problems. For instance, patients with severe tremor (but not other parkinsonian signs) can be effectively treated with thalamic DBS at the border between the thalamic nucleus ventralis oralis and the ventral intermediate nucleus [see, e.g., 71, 72, 73]. However, even in patients with tremor-predominant parkinsonism, DBS is most commonly done in the STN or GPi rather than the thalamus, because of the expectation that such patients will develop significant additional parkinsonian signs which would not be adequately treated with the thalamic DBS.
While DBS of STN, GPi and thalamic targets is well established in the treatment of Parkinson’s disease, DBS of other targets, including the PPN, caudal zona incerta and cortex remains experimental. Following encouraging studies in MPTP-treated monkeys [74], stimulation of the PPN has been explored in patients with Parkinson’s disease with levodopa-unresponsive gait and balance problems. The DBS target in the PPN overlaps or is part of what has been described as the “locomotion center” in the pons [75, 76]. Moderately positive effects of low-frequency (10–25 Hz) stimulation of the PPN on balance problems and falls have been described in some parkinsonian patients [77–82], but questions remain as to the exact position of the electrodes-and the degree and durability of improvement.
DBS of the caudal zona incerta region has been shown to be efficacious in essential tremor and other forms of tremor, especially those with a prominent proximal limb involvement, as well as Parkinson’s disease and possibly dystonia [83–89]. The experience with this target remains relatively limited at this time, but it has been speculated that it may offer benefits over other DBS procedures in terms of a smaller incidence of cognitive or emotional side effects [84, 90]. As in the case of the PPN target, the exact location of the most effective electrode position remains to be determined.
The mechanisms of action of DBS in Parkinson’s disease and other conditions remain controversial. When the procedure was first introduced, the clinical similarities between the effects of DBS and lesioning of the same target prompted the belief that DBS works similar to lesioning procedures, i.e., by inactivating the stimulated tissue, through mechanisms such as depolarization block, or the local release of inhibitory transmitters. However, electrophysiologic recording studies in primates and patients have demonstrated that DBS has multiple actions on cell bodies and fibers, both afferent and efferent, and that such actions may differ with distance from the stimulation lead [91]. Furthermore, stimulation parameters, such as the pulse duration, strongly determine which tissue elements (cell bodies or axons) are stimulated. Modeling studies have shown that STN-DBS may simultaneously inhibit cell bodies of STN neurons through activation of local GABA release from GPe afferents, while directly activating axons of nearby neurons [92]. Stimulation in the STN has been demonstrated to evoke complex excitatory and inhibitory effects in the GPi, one of the primary recipients of STN efferents, and may alter oscillatory resonance characteristics of the STN-GPi network. In addition, STN stimulation may have prominent effects on nearby pallidothalamic fibers, which may directly affect thalamic activities. Finally, recent electrophysiological and optogenetic studies in rodents have suggested that STN stimulation may influence cortical activity via antidromic activation of the hyperdirect cortico-subthalamic pathway [93, 94].
The consequences of electrical stimulation in STN or GPi on cortical activity have also been explored in patients with Parkinson’s disease [95–101]. STN-DBS was shown to normalize intracortical inhibitory mechanisms in transcranial magnetic stimulation studies [100, 102], and functional imaging studies have shown that STN-DBS induces widespread normalization of activity in frontal motor areas both at rest and with movement tasks [e.g., 103]. Several PET studies in the resting state have found that STN-DBS results in increased metabolic activity in the pallidum, thalamus and substantia nigra, and decreased metabolic activity in the pre-central motor fields [104, 105].
Although the effects of DBS are incompletely understood, it appears that a major mechanism whereby high frequency stimulation (and lesioning) works is to override or replace the abnormal basal ganglia output patterns with a basal ganglia output that is more tolerable in the downstream areas. Both, ablation and stimulation therapies may therefore lead to the removal of abnormal basal ganglia activity, acting as an ‘informational lesion’ [106]. A recent study of changes in neuronal activity recorded in the thalamus of monkeys undergoing DBS in the STN indicated that a measure of entropy was decreased in thalamic neurons, reflecting an increased signal to noise ratio [95].
Given its substantial cost and other logistical issues, it is fortunate, that DBS is only one of several neurosurgical treatments for Parkinson’s disease. While DBS is by far the most commonly used procedure in the United States and other developed countries, ablative procedures, such as pallidotomy or thalamotomy are still widely used in developing countries, where access, financial resources and trained personnel are lacking. An important advance in this field has been the recent demonstration that STN lesions are effective in the treatment of Parkinson’s disease [107–112]. The STN target was chosen based on the results of studies that explored the effects of lesions of the STN on parkinsonism in MPTP-treated primates [113, 114]. Although the development of persistent hemi-chorea in a small percentage of patients is a potential complication, the clinical results are generally similar between STN-DBS and STN lesions.
Another recent surgical approach to modulate the indirect pathway at the level of the STN is the use of viral transfection of portions of the STN with the gene for the GABA-synthesizing enzyme, glutamic acid decarboxylase. This approach was designed to change the balance of excitatory and inhibitory influences within the STN in favor of inhibition. Positive results of a small pilot study of this method were recently published in an industry-sponsored phase-II trial [115], showing that the viral transfection is safe and potentially beneficial. The documented effectiveness of this therapy was, however, inferior to that of STN-DBS or ablation.
4.2. Dystonia
In dystonia, the third most common movement disorder, movements are slowed and disrupted by co-contraction of agonist and antagonist muscles, and by recruitment of inappropriate muscle groups (overflow), leading to abnormal movements and postures. Dystonia is a heterogeneous disorder, including primary and secondary forms. The latter are cases in which dystonia is caused by focal brain damage (to regions such as the putamen, thalamus, cortex or cerebellum), exposure to drugs (e.g., neuroleptics), or by other factors. Dystonia is often seen as a component of other disorders such as Parkinson’s disease, Huntington’s disease, mitochondrial disorders, or metabolic disturbances. In adults, dystonia is most commonly an idiopathic focal disease, involving the neck (cervical dystonia), eye lids (blepharospasm), or vocal apparatus (spasmodic dysphonia), whereas in children and young adults, generalized inherited forms of dystonia are more common, often resulting from genetic diseases such as idiopathic torsion dystonia [DYT1, 116].
In terms of the basal ganglia involvement in this process, there is evidence suggesting that some forms of dystonia may result from disturbances in dopaminergic transmission [117]. For instance, dystonia may develop in normal individuals treated with dopamine-receptor blocking agents or delayed, as tardive dystonia, and can be a sign of other diseases with disturbed dopamine metabolism, such as Parkinson’s disease, DOPA-responsive Dystonia (DRD), or DYT1 [118–123]. Transient dystonia has also been described to occur in monkeys treated with the dopaminergic neurotoxin, MPTP [124–126].
Studies in primate models have shown that dystonia may be associated with a reduction of activity along the putamen-GPe connection, and increased inhibition of STN and GPi by GPe efferents [127, 128]. Pharmacologic studies have suggested that there is a relative increase in the activity of striatal neurons of the direct pathway over those that give rise to the indirect pathway in dystonia [129, 130]. In (partial) agreement with the animal studies, single-cell recording studies in patients undergoing functional neurosurgical treatments have shown that discharge rates in GPe and GPi are low [131–136]. Other studies have shown the emergence of low-frequency oscillations in single-cell and local field potential activities in the basal ganglia or thalamus [134, 135, 137–139]. As is the case with Parkinson’s disease, the relative importance of the rate and pattern changes in the basal ganglia is not clear.
Although, primary dystonia is regarded as a disorder of the basal ganglia and its connections, recent evidence in animal and human studies has implicated an involvement of the cerebellum in addition to pathways through the basal ganglia [140–142]. Thus, recent neuroimaging studies have suggested that some forms of primary dystonia involve both the basal ganglia-thalamocortical and cerebellum-thalamocortical pathways [143, 144]. In the autosomal dominant DYT1 and DYT6 dystonias, which both have reduced penetrance (approximately 30% penetrance in DYT1 and 60% in DYT6), there is imaging evidence that in “manifesting” and “non-manifesting” gene carriers the connectivity in the cerebellum is disturbed. The lowered penetrance in DYT1 and DYT 6 carriers appears to result from an additional abnormality in thalamocortical projections which may be protective in these cases [143–145]. The same type of disturbance that has been shown in human carriers of DYT1 dystonia has also been shown in a knock-in rodent model of the disease [146]. The structural details corresponding to these imaging finding have not yet been worked out.
Interplay between cerebellar and basal ganglia circuits in the pathophysiology of dystonia is also suggested by the results of a recent study in an animal model of rapid onset dystonia-parkinsonism [RDP, 142]. RDP is a hereditary disorder caused by a mutation in the gene for the alpha-3 isoform of the Na+/K+ ATPase pump. Patients with this disorder are (relatively) asymptomatic for years but then rapidly develop persistent dystonia and parkinsonism after a period of stress. Calderon et al. [142] showed that infusions of ouabain, a blocker of the mutated isoform of the Na+/K+ ATPase, into the cerebellum produced dystonic posturing in mice. Lesioning the centrolateral thalamic nucleus (CL) in these animals prevented the cerebellar-induced dystonia. Because CL is known to be a way station of a disynaptic pathway linking cerebellum and basal ganglia in mice [33], this suggests that the cerebellar infusions produced their dystonic effect via a trans-basal ganglia pathway. The dystonic effects of the cerebellar ouabain infusions were seen even in animals whose motor cortex was (partially) disabled by tetrodotoxin infusions. Together, these results suggest that the dystonic posturing was mediated by a transthalamic pathway linking cerebellum and the contralateral basal ganglia, and then involved descending basal ganglia projections.
The insight that some forms of dystonia may arise from abnormalities in the basal ganglia and the past experience with ablative basal ganglia procedures in dystonia patients has lead to several trials of GPi-DBS in cases of advanced intractable dystonia. GPi-DBS was found to work well, specifically in cases of primary generalized dystonia [147–149], but to be less effective for the secondary dystonias [e.g., 150, 151]. Other indications for GPi-DBS are treatment resistant severe cervical and other focal dystonias and tardive dystonia [147, 152–156]. DBS for primary dystonia has been shown to have lasting benefit [157] and to improve the quality of life [158–161]. GPi-DBS for dystonia has few (if any) cognitive side effects [162].
Many forms of dystonia may be associated with a decrease in cortical inhibition and abnormal sensorimotor integration and changes in neuroplasticity [reviewed in 163, 164, 165]. These features may be altered by GPi-DBS. Thus GPi-DBS has been shown in PET activation studies to normalize motor cortical activity in dystonia [166], and to enhance motor cortex excitability (without changing intracortical inhibition) through modulation of thalamocortical projections [144, 167]. The possibility that GPi-DBS may also affect brain plasticity is suggested by the observation that the beneficial effects of GPi-DBS in dystonia are often delayed by weeks or months [168]. Recent transcortical magnetic stimulation of the effects of pallidal DBS [169] have shown that, prior to the onset of DBS, inhibition was reduced and plasticity increased in dystonic patients (compared with controls). During the course of DBS, short-latency intracortical inhibition gradually increased toward normal levels in parallel with the patients' clinical benefit. A measure of synaptic plasticity was found to be absent 1 month after initiation of DBS, and gradually increased over the following months toward levels observed in healthy individuals. These observations support the notion that a normalization of plasticity may be a key determinant of long-term therapeutic effects of DBS in dystonia.
DBS at other locations, particularly the STN, has also recently been explored [170–176]. Compared to GPi-DBS, STN-DBS may offer the advantage of more rapid improvement for dystonia, and may require lower stimulation voltages, and thus lower energy consumption.
Although the thalamic ventral intermediate nucleus was a favored target for ablative treatments for dystonia in the past, thalamic DBS is rarely done, for reasons that are not entirely clear. Exceptions to this statement are writer’s cramp, a focal occupational form of hand dystonia that appears to be uniquely sensitive to thalamotomy or thalamic DBS [177, 178], and myoclonus-dystonia syndrome in which addition of thalamic DBS to GPi-DBS may help patients who are refractory to GPi-DBS alone [179, 180].
Although early improvement (days to weeks) after programming is sometimes seen with pain and mobile dystonia [181–183], postoperative programming for dystonia has proven to be challenging, because benefits may take weeks to become evident and 6–12 months to reach peak. Also, in the past, higher voltages and wider pulse widths were used for GPi-DBS in dystonia than used for the treatment of Parkinson’s disease, resulting in a shortening of battery life. Currently, stimulation parameters are chosen that are similar to those used for patients with Parkinson’s disease [184, 185]. It has been shown that stimulation with lower frequencies (60 Hz) may be effective in primary generalized dystonia [186–188].
