Abstract
Biological effects of wear products (particles and metal ions) generated by metal-on-metal (MoM) hip replacements made of CoCrMo alloy remain a major cause of concern. Periprosthetic osteolysis, potential hypersensitivity response and pseudotumour formation are possible reactions that can lead to early revisions. To accurately analyse the biological response to wear particles from MoM implants, the exact nature of these particles needs to be characterized. Most previous studies used energy-dispersive X-ray spectroscopy (EDS) analysis for characterization. The present study used energy filtered transmission electron microscopy (TEM) and electron diffraction pattern analysis to allow for a more precise determination of the chemical composition and to gain knowledge of the crystalline structure of the wear particles.
Particles were retrieved from two different test rigs: a reciprocating sliding wear tribometer (CoCrMo cylinder vs. bar) and a hip simulator according to ISO 14242-1 (CoCrMo head vs. CoCrMo cup). All tests were conducted in bovine serum. Particles were retrieved from the test medium using a previously published enzymatic digestion protocol.
Particles isolated from tribometer samples had a size of 100 – 500 nm. Diffraction pattern analysis clearly revealed the lattice structure of strain induced hcp ε-martensite. Hip simulator samples revealed numerous particles of 15 – 30 nm and 30 – 80 nm size. Most of the larger particles appeared to be only partially oxidized and exhibited cobalt locally. The smallest particles were Cr2O3 with no trace of cobalt. It optically appeared that these Cr2O3 particles were flaking off the surface of larger particles that depicted a very high intensity of oxygen, as well as chromium, and only background noise of cobalt. The particle size difference between the two test rigs is likely related to the conditions of the two tribosystems, in particular the difference in the sample geometry and in the type of sliding (reciprocating vs. multidirectional).
Results suggest that there may be a critical particle size at which chromium oxidation and cobalt ionization is accelerated. Since earlier studies have shown that wear particles are covered by organic residue which may act as a passive layer inhibiting further oxidation, it would suggest that this organic layer may be removed during the particle isolation process, resulting in a change of the particle chemical composition due to their pyrophoric properties. However, prior to being isolated from the serum lubricant, particles remain within the contact area of head and cup as a third-body. It is therefore possible that during that time, particles may undergo significant transformation and changes in chemical composition in the contact area of the head and cup within the tribological interface due to mechanical interaction with surface asperities.
Keywords: Wear particles, hip joint, CoCrMo, Wear, TEM
1. Introduction
Total hip replacements (THR) made from CoCrMo alloy are used since more than 50 years [1, 2]. Due to the great success of metal-on-polyethylene (MoP) articulations in the 1970s and 1980s, the number of metal-on-metal (MoM) implants declined strongly. However, since osteolysis triggered by polyethylene wear debris was determined to be a major cause of implant loosening and failure, MoM bearings gained renewed interest as an alternative bearing material because of their lower volumetric wear rate [3, 4]. The overall good clinical performance of early MoM articulations in the 1960s has proven CoCrMo to be a competent bearing material for orthopaedic applications [5, 6] with studies reporting little to no osteolysis around MoM implants [2, 7]. During the running-in phase, the linear wear rate has been determined to be as low as 25 – 35 μm/yr, decreasing to 1 – 5 μm/yr during the steady-state phase [8]. The volumetric wear rate after running-in phase has been reported to be 0.3 μm3/yr, which is approximately 100 times lower than in MoP couples [8, 9]. However, the total number of particles generated within one year in MoM THRs lies between 6.7 × 1012 and 2.5 × 1014, which is 13 to 500-fold greater than in MoP THRs [10].
Recently, there has been a great concern regarding the potential biological effects of metal nanoparticles and ions [10, 11]. Although no apparent toxic effect was determined, it was reported that large amounts of wear particles generated in MoM THR migrate to the liver, spleen and abdominal lymph nodes [12]. A recent in vitro study has shown that CoCrMo nanoparticles may cause damage to DNA and chromosomes [13]. The tested particles, however, were generated under different conditions than in a THR and may differ in shape and composition from those generated in vivo. Other studies showed a potential hypersensitivity response to metal wear products [7, 14]. Finally, recent reports on so called pseudotumors have generated a growing concern [15, 16]. These pseudotumors are described as soft tissue masses or fluid-filled bursa leading to early implant failures. Although their causes are still not well understood, they could be due to excessive wear, metal hypersensitivity or a combination of the two [15] [17].
Many studies have been conducted to determine the exact size, morphology and chemical composition of MoM wear particles generated in MoM hip prostheses [18–23]. There is a general agreement that the particle size ranges from 30 to 70 nm. Some authors also reported larger particles linked to the occurrence of agglomeration [16, 17, 20]. The shape of wear particles appears to slightly vary from study to study, including round, oval and needle shapes. Regarding the chemical composition, most studies reported that the majority of particles were chromium-oxide, whereas a minority appeared to be of CoCrMo alloy or carbide origin [19, 20]. Since the alloy consists mostly of cobalt (~ 64 %), this suggests that Co would dissolve and leave the body through the urine, whereas mainly oxidized chromium would remain within the organism. Another possibility may be that the chromium oxide particles originate from the passive layer initially covering the implant surface. Finally, it is also possible that the chromium-oxide particles still exhibit a cobalt core that would not have been detected in previous studies using only energy dispersive X-ray spectroscopy (EDS) analysis for chemical characterization. Indeed, EDS is limited for the quantitative analysis of light elements like oxygen or carbon. Hence, the present study used energy filtered transmission electron microscopy (TEM) and electron diffraction pattern analysis to allow for a more precise determination of the chemical composition and to gain knowledge of the crystalline structure of the wear particles.
