Abstract
Exaggerated inflammatory responses and the resultant increases in alveolar-capillary permeability underlie the pathogenesis of acute lung injury during sepsis. This study examined the functions of aldose reductase (AR) in mediating acute lung inflammation. Transgenic mice expressing human AR (ARTg) were used to study the functions of AR since mice have low intrinsic AR activity. In a mild cecal ligation and puncture model, ARTg mice demonstrated an enhanced AR activity and a greater inflammatory response as evaluated by circulating cytokine levels, neutrophil accumulation in the lungs, and activation of Rho kinase in lung endothelial cells (ECs). Compared with WT lung cells, ARTg lung cells produced more IL-6 and showed augmented JNK activation in response to LPS stimulation ex vivo. In human neutrophils, AR activity was required for fMLP-included CD11b activation and up-regulation, respiratory burst, and shape changes. In human pulmonary microvascular ECs, AR activity was required for TNF-α-induced activation of the Rho kinase/MKK4/JNK pathway and IL-6 production, but not p38 activation or ICAM-1 expression. Importantly, AR activity in both human neutrophils and ECs was required for neutrophil adhesion to TNF-α-stimulated ECs. These data demonstrate a novel role for AR in regulating the signaling pathways leading to neutrophil-EC adhesion during acute lung inflammation.
Sepsis and subsequent multiple organ failure such as the development of acute respiratory distress syndrome (ARDS)5 and acute lung injury (ALI) remain the leading cause of mortality in patients in the intensive care units (1). The exaggerated inflammatory responses of the host following infection and the resultant increases in alveolar-capillary permeability and edema formation underlie the pathogenesis of ARDS and ALI (2). Systemic or locally produced inflammatory mediators and/or intrapulmonary sequestrated neutrophils may all target pulmonary endothelium to induce intracellular signaling events, resulting in endothelial barrier dysfunction. Therapeutic interventions targeting cytokines such as TNF-α and IL-1β, endotoxins, or neutrophil functions have yet to impart significant improvement in clinical outcomes for patients with sepsis and ARDS/ALI. In this context, our study focused on the impact of upstream events of inflammatory signals in sepsis. Specifically, we have focused on the role of the polyol pathway in mediating inflammatory signaling in sepsis.
The polyol pathway includes the rate-limiting enzyme aldose reductase (AR) and sorbitol dehydrogenase (SDH). The substrates for AR include glucose and other saturated and unsaturated aldehydes (3, 4). Substrate flux via AR requires NADPH, while SDH requires NAD+. NADPH is also required for glutathione reductase activity, which is a major antioxidant mechanism in many cells, including endothelial cells (ECs). Therefore, an increased flux of glucose via AR competes with glutathione reductase for NADPH, resulting in a redox imbalance and oxidative stress (5). Experimental evidence has indeed linked flux via AR to increases in oxidative stress in hyperglycemic and ischemic states (6–8). AR has been implicated in the development of microvascular dysfunction during diabetes (8, 9). In addition, AR has been shown to mediate ischemia-reperfusion injury in both diabetic and nondiabetic animals (8, 10). Recently, inhibition of AR was shown to prevent inflammatory cytokine generation and cardiac dysfunction induced by LPS in mice (11).
The potential link between increased flux via AR and oxidative stress led us to hypothesize that increased flux via AR may promote inflammation by modulating signaling pathways that depend on reactive oxygen species. Both acute and sustained activation of JNK, for example, requires production of reactive oxygen species and plays important roles in regulating the expression of inflammatory cytokines such as IL-6, thereby promoting acute inflammation (12–16). In this study, we induced a clinically relevant model of sepsis by the cecal ligation and puncture (CLP) protocol to address the role of AR in mediating inflammatory signaling in the murine lung. CLP-induced sepsis is polymicrobial in nature, slow in onset, and prolonged in course. Since AR activity in the mouse is known to be severalfold less than that in the rat and humans, transgenic mice expressing human AR (ARTg) and their nontransgenic littermates were used to test the hypothesis that AR plays important roles in regulating the progression of pulmonary inflammatory responses in sepsis. The acute inflammatory responses were evaluated by measuring systemic cytokine expression, neutrophil infiltration into the lungs, and activation of lung ECs. Furthermore, we investigated the signaling mechanisms that are regulated by AR during inflammation in human neutrophils and human pulmonary microvascular ECs.