5. Neuropsychiatric Disorders
5.1. General aspects
Given the success of DBS for movement disorders, DBS is now also being explored as a treatment for patients with severe, medication-resistant psychiatric disorders, such as OCD, major depression and TS. DBS for TS, a disorder with combined neurologic and psychiatric symptoms and signs, is discussed together with the two other disorders, because the psychiatric “comorbidities” in TS often comprise a large part of the burden of the disorder.
DBS has been explored for these disorders for several reasons, including failure of conventional drug treatments in some of the most severely affected individuals, a greater understanding of the disordered neuronal networks in these disorders, and the aforementioned advantages of DBS treatment (in comparison with the previously used ablative approaches). In most cases, the targets discovered empirically for ablative studies have also been utilized for DBS. However, the selection of DBS targets for these disorders is best understood within the framework of the concept that abnormalities in segregated basal ganglia circuits result in circuit-specific disease manifestations. Neuropsychiatric disorders appear to reflect disturbances of neural activity within the associative and/or limbic basal ganglia circuits [see above, figure 1, and ref. 189, 190, 191].
It should be emphasized that, in distinction to the use of DBS in movement disorders, the use of DBS for neuropsychiatric conditions remains strictly experimental, because of technical and ethical considerations. The public distrust generated by the past excesses of the psychosurgery era of the 1950’s and 60’s and the unique vulnerability of patients with psychiatric disorders creates an ethical mandate to establish and sustain the public understanding and trust in techniques such as DBS, that target mood, motivation, and behavior at the highest level.
In this context it is perhaps important to mention that the FDA has recently granted a Humanitarian Device Exemption (HDE) for the use of DBS in patients with OCD. The HDE allows manufactures, under certain conditions, to market a device without requiring normal safety and efficacy data based on large blinded placebo-controlled clinical trials. The HDE process was established to encourage the development of devices for treatment of uncommon conditions, provides substantial financial benefits to device manufacturers and gives patients and physicians access to the technique without requiring rigorous demonstration of the device’s safety and effectiveness for a specific disorder. In such cases safety is assumed, based on the experience with the use of DBS in other disorders with a different target. The use of the HDE for OCD and other disorders has been criticized on the grounds that it may put patients at risk by bypassing rigorous clinical testing prior to wide-spread use, and it has been argued that OCD is not a rare disease, as discussed below.
Most importantly, it is possible that DBS of nodes of the limbic circuitry has outcomes and side effects different from those expected from circuit analysis alone. Thus, it is well recognized that psychiatric disorders differ fundamentally from movement disorders in that the patient’s behavior, as well as social and family interactions have often been disturbed for a long period of time, and any reversal of mood or behavior may require significant postoperative psychiatric rehabilitation and take a longer period of time before clear improvements in symptom severity, functioning and quality of life occur. This also means that the adverse effects of undetected DBS failure are potentially far more severe in patients with psychiatric conditions than in patients with movement disorders, because of the risk of mood changes and potential suicide attempts. Such beneficial or detrimental effects can only be determined by careful long term controlled clinical trials [192].
5.2 Tourette’s syndrome
TS is a familial, neurologic disorder characterized by the childhood onset of motor and vocal tics which typically peak in preadolescence and decline in the later teenage years. Motor tics are rapid, stereotyped movements such as eye blinking, head and orofacial movements, vocalizations such as throat clearing, coughing, or grunting, or complex behavioral acts and utterances, including coprolalia. As discussed, a high percentage of TS patients also suffer from disabling psychiatric comorbidities including OCD, attention-deficit hyperactivity disorder, depression and psychosocial difficulties. Based on neuroimaging and other studies [193], TS may involve abnormalities in both, the limbic and motor circuitry, accounting for the complex constellation of motor and psychiatric symptoms.
In a small proportion of TS patients, severe symptoms persist into adulthood. Although the treatment of tics with neuroleptics and other drugs and behavioral therapy is often helpful, weight gain and the risk of inducing tardive dyskinesia with neuroleptics are significant factors for patients. Most patients improve over time, but some continue to experience severe symptoms. Over the past decade, the use of DBS has been explored as treatment in such patients in small pilot studies [191, 194–196]. Several targets have been used, including the medial thalamus, in the region of the CM, the substantia periventricularis, and the nucleus ventro-oralis internus, [197–205], based on earlier lesioning studies by Hassler and Dieckmann [206–208]. In a large study including 18 cases of treatment refractory TS who underwent bilateral DBS in the region of the CM/Pf, Servello et al. reported that tics, OCD, self-injurious behaviors, and anxiety decreased with DBS [199]. The same group subsequently published the 2-year outcome for 15 of these patients, demonstrating a 53% improvement in tic severity and sustained improvement in compulsions, anxiety, depression and quality of life [209]. A recent double-blind study confirmed that DBS at the thalamic target lowers the severity of tics, but reported a relative high incidence of adverse effects (including a reduced sense of energy in all treated patients) [210].
Other surgical targets to treat TS include the motor and limbic portions of GPi [197, 211, 212], and the anterior limb of the internal capsule, close to the ventral striatum [213–215]. The rationale for using GPi-DBS in TS is that stimulation of the sensorimotor territory of GPi was shown to ameliorate hyperkinetic states [211, 212]. Reports of a small number of patients with electrodes placed simultaneously in the (motor) pallidal target and the medial thalamus suggest that comparable effects can be obtained with stimulation of either site, with no additional benefit from simultaneous stimulation [212].
Although persistent serious adverse effects are rare in TS patients treated with DBS, surgery-related complications (e.g., bleeding, infection) as well as stimulation-related side effects, such as sedation, anxiety, and altered mood have been seen. The high prevalence of psychiatric comorbidities presents an additional specific challenge for the implementation of DBS in patients with TS. There is general agreement that DBS should only be used in adult, treatment resistant, and severely affected patients, preferably in the context of controlled trials [194–196, 216]. DBS for TS remains experimental and should only be carried out as part of studies in clinical research centers with the resources to conduct such trials with institutional review board approval.
5.3 Obsessive Compulsive Disorder
OCD is a relatively common disorder, affecting up to 2% of the population, and is characterized by the presence of intrusive and recurrent thoughts (obsessions), compulsive behaviors and rituals that can severely disrupt behavior. Although medications and behavioral therapies are effective in many patients, approximately 10% have refractory symptoms and may be considered for surgical treatments [217]. Neurosurgical treatments of OCD have been carried out for many years with lesions of empirically defined targets, e.g., the cingulate gyrus and the anterior limb of the internal capsule. Lesions such as anterior capsulotomy may, indeed, benefit a significant proportion (35–70%) of patients. However, the destructive character and irreversibility of lesions are not acceptable to many patients [see, e.g., ref. 218].
Functional imaging studies in OCD patients have demonstrated abnormalities in the activity of limbic basal ganglia–thalamocortical projection systems, including the orbitofrontal cortex and the anterior cingulate cortex, the caudate nucleus and the thalamus [219]. Consequently, a number of different DBS targets within the limbic loop have been examined. The anterior limb of the internal capsule is one of these targets. In general, the benefits of DBS of this area are seen in approximately two thirds of OCD patients, and are long-lasting [218, 220, 221]. A large multi-center study by Greenberg et al. [221] identified the junction of the anterior capsule, anterior commissure and posterior ventral striatum as the most effective DBS target within this region. In this study, the reported adverse events included forgetfulness (31% of patients) and word-finding difficulties (19%).
This brain region receives afferents from limbic areas, such as the amygdala, the orbitofrontal cortex and the medial prefrontal cortex, and sends efferents to mesolimbic and prefrontal areas, and to the cingulate cortex, striatum, pallidum, and thalamus [222–224]. A DBS lead spanning this region is capable of activating any of these structures, depending on the lead geometry and stimulation parameters used. Imaging studies have, in fact, shown that DBS of the anterior limb of the internal capsule in OCD patients influences the activity in the nearby limbic ventral striatum [e.g., 214], which may then act to increase brain activity predominately in limbic areas of cortex, basal ganglia and thalamus [225]. Involvement of the ventral striatum in the beneficial effects is also supported by the observation that direct stimulation of this region can have comparable long lasting benefits in OCD [226].
The nucleus accumbens, a subregion of the ventral striatum, has been specifically targeted in a double-blind cross-over study [218]. In its blinded phase, this study found a 25% improvement in OCD symptoms, as well as significant reductions in depression and anxiety. Interestingly, depression improved within seconds of stimulation, while anxiety responded in minutes, obsessions in days and compulsions in months. Other than mild forgetfulness and word finding problems, there were no permanent adverse effects.
Based on studies indicating that inadvertent stimulation of the medial (limbic) portion of the STN in patients with Parkinson’s disease with co-existent OCD can reduce obsessive-compulsive symptoms [227, 228], the medial portion of the STN has recently been targeted for OCD treatment [229, 230]. According to imaging studies, medial STN stimulation alters prefrontal cortical activity [231]. In a recent study of 8 patients in a 10 month, crossover, double-blind study of STN-DBS for OCD, a positive outcome was observed for compulsions, while depression and anxiety did not respond [230]. The authors reported a relatively high rate of serious adverse events, including one intracerebral hemorrhage and two infections.
5.4. Treatment-resistant depression
Major depression is one of the most severe and prevalent neuropsychiatric disorders and the most common cause of disability, with a prevalence of about 5% [232]. Up to 30% of patients with chronic major depression fail to respond to traditional antidepressants, behavioral therapy, or other established treatments such as vagal nerve stimulation or electroconvulsive therapy [233]. A failure to respond to these conventional treatments constitutes TRD.
Clues to the potential for DBS of the basal ganglia and related structures to modulate mood and behavior have come from observations in a small number of parkinsonian patients in whom severe, but reversible, depression was reported in response to STN-DBS [66, 69]. In these cases the cause of the mood change appeared to be inadvertent stimulation of the underlying ‘limbic’ SNr. Other effects such as hypomania, merriment, and laughter have been reported with stimulation in the STN, GPi, and in the zona incerta. Although largely anecdotal, these findings indicate that focal stimulation of portions of the basal ganglia circuitry can profoundly alter mood and emotional expression, and suggested that DBS of nodes of the limbic circuitry could potentially be used to modulate mood.
The knowledge gained from imaging studies in patients with severe depression [234–238] has recently led to the identification of the cingulate cortical area 25 as a potential target for DBS treatment (figure 2A). Positron emission tomography (PET) studies showed that clinical improvements of depression coincided with a decrease in activity in this area [236, 239–241]. In a trial of stimulation in area 25 in patients with TRD, significant clinical benefits were seen, with response rates of up to 75% three years after implantation, as measured by changes in the Hamilton Depression Rating Scale [242, 243]. Progressive improvements in physical health and social functioning were also seen. DBS was associated with specific changes in the metabolic activity localized to cortical and limbic circuits implicated in the pathogenesis of depression. While no significant direct adverse events were reported, two patients committed suicide.
Figure 2.
DBS targets for treatment of severe TRD. A. Target in the sugbgenual cortical area 25, as identified in depression patients with induction of sadness accompanying the recollection of a sad memory, using PET to measure cerebral blood flow changes. Areas in red represent a significant increase in blood flow, while areas in blue show decreased blood flow. B. MRI image showing the DBS lead position in a TRD patient with DBS of the anterior limb of the internal capsule/ventral striatum target. C. Sagittal view of fluoro-deoxyglucose positron emission tomography (FDG-PET) imaging of the metabolic effects of DBS in the lateral habenula for treatment of TRD. The position of the electrode tip position is marked in green. This figure is a compilation of previously published images. The image under A. was published in ref. [237], the one under B. by ref. [247], and the image under C. stems from ref. [245]. All images were used with permission.
As was the case for DBS for movement disorders, other DBS targets for depression were chosen based on those previously identified empirically for ablation (figure 2B/C). These include the anterior limb of the internal capsule/ventral striatum, the nucleus accumbens (see below), as well as the inferior thalamic peduncle [244] and the lateral habenula [245]. The anterior limb of the internal capsule/ventral striatum target also grew out of clues from earlier ablation and DBS studies of this target for treatment of OCD (see section 5.3), which showed improvement in depression, independent of the effects on OCD. In a recent study of DBS of this target in TRD patients, Malone and colleagues reported a reduction of depression scores by 44% and 57% in 15 patients who were evaluated at 1 and 2-years post-operatively, respectively [246, 247], with 40% achieving remission. Similar findings were reported in 10 patients with DBS of the nucleus accumbens which resulted in a 36% mean reduction of depression scores, with over half of the patients achieving a reduction of 50%, and a 30% remission rate [248]. According to 2-deoxy-glucose PET data, DBS of the nucleus accumbens results in decreased metabolism in the subgenual cingulate gyrus and in prefrontal regions, including the orbital prefrontal cortex. Stimulation in this area led to a significant number of reversible complications and side effects, including pain, sweating, paresthesias, agitation, hypomania and psychotic symptoms. One patient committed and another attempted suicide.