Regarding the generation of particles from in-vitro studies and the biological response to these particles, it is essential to precisely mimic the tribological conditions as they occur in the artificial joint in-vivo. Changes to the operating variables as well as in the environment may have significant effects on the size and chemical composition of the generated wear particles. It is therefore necessary to show that particles isolated from testing fluid are really from the same nature than those occurring in-vivo. The present study aimed to characterize and compare the composition of wear particles generated by two different tribosystems (a reciprocating sliding wear tribometer and a hip simulator).
2. Materials and Methods
2.1. Samples
Three groups of particle samples were available for analysis.
The first group of particles was generated in a reciprocating sliding wear test rig with cylinder-on-bar configuration (Fig. 1). All samples were made from high carbon wrought CoCrMo alloy. A bar (80 × 10 × 10 mm) reciprocated on a vertical axis while two cylinders (ø 10 × 15 mm) applied horizontally the normal force (Fig. 2). A triangle signal (6 Hz) was used in order to minimize stick slip. The test was performed in new born calf serum (30 g/l protein content) at 37 °C. Even though the unidirectional reciprocating sliding motion does not represent the circumstances in a hip joint, it allows for a similar nominal contact pressure. The normal and friction force were recorded during the test. Furthermore, the cylinders and the bar were electrically isolated from the sample holders in order to prevent the formation of a galvanic element in the testing fluid. Both bar and cylinders, were polished (Ra = 0.04). The operating variables are given in Tab. 1. Wear particles were retrieved from the test period of 0.1 – 1 million cycles. Particles from this group are referred to as CoB (cylinder-on-bar) particles.
Fig. 1.

(a) Test setup of cylinder-on-bar tribometer; (b) schematic setup of cylinders and bar sample
Fig. 2.

Bar and cylinder samples made from hc CoCrMo
Tab. 1.
Operating variables for cylinder-on-bar tribometer test procedure
| normal force | 25 N |
| nominal surface pressure | 135 MPa |
| relative velocity (frequency) | 6 Hz, triangle signal |
| stroke | 3 mm |
| ambient temperature | 37 °C |
| motion | reciprocating sliding |
| test duration | 0.1 – 2 million cycles (MC) |
| test medium | bovine serum test fluid (30g/l protein) |
The second group of particles was generated in a hip simulator according to ISO 14242-1. The tests were conducted at Endolab GmbH (Thansau/Rosenheim, Germany). The tested samples were MoM hip joints made of high carbon CoCrMo wrought alloy (head and cup) with a 28 mm head diameter. Particles were only available from the 0.5 – 1 million cycles test period. The tests were conducted in newborn calf serum (30 g/l protein content) at 37 °C. Particles from this group are referred to as HS (hip simulator) particles.
The third group was commercially available chromium oxide-particles (Sigma-Aldrich, St. Louis, USA) which were used as reference samples. The particles were available in powder form with a nominal average size of 60 nm. The powder had a matt green color as it is typical for this type of chromium oxide (Cr2O3).
2.2. Particle isolation
The testing fluid (bovine serum) containing the wear particles was stored at −20 °C until needed for further analysis. Proteins disturb the image contrast, increase the degree of agglomeration and inhibit the identification of single particles. Therefore, several different protocols have been described in the literature [18–23] to isolate particles from the protein rich environment of the testing fluid. Compared to polyethylene particles, the isolation of metallic wear particles requires a more sophisticated protocol using enzymatic digestion to remove organic components, especially proteins, without damaging the particles. Particles in the present study were isolated following the protocol developed by Catelas et al [18, 19, 24], as briefly described below, with minor changes. This protocol was shown to minimize particle damage [18, 24]. It consists of several washing as well as incubation steps with two different enzymes, papain and proteinase K. Papain is a cysteine protease enzyme. Its main mechanism of action is through breaking of peptide bonds. Proteinase K is a broad spectrum serine protease. This enzyme is known for its protein denaturing abilities, especially in presence of reagents like etylenediaminetetraacetic acid (EDTA).