Materials and Methods
Mice, AR activity, and CLP
All of the animal studies were performed with the approval of the Institutional Animal Care and Use Committee at Columbia University. Mice between 12 and 15 wk of age were used for the experiments. Mice transgenic for human AR (ARTg) driven by the MHC class I promoter were backcrossed 10 generations to the C57BL6 background as previously described (10).
Wild-type (WT) mice and ARTg mice were subdivided into three groups consisting of control, sham, and CLP. The CLP procedure was performed as described previously (17). Briefly, animals were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg). The appendix was identified and exteriorized along with the cecum. Cecum was ligated ~1 cm from the ileocecal junction and a single puncture was made in the cecum using a 22-gauge needle with the expression of a small amount of cecal content into the abdominal cavity. The cecum along with the appendix was replaced into the abdominal cavity and the anterior abdominal wall was closed in layers. Sham animals were subjected to a laparotomy consisting of opening the anterior abdominal wall and peritoneal cavity followed by closure of the abdominal wall in layers. The animals were sacrificed 24 h later to evaluate acute inflammatory responses. This model of CLP did not induce significant mortality within 24 h when compared with animals treated with the sham procedure.
AR activity in the lungs of untreated, sham-operated and CLP-treated WT C57BL6 mice and mice expressing human AR (ARTg) was evaluated by measuring NADPH consumption using D-glyceraldehyde as a substrate (10). One unit of AR activity is defined as nanomoles of NADPH consumed per milligram of lung tissue proteins per minute.
Evaluation of acute inflammatory responses in mice in vivo
The acute inflammatory responses following CLP were evaluated by measuring 1) circulating inflammatory mediators including IL-6, TNF-α, and C-reactive protein in plasma using ELISA, 2) neutrophil content in the bone marrow, 3) neutrophil accumulation in the lungs, and 4) the activity of Rho kinase in lung ECs.
The circulating IL-6 and TNF-α levels were measured in plasma using an ELISA kit according to the manufacturer’s instructions (eBioscience). Glucose levels in blood were measured by using a Nova Biomedical PHOx plus L analyzer.
To measure neutrophil content in the bone marrow, femurs were obtained from WT or AR-expressing mice, and their marrow cavities were flushed using ice-cold PBS. The single-cell suspension was analyzed for neutrophil content by staining for GR1, a cell surface marker for murine neutrophils, and CD11b using flow cytometry (Abs were obtained from eBioscience). The proportion of cells positively stained for GR1 and CD11b was determined using a FACSCalibur (BD Biosciences).
To evaluate neutrophil accumulation in the lungs, the single lung cell suspension was obtained by protease digestion and the proportion of neutrophils (GR1+) was determined by flow cytometry using protocols as recently described (11).
To evaluate the activity of Rho kinase in lung ECs following CLP, lung ECs were freshly isolated from lung single cell suspensions and Rho kinase activity in freshly isolated lung ECs was evaluated by measuring the phosphorylation state of a major Rho kinase substrate, myosin phosphatase targeting subunit 1 (MYPT-1), at threonine 853 by immunoblotting (Upstate Biotechnology) as recently described (18).
Evaluation of the effect of human AR expression on inflammatory responses ex vivo
The effect of human AR expression was examined ex vivo using cells isolated from untreated WT and ARTg mice. Bone marrow-derived neutrophils from untreated WT or ARTg mice were stimulated ex vivo using vehicle or fMLP, and cell surface expression of CD11b or shape changes were evaluated using flow cytometry.
In addition, single lung cells were isolated from WT mice and ARTg mice and these freshly isolated lung cells were stimulated using LPS. The supernatants were collected for cytokine measurement (TNF-α and IL-6) using ELISA, and the cell pellets were collected to evaluate JNK activation using immunoblot.