It seems that the side effects of DBS for TRD are generally similar to those of DBS in movement disorder cases. Overall, stimulation side effects may be less frequent with the area 25 target than with the ventral striatal region target, but it is premature to judge which of the different targets might be best for depression, given the preliminary nature of the studies. A large placebo response seems unlikely in patients undergoing DBS for TRD, in light of the fact that the patients treated with DBS have a long history of failing other types of treatment, show sustained clinical DBS responses over years, need specific stimulation parameters for maximal benefit, and a loose clinical benefits following cessation or inadvertent device malfunction. Nonetheless, a careful double-blind appraisal of the effects and side effects is required for FDA approval, and needs to be done before the procedures can be recommended for use on a wider scale.
Other areas of future research in the area of the use of DBS in depression should be the optimization of stimulation parameters and electrode configurations, the study of the nature and duration of remissions and long-term outcomes, and the identification of optimal stimulation targets. We also need to determine what the critical structures in the target are that are being stimulated. For instance, while the area 25 target was selected for stimulation because of changes in the metabolic activity of this region, the DBS benefits may result from stimulation of subcortical white matter tracts, which connect to multiple other cortical and subcortical areas. It is possible that DBS at multiple nodes of the circuitry may eventually prove more effective than single-site stimulation, and that some sites may prove more appropriate for certain types of patients and clinical features.
6. Future Directions for DBS
DBS has been utilized for movement disorders and neuropsychiatric disorders for nearly two decades, but the technology has remained largely unchanged. In part, the slow technical progress may reflect the overall success of the currently available technique. However, rechargeable batteries, programming devices with closed loop capabilities are now in development or becoming available. Future improvements will also be achieved with: 1) better techniques of optimizing electrode placement, such as the use of high-field strength MRI for pre-operative localization [249] and improved electrophysiologic recording techniques [250], 2) the use of different electrode designs which may help to sculpt the electrical field, 3) more versatile programming devices with greater control of stimulation patterns, and 4) improved clinical programming techniques or algorithms.
Furthermore, a better understanding of the pathophysiologic basis of the different disorders and the effects of DBS on the networks, utilizing neuroimaging and neurophysiologic approaches, may help us to identify new targets for these disorders. For instance, the aforementioned studies suggesting that cerebellar dysfunction may contribute to the manifestations of so-called basal ganglia disorders may lead not only to new pathophysiologic models of these diseases, but may also provide us with new DBS targets to help patients with these disorders.
7. Conclusions
Since its introduction to the treatment of tremor and Parkinson’s disease almost 20 years ago, DBS has become an accepted treatment for movement disorders, and has been used in over 70,000 patients. These successes have encouraged clinicians to explore the use of DBS in many other neurologic and psychiatric disorders. The clear therapeutic effects of these procedures, directed at multiple nodes of the motor and limbic circuits supports the notion that movement disorders result from disturbances within the motor circuit, while neuropsychiatric disorders such as OCD, and TRD may result from disturbances involving the limbic basal ganglia-thalamocortical circuitry. Some disorders such as Parkinson’s and TS involve dysfunction of more than one of the circuits. The seemingly indiscriminate effectiveness of electrical stimulation across a wide spectrum of disorders with greatly varying phenotypes and underlying pathophysiologies argues against disease-specific effects, and in favor of circuit selective effects. It is likely that DBS primarily acts to override and replace abnormal subcortical or cortical signals, whatever their distinctive features may be, allowing the otherwise relatively intact systems to function more normally.
DBS is not without risks, primarily related to the surgery itself. The extension of DBS to psychiatric disorders presents unique scientific, clinical, ethical and regulatory challenges. The unique vulnerabilities of patients with psychiatric disorders call for extraordinary care and caution in the extension of DBS to patients suffering from some of the most disruptive disorders affecting mankind, both for the sake of individuals and the realization of the enormous potential of this highly innovative technique.
Acknowledgements
The preparation of this article was supported through grants from the NIH/NINDS (R01-NS054976, R01-NS071074 and P50-NS071669 (TW)), and by NIH/NCRR grant RR-000165 (Yerkes National Primate Center).
Abbreviations
- ADL
Activities of daily living
- CM
centromedian nucleus of the thalamus
- CMA
cingulate motor area
- DBS
deep brain stimulation
- DRD
DOPA-responsive Dystonia
- DYT1
idiopathic torsion dystonia
- GPe
external segment of the globus pallidus
- GPi
internal pallidal segment
- HDE
Humanitarian Device Exemption
- M1
primary motor cortex
- MD
mediodorsal nucleus of thalamus
- OCD
obsessive compulsive disorder
- PET
Positron emission tomography
- Pf
parafascicular nucleus
- PMC
premotor cortex
- PPN
pedunculopontine nucleus
- RDP
rapid onset dystonia dystonia-parkinsonism
- SMA
supplementary motor area
- SNc
substantia nigra pars compacta
- SNr
substantia nigra pars reticulata
- STN
subthalamic nucleus
- TRD
treatment-resistant depression
- TS
Tourette syndrome
- UPDRS
United Parkinson’s disease rating scale
- VA
ventral anterior nucleus of the thalamus
- VL
ventrolateral nucleus of the thalamus
- VS
ventral striatum
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 2.Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neuroscience. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- 3.Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, `prefrontal' and `limbic' functions. Prog. Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- 4.Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamocortical loop. Brain Res Brain Res Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- 5.Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Rev. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- 6.Parent A. Extrinsic connections of the basal ganglia. Trends Neurosci. 1990;13:254–258. doi: 10.1016/0166-2236(90)90105-j. [DOI] [PubMed] [Google Scholar]
- 7.Haber SN, Kunishio K, Mizobuchi M, Lynd-Balta E. The orbital and medial prefrontal circuit through the primate basal ganglia. J. Neurosci. 1995;15:4851–4867. doi: 10.1523/JNEUROSCI.15-07-04851.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoover JE, Strick PL. Multiple output channels in the basal ganglia. Science. 1993;259:819–821. doi: 10.1126/science.7679223. [DOI] [PubMed] [Google Scholar]
- 9.Turner RS, Grafton ST, Votaw JR, Delong MR, Hoffman JM. Motor subcircuits mediating the control of movement velocity: a PET study. J. Neurophysiol. 1998;80:2162–2176. doi: 10.1152/jn.1998.80.4.2162. [DOI] [PubMed] [Google Scholar]
- 10.Schell GR, Strick PL. The origin of thalamic inputs to the arcuate premotor and supplementary motor areas. J. Neurosci. 1984;4:539–560. doi: 10.1523/JNEUROSCI.04-02-00539.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inase M, Tanji J. Thalamic distribution of projection neurons to the primary motor cortex relative to afferent terminal fields from the globus pallidus in the macaque monkey. J. Comp. Neurol. 1995;353:415–426. doi: 10.1002/cne.903530309. [DOI] [PubMed] [Google Scholar]
- 12.Shink E, Bevan MD, Bolam JP, Smith Y. The subthalamic nucleus and the external pallidum: two tightly interconnected structures that control the output of the basal ganglia in the monkey. Neurosci. 1996;73:335–357. doi: 10.1016/0306-4522(96)00022-x. [DOI] [PubMed] [Google Scholar]
- 13.Smith Y, Bevan MD, Shink E, Bolam JP. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neurosci. 1998;86:353–387. doi: 10.1016/s0306-4522(98)00004-9. [DOI] [PubMed] [Google Scholar]
- 14.Nambu A, Tokuno H, Takada M. Functional significance of the cortico-subthalamo-pallidal 'hyperdirect' pathway. Neurosci. Res. 2002;43:111–117. doi: 10.1016/s0168-0102(02)00027-5. [DOI] [PubMed] [Google Scholar]
- 15.Hartmann-von Monakow K, Akert K, Kunzle H. Projections of the precentral motor cortex and other cortical areas of the frontal lobe to the subthalamic nucleus in the monkey. Exp. Brain Res. 1978;33:395–403. doi: 10.1007/BF00235561. [DOI] [PubMed] [Google Scholar]
- 16.Nambu A, Tokuno H, Hamada I, Kita H, Imanishi M, Akazawa T, et al. Excitatory cortical inputs to pallidal neurons via the subthalamic nucleus in the monkey. J. Neurophysiol. 2000;84:289–300. doi: 10.1152/jn.2000.84.1.289. [DOI] [PubMed] [Google Scholar]
- 17.Smith Y, Raju DV, Pare JF, Sidibe M. The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends Neurosci. 2004;27:520–527. doi: 10.1016/j.tins.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Sidibe M, Bevan MD, Bolam JP, Smith Y. Efferent connections of the internal globus pallidus in the squirrel monkey: I. Topography and synaptic organization of the pallidothalamic projection. J. Comp. Neurol. 1997;382:323–347. [PubMed] [Google Scholar]
- 19.Nanda B, Galvan A, Smith Y, Wichmann T. Effects of stimulation of the centromedian nucleus of the thalamus on the activity of striatal cells in awake rhesus monkeys. Eur. J. Neurosci. 2009;29:588–598. doi: 10.1111/j.1460-9568.2008.06598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith Y, Parent A. Differential connections of caudate nucleus and putamen in the squirrel monkey (Saimiri sciureus) Neurosci. 1986;18:347–371. doi: 10.1016/0306-4522(86)90159-4. [DOI] [PubMed] [Google Scholar]
- 21.Sadikot AF, Parent A, Francois C. Efferent connections of the centromedian and parafascicular thalamic nuclei in the squirrel monkey: a PHA-L study of subcortical projections. J. Comp. Neurol. 1992;315:137–159. doi: 10.1002/cne.903150203. [DOI] [PubMed] [Google Scholar]
- 22.Minamimoto T, Hori Y, Kimura M. Complementary process to response bias in the centromedian nucleus of the thalamus. Science. 2005;308:1798–1801. doi: 10.1126/science.1109154. [DOI] [PubMed] [Google Scholar]
- 23.Minamimoto T, Hori Y, Kimura M. Roles of the thalamic CM-PF complex-Basal ganglia circuit in externally driven rebias of action. Brain Res Bull. 2009;78:75–79. doi: 10.1016/j.brainresbull.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 24.Harnois C, Filion M. Pallidofugal projections to thalamus and midbrain: a quantitative antidromic activation study in monkeys and cats. Exp. Brain Res. 1982;47:277–285. doi: 10.1007/BF00239387. [DOI] [PubMed] [Google Scholar]