Summary of particle isolation procedure (see [19] for more details): The amount of testing fluid retrieved from the laboratory tests ranged from 60 ml (hip simulator tests) up to 300 ml (sliding wear test rig). Fluid samples were aliquoted into 40 ml tubes and centrifuged at 18,000 × g for 15 minutes using a Sorvall RC-5B superspeed centrifuge (particles from the different aliquots of the same testing fluid sample were recombined after the first overnight incubation). Supernatants were discarded except 1.5 ml in order to resuspend the pellets and transfer them in microcentrifuge tubes. All centrifugations were performed for 10 minutes at 18,000 × g. First, the pellets were resuspended in 2.5 % Sodium Dodecyl Sulfate (SDS) (v/v distilled water) and boiled for 10 minutes. This was followed by one wash in 80 % acetone and three washes in 1 ml of 250 mM sodium phosphate buffer solution (PBS) containing 25 mM EDTA at pH 7.4. After 30 seconds of sonification, papain (3.2 U in 1 ml PBS/EDTA) was added. The samples were then incubated overnight on an Eppendorf thermo-mixer at 65 °C under slight motion. After the incubation, pellets from aliquots of the same initial fluid sample were combined in microcentrifuge tubes. Pellets were then resuspended in 2.5% SDS, boiled again for 10 min and then washed twice in 1 ml of 50 mM Tris-HCl pH 7.6. Before adding proteinase K (3 U in 1 ml Tris-HCl), the pellets were sonicated for 30 seconds. A second overnight incubation was conducted at 55 °C under slight motion. After the incubation, the samples were boiled again in 2.5% SDS. The pellets were then washed once in 1ml of 50 mM Tris-HCl, once in 3 % SDS (v/v 80 % acetone) and once in distilled, deionized water. At the end of the isolation protocol, the pelleted particles were stored in 100 % ethanol at 4 °C. In a few cases, the pellets still contained denatured organic components. For those cases, the second overnight incubation was repeated. This may have been due to the use of 40 ml initial aliquots instead of 15 ml as previously described in [19].
Particles were then embedded in epoxy resin for TEM analysis as described by Catelas et al. [18, 19]. Prior to embedding, the particles were centrifuged for 20 minutes at 18,000 × g. The supernatant was removed and the particles were resuspended in a solution of 0.5 ml acetone and 0.5 ml liquid epoxy resin (Epoxy 3000, Cloeren Technology GmbH, Wegberg, Germany). Microcentrifuge tubes were then placed on a thermomixer overnight under slight motion at room temperature to allow pellet infiltration by the resin. The tubes were further centrifuged for 20 minutes at 18,000 × g and vacuumed for 1 hour to evaporate the acetone. One ml of epoxy resin was added and the tubes were placed in a vacuum chamber for 1 hour to remove potential air bubbles and trace of remaining acetone. The samples were then placed in an oven at 60 °C for one hour to harden the epoxy resin. Once hardened, the transparent epoxy cone at the tip of the tubes showed the embedded particles. Approximately 100 nm thick sections were cut using a diamond knife and placed on carbon film coated copper grid nets (formvar carbon-film S162-4, Plano GmbH, Wetzlar, Germany) for TEM analysis. It was mandatory to place the samples on carbon film grid nets in order to provide sufficient stability under high TEM voltage. Particles placed on pure copper grid nets were not stable under an electron beam higher than 120 kV.
The purchased particles were only suspended in ethanol. After centrifugation they were embedded in epoxy resin and prepared for TEM analysis as described above.
2.3. Microscopy
Particles were visualized under TEM. Two different TEMs were employed: An EM 400 (Philips, Eindhoven Netherlands) with a voltage of 120 kV and a Tecnai F20 (FEI, Eindhoven, Netherlands) with a voltage of 200 kV. The latter also allowed for electron dispersive x-ray spectroscopy (EDS) and energy filtered TEM mode (EFTEM). EDS analysis was used to determine the chemical composition of the particles. If the sections were thin enough, EFTEM mapping could be employed to visualize the element distribution. Electron diffraction pattern analysis was used in order to gain information about the particle lattice structures. The number of analyzed particles for each section varied from 10–50. Eight sections were viewed with TEM for both CoB and HS samples, and two sections for the commercially available chromium oxide particles.
For comparison, TEM analysis was also performed in the subsurface zone of the bar samples prior to and after testing. The necessary cross section preparation method is described in [25, 26].
3. Results
3.1. Particles from the cylinder-on-bar tribometer
The wear mechanisms observed on bar samples tested in the reciprocating sliding wear tribometer differed from those described in literature for retrieved MoM hip joints [27]. Grooves in sliding direction were a result of three-body abrasive wear during running-in caused by carbides (Fig. 3). TEM images demonstrated a nano-crystalline (nc) subsurface zone prior to testing as it was reported to form in MoM hip joint in-situ [27, 28]. After 1 million cycles of testing, a nano-crystalline zone could be observed as well (Fig. 4). It appeared that, similar to MoM hip joints, this nc-zone was a result of ongoing grain refinement and fcc → hcp phase transformation.
Fig. 3.

Grooves in sliding direction on the bar sample after testing; dark spots refer to subsurface carbides
Fig. 4.
Nano-crystalline subsurface zone of CoCrMo bar (a) prior to and (b) after testing in cylinder-on-bar tribometer
The CoB particles had a long shape with a length of 300 – 800 nm and a width of 100 – 300 nm (Fig. 5). The result of the electron diffraction pattern analysis revealed clearly the hexagonal closed packed ε-martensite. The same lattice structure and chemical composition was observed as in the subsurface zone of the articulating surfaces. The thickness of these particles was too large to perform an accurate EFTEM analysis. However, the lattice structure clearly indicated a CoCrMo solid solution. Thus, extensive oxidation can be excluded.