Human pulmonary microvascular endothelial cells, pharmacologic inhibitors, and activation of signaling pathways
Human pulmonary microvascular ECs (Cambrex) were grown on dishes precoated with 4 μg/ml fibronectin. Confluent ECs were treated with either vehicle (PBS containing 0.1% BSA) or 20 ng/ml recombinant human TNF-α for 5 min to 24 h by adding vehicle or a stock solution directly to EC cultures (12).
The role of AR was evaluated by pretreatment with a specific pharmacologic inhibitor, zopolrestat, for 24 h at the indicated concentration. The activity of JNK, p38, MKK4, and Rho kinase following TNF-α stimulation was evaluated by measuring the amount of phosphorylated and active forms of these kinases or their downstream effectors: phosphorylated JNK (threonine 183/tyrosine 185), p38 (threonine 180/tyrosine 182), MKK4 (serine 257/threonine 261), and myosin L chain (threonine 18/serine 19) (Cell Signaling), respectively. The expression of ICAM-1 on the EC surface was evaluated using flow cytometry (eBioscience). IL-6 production was evaluated using ELISA (eBioscience).
Human neutrophil isolation, neutrophil responses to chemoattractants, and neutrophil-endothelial cell adhesion
Blood was drawn from healthy human volunteers by venipuncture after obtaining informed consent. Human neutrophils were isolated using Histopaque density gradients (Sigma-Aldrich) according to the manufacturer’s protocols. The purity of isolated neutrophils was >95%. The role of AR in regulating neutrophil responses was examined by treating purified human neutrophils with vehicle or 200 μM zopolrestat for 30 min at room temperature.
The effect of AR inhibition on the up-regulation of CD11b on the neutrophil surface was evaluated using flow cytometry. Neutrophils treated with vehicle or zopolrestat were stimulated with control or 1 μM fMLP, and total cell surface CD11b as well as the active CD11b epitope was evaluated using an Ab that recognizes total CD11b (clone ICRF44) or CD11b activation epitope (clone CBRM1/5; eBioscience).
The effect of AR inhibition on superoxide production and respiratory burst was evaluated using a cytochrome c reduction assay (19). Neutrophils were incubated in 80 μM cytochrome c solution in the absence or presence of 150 U/ml superoxide dismutase. Either vehicle or 1 μM fMLP was added to each group, and absorbance at 550 nm was measured for 20 min at 37°C at a 1-min interval. The rate of superoxide dismutase-inhibitable reduction of ferricytochrome c at 550 nm was measured, and release of O2− was calculated as nanomoles of superoxide produced per 10 million cells per minute using an extinction coefficient of 22 nM−1cm−1.
The effect of AR inhibition on neutrophil-EC adhesion was evaluated by pretreating ECs or neutrophils with zopolrestat. Confluent ECs plated onto 96-well plates were treated with 20 ng/ml TNF-α along with vehicle or 200 μM zopolrestat for 24 h. After two washes with HBSS containing 1.2 mM Ca2+, 0.4 mM Mg2+, and 5.5 mM glucose, neutrophils treated with vehicle or zopolrestat were added to the ECs. The plate was gently centrifuged at 200 rpm/min for 2 min and neutrophils were allowed to adhere for 15 min. Nonadherent neutrophils were washed and the cells were fixed with 10% buffered formalin. The number of neutrophils that adhered was counted using an inverted microscope.
The effect of AR inhibition on neutrophil spreading on ECs during neutrophil-EC adhesion was evaluated using confocal microscopy. Neutrophils were labeled with 5 μg/ml CFSE, and neutrophils or ECs were treated with zopolrestat and neutrophils were allowed to adhere to ECs for 15 min as described above. The cells were washed and fixed with paraformaldehyde and stained for F-actin using rhodamine-phalloidin. Confocal images of different fields were acquired, and the projection area of neutrophils was quantitated using NIH ImageJ.
The effect of AR inhibition on neutrophil CD11b expression during neutrophil-EC adhesion was evaluated using flow cytometry. Neutrophils or ECs were treated with zopolrestat and neutrophils were allowed to adhere to ECs for 15 min as described above. Neutrophils that remained in the supernatant (nonadherent neutrophils) were removed, and neutrophils that were adherent to ECs were detached by cold EDTA containing PBS. The CD11b expression was evaluated using flow cytometry.