- 25.Rye DB, Lee HJ, Saper CB, Wainer BH. Medullary and spinal efferents of the pedunculopontine tegmental nucleus and adjacent mesopontine tegmentum in the rat. J. Comp. Neurol. 1988;269:315–341. doi: 10.1002/cne.902690302. [DOI] [PubMed] [Google Scholar]
- 26.Mena-Segovia J, Bolam JP, Magill PJ. Pedunculopontine nucleus and basal ganglia: distant relatives or part of the same family? Trends Neurosci. 2004;27:585–588. doi: 10.1016/j.tins.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 27.May PJ, McHaffie JG, Stanford TR, Jiang H, Costello MG, Coizet V, et al. Tectonigral projections in the primate: a pathway for pre-attentive sensory input to midbrain dopaminergic neurons. Eur. J. Neurosci. 2009;29:575–587. doi: 10.1111/j.1460-9568.2008.06596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu P, Basso MA. Substantia nigra stimulation influences monkey superior colliculus neuronal activity bilaterally. J. Neurophysiol. 2008;100:1098–1112. doi: 10.1152/jn.01043.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaneda K, Isa K, Yanagawa Y, Isa T. Nigral inhibition of GABAergic neurons in mouse superior colliculus. J. Neurosci. 2008;28:11071–11078. doi: 10.1523/JNEUROSCI.3263-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hikosaka O. Basal ganglia mechanisms of reward-oriented eye movement. Ann N Y Acad Sci. 2007;1104:229–249. doi: 10.1196/annals.1390.012. [DOI] [PubMed] [Google Scholar]
- 31.Hoshi E, Tremblay L, Feger J, Carras PL, Strick PL. The cerebellum communicates with the basal ganglia. Nature NeuroScience. 2005;8:1491–1493. doi: 10.1038/nn1544. [DOI] [PubMed] [Google Scholar]
- 32.Bostan AC, Dum RP, Strick PL. The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci U S A. 2010;107:8452–8456. doi: 10.1073/pnas.1000496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ichinohe N, Mori F, Shoumura K. A di-synaptic projection from the lateral cerebellar nucleus to the laterodorsal part of the striatum via the central lateral nucleus of the thalamus in the rat. Brain Res. 2000;880:191–197. doi: 10.1016/s0006-8993(00)02744-x. [DOI] [PubMed] [Google Scholar]
- 34.Akkal D, Dum RP, Strick PL. Supplementary motor area and presupplementary motor area: targets of basal ganglia and cerebellar output. J. Neurosci. 2007;27:10659–10673. doi: 10.1523/JNEUROSCI.3134-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiology of Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 36.Lim SY, Lang AE. The nonmotor symptoms of Parkinson's disease--an overview. Mov Disord. 2010;25(Suppl 1):S123–S130. doi: 10.1002/mds.22786. [DOI] [PubMed] [Google Scholar]
- 37.Crossman AR, Mitchell IJ, Sambrook MA. Regional brain uptake of 2-deoxyglucose in N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism in the macaque monkey. Neuropharmacology. 1985;24:587–591. doi: 10.1016/0028-3908(85)90070-x. [DOI] [PubMed] [Google Scholar]
- 38.Schwartzman RJ, Alexander GM. Changes in the local cerebral metabolic rate for glucose in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) primate model of Parkinson's disease. Brain Res. 1985;358:137–143. doi: 10.1016/0006-8993(85)90957-6. [DOI] [PubMed] [Google Scholar]
- 39.Filion M, Tremblay L, Bedard PJ. Abnormal influences of passive limb movement on the activity of globus pallidus neurons in parkinsonian monkeys. Brain Res. 1988;444:165–176. doi: 10.1016/0006-8993(88)90924-9. [DOI] [PubMed] [Google Scholar]
- 40.Miller WC, DeLong MR. Altered tonic activity of neurons in the globus pallidus and subthalamic nucleus in the primate MPTP model of parkinsonis. In: Carpenter MB, Jayaraman A, editors. The Basal Ganglia II. New York: Plenum Press; 1987. pp. 415–427. [Google Scholar]
- 41.Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J. Neurophysiol. 1994;72:507–520. doi: 10.1152/jn.1994.72.2.507. [DOI] [PubMed] [Google Scholar]
- 42.Galvan A, Wichmann T. Pathophysiology of parkinsonism. Clin. Neurophysiol. 2008;119:1459–1474. doi: 10.1016/j.clinph.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dogali M, Beric A, Sterio D, Eidelberg D, Fazzini E, Takikawa S, et al. Anatomic and physiological considerations in pallidotomy for Parkinson's disease. Stereotact Funct Neurosurg. 1994;62:53–60. doi: 10.1159/000098597. [DOI] [PubMed] [Google Scholar]
- 44.Lozano A, Hutchison W, Kiss Z, Tasker R, Davis K, Dostrovsky J. Methods for microelectrode-guided posteroventral pallidotomy. J. Neurosurg. 1996;84:194–202. doi: 10.3171/jns.1996.84.2.0194. [DOI] [PubMed] [Google Scholar]
- 45.Vitek JL, Kaneoke Y, Turner R, Baron M, Bakay R, DeLong M. Neuronal activity in the internal (GPi) and external (GPe) segments of the globus pallidus (GP) of parkinsonian patients is similar to that in the MPTP-treated primate model of parkinsonism. Soc.Neurosci.Abstr. 1993;19:1584. [Google Scholar]
- 46.Filion M, Tremblay L. Abnormal spontaneous activity of globus pallidus neurons in monkeys with MPTP-induced parkinsonism. Brain Res. 1991;547:142–151. [PubMed] [Google Scholar]
- 47.Hammond C, Bergman H, Brown P. Pathological synchronization in Parkinson's disease: networks, models and treatments. Trends Neurosci. 2007;30:357–364. doi: 10.1016/j.tins.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 48.Wichmann T, DeLong MR. Basal ganglia discharge abnormalities in Parkinson's disease. J Neural Transm. 2006;(Suppl):21–25. doi: 10.1007/978-3-211-45295-0_5. [DOI] [PubMed] [Google Scholar]
- 49.Gatev P, Darbin O, Wichmann T. Oscillations in the basal ganglia under normal conditions and in movement disorders. Mov Disord. 2006;21:1566–1577. doi: 10.1002/mds.21033. [DOI] [PubMed] [Google Scholar]
- 50.Weinberger M, Hutchison WD, Dostrovsky JO. Pathological subthalamic nucleus oscillations in PD: can they be the cause of bradykinesia and akinesia? Exp. Neurol. 2009;219:58–61. doi: 10.1016/j.expneurol.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 51.Timmermann L, Fink GR. Modulating pathological oscillatory activity in Parkinson's disease: What's the rhythm? Exp. Neurol. 2009;215:209–211. doi: 10.1016/j.expneurol.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 52.Timmermann L, Florin E, Reck C. Pathological cerebral oscillatory activity in Parkinson's disease: a critical review on methods, data and hypotheses. Expert Rev Med Devices. 2007;4:651–661. doi: 10.1586/17434440.4.5.651. [DOI] [PubMed] [Google Scholar]
- 53.Eusebio A, Brown P. Oscillatory activity in the basal ganglia. Parkinsonism Relat Disord. 2007;13(Suppl 3):S434–S436. doi: 10.1016/S1353-8020(08)70044-0. [DOI] [PubMed] [Google Scholar]
- 54.Brown P. Abnormal oscillatory synchronisation in the motor system leads to impaired movement. Curr. Opin. Neurobiol. 2007;17:656–664. doi: 10.1016/j.conb.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 55.Brown P. Bad oscillations in Parkinson's disease. J Neural Transm Suppl. 2006:27–30. doi: 10.1007/978-3-211-45295-0_6. [DOI] [PubMed] [Google Scholar]
- 56.Wider C, Pollo C, Bloch J, Burkhard PR, Vingerhoets FJ. Long-term outcome of 50 consecutive Parkinson's disease patients treated with subthalamic deep brain stimulation. Parkinsonism Relat Disord. 2008;14:114–119. doi: 10.1016/j.parkreldis.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 57.Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schafer H, Botzel K, et al. A randomized trial of deep-brain stimulation for Parkinson's disease. N Engl J Med. 2006;355:896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- 58.Ostergaard K, Sunde NA. Evolution of Parkinson's disease during 4 years of bilateral deep brain stimulation of the subthalamic nucleus. Mov Disord. 2006;21:624–631. doi: 10.1002/mds.20776. [DOI] [PubMed] [Google Scholar]
- 59.Rodrigues JP, Walters SE, Watson P, Stell R, Mastaglia FL. Globus pallidus stimulation improves both motor and nonmotor aspects of quality of life in advanced Parkinson's disease. Mov Disord. 2007;22:1866–1870. doi: 10.1002/mds.21427. [DOI] [PubMed] [Google Scholar]
- 60.Volkmann J. Deep brain stimulation for the treatment of Parkinson's disease. J Clin Neurophysiol. 2004;21:6–17. doi: 10.1097/00004691-200401000-00003. [DOI] [PubMed] [Google Scholar]
- 61.Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ, Jr, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled tria. JAMA. 2009;301:63–73. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rodriguez-Oroz MC, Zamarbide I, Guridi J, Palmero MR, Obeso JA. Efficacy of deep brain stimulation of the subthalamic nucleus in Parkinson's disease 4 years after surgery: double blind and open label evaluation. J Neurol Neurosurg Psychiatry. 2004;75:1382–1385. doi: 10.1136/jnnp.2003.031294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parsons TD, Rogers SA, Braaten AJ, Woods SP, Troster AI. Cognitive sequelae of subthalamic nucleus deep brain stimulation in Parkinson's disease: a meta-analysis. Lancet Neurol. 2006;5:578–588. doi: 10.1016/S1474-4422(06)70475-6. [DOI] [PubMed] [Google Scholar]
- 64.Troster AI, Fields JA, Wilkinson SB, Pahwa R, Miyawaki E, Lyons KE, et al. Unilateral pallidal stimulation for Parkinson's disease: neurobehavioral functioning before and 3 months after electrode implantation. Neurology. 1997;49:1078–1083. doi: 10.1212/wnl.49.4.1078. [DOI] [PubMed] [Google Scholar]
- 65.Anderson VC, Burchiel KJ, Hogarth P, Favre J, Hammerstad JP. Pallidal vs subthalamic nucleus deep brain stimulation in Parkinson disease. Arch Neurol. 2005;62:554–560. doi: 10.1001/archneur.62.4.554. [DOI] [PubMed] [Google Scholar]
- 66.Temel Y, Kessels A, Tan S, Topdag A, Boon P, Visser-Vandewalle V. Behavioural changes after bilateral subthalamic stimulation in advanced Parkinson disease: a systematic review. Parkinsonism Relat Disord. 2006;12:265–272. doi: 10.1016/j.parkreldis.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 67.Voon V, Krack P, Lang AE, Lozano AM, Dujardin K, Schupbach M, et al. A multicentre study on suicide outcomes following subthalamic stimulation for Parkinson's disease. Brain. 2008;131:2720–2728. doi: 10.1093/brain/awn214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stefurak T, Mikulis D, Mayberg H, Lang AE, Hevenor S, Pahapill P, et al. Deep brain stimulation for Parkinson's disease dissociates mood and motor circuits: a functional MRI case study. Mov Disord. 2003;18:1508–1516. doi: 10.1002/mds.10593. [DOI] [PubMed] [Google Scholar]
- 69.Bejjani BP, Damier P, Arnulf I, Thivard L, Bonnet AM, Dormont D, et al. Transient acute depression induced by high-frequency deep-brain stimulation. N Engl J Med. 1999;340:1476–1480. doi: 10.1056/NEJM199905133401905. [DOI] [PubMed] [Google Scholar]