Fig. 5.
Bright field images and electron diffraction pattern of CoB particles; electron diffraction pattern analysis revealed hcp lattice of ε-martensite
3.2. Particles from hip simulator
Three different types of HS particles were identified. The first type (type I) of HS particle consisted mostly of oxygen and chromium, but exhibited cobalt locally. The second type (type II) of HS particles exhibited only chromium and oxygen. Both, type I and II, had a particle size of 30 – 80 nm. In Fig. 6 a type I and type II particle are shown located next to each other. EFTEM mapping not only displayed the chemical composition but also the exact location of single elements (Fig. 7). It can be seen that the type II particles had a very high intensity in the oxygen map. A strong signal was also received from chromium, but the cobalt map showed only background noise. The type I particles exhibited similar appearances on one side, whereas on the other side the cobalt map showed a relatively high intensity. Hence, the cobalt did not form a core but appeared localized at one side of the type I particles. An attempt of taking a diffraction pattern image by TEM failed. Only an amorphous pattern occurred (Fig. 8). However, crystalline structures could be observed in the cobalt rich area when using high resolution mode (Fig. 8). Judging from the bright field image, it appeared that smaller particles were flaking off the surface of the type II particles, generating a third type of particles (type III).
Fig. 6.
Bright field image of single type I and type II HS particles. EDS revealed high peaks for chromium and oxygen in both types. Type I particles additionally exhibited peaks of cobalt and molybdenum
Fig. 7.
EFTEM-mapping of the elements chromium (Cr), cobalt (Co), oxygen (O) and carbon (C) on type I and II HS particles
Fig. 8.
(a) Electron diffraction pattern of type I HS particle exhibited no crystalline structure, (b) high resolution imaging revealed crystalline structures in the cobalt rich areas of type I HS particles
These type III particles consisted dominantly of chromium and oxygen and were smaller in size, ranging from 5 to 15 nm (Fig. 9). Accumulations of these predominantly occurring particles were found in every epoxy section. The diffraction pattern of type III particles (Fig. 10) revealed the lattice structure of Cr2O3.
Fig. 9.
Bright field (BF) image and EFTEM-mapping of the elements chromium cobalt and oxygen on type III HS particles
Fig. 10.
bright field (BF) and dark field image (DF) of type III HS particles and the corresponding diffraction pattern
Finally, bright field images visualized defects in the lattice structure, suggesting that these type III particles had been exposed to high shear stress.
3.3. Purchased particles
Numerous particles could be visualized by TEM. The particle size varied largely. Even though the majority of particles appeared to have a size of 40 – 80 nm, many particles exhibited dimensions of 100 – 200 nm (Fig. 11). The diffraction pattern of these particles gave a marked ring pattern which pointed clearly to the structure of Cr2O3. So, the structure was identical to those observed with the type III HS particles. Bright field images showed that the particle surface of the commercially available particles was much smoother than that of type III HS particles retrieved from the testing fluid. This can be explained by the generation process of these particles. In contrast to particles generated during wear tests, commercially available chromium-oxide particles did not undergo mechanical load and tribological stresses. Therefore, the surface exhibited no defects and the particle structure remained defect-free.
Fig. 11.
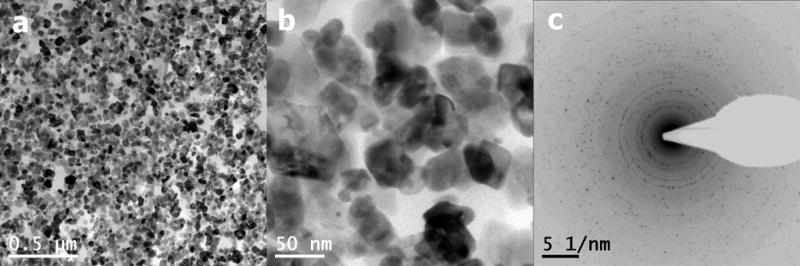
(a,b) Bright field images and (c) electron diffraction pattern of purchased Cr2O3-particles
4. Discussion
Particles generated in a given tribosystem are mostly suspected to have the same structure and chemical composition as the material close to the worn surfaces. This was verified for CoB particles. Even though the thickness of these particles was too large to perform an accurate analysis of the chemical composition, the results of the electron diffraction pattern analysis confirm the same crystal structure as the deformed CoCrMo alloy. Thus, the particles were not oxidized although a thin oxide film of 1 to 2 nm had most likely formed on the surface but could not be visualized. The fact that the particle size was significantly larger than the crystal size in the subsurface zone of the bar sample suggests that different wear mechanisms must have been active as it is the case for MoM hip joints [29, 30]. This is mainly linked to the test conditions (reciprocating vs. multidirectional sliding) and the fact that body and counter body had non-conforming surfaces.