Statistical analysis
Data were analyzed using the Student t test or one-way ANOVA followed by post hoc comparisons (least significant difference test). A p <0.05 is considered significant. The data are expressed as the mean value ±SEM.
Results
Expression of human AR results in enhanced inflammatory responses induced by CLP
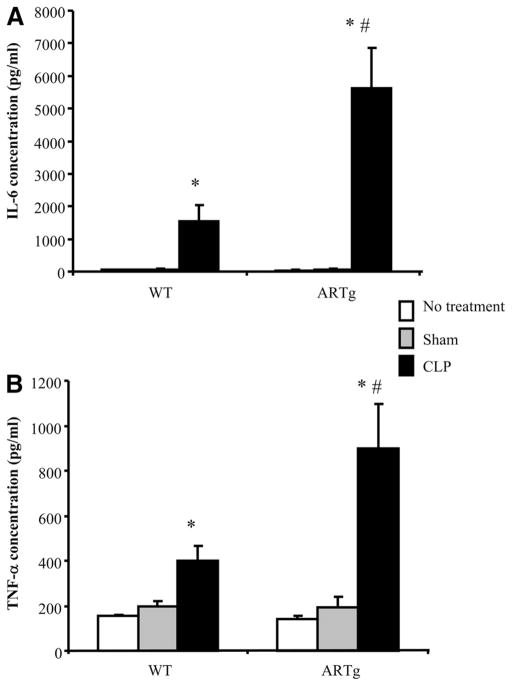
The acute inflammatory responses during the early phase of CLP-induced injury were compared between WT mice and mice expressing human AR (ARTg mice). In WT mice, circulating IL-6 levels increased significantly 24 h following the onset of CLP (Fig. 1A). This increase was significantly higher in ARTg mice vs WT mice (Fig. 1A). Similarly, increases in circulating TNF-α following CLP were significantly greater in ARTg mice compared with WT mice (Fig. 1B). In animals that received no operations or in sham-operated animals, the basal levels of these cytokines were similar between WT and ARTg mice. As has been shown in previous studies (20), the blood glucose level decreased following CLP and no difference was observed between WT and ARTg mice, either basally or following CLP (data not shown).
FIGURE 1.
Circulating IL-6 and TNF-α levels during CLP in WT mice and mice expressing human AR (ARTg). The expression of IL-6 (A) and TNF-α(B) was measured using ELISA. □, Mice receiving no treatment;
 , mice receiving sham procedure; and ■, mice receiving CLP. Data represent mean ± SEM (n =4–9 individual animals each group). *, p < 0.05 when compared with mice that received no treatment or sham-operated; #, p < 0.05 when compared with WT mice in the corresponding group.
, mice receiving sham procedure; and ■, mice receiving CLP. Data represent mean ± SEM (n =4–9 individual animals each group). *, p < 0.05 when compared with mice that received no treatment or sham-operated; #, p < 0.05 when compared with WT mice in the corresponding group.
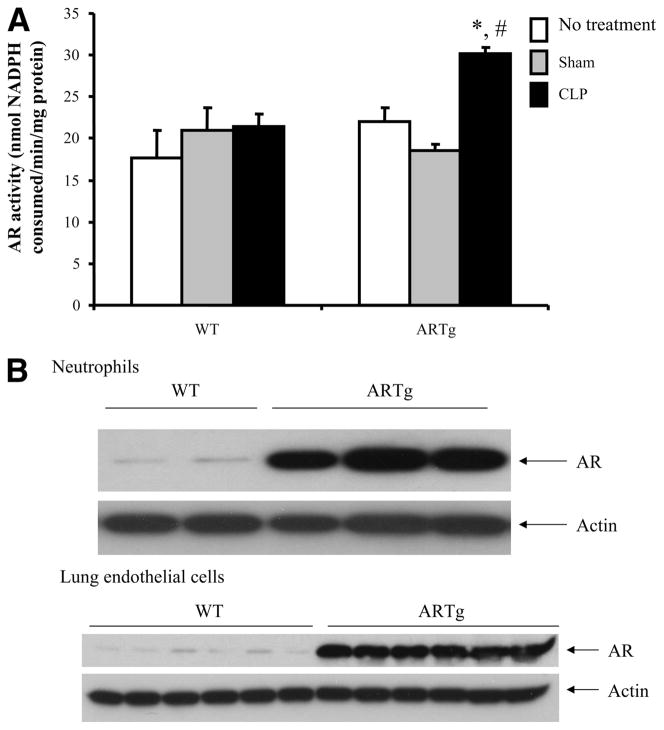
The activity of AR in the lungs of WT mice and ARTg mice following CLP was evaluated. In WT mice, which are known to exhibit low activity of AR, induction of CLP did not increase the activity of AR (Fig. 2A). However, in ARTg mice, the activity of AR was significantly increased following CLP (Fig. 2A). Examination of AR expression in neutrophils and in lung ECs showed that AR was expressed in both cell types in WT mice and that AR expression was markedly increased in both cell types in ARTg mice (Fig. 2B).