- 70.Bronstein JM, Tagliati M, Alterman RL, Lozano AM, Volkmann J, Stefani A, et al. Deep Brain Stimulation for Parkinson Disease: An Expert Consensus and Review of Key Issues. Arch Neurol. 2010 doi: 10.1001/archneurol.2010.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kumar R, Lozano AM, Sime E, Lang AE. Long-term follow-up of thalamic deep brain stimulation for essential and parkinsonian tremor. Neurology. 2003;61:1601–1604. doi: 10.1212/01.wnl.0000096012.07360.1c. [DOI] [PubMed] [Google Scholar]
- 72.Ondo W, Jankovic J, Schwartz K, Almaguer M, Simpson RK. Unilateral thalamic deep brain stimulation for refractory essential tremor and Parkinson's disease tremor. Neurology. 1998;51:1063–1069. doi: 10.1212/wnl.51.4.1063. [DOI] [PubMed] [Google Scholar]
- 73.Obeso JA, Rodriguez MC, Gorospe A, Guridi J, Alvarez L, Macias R. Surgical treatment of Parkinson's disease. Baillieres Clinical Neurology. 1997;6:125–145. [PubMed] [Google Scholar]
- 74.Nandi D, Liu X, Winter JL, Aziz TZ, Stein JF. Deep brain stimulation of the pedunculopontine region in the normal non-human primate. J Clin Neurosci. 2002;9:170–174. doi: 10.1054/jocn.2001.0943. [DOI] [PubMed] [Google Scholar]
- 75.Jahn K, Deutschlander A, Stephan T, Kalla R, Wiesmann M, Strupp M, et al. Imaging human supraspinal locomotor centers in brainstem and cerebellum. Neuroimage. 2008;39:786–792. doi: 10.1016/j.neuroimage.2007.09.047. [DOI] [PubMed] [Google Scholar]
- 76.Tsang EW, Hamani C, Moro E, Mazzella F, Poon YY, Lozano AM, et al. Involvement of the human pedunculopontine nucleus region in voluntary movements. Neurology. 2010;75:950–959. doi: 10.1212/WNL.0b013e3181f25b35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Plaha P, Gill SS. Bilateral deep brain stimulation of the pedunculopontine nucleus for Parkinson's disease. Neuroreport. 2005;16:1883–1887. doi: 10.1097/01.wnr.0000187637.20771.a0. [DOI] [PubMed] [Google Scholar]
- 78.Stefani A, Lozano AM, Peppe A, Stanzione P, Galati S, Tropepi D, et al. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson's disease. Brain. 2007;130:1596–1607. doi: 10.1093/brain/awl346. [DOI] [PubMed] [Google Scholar]
- 79.Lozano AM, Snyder BJ. Deep brain stimulation for parkinsonian gait disorders. J Neurol. 2008;255(Suppl 4):30–31. doi: 10.1007/s00415-008-4005-6. [DOI] [PubMed] [Google Scholar]
- 80.Moro E, Hamani C, Poon YY, Al-Khairallah T, Dostrovsky JO, Hutchison WD, et al. Unilateral pedunculopontine stimulation improves falls in Parkinson's disease. Brain. 2010;133:215–224. doi: 10.1093/brain/awp261. [DOI] [PubMed] [Google Scholar]
- 81.Ferraye MU, Debu B, Fraix V, Goetz L, Ardouin C, Yelnik J, et al. Effects of pedunculopontine nucleus area stimulation on gait disorders in Parkinson's disease. Brain. 2010;133:205–214. doi: 10.1093/brain/awp229. [DOI] [PubMed] [Google Scholar]
- 82.Peppe A, Pierantozzi M, Chiavalon C, Marchetti F, Caltagirone C, Musicco M, et al. Deep brain stimulation of the pedunculopontine tegmentum and subthalamic nucleus: effects on gait in Parkinson's disease. Gait Posture. 2010;32:512–518. doi: 10.1016/j.gaitpost.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 83.Blomstedt P, Fytagoridis A, Tisch S. Deep brain stimulation of the posterior subthalamic area in the treatment of tremor. Acta Neurochir.(Wien) 2009;151:31–36. doi: 10.1007/s00701-008-0163-7. [DOI] [PubMed] [Google Scholar]
- 84.Blomstedt P, Sandvik U, Fytagoridis A, Tisch S. The posterior subthalamic area in the treatment of movement disorders: past, present, and future. Neurosurgery. 2009;64:1029–1038. doi: 10.1227/01.NEU.0000345643.69486.BC. discussion 38-42. [DOI] [PubMed] [Google Scholar]
- 85.Kitagawa M, Murata J, Kikuchi S, Sawamura Y, Saito H, Sasaki H, et al. Deep brain stimulation of subthalamic area for severe proximal tremor. Neurology. 2000;55:114–116. doi: 10.1212/wnl.55.1.114. [DOI] [PubMed] [Google Scholar]
- 86.Murata J, Kitagawa M, Uesugi H, Saito H, Iwasaki Y, Kikuchi S, et al. Electrical stimulation of the posterior subthalamic area for the treatment of intractable proximal tremor. J. Neurosurg. 2003;99:708–715. doi: 10.3171/jns.2003.99.4.0708. [DOI] [PubMed] [Google Scholar]
- 87.Kitagawa M, Murata J, Uesugi H, Kikuchi S, Saito H, Tashiro K, et al. Two-year follow-up of chronic stimulation of the posterior subthalamic white matter for tremor-dominant Parkinson's disease. Neurosurgery. 2005;56:281–289. doi: 10.1227/01.neu.0000148167.49105.a3. discussion -9. [DOI] [PubMed] [Google Scholar]
- 88.Blomstedt P, Sandvik U, Tisch S. Deep brain stimulation in the posterior subthalamic area in the treatment of essential tremor. Mov Disord. 2010;25:1350–1356. doi: 10.1002/mds.22758. [DOI] [PubMed] [Google Scholar]
- 89.Plaha P, Ben-Shlomo Y, Patel NK, Gill SS. Stimulation of the caudal zona incerta is superior to stimulation of the subthalamic nucleus in improving contralateral parkinsonism. Brain. 2006;129:1732–1747. doi: 10.1093/brain/awl127. [DOI] [PubMed] [Google Scholar]
- 90.Fytagoridis A, Blomstedt P. Complications and side effects of deep brain stimulation in the posterior subthalamic area. Stereotact Funct Neurosurg. 2010;88:88–93. doi: 10.1159/000271824. [DOI] [PubMed] [Google Scholar]
- 91.Kringelbach ML, Jenkinson N, Owen SL, Aziz TZ. Translational principles of deep brain stimulation. Nat Rev Neurosci. 2007;8:623–635. doi: 10.1038/nrn2196. [DOI] [PubMed] [Google Scholar]
- 92.McIntyre CC, Savasta M, Walter BL, Vitek JL. How does deep brain stimulation work? Present understanding and future questions. Journal of Clinical Neurophysiology. 2004;21:40–50. doi: 10.1097/00004691-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 93.Li S, Arbuthnott GW, Jutras MJ, Goldberg JA, Jaeger D. Resonant antidromic cortical circuit activation as a consequence of high-frequency subthalamic deep-brain stimulation. J. Neurophysiol. 2007;98:3525–3537. doi: 10.1152/jn.00808.2007. [DOI] [PubMed] [Google Scholar]
- 94.Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324:354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guo Y, Rubin JE, McIntyre CC, Vitek JL, Terman D. Thalamocortical relay fidelity varies across subthalamic nucleus deep brain stimulation protocols in a data-driven computational model. J. Neurophysiol. 2008;99:1477–1492. doi: 10.1152/jn.01080.2007. [DOI] [PubMed] [Google Scholar]
- 96.Anderson ME, Postupna N, Ruffo M. Effects of high-frequency stimulation in the internal globus pallidus on the activity of thalamic neurons in the awake monkey. J. Neurophysiol. 2003;89:1150–1160. doi: 10.1152/jn.00475.2002. [DOI] [PubMed] [Google Scholar]
- 97.Kuriakose R, Saha U, Castillo G, Udupa K, Ni Z, Gunraj C, et al. The nature and time course of cortical activation following subthalamic stimulation in Parkinson's disease. Cereb Cortex. 2010;20:1926–1936. doi: 10.1093/cercor/bhp269. [DOI] [PubMed] [Google Scholar]
- 98.Dejean C, Hyland B, Arbuthnott G. Cortical effects of subthalamic stimulation correlate with behavioral recovery from dopamine antagonist induced akinesia. Cereb Cortex. 2009;19:1055–1063. doi: 10.1093/cercor/bhn149. [DOI] [PubMed] [Google Scholar]
- 99.Grill WM, Cantrell MB, Robertson MS. Antidromic propagation of action potentials in branched axons: implications for the mechanisms of action of deep brain stimulation. Journal of Computational NeuroScience. 2007 doi: 10.1007/s10827-007-0043-9. [DOI] [PubMed] [Google Scholar]
- 100.Cunic D, Roshan L, Khan FI, Lozano AM, Lang AE, Chen R. Effects of subthalamic nucleus stimulation on motor cortex excitability in Parkinson's disease. Neurology. 2002;58:1665–1672. doi: 10.1212/wnl.58.11.1665. [DOI] [PubMed] [Google Scholar]
- 101.Devergnas A, Wichmann T. Cortical Potentials Evoked by Deep Brain Stimulation in the Subthalamic Area. Frontiers in NeuroScience. 2011 doi: 10.3389/fnsys.2011.00030. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tisch S, Rothwell JC, Bhatia KP, Quinn N, Zrinzo L, Jahanshahi M, et al. Pallidal stimulation modifies after-effects of paired associative stimulation on motor cortex excitability in primary generalised dystonia. Exp. Neurol. 2007;206:80–85. doi: 10.1016/j.expneurol.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 103.Grafton ST, Turner RS, Desmurget M, Bakay R, Delong M, Vitek J, et al. Normalizing motor-related brain activity: Subthalamic nucleus stimulation in Parkinson disease. Neurology. 2006;66:1192–1199. doi: 10.1212/01.wnl.0000214237.58321.c3. [DOI] [PubMed] [Google Scholar]
- 104.Karimi M, Golchin N, Tabbal SD, Hershey T, Videen TO, Wu J, et al. Subthalamic nucleus stimulation-induced regional blood flow responses correlate with improvement of motor signs in Parkinson disease. Brain. 2008;131:2710–2719. doi: 10.1093/brain/awn179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhao YB, Sun BM, Li DY, Wang QS. Effects of bilateral subthalamic nucleus stimulation on resting-state cerebral glucose metabolism in advanced Parkinson's disease. Chin Med J (Engl) 2004;117:1304–1308. [PubMed] [Google Scholar]
- 106.Grill WM, Snyder AN, Miocinovic S. Deep brain stimulation creates an informational lesion of the stimulated nucleus. Neuroreport. 2004;15:1137–1140. doi: 10.1097/00001756-200405190-00011. [DOI] [PubMed] [Google Scholar]
- 107.Parkin S, Nandi D, Giladi N, Joint C, Gregory R, Bain P, et al. Lesioning the subthalamic nucleus in the treatment of Parkinson's disease. Stereotactic & Functional Neurosurgery. 2001;77:68–72. doi: 10.1159/000064599. [DOI] [PubMed] [Google Scholar]
- 108.Alvarez L, Macias R, Guridi J, Lopez G, Alvarez E, Maragoto C, et al. Dorsal subthalamotomy for Parkinson's disease. Mov Disord. 2001;16:72–78. doi: 10.1002/1531-8257(200101)16:1<72::aid-mds1019>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 109.Alvarez L, Macias R, Lopez G, Alvarez E, Pavon N, Rodriguez-Oroz MC, et al. Bilateral subthalamotomy in Parkinson's disease: initial and long-term response. Brain. 2005;128:570–583. doi: 10.1093/brain/awh397. [DOI] [PubMed] [Google Scholar]
- 110.Alvarez L, Macias R, Pavon N, Lopez G, Rodriguez-Oroz MC, Rodriguez R, et al. Therapeutic efficacy of unilateral subthalamotomy in Parkinson's disease: results in 89 patients followed for up to 36 months. J Neurol Neurosurg Psychiatry. 2009;80:979–985. doi: 10.1136/jnnp.2008.154948. [DOI] [PubMed] [Google Scholar]
- 111.Patel NK, Heywood P, O'Sullivan K, McCarter R, Love S, Gill SS. Unilateral subthalamotomy in the treatment of Parkinson's disease. Brain. 2003;126:1136–1145. doi: 10.1093/brain/awg111. [DOI] [PubMed] [Google Scholar]
- 112.Su PC, Tseng HM, Liu HM, Yen RF, Liou HH. Subthalamotomy for advanced Parkinson disease. J Neurosurg. 2002;97:598–606. doi: 10.3171/jns.2002.97.3.0598. [DOI] [PubMed] [Google Scholar]
- 113.Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436–1438. doi: 10.1126/science.2402638. [DOI] [PubMed] [Google Scholar]
- 114.Aziz TZ, Peggs D, Sambrook MA, Crossman AR. Lesion of the subthalamic nucleus for the alleviation of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism in the primate. Mov Disord. 1991;6:288–292. doi: 10.1002/mds.870060404. [DOI] [PubMed] [Google Scholar]