Different results could however be expected for wear particles generated from tribosystems operating under ultra-low wear conditions such as MoM hip joints. In such systems, particles have been shown to be generated from an in-situ formed tribolayer rather than the underlying deformed subsurface zones [30–32]. Thus, the wear particle material is altered by strain, mechanical-mixing and tribo-chemical reactions prior to its detachment from the surface. This tribolayer has been well described in the literature [28, 29]. Büscher et al. reported the in-situ formed nc-zone in MoM hip joints as key factor for the generation of wear debris within the nm-range [27]. Interestingly, the grain size in that zone strongly correlates with the observed size of type I and II particles in the present study. Wimmer et al. further pointed out the importance of denatured proteins forming a tribofilm upon the bearing surfaces [25, 31]. Due to mechanical-mixing carbon-rich material from organic origin would get incorporated between the crystals of the nc-zone forming a metallo-organic composite (tribolayer) [26, 33]. In addition, plastic flow due to the displacement and/or rolling of entire nano-size grains would allow for low wear under normal sliding conditions. Fig. 12 depicts how single particles can be embedded within the carbon rich material. However, the exact mechanism of particle detachment is yet unclear. Also the surface state of such particles is unknown. A recent study has shown particles that were not completely oxidized and still exhibited both cobalt and chromium in the joint capsule [34]. Phosphate and carbon groups were found to bond to the surface of these particles. Furthermore, it was shown that organic tribofilms on bulk surfaces provide a type of passivation, but not by means of an oxide film [35]. Thus, wear particles might be protected by organic films against chemical alteration.
Fig. 12.

Mechanical-mixed subsurface zone of a MoM hip joint femoral heads’ bearing surface: single grains embedded in carbon rich zone
With regards to the analysis of HS particles isolated in the present study, it should be underlined that only serum samples from simulator tests within the transition from the running-in to the steady state regime were available (0.5 – 1 million cycles). Hence, the amount of particles was significantly lower than it would have been expected for samples from running-in wear (0 – 0.5 million cycles). The majority of the particles appeared to be chromium oxide, which corroborates with most literature studies [19, 22, 36, 37]. However, particles containing cobalt (type I particles) were also found, similar to those described by Catelas et al. [19], who also stated that the number of such particles was relatively low under steady-state conditions. Interestingly, results from the present study suggest that cobalt would not form the core of type I HS particles but instead, it would only be present locally, on one side of the particles. Indeed, the type I particles exhibited a crystalline structure only in this specific area whereas the rest of the particles was amorphous. Hence, these results ruled out the possibility that the chromium oxide particles described in previous studies using only EDS exhibited a cobalt core. The type II particles showed a comparable appearance and size but consisted only of chromium and oxygen and were completely amorphous. The surface of these particles appeared to be torn with particles flaking off giving rise to a third type of HS particles (type III) which depicted the crystalline structure of Cr2O3. Earlier studies suggested that particles consisting only of chromium and oxygen may originate from the passive oxide film on the bearing surface, which would get disrupted during sliding. However, such oxide film, which most likely exhibits a thickness of 2 to maximum 10 nm [38], may be too thin to be at the origin of the tribologically generated type III particles. Commercially available Cr2O3 particles were completely crystalline and showed no lattice defects in contrast to type III HS particles. This indicates that type III HS particles were likely subjected to high mechanical and chemical reactions enabled by strain and protein interaction.
Under bulk conditions, one would expect the surface of CoCrMo to form a protective oxide film inhibiting chemical alteration. This appeared to be the case for the rather large CoB particles as no alteration of the lattice structure was observed compared to the nano-crystalline subsurface zone of head and cup. Thus, the particles surface must have been covered with a thin oxide film preventing alteration of the lattice structure and the chemical composition. However, nanoparticles are known to possess unique properties like the decrease of the melting temperature [39] or reversible transition to the amorphous state under load [32, 40]. Usually, so called size effects are well known for particles being smaller than 20 nm. Even though type I and II HS particles were considerably larger than 20 nm, several parameters may have interfered. First, the surface/volume ratio was very high compared to larger particles (> 100 nm) as for example the observed CoB particles. Second, originating from the nc-zone, the particles were exposed to high shear stress as depicted by a high density of lattice defects in type III HS particles, and thus, had a larger inner energy and were highly reactive. Also the transformation to the amorphous state in type I and II HS particles may be related to this effect. Third, due to the curvature of the particles, there would be more vacancies at the surface, as well as a higher degree of lattice strain, both contributing to a high diffusion rate of elements like oxygen and cobalt. Taking all these facts into account, it appears that particles generated from the nc-zone could likely exhibit pyrophoric properties.