FIGURE 2.
A, AR activity was enhanced in the lungs of ARTg mice following CLP. Data represent mean ± SEM (n = 4–8 individual animals in each group). *, p < 0.05 when compared with sham-operated animals; #, p < 0.05 when compared with WT mice following CLP. B, AR expression was enhanced in both neutrophils and lung ECs of ARTg mice. Neutrophils were isolated from the bone marrow and ECs were isolated from mouse lungs. The expression of AR was examined using immunoblot.
In mice, the bone marrow contains the majority of total body neutrophils and only a small fraction of the total bone marrow neutrophils is released into circulation at steady state (21). In untreated mice or sham-operated mice, ~20% of total extracted bone marrow cells were positive for neutrophil markers GR1 and CD11b. The expression levels of CD11b and GR1 on mature vs immature neutrophils vary in the bone marrow. The more mature neutrophils express high levels of GR1, while the least mature ones express low levels of GR1 (22). Following CLP, the percentage of total neutrophils in the bone marrow decreased, indicating the release of bone marrow neutrophils in this early phase of inflammatory responses (Fig. 3A). Both mature neutrophils (high GR1 expressing) and immature neutrophils (low GR1 expressing) were released (Fig. 3A). This release response was similar between WT and ARTg mice (Fig. 3A).
FIGURE 3.
The role of human AR expression in regulating neutrophil release from the bone marrow (A), neutrophil accumulation in the lung (B), and Rho kinase activation in lung ECs (C). A, Neutrophil numbers similarly decreased in the bone marrow during CLP in WT and ARTg mice. Neutrophil number was evaluated by measuring percent CD11b+GR1+ cells in the bone marrow using flow cytometry. B, Neutrophil infiltration was increased in the lungs of ARTg mice. Accumulation of total neutrophils or immature neutrophils in the lung was evaluated by measuring GR1- expressing or low GR1-expressing cells using flow cytometry. □, Mice receiving no treatment;
 , mice receiving sham procedures; and ■, mice receiving CLP. Data represent mean ± SEM (n = 4–8 individual animals in each group). *, p < 0.05 when compared with mice that received no treatment or sham-operated; #, p < 0.05 when compared with WT mice in the corresponding group. C, Lung ECs were isolated from untreated control mice, sham-operated mice, or mice following CLP for 24 h. Rho kinase activation in freshly isolated lung ECs was examined by measuring the amount of phosphorylated myosin phosphatase targeting subunit 1 (Pi-MYPT; endogenous, 130 kDa), a major Rho kinase substrate. Total ezrin, radixin, and moesin (ERM, 80 kDa) was examined as a loading control. Each lane represents samples obtained from different individual animals.
, mice receiving sham procedures; and ■, mice receiving CLP. Data represent mean ± SEM (n = 4–8 individual animals in each group). *, p < 0.05 when compared with mice that received no treatment or sham-operated; #, p < 0.05 when compared with WT mice in the corresponding group. C, Lung ECs were isolated from untreated control mice, sham-operated mice, or mice following CLP for 24 h. Rho kinase activation in freshly isolated lung ECs was examined by measuring the amount of phosphorylated myosin phosphatase targeting subunit 1 (Pi-MYPT; endogenous, 130 kDa), a major Rho kinase substrate. Total ezrin, radixin, and moesin (ERM, 80 kDa) was examined as a loading control. Each lane represents samples obtained from different individual animals.