- 115.Lewitt PA, Rezai AR, Leehey MA, Ojemann SG, Flaherty AW, Eskandar EN, et al. AAV2-GAD gene therapy for advanced Parkinson's disease: a double-blind, sham-surgery controlled, randomised trial. Lancet Neurol. 2011;10:309–319. doi: 10.1016/S1474-4422(11)70039-4. [DOI] [PubMed] [Google Scholar]
- 116.Ozelius LJ, Hewett J, Kramer P, Bressman SB, Shalish C, deLeon D, et al. Fine localization of the torsion dystonia gene (DYT1) on human chromosome 9q34: YAC map and linkage disequilibrium. Genome Research. 1997;7:483–494. doi: 10.1101/gr.7.5.483. [DOI] [PubMed] [Google Scholar]
- 117.Wichmann T. Commentary: Dopaminergic dysfunction in DYT1 dystonia. Exp. Neurol. 2008;212:242–246. doi: 10.1016/j.expneurol.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Augood SJ, Hollingsworth Z, Albers DS, Yang L, Leung JC, Muller B, et al. Dopamine transmission in DYT1 dystonia: a biochemical and autoradiographical study. Neurology. 2002;59:445–448. doi: 10.1212/wnl.59.3.445. [DOI] [PubMed] [Google Scholar]
- 119.Balcioglu A, Kim MO, Sharma N, Cha JH, Breakefield XO, Standaert DG. Dopamine release is impaired in a mouse model of DYT1 dystonia. J. Neurochem. 2007;102:783–788. doi: 10.1111/j.1471-4159.2007.04590.x. [DOI] [PubMed] [Google Scholar]
- 120.Zhao Y, Decuypere M, Ledoux MS. Abnormal motor function and dopamine neurotransmission in DYT1 DeltaGAG transgenic mice. Exp. Neurol. 2008;210:719–730. doi: 10.1016/j.expneurol.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pisani A, Bernardi G, Ding J, Surmeier DJ. Re-emergence of striatal cholinergic interneurons in movement disorders. Trends Neurosci. 2007;30:545–553. doi: 10.1016/j.tins.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 122.Pisani A, Martella G, Tscherter A, Bonsi P, Sharma N, Bernardi G, et al. Altered responses to dopaminergic D2 receptor activation and N-type calcium currents in striatal cholinergic interneurons in a mouse model of DYT1 dystonia. Neurobiol Dis. 2006;24:318–325. doi: 10.1016/j.nbd.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 123.Sciamanna G, Bonsi P, Tassone A, Cuomo D, Tscherter A, Viscomi MT, et al. Impaired striatal D2 receptor function leads to enhanced GABA transmission in a mouse model of DYT1 dystonia. Neurobiol Dis. 2009;34:133–145. doi: 10.1016/j.nbd.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Perlmutter JS, Tempel LW, Black KJ, Parkinson D, Todd RD. MPTP induces dystonia and parkinsonism. Clues to the pathophysiology of dystoni. Neurology. 1997;49:1432–1438. doi: 10.1212/wnl.49.5.1432. [DOI] [PubMed] [Google Scholar]
- 125.Tabbal SD, Mink JW, Antenor JA, Carl JL, Moerlein SM, Perlmutter JS. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced acute transient dystonia in monkeys associated with low striatal dopamine. Neurosci. 2006;141:1281–1287. doi: 10.1016/j.neuroscience.2006.04.072. [DOI] [PubMed] [Google Scholar]
- 126.Ma Y, Hu X, Jinnah HA, Galvan A, Smith Y, Wichmann T. Induction of abnormal movements by MPTP treatment in infant rhesus monkeys. Soc. Neurosci. Abstr. 2010 [Google Scholar]
- 127.Mitchell IJ, Luquin R, Boyce S, Clarke CE, Robertson RG, Sambrook MA, et al. Neural mechanisms of dystonia: evidence from a 2-deoxyglucose uptake study in a primate model of dopamine agonist-induced dystonia. Mov Disord. 1990;5:49–54. doi: 10.1002/mds.870050113. [DOI] [PubMed] [Google Scholar]
- 128.Hantraye P, Riche D, Maziere M, Isacson O. A primate model of Huntington's disease: behavioral and anatomical studies of unilateral excitotoxic lesions of the caudate-putamen in the baboon. Exp. Neurol. 1990;108:91–104. doi: 10.1016/0014-4886(90)90014-j. [DOI] [PubMed] [Google Scholar]
- 129.Gerlach J, Hansen L. Clozapine and D1/D2 antagonism in extrapyramidal functions. Br.J.Pychiatr. 1997;17(Suppl):34–37. [PubMed] [Google Scholar]
- 130.Casey DE. Dopamine D1 (SCH 23390) and D2 (haloperidol) antagonists in drug-naive monkeys. Psychopharmacol. (Berlin) 1992;107:18–22. doi: 10.1007/BF02244960. [DOI] [PubMed] [Google Scholar]
- 131.Vitek JL, Chockkan V, Zhang JY, Kaneoke Y, Evatt M, DeLong MR, et al. Neuronal activity in the basal ganglia in patients with generalized dystonia and hemiballismus. Ann. Neurol. 1999;46:22–35. doi: 10.1002/1531-8249(199907)46:1<22::aid-ana6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 132.Vitek JL. Pathophysiology of dystonia: a neuronal model. Mov Disord. 2002;17(Suppl 3):S49–S62. doi: 10.1002/mds.10142. [DOI] [PubMed] [Google Scholar]
- 133.Lenz FA, Suarez JI, Metman LV, Reich SG, Karp BI, Hallett M, et al. Pallidal activity during dystonia: somatosensory reorganisation and changes with severity. J Neurol Neurosurg Psychiatry. 1998;65:767–770. doi: 10.1136/jnnp.65.5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhuang P, Li Y, Hallett M. Neuronal activity in the basal ganglia and thalamus in patients with dystonia. Clin Neurophysiol. 2004;115:2542–2557. doi: 10.1016/j.clinph.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 135.Starr PA, Rau GM, Davis V, Marks WJ, Jr, Ostrem JL, Simmons D, et al. Spontaneous pallidal neuronal activity in human dystonia: comparison with Parkinson's disease and normal macaqu. J. Neurophysiol. 2005;93:3165–3176. doi: 10.1152/jn.00971.2004. [DOI] [PubMed] [Google Scholar]
- 136.Tang JK, Moro E, Mahant N, Hutchison WD, Lang AE, Lozano AM, et al. Neuronal firing rates and patterns in the globus pallidus internus of patients with cervical dystonia differ from those with Parkinson's disease. J. Neurophysiol. 2007;98:720–729. doi: 10.1152/jn.01107.2006. [DOI] [PubMed] [Google Scholar]
- 137.Lenz FA, Jaeger CJ, Seike MS, Lin YC, Reich SG, DeLong MR, et al. Thalamic single neuron activity in patients with dystonia: dystonia-related activity and somatic sensory reorganization. J. Neurophysiol. 1999;82:2372–2392. doi: 10.1152/jn.1999.82.5.2372. [DOI] [PubMed] [Google Scholar]
- 138.Silberstein P, Kuhn AA, Kupsch A, Trottenberg T, Krauss JK, Wohrle JC, et al. Patterning of globus pallidus local field potentials differs between Parkinson's disease and dystonia. Brain. 2003;126:2597–2608. doi: 10.1093/brain/awg267. [DOI] [PubMed] [Google Scholar]
- 139.Chen CC, Kuhn AA, Hoffmann KT, Kupsch A, Schneider GH, Trottenberg T, et al. Oscillatory pallidal local field potential activity correlates with involuntary EMG in dystonia. Neurology. 2006;66:418–420. doi: 10.1212/01.wnl.0000196470.00165.7d. [DOI] [PubMed] [Google Scholar]
- 140.Jinnah HA, Hess EJ. A new twist on the anatomy of dystonia: the basal ganglia and the cerebellum? Neurology. 2006;67:1740–1741. doi: 10.1212/01.wnl.0000246112.19504.61. [DOI] [PubMed] [Google Scholar]
- 141.Neychev VK, Fan X, Mitev VI, Hess EJ, Jinnah HA. The basal ganglia and cerebellum interact in the expression of dystonic movement. Brain. 2008;131:2499–2509. doi: 10.1093/brain/awn168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Calderon DP, Fremont R, Kraenzlin F, Khodakhah K. The neural substrates of rapid-onset Dystonia-Parkinsonism. Nat Neurosci. 2011;14:357–365. doi: 10.1038/nn.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Carbon M, Niethammer M, Peng S, Raymond D, Dhawan V, Chaly T, et al. Abnormal striatal and thalamic dopamine neurotransmission: Genotype-related features of dystonia. Neurology. 2009;72:2097–2103. doi: 10.1212/WNL.0b013e3181aa538f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Niethammer M, Carbon M, Argyelan M, Eidelberg D. Hereditary dystonia as a neurodevelopmental circuit disorder: Evidence from neuroimaging. Neurobiol Dis. 2011;42:202–209. doi: 10.1016/j.nbd.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Argyelan M, Carbon M, Niethammer M, Ulug AM, Voss HU, Bressman SB, et al. Cerebellothalamocortical connectivity regulates penetrance in dystonia. J. Neurosci. 2009;29:9740–9747. doi: 10.1523/JNEUROSCI.2300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ulug AM, Vo A, Argyelan M, Tanabe L, Schiffer WK, Dewey S, et al. Cerebellothalamocortical pathway abnormalities in torsinA DYT1 knock-in mice. Proc Natl Acad Sci U S A. 2011;108:6638–6643. doi: 10.1073/pnas.1016445108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Eltahawy HA, Saint-Cyr J, Poon YY, Moro E, Lang AE, Lozano AM. Pallidal deep brain stimulation in cervical dystonia: clinical outcome in four cases. Can J Neurol Sci. 2004;31:328–332. doi: 10.1017/s0317167100003401. [DOI] [PubMed] [Google Scholar]
- 148.Coubes P, Cif L, El Fertit H, Hemm S, Vayssiere N, Serrat S, et al. Electrical stimulation of the globus pallidus internus in patients with primary generalized dystonia: long-term results. J. Neurosurg. 2004;101:189–194. doi: 10.3171/jns.2004.101.2.0189. [DOI] [PubMed] [Google Scholar]
- 149.Vidailhet M, Vercueil L, Houeto JL, Krystkowiak P, Benabid AL, Cornu P, et al. Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med. 2005;352:459–467. doi: 10.1056/NEJMoa042187. [DOI] [PubMed] [Google Scholar]
- 150.Cif L, El Fertit H, Vayssiere N, Hemm S, Hardouin E, Gannau A, et al. Treatment of dystonic syndromes by chronic electrical stimulation of the internal globus pallidus. J Neurosurg Sci. 2003;47:52–55. [PubMed] [Google Scholar]
- 151.Kupsch A, Benecke R, Muller J, Trottenberg T, Schneider GH, Poewe W, et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med. 2006;355:1978–1990. doi: 10.1056/NEJMoa063618. [DOI] [PubMed] [Google Scholar]
- 152.Trottenberg T, Volkmann J, Deuschl G, Kuhn AA, Schneider GH, Muller J, et al. Treatment of severe tardive dystonia with pallidal deep brain stimulation. Neurology. 2005;64:344–346. doi: 10.1212/01.WNL.0000149762.80932.55. [DOI] [PubMed] [Google Scholar]
- 153.Loher TJ, Hasdemir MG, Burgunder JM, Krauss JK. Long-term follow-up study of chronic globus pallidus internus stimulation for posttraumatic hemidystonia. J. Neurosurg. 2000;92:457–460. doi: 10.3171/jns.2000.92.3.0457. [DOI] [PubMed] [Google Scholar]
- 154.Pretto TE, Dalvi A, Kang UJ, Penn RD. A prospective blinded evaluation of deep brain stimulation for the treatment of secondary dystonia and primary torticollis syndromes. J. Neurosurg. 2008;109:405–409. doi: 10.3171/JNS/2008/109/9/0405. [DOI] [PubMed] [Google Scholar]
- 155.Sako W, Goto S, Shimazu H, Murase N, Matsuzaki K, Tamura T, et al. Bilateral deep brain stimulation of the globus pallidus internus in tardive dystonia. Mov Disord. 2008;23:1929–1931. doi: 10.1002/mds.22100. [DOI] [PubMed] [Google Scholar]
- 156.Houser M, Waltz T. Meige syndrome and pallidal deep brain stimulation. Mov Disord. 2005;20:1203–1205. doi: 10.1002/mds.20522. [DOI] [PubMed] [Google Scholar]
- 157.Loher TJ, Capelle HH, Kaelin-Lang A, Weber S, Weigel R, Burgunder JM, et al. Deep brain stimulation for dystonia: outcome at long-term follow-up. J Neurol. 2008;255:881–884. doi: 10.1007/s00415-008-0798-6. [DOI] [PubMed] [Google Scholar]
- 158.Mueller J, Skogseid IM, Benecke R, Kupsch A, Trottenberg T, Poewe W, et al. Pallidal deep brain stimulation improves quality of life in segmental and generalized dystonia: results from a prospective, randomized sham-controlled trial. Mov Disord. 2008;23:131–134. doi: 10.1002/mds.21783. [DOI] [PubMed] [Google Scholar]