One may question if the particle isolation procedure may alter the particle shape (through the centrifugation steps) or chemical composition (through the elimination of the denatured proteins covering the particle surface). Because of the small size of the particles, the applied pressure on the particles under 18,000 × g is negligible. Therefore, it is unlikely that the particle morphology would be altered due to the centrifugation process. On the other hand, it is possible that through the removal of the organic components to isolate the particles from the new born calf serum, the protective organic film that initially covers the particles would also be removed, hence possibly leading to a change in the chemical composition of the particles. However, when looking at the effects of the isolation protocol on wear particles isolated from 95% serum, Catelas et al. did not show any significant levels of Co ions released in solution after applying the enzymatic treatments on the particles, and also showed that organic serum components adhering to the particles did not seem to be removed during the isolation procedure [24]. Hence, the origin of the large amount of chromium oxide particles still remains uncertain. An alternative scenario of particle chemical alteration may be linked to their dwell period within the contact area of the bearing surfaces. Acting as third-body, such particles are too small in size to cause damage to the surface. However, before particles exit the tribological interface, they may be exposed to high mechanical load when trapped between surface asperities of head and cup during contact. A protective film of denatured proteins could be partly disrupted allowing for oxidation. This, however, contrasts with the recent observations in [34], showing particles with a high amount of cobalt within the tissue of the joint capsule. Hence, at this point, the exact pathways of particles generation and detachment into the joint environment still remain unclear.
5. Summary and Outlook
Wear particles generated from CoCrMo alloy in two different tribometers were analyzed. Particles retrieved from a cylinder-on-bar-tribometer were about ten times larger than particles retrieved from a hip simulator. They exhibited the exact same chemical composition and lattice structure as the near-surface material. These particles would detach from below the nc-zone due to the more severe tribological conditions of the test setup.
The hip simulator particles had a size range from 5 – 70 nm. Three different types of particles were identified by means of size, chemical composition and lattice structure. Such particles would originate from the nc-zone or, more precisely, the mechanically-mixed zone. A possible pathway would be that a single grain within the nc-zone is transported due to the plastic flow of entire grains to the mechanically-mixed zone where carbon-rich denatured organic material as well as phosphates settle at the grain boundaries. During ongoing plastic flow, such grain could be transported to the uppermost surface where it would stick in a film of denatured proteins. Eventually, the grain would be pushed out from the tribological interface and form a single particle. At this point, the particle would still exhibit a relatively high amount of cobalt as well as chromium, and be protected by a shell of organic material [34, 41]. In that case, it would suggest that the particle isolation protocol may have removed the organic protective film on the particle surface, resulting in the activation of size effects that would lead to an increased pyrophoric behavior of the particles. However, at this point, it is still unclear which alterations a wear particle goes through between its generation and its detachement in the joint environment. It is possible that particles undergo already significant transformation and changes in chemical composition in the contact area of the head and cup within the tribological interface leading to the disruption of the organic protective film and thus particle oxidation. This remains to be further investigated.
In order to gain more precise information about the nature of wear particles generated in MoM hip joints, it is essential to analyze particles from periprosthetic tissues using TEM. In addition, EFTEM mapping and EELS (electron energy loss spectroscopy) need to be applied for more accurate determination of the particles chemical composition. Finally, in order to determine whether particles are crystalline or not and to gain knowledge of the occurring lattice structures, electron diffraction pattern analysis needs to be applied as well.
Acknowledgments
The authors would like to acknowledge Ronnie Seeger, Birgit Gleising, Marco Joeken and Priska Stemmer for their support and excellent sample preparation. Further, we would like to thank Prof. W. Dudzinski (Technical University, Wroclaw, Poland) for preparing microtome foils, Prof. Ralf Küppers and Heike Heise (Institut für Zellforschung, Universitätsklinikum Essen, Germany) for providing the high performance centrifuge and Prof. Michael Farle (Faculty of Physics, AG Farle, University Duisburg-Essen, Germany) for providing access to the high resolution TEM. A part of this work was sponsored by NIH under grant RC2 AR058993-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McKee GK, Watson-Farrar J. Replacement of Arthritic Hips by the McKee-Farrar Prosthesis. J Bone Joint Surg Br. 1966;48-B:245. [PubMed] [Google Scholar]