- 159.Skogseid IM. Pallidal deep brain stimulation is effective, and improves quality of life in primary segmental and generalized dystonia. Acta Neurol Scand Suppl. 2008;188:51–55. doi: 10.1111/j.1600-0404.2008.01032.x. [DOI] [PubMed] [Google Scholar]
- 160.Blahak C, Wohrle JC, Capelle HH, Bazner H, Grips E, Weigel R, et al. Health-related quality of life in segmental dystonia is improved by bilateral pallidal stimulation. J Neurol. 2008;255:178–182. doi: 10.1007/s00415-008-0614-3. [DOI] [PubMed] [Google Scholar]
- 161.Valldeoriola F, Regidor I, Minguez-Castellanos A, Lezcano E, Garcia-Ruiz P, Rojo A, et al. Efficacy and safety of pallidal stimulation in primary dystonia: results of the Spanish multicentric study. J Neurol Neurosurg Psychiatry. 2010;81:65–69. doi: 10.1136/jnnp.2009.174342. [DOI] [PubMed] [Google Scholar]
- 162.Pillon B, Ardouin C, Dujardin K, Vittini P, Pelissolo A, Cottencin O, et al. Preservation of cognitive function in dystonia treated by pallidal stimulation. Neurology. 2006;66:1556–1558. doi: 10.1212/01.wnl.0000216131.41563.24. [DOI] [PubMed] [Google Scholar]
- 163.Breakefield XO, Blood AJ, Li Y, Hallett M, Hanson PI, Standaert DG. The pathophysiological basis of dystonias. Nat Rev Neurosci. 2008;9:222–234. doi: 10.1038/nrn2337. [DOI] [PubMed] [Google Scholar]
- 164.Quartarone A, Pisani A. Abnormal plasticity in dystonia: Disruption of synaptic homeostasis. Neurobiol Dis. 2011;42:162–170. doi: 10.1016/j.nbd.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 165.Hallett M. Neurophysiology of dystonia: The role of inhibition. Neurobiol Dis. 2011;42:177–184. doi: 10.1016/j.nbd.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Detante O, Vercueil L, Thobois S, Broussolle E, Costes N, Lavenne F, et al. Globus pallidus internus stimulation in primary generalized dystonia: a H215O PET study. Brain. 2004;127:1899–1908. doi: 10.1093/brain/awh213. [DOI] [PubMed] [Google Scholar]
- 167.Kuhn AA, Meyer BU, Trottenberg T, Brandt SA, Schneider GH, Kupsch A. Modulation of motor cortex excitability by pallidal stimulation in patients with severe dystonia. Neurology. 2003;60:768–774. doi: 10.1212/01.wnl.0000044396.64752.4c. [DOI] [PubMed] [Google Scholar]
- 168.Krauss JK, Loher TJ, Pohle T, Weber S, Taub E, Barlocher CB, et al. Pallidal deep brain stimulation in patients with cervical dystonia and severe cervical dyskinesias with cervical myelopathy. J Neurol Neurosurg Psychiatry. 2002;72:249–256. doi: 10.1136/jnnp.72.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ruge D, Tisch S, Hariz MI, Zrinzo L, Bhatia KP, Quinn NP, et al. Deep brain stimulation effects in dystonia: Time course of electrophysiological changes in early treatment. Mov Disord. 2011 doi: 10.1002/mds.23731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pastor-Gomez J, Hernando-Requejo V, Luengo-Dos Santos A, Pedrosa-Sanchez M, Sola RG. [Treatment of a case of generalised dystonia using subthalamic stimulation] Rev Neurol. 2003;37:529–531. [PubMed] [Google Scholar]
- 171.Sun B, Chen S, Zhan S, Le W, Krahl SE. Subthalamic nucleus stimulation for primary dystonia and tardive dystonia. Acta Neurochir Suppl. 2007;97:207–214. doi: 10.1007/978-3-211-33081-4_23. [DOI] [PubMed] [Google Scholar]
- 172.Benabid AL, Koudsie A, Benazzouz A, Vercueil L, Fraix V, Chabardes S, et al. Deep brain stimulation of the corpus luysi (subthalamic nucleus) and other targets in Parkinson's disease. Extension to new indications such as dystonia and epileps. J Neurol. 2001;248(Suppl 3):37–47. doi: 10.1007/pl00007825. [DOI] [PubMed] [Google Scholar]
- 173.Lyons KE, Pahwa R. Effects of bilateral subthalamic nucleus stimulation on sleep, daytime sleepiness, and early morning dystonia in patients with Parkinson disease. J. Neurosurg. 2006;104:502–505. doi: 10.3171/jns.2006.104.4.502. [DOI] [PubMed] [Google Scholar]
- 174.Chou KL, Hurtig HI, Jaggi JL, Baltuch GH. Bilateral subthalamic nucleus deep brain stimulation in a patient with cervical dystonia and essential tremor. Mov Disord. 2005;20:377–380. doi: 10.1002/mds.20341. [DOI] [PubMed] [Google Scholar]
- 175.Ostrem JL, Racine CA, Glass GA, Grace JK, Volz MM, Heath SL, et al. Subthalamic nucleus deep brain stimulation in primary cervical dystonia. Neurology. 2011;76:870–878. doi: 10.1212/WNL.0b013e31820f2e4f. [DOI] [PubMed] [Google Scholar]
- 176.Kleiner-Fisman G, Liang GS, Moberg PJ, Ruocco AC, Hurtig HI, Baltuch GH, et al. Subthalamic nucleus deep brain stimulation for severe idiopathic dystonia: impact on severity, neuropsychological status, and quality of life. J Neurosurg. 2007;107:29–36. doi: 10.3171/JNS-07/07/0029. [DOI] [PubMed] [Google Scholar]
- 177.Fukaya C, Katayama Y, Kano T, Nagaoka T, Kobayashi K, Oshima H, et al. Thalamic deep brain stimulation for writer's cramp. J. Neurosurg. 2007;107:977–982. doi: 10.3171/JNS-07/11/0977. [DOI] [PubMed] [Google Scholar]
- 178.Taira T, Hori T. Stereotactic ventrooralis thalamotomy for task-specific focal hand dystonia (writer's cramp) Stereotact Funct Neurosurg. 2003;80:88–91. doi: 10.1159/000075165. [DOI] [PubMed] [Google Scholar]
- 179.Gruber D, Kuhn AA, Schoenecker T, Kivi A, Trottenberg T, Hoffmann KT, et al. Pallidal and thalamic deep brain stimulation in myoclonus-dystonia. Mov Disord. 2010;25:1733–1743. doi: 10.1002/mds.23312. [DOI] [PubMed] [Google Scholar]
- 180.Kuncel AM, Turner DA, Ozelius LJ, Greene PE, Grill WM, Stacy MA. Myoclonus and tremor response to thalamic deep brain stimulation parameters in a patient with inherited myoclonus-dystonia syndrome. Clin Neurol Neurosurg. 2009;111:303–306. doi: 10.1016/j.clineuro.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Vidailhet M, Vercueil L, Houeto JL, Krystkowiak P, Lagrange C, Yelnik J, et al. Bilateral, pallidal, deep-brain stimulation in primary generalised dystonia: a prospective 3 year follow-up study. Lancet Neurol. 2007;6:223–229. doi: 10.1016/S1474-4422(07)70035-2. [DOI] [PubMed] [Google Scholar]
- 182.Ostrem JL, Marks WJ, Jr, Volz MM, Heath SL, Starr PA. Pallidal deep brain stimulation in patients with cranial-cervical dystonia (Meige syndrome) Mov Disord. 2007;22:1885–1891. doi: 10.1002/mds.21580. [DOI] [PubMed] [Google Scholar]
- 183.Kiss ZH, Doig-Beyaert K, Eliasziw M, Tsui J, Haffenden A, Suchowersky O. The Canadian multicentre study of deep brain stimulation for cervical dystonia. Brain. 2007;130:2879–2886. doi: 10.1093/brain/awm229. [DOI] [PubMed] [Google Scholar]
- 184.Hung SW, Hamani C, Lozano AM, Poon YY, Piboolnurak P, Miyasaki JM, et al. Long-term outcome of bilateral pallidal deep brain stimulation for primary cervical dystonia. Neurology. 2007;68:457–459. doi: 10.1212/01.wnl.0000252932.71306.89. [DOI] [PubMed] [Google Scholar]
- 185.Vercueil L, Houeto JL, Krystkowiak P, Lagrange C, Cassim F, Benazzouz A, et al. Effects of pulse width variations in pallidal stimulation for primary generalized dystonia. J Neurol. 2007 doi: 10.1007/s00415-007-0578-8. [DOI] [PubMed] [Google Scholar]
- 186.Alterman RL, Miravite J, Weisz D, Shils JL, Bressman SB, Tagliati M. Sixty hertz pallidal deep brain stimulation for primary torsion dystonia. Neurology. 2007;69:681–688. doi: 10.1212/01.wnl.0000267430.95106.ff. [DOI] [PubMed] [Google Scholar]
- 187.Alterman RL, Shils JL, Miravite J, Tagliati M. Lower stimulation frequency can enhance tolerability and efficacy of pallidal deep brain stimulation for dystonia. Mov Disord. 2007;22:366–368. doi: 10.1002/mds.21274. [DOI] [PubMed] [Google Scholar]
- 188.Isaias IU, Alterman RL, Tagliati M. Deep brain stimulation for primary generalized dystonia: long-term outcomes. Arch Neurol. 2009;66:465–470. doi: 10.1001/archneurol.2009.20. [DOI] [PubMed] [Google Scholar]
- 189.DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64:20–24. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- 190.Bonelli RM, Cummings JL. Frontal-subcortical circuitry and behavior. Dialogues Clin Neurosci. 2007;9:141–151. doi: 10.31887/DCNS.2007.9.2/rbonelli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Krack P, Hariz MI, Baunez C, Guridi J, Obeso JA. Deep brain stimulation: from neurology to psychiatry? Trends Neurosci. 2010;33:474–484. doi: 10.1016/j.tins.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 192.Fins JJ, Mayberg HS, Nuttin B, Kubu CS, Galert T, Sturm V, et al. Misuse of the FDA’s Humanitarian Device Exemption in deep brain stimulation for obsessive-compulsive disorder. Health Affairs. 2011;30:302–311. doi: 10.1377/hlthaff.2010.0157. [DOI] [PubMed] [Google Scholar]
- 193.Kopell BH, Greenberg BD. Anatomy and physiology of the basal ganglia: Implications for DBS in psychiatry. Neuroscience and Biobehavioral Reviews. 2008;32:408–422. doi: 10.1016/j.neubiorev.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 194.Muller-Vahl KR, Cath DC, Cavanna AE, Dehning S, Porta M, Robertson MM, et al. European clinical guidelines for Tourette syndrome and other tic disorders. Part IV: deep brain stimulation. Eur Child Adolesc Psychiatry. 2011;20:209–217. doi: 10.1007/s00787-011-0166-4. [DOI] [PubMed] [Google Scholar]
- 195.Hariz MI, Robertson MM. Gilles de la Tourette syndrome and deep brain stimulation. Eur J Neurosci. 2010;32:1128–1134. doi: 10.1111/j.1460-9568.2010.07415.x. [DOI] [PubMed] [Google Scholar]
- 196.Mink JW. Clinical review of DBS for Tourette Syndrome. Front Biosci (Elite Ed) 2009;1:72–76. doi: 10.2741/E8. [DOI] [PubMed] [Google Scholar]
- 197.Houeto JL, Karachi C, Mallet L, Pillon B, Yelnik J, Mesnage V, et al. Tourette's syndrome and deep brain stimulation. J Neurol Neurosurg Psychiatry. 2005;76:992–995. doi: 10.1136/jnnp.2004.043273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Shields DC, Cheng ML, Flaherty AW, Gale JT, Eskandar EN. Microelectrode-guided deep brain stimulation for Tourette syndrome: within-subject comparison of different stimulation sites. Stereotact Funct Neurosurg. 2008;86:87–91. doi: 10.1159/000112429. [DOI] [PubMed] [Google Scholar]
- 199.Servello D, Porta M, Sassi M, Brambilla A, Robertson MM. Deep brain stimulation in 18 patients with severe Gilles de la Tourette syndrome refractory to treatment: the surgery and stimulation. J Neurol Neurosurg Psychiatry. 2008;79:136–142. doi: 10.1136/jnnp.2006.104067. [DOI] [PubMed] [Google Scholar]
- 200.Bajwa RJ, de Lotbiniere AJ, King RA, Jabbari B, Quatrano S, Kunze K, et al. Deep brain stimulation in Tourette's syndrome. Mov Disord. 2007;22:1346–1350. doi: 10.1002/mds.21398. [DOI] [PubMed] [Google Scholar]
- 201.Visser-Vandewalle V, Ackermans L, van der Linden C, Temel Y, Tijssen MA, Schruers KR, et al. Deep brain stimulation in Gilles de la Tourette's syndrome. Neurosurgery. 2006;58:E590. doi: 10.1227/01.NEU.0000207959.53198.D6. [DOI] [PubMed] [Google Scholar]
- 202.Visser-Vandewalle V, Temel Y, van der Linden C, Ackermans L, Beuls E. Deep brain stimulation in movement disorders. The applications reconsidered. Acta Neurol Belg. 2004;104:33–36. [PubMed] [Google Scholar]