- 2.Semlitsch M. 30 Jahre CoCrMoC Metall/Metall-Gleitpaarung bei Hüftgelenkendoprothesen mit wenig Verschleiβ. In: Schmidt M, editor. Die Metallpaarung ≪Metasul≫ in der Hüftendoprothetik. Bern: Verlag Hans Huber; 1995. [Google Scholar]
- 3.Ingham E, Fisher J. The role of macrophages in osteolysis of total joint replacement. Biomaterials. 2005;26:1271. doi: 10.1016/j.biomaterials.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 4.Schmalzried TP, Szuszczewicz ES, Akizuki KH, Petersen TD, Amstutz HC. Factors Correlating With Long Term Survival of McKee-Farrar Total Hip Prostheses. Clinical Orthopaedics and Related Research. 1996;329:S48. doi: 10.1097/00003086-199608001-00005. [DOI] [PubMed] [Google Scholar]
- 5.Brown SR, Davies WA, DeHeer DH, Swanson AB. Long-Term Survival of McKee-Farrar Total Hip Prostheses. Clinical Orthopaedics and Related Research. 2002;402:157. doi: 10.1097/00003086-200209000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Jacobsson S-A, Djerf K, Wahlström O. 20-Year Results of McKee-Farrar Versus Charnley Prosthesis. Clinical Orthopaedics and Related Research. 1996;329:S60. doi: 10.1097/00003086-199608001-00006. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs JJ, Hallab NJ. Loosening and osteolysis associated with metal-on-metal bearings: A local effect of metal hypersensitivity? J Bone Joint Surg Am. 2006;88:1171. doi: 10.2106/JBJS.F.00453. [DOI] [PubMed] [Google Scholar]
- 8.Kim RH, Dennis DA, Carothers JT. Metal-on-Metal Total Hip Arthroplasty. The Journal of Arthroplasty. 2008;23:44. doi: 10.1016/j.arth.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Dowson D, Hardaker C, Flett M, Isaac GH. A hip joint simulator study of the performance of metal-on-metal joints: Part I: The role of materials. The Journal of Arthroplasty. 2004;19:118. doi: 10.1016/j.arth.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Ingham E, Fisher J. Biological reactions to wear debris in total joint replacement. Proceedings of the Institution of Mechanical Engineers, Part H: Journal of Engineering in Medicine. 2000;214:21. doi: 10.1243/0954411001535219. [DOI] [PubMed] [Google Scholar]
- 11.Brodner W, Bitzan P, Meisinger V, Kaider A, Gottsauner-Wolf F, Kotz R. Serum Cobalt Levels After Metal-on-Metal Total Hip Arthroplasty. J Bone Joint Surg Am. 2003;85:2168. doi: 10.2106/00004623-200311000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Urban RM, Jacobs JJ, Tomlinson MJ, Gavrilovic J, Black J, Peoc’h M. Dissemination of Wear Particles to the Liver, Spleen, and Abdominal Lymph Nodes of Patients with Hip or Knee Replacement. J Bone Joint Surg Am. 2000;82:457. doi: 10.2106/00004623-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Bhabra G, Sood A, Fisher B, Cartwright L, Saunders M, Evans WH, Surprenant A, Lopez-Castejon G, Mann S, Davis SA, Hails LA, Ingham E, Verkade P, Lane J, Heesom K, Newson R, Case CP. Nanoparticles can cause DNA damage across a cellular barrier. Nat Nano. 2009;4:876. doi: 10.1038/nnano.2009.313. [DOI] [PubMed] [Google Scholar]
- 14.Willert H-G, Buchhorn GH, Fayyazi A, Flury R, Windler M, Koster G, Lohmann CH. Metal-on-Metal Bearings and Hypersensitivity in Patients with Artificial Hip Joints. A Clinical and Histomorphological Study. J Bone Joint Surg Am. 2005;87:28. doi: 10.2106/JBJS.A.02039pp. [DOI] [PubMed] [Google Scholar]
- 15.Clayton RAE, Beggs I, Salter DM, Grant MH, Patton JT, Porter DE. Inflammatory Pseudotumor Associated with Femoral Nerve Palsy Following Metal-on-Metal Resurfacing of the Hip. A Case Report. J Bone Joint Surg Am. 2008;90:1988. doi: 10.2106/JBJS.G.00879. [DOI] [PubMed] [Google Scholar]
- 16.Pandit H, Glyn-Jones S, McLardy-Smith P, Gundle R, Whitwell D, Gibbons CLM, Ostlere S, Athanasou N, Gill HS, Murray DW. Pseudotumours associated with metal-on-metal hip resurfacings. J Bone Joint Surg Br. 2008;90-B:847. doi: 10.1302/0301-620X.90B7.20213. [DOI] [PubMed] [Google Scholar]
- 17.Campbell P, Ebramzadeh E, Nelson S, Takamura K, De Smet K, Amstutz H. Histological Features of Pseudotumor-like Tissues From Metal-on-Metal Hips. Clinical Orthopaedics and Related Research®. 2010;468:2321. doi: 10.1007/s11999-010-1372-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Catelas I, Bobyn JD, Medley JB, Krygier JJ, Zukor DJ, Petit A, Huk OL. Effects of digestion protocols on the isolation and characterization of metal-metal wear particles. I. Analysis of particle size and shape. Journal of Biomedical Materials Research. 2001;55:320. doi: 10.1002/1097-4636(20010605)55:3<320::aid-jbm1020>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Catelas I, Bobyn JD, Medley JB, Krygier JJ, Zukor DJ, Huk OL. Size, shape, and composition of wear particles from metal-metal hip simulator testing: Effects of alloy and number of loading cycles. Journal of Biomedical Materials Research Part A. 2003;67A:312. doi: 10.1002/jbm.a.10088. [DOI] [PubMed] [Google Scholar]