- 203.Ackermans L, Temel Y, Visser-Vandewalle V. Deep brain stimulation in Tourette's Syndrome. Neurotherapeutics. 2008;5:339–344. doi: 10.1016/j.nurt.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Temel Y, Visser-Vandewalle V. Surgery in Tourette syndrome. Mov Disord. 2004;19:3–14. doi: 10.1002/mds.10649. [DOI] [PubMed] [Google Scholar]
- 205.Maciunas RJ, Maddux BN, Riley DE, Whitney CM, Schoenberg MR, Ogrocki PJ, et al. Prospective randomized double-blind trial of bilateral thalamic deep brain stimulation in adults with Tourette syndrome. J. Neurosurg. 2007;107:1004–1014. doi: 10.3171/JNS-07/11/1004. [DOI] [PubMed] [Google Scholar]
- 206.Hassler R, Dieckmann G. Stereotaxic treatment of tics and inarticulate cries or coprolalia considered as motor obsessional phenomena in Gilles de la Tourette’s disease. Rev Neurol (Paris) 1970;123:89–100. [PubMed] [Google Scholar]
- 207.Hassler R, Dieckmann G. Relief of obsessive-compulsive disorders, phobias and tics by stereotactic coagulations of the rostral intralaminar and medial-thalamic nucle. In: Laitinen LV, Livingston K, editors. Surgical approaches in psychiatry. Proceedings of the Third International Congress of Psychosurgery. Cambridge, UK: Garden City Press; 1973. pp. 206–212. [Google Scholar]
- 208.Hassler R, Dieckmann G. Stereotaxic treatment of tics and inarticulate cries or coprolalia considered as motor obsessional phenomena in Gilles de la Tourette’s disease. Rev Neurol (Paris) 1997;123:89–100. [PubMed] [Google Scholar]
- 209.Porta M, Brambilla A, Cavanna AE, Servello D, Sassi M, Rickards H, et al. Thalamic deep brain stimulation for treatment-refractory Tourette syndrome: two-year outcome. Neurology. 2009;73:1375–1380. doi: 10.1212/WNL.0b013e3181bd809b. [DOI] [PubMed] [Google Scholar]
- 210.Ackermans L, Duits A, van der Linden C, Tijssen M, Schruers K, Temel Y, et al. Double-blind clinical trial of thalamic stimulation in patients with Tourette syndrome. Brain. 2011;134:832–844. doi: 10.1093/brain/awq380. [DOI] [PubMed] [Google Scholar]
- 211.Diederich NJ, Kalteis K, Stamenkovic M, Pieri V, Alesch F. Efficient internal pallidal stimulation in Gilles de la Tourette syndrome: A case report. Mov Disord. 2005;20:1496–1499. doi: 10.1002/mds.20551. [DOI] [PubMed] [Google Scholar]
- 212.Ackermans L, Temel Y, Cath D, van der Linden C, Bruggeman R, Kleijer M, et al. Deep brain stimulation in Tourette's syndrome: two targets? Mov Disord. 2006;21:709–713. doi: 10.1002/mds.20816. [DOI] [PubMed] [Google Scholar]
- 213.Flaherty AW, Williams ZM, Amirnovin R, Kasper E, Rauch SL, Cosgrove GR, et al. Deep brain stimulation of the anterior internal capsule for the treatment of Tourette syndrome: technical case report. Neurosurgery. 2005;57:E403. doi: 10.1227/01.neu.0000176854.24694.95. discussion E. [DOI] [PubMed] [Google Scholar]
- 214.Nuttin BJ, Gabriels LA, Cosyns PR, Meyerson BA, Andreewitch S, Sunaert SG, et al. Long-term electrical capsular stimulation in patients with obsessive-compulsive disorder. Neurosurgery. 2003;52:1263–1272. doi: 10.1227/01.neu.0000064565.49299.9a. discussion 72-4. [DOI] [PubMed] [Google Scholar]
- 215.Nuttin B, Cosyns P, Demeulemeester H, Gybels J, Meyerson B. Electrical stimulation in anterior limbs of internal capsules in patients with obsessive-compulsive disorder. Lancet. 1999;354:1526. doi: 10.1016/S0140-6736(99)02376-4. [DOI] [PubMed] [Google Scholar]
- 216.Welter ML, Grabli D, Vidailhet M. Deep brain stimulation for hyperkinetics disorders: dystonia, tardive dyskinesia, and tics. Curr Opin Neurol. 2010;23:420–425. doi: 10.1097/WCO.0b013e32833b7798. [DOI] [PubMed] [Google Scholar]
- 217.Denys D. Pharmacotherapy of obsessive-compulsive disorder and obsessive-compulsive spectrum disorders. Psychiatr Clin North Am. 2006;29:553–584. doi: 10.1016/j.psc.2006.02.013. xi. [DOI] [PubMed] [Google Scholar]
- 218.Denys D, Mantione M, Figee M, van den Munckhof P, Koerselman F, Westenberg H, et al. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 2010;67:1061–1068. doi: 10.1001/archgenpsychiatry.2010.122. [DOI] [PubMed] [Google Scholar]
- 219.Haynes WI, Mallet L. High-frequency stimulation of deep brain structures in obsessive-compulsive disorder: the search for a valid circuit. Eur J Neurosci. 2010;32:1118–1127. doi: 10.1111/j.1460-9568.2010.07418.x. [DOI] [PubMed] [Google Scholar]
- 220.Greenberg BD, Malone DA, Friehs GM, Rezai AR, Kubu CS, Malloy PF, et al. Three-Year Outcomes in Deep Brain Stimulation for Highly Resistant Obsessive-Compulsive Disorder. Neuropsychopharm. 2006;31:2384–2393. doi: 10.1038/sj.npp.1301165. [DOI] [PubMed] [Google Scholar]
- 221.Greenberg BD, Gabriels LA, Malone DA, Jr, Rezai AR, Friehs GM, Okun MS, et al. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experienc. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Heimer L. The legacy of the silver methods and the new anatomy of the basal forebrain: implications for neuropsychiatry and drug abuse. Scand J Psychol. 2003;44:189–201. doi: 10.1111/1467-9450.00336. [DOI] [PubMed] [Google Scholar]
- 223.Nauta WJ, Domesick VB. Afferent and efferent relationships of the basal ganglia. Ciba Foundation Symposium. 1984;107:3–29. doi: 10.1002/9780470720882.ch2. [DOI] [PubMed] [Google Scholar]
- 224.Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J. Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Rauch SL, Dougherty DD, Malone D, Rezai A, Friehs G, Fischman AJ, et al. A functional neuroimaging investigation of deep brain stimulation in patients with obsessive-compulsive disorder. J. Neurosurg. 2006;104:558–565. doi: 10.3171/jns.2006.104.4.558. [DOI] [PubMed] [Google Scholar]
- 226.Aouizerate B, Cuny E, Martin-Guehl C, Guehl D, Amieva H, Benazzouz A, et al. Deep brain stimulation of the ventral caudate nucleus in the treatment of obsessive-compulsive disorder and major depression. Case report. J. Neurosurg. 2004;101:682–686. doi: 10.3171/jns.2004.101.4.0682. [DOI] [PubMed] [Google Scholar]
- 227.Mallet L, Mesnage V, Houeto JL, Pelissolo A, Yelnik J, Behar C, et al. Compulsions, Parkinson's disease, and stimulation. Lancet. 2002;360:1302–1304. doi: 10.1016/S0140-6736(02)11339-0. [DOI] [PubMed] [Google Scholar]
- 228.Fontaine D, Mattei V, Borg M, von Langsdorff D, Magnie MN, Chanalet S, et al. Effect of subthalamic nucleus stimulation on obsessive-compulsive disorder in a patient with Parkinson disease. Case report. J. Neurosurg. 2004;100:1084–1086. doi: 10.3171/jns.2004.100.6.1084. [DOI] [PubMed] [Google Scholar]
- 229.Baup N, Grabli D, Karachi C, Mounayar S, Francois C, Yelnik J, et al. High-frequency stimulation of the anterior subthalamic nucleus reduces stereotyped behaviors in primates. J. Neurosci. 2008;28:8785–8788. doi: 10.1523/JNEUROSCI.2384-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Mallet L, Polosan M, Jaafari N, Baup N, Welter ML, Fontaine D, et al. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med. 2008;359:2121–2134. doi: 10.1056/NEJMoa0708514. [DOI] [PubMed] [Google Scholar]
- 231.Le Jeune F, Verin M, N'Diaye K, Drapier D, Leray E, Du Montcel ST, et al. Decrease of prefrontal metabolism after subthalamic stimulation in obsessive-compulsive disorder: a positron emission tomography study. Biol Psychiatry. 2010;68:1016–1022. doi: 10.1016/j.biopsych.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 232.Prince M, Patel V, Saxena S, Maj M, Maselko J, Phillips MR, et al. No health without mental health. Lancet. 2007;370:859–877. doi: 10.1016/S0140-6736(07)61238-0. [DOI] [PubMed] [Google Scholar]
- 233.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 234.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Drevets WC. Orbitofrontal cortex function and structure in depression. Ann N Y Acad Sci. 2007;1121:499–527. doi: 10.1196/annals.1401.029. [DOI] [PubMed] [Google Scholar]
- 236.Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- 237.Hamani C, Mayberg H, Stone S, Laxton A, Haber S, Lozano AM. The subcallosal cingulate gyrus in the context of major depression. Biol Psychiatry. 2011;69:301–308. doi: 10.1016/j.biopsych.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 238.Mayberg HS. Positron emission tomography imaging in depression: a neural systems perspective. Neuroimaging Clin N Am. 2003;13:805–815. doi: 10.1016/s1052-5149(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 239.Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 240.Cohen OS, Hassin-Baer S, Spiegelmann R. Deep brain stimulation of the internal globus pallidus for refractory tardive dystonia. Parkinsonism Relat Disord. 2007;13:541–544. doi: 10.1016/j.parkreldis.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 241.Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharm. 2008;33:368–377. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- 242.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 243.Kennedy SH, Giacobbe P, Rizvi SJ, Placenza FM, Nishikawa Y, Mayberg HS, et al. Deep Brain Stimulation for Treatment-Resistant Depression: Follow-Up After 3 to 6 Years. Am J Psychiatry. 2011 doi: 10.1176/appi.ajp.2010.10081187. [DOI] [PubMed] [Google Scholar]
- 244.Jimenez F, Velasco F, Salin-Pascual R, Hernandez JA, Velasco M, Criales JL, et al. A patient with a resistant major depression disorder treated with deep brain stimulation in the inferior thalamic peduncle. Neurosurgery. 2005;57:585–593. doi: 10.1227/01.neu.0000170434.44335.19. discussion -93. [DOI] [PubMed] [Google Scholar]
- 245.Sartorius A, Kiening KL, Kirsch P, von Gall CC, Haberkorn U, Unterberg AW, et al. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol Psychiatry. 2010;67:e9–e11. doi: 10.1016/j.biopsych.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 246.Malone DA., Jr Use of deep brain stimulation in treatment-resistant depression. Cleve Clin J Med. 2010;77(Suppl 3):S77–S80. doi: 10.3949/ccjm.77.s3.14. [DOI] [PubMed] [Google Scholar]
- 247.Malone DA, Jr, Dougherty DD, Rezai AR, Carpenter LL, Friehs GM, Eskandar EN, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depressio. Biol Psychiatry. 2009;65:267–275. doi: 10.1016/j.biopsych.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Bewernick BH, Hurlemann R, Matusch A, Kayser S, Grubert C, Hadrysiewicz B, et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 2010;67:110–116. doi: 10.1016/j.biopsych.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 249.Cho ZH, Min HK, Oh SH, Han JY, Park CW, Chi JG, et al. Direct visualization of deep brain stimulation targets in Parkinson disease with the use of 7-tesla magnetic resonance imaging. J Neurosurg. 2010;113:639–647. doi: 10.3171/2010.3.JNS091385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Zaidel A, Spivak A, Grieb B, Bergman H, Israel Z. Subthalamic span of beta oscillations predicts deep brain stimulation efficacy for patients with Parkinson's disease. Brain. 2010;133:2007–2021. doi: 10.1093/brain/awq144. [DOI] [PubMed] [Google Scholar]
- 251.Wichmann T, DeLong MR. Deep-Brain Stimulation for Neurologic and Psychiatric Disorders. In: Steiner H, Tseng K, editors. Handbook of Basal Ganglia Structure and Function. London: Academic Press (Elsevier); 2010. pp. 659–681. [Google Scholar]