- 20.Doorn PF, Campbell PA, Worrall J, Benya PD, McKellop HA, Amstutz HC. Metal wear particle characterization from metal on metal total hip replacements: Transmission electron microscopy study of periprosthetic tissues and isolated particles. Journal of Biomedical Materials Research. 1998;42:103. doi: 10.1002/(sici)1097-4636(199810)42:1<103::aid-jbm13>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 21.Firkins PJ, Tipper JL, Saadatzadeh MR, Ingham E, Stone MH, Farrar R, Fisher J. Quantitative analysis of wear and wear debris from metal-on-metal hip prostheses tested in a physiological hip joint simulator. Bio-Medical Materials and Engineering. 2001;11:143. [PubMed] [Google Scholar]
- 22.Billi F, Benya P, Ebramzadeh E, Campbell P, Chan F, McKellop HA. Metal wear particles: What we know, what we do not know, and why. SAS Journal. 2009;3:133. doi: 10.1016/j.esas.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown C, Williams S, Tipper J, Fisher J, Ingham E. Characterisation of wear particles produced by metal on metal and ceramic on metal hip prostheses under standard and microseparation simulation. Journal of Materials Science: Materials in Medicine. 2007;18:819. doi: 10.1007/s10856-006-0015-z. [DOI] [PubMed] [Google Scholar]
- 24.Catelas I, Bobyn JD, Medley JJ, Zukor DJ, Petit A, Huk OL. Effects of digestion protocols on the isolation and characterization of metal-metal wear particles. II. Analysis of ion release and particle composition. Journal of Biomedical Materials Research. 2001;55:330. doi: 10.1002/1097-4636(20010605)55:3<330::aid-jbm1021>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 25.Pourzal R, Theissmann R, Williams S, Gleising B, Fisher J, Fischer A. Subsurface changes of a MoM hip implant below different contact zones. Journal of the Mechanical Behavior of Biomedical Materials. 2009;2:186. doi: 10.1016/j.jmbbm.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Büscher R. Materials Sceine and Engineering. Ph.D. Duisburg: University Duisburg-Essen; 2005. Gefügebildung und Partikelbildung in künstlichen Metall/Metall-Hüftgelenken. [Google Scholar]
- 27.Büscher R, Gleising B, Dudzinski W, Fischer A. Transmission electron microscopy examinations on explanted metal-on-metal hip joints. Praktische Metallographie. 2005;42:15. [Google Scholar]
- 28.Büscher R, Fischer A. The pathways of dynamic recrystallization in all-metal hip joints. Wear. 2005:887. [Google Scholar]
- 29.Wimmer MA, Loos J, Heitkemper M, Fischer A. The Acting Wear Mechanisms on Metal-On-Metal Hip Joint Bearings - In-Vitro Results. Wear. 2001;250:129. [Google Scholar]
- 30.Godet M. The third-body approach: A mechanical view of wear. Wear. 1984;100:437. [Google Scholar]
- 31.Wimmer MA, Sprecher C, Hauert R, Täger G, Fischer A. Tribochemical Reaction on Metal-On-Metal Hip Joint Bearings - A Comparison between in-vitro and in-vivo Results. Wear. 2003;255:1007. [Google Scholar]
- 32.Scherge M, Martin JM, Pöhlmann K. Characterization of wear debris of systems operated under low wear-rate conditions. Wear. 2006;260:458. [Google Scholar]
- 33.Pourzal R, Theissmann R, Morlock M, Fischer A. Micro-structural alterations within different areas of articulating surfaces of a metal-on-metal hip resurfacing system. Wear. 2009;267:689. [Google Scholar]
- 34.Goode AE, Karunakaran C, Hart A, Porter A, Ryan MP, McComb DW. Correlative Microscopy of Nanometre Sized Particles in Human Tissue Surrounding Hip Replacements. MRS Fall Meeting; Boston, USA. 2009. [Google Scholar]
- 35.Schymura M, Mathew MT, Pourzal R, Wimmer M, Fischer A. EUROCORR. Nice; France: 2009. Influence of Protein and Micro-Structure on the Passive Film Formation on MoM Hip Replacements. [Google Scholar]
- 36.Catelas I, Medley JB, Campbell PA, Huk OL, Bobyn JD. Comparison of in vitro with in vivo characteristics of wear particles from metal–metal hip implants. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2004;70B:167. doi: 10.1002/jbm.b.20036. [DOI] [PubMed] [Google Scholar]
- 37.Catelas I, Campbell P, Bobyn J, Medley J, Huk O. Wear particles from metal-on-metal total hip replacements: effects of implant design and implantation time. The Proceedings of the Insitution of Mechanical Engineers. 2006;220:195. doi: 10.1243/09544119JEIM112. [DOI] [PubMed] [Google Scholar]
- 38.Gilbert JL. Metals. In: Callaghan JJ, Rosenberg AA, Rubash HE, editors. The Adult Hip. Vol. 1. Philadelphia: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 39.Schierning G, Theissmann R, Wiggers H, Sudfeld D, Ebbers A, Franke D, Witusiewicz, Apel M. Microcrystalline silicon formation by silicon nanoparticles. Journal of applied physics. 2008:103. [Google Scholar]
- 40.Mosey NJ, Muser MH, Woo TK. Molecular Mechanisms for the Functionality of Lubricant Additives. Science. 2005;307:1612. doi: 10.1126/science.1107895. [DOI] [PubMed] [Google Scholar]
- 41.Vasnyov S, Speller S. Institute for Molecules and Materials. Radboud University; Nijmegen, Netherlands: com: 2010. [Google Scholar]