The accumulation of neutrophils in the lung tissues was then examined by measuring percent GR1+ cells in lung single cell suspensions using flow cytometry. In untreated or sham animals, there was a marginated pool of neutrophils in the lungs as has been shown in previous studies (23), and no difference was observed between WT and ARTg mice (Fig. 3B). This mild model of CLP did not induce neutrophil recruitment into the lungs of WT mice (Fig. 3B). In ARTg mice, however, significant neutrophil infiltration in the lungs was observed (Fig. 3B). This increase in neutrophils in the lungs of ARTg mice was totally accounted for by the increase in GR1low immature neutrophils (Fig. 3B).
Lung endothelium is a major target of inflammatory cytokines during sepsis and plays an essential role in regulating permeability and leukocyte trafficking. Sequestered neutrophils further signal into ECs and result in EC injury. One of the key signaling pathways that plays important roles in regulating EC cytoskeleton remodeling, permeability increases, and leukocyte trafficking is the Rho kinase pathway (12, 18, 24, 25). To determine whether the enhanced inflammatory responses observed in ARTg mice are linked to enhanced activation of the Rho kinase pathway in lung ECs, ECs were isolated from mouse lungs for Rho kinase evaluation. In WT mice, CLP induced a 2-fold increase in Rho kinase activity in lung ECs when compared with untreated or sham-operated mice. CLP-induced activation of Rho kinase in lung ECs was enhanced in ARTg mice (Fig. 3C). The fold increase in Rho kinase activity induced by CLP over sham-operated animals measured 2.0 ± 0.4 and 3.2 ± 0.4 in WT and ARTg mice, respectively (p < 0.05), consistent with the enhanced inflammatory responses observed in these animals.
Expression of human AR augments LPS-induced cytokine production from mouse lung cells ex vivo
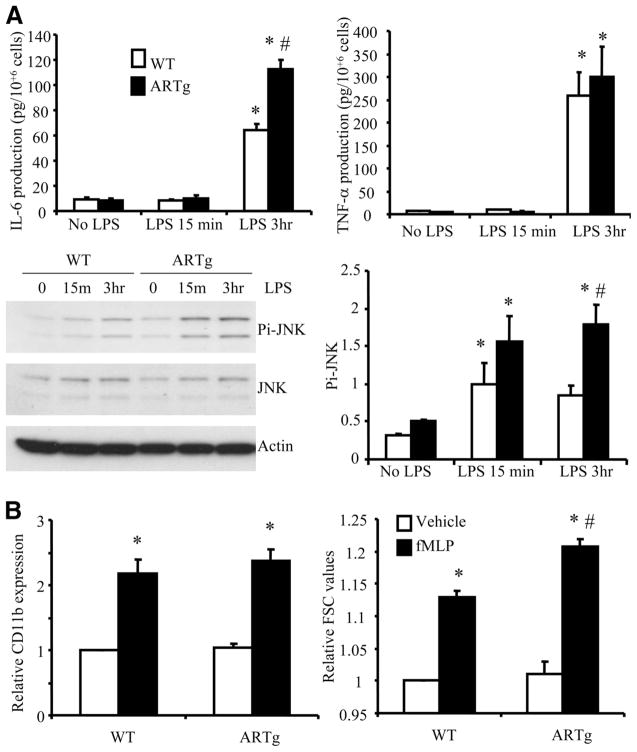
To directly address whether expression of human AR influences lung inflammation, an ex vivo approach was taken to evaluate the responses of lung cells to LPS stimulation. Lung cells were isolated from untreated WT and ARTg mice and stimulated ex vivo with LPS. LPS challenge induced IL-6 production from WT lung cells and this response was significantly augmented in ARTg lung cells (Fig. 4A). In contrast, TNF-α production was not significantly different between WT and ARTg lung cells exposed to LPS challenge. These data suggest that human AR expression may alter specific signaling pathways leading to IL-6 expression but not TNF-α production in the lung. Since IL-6 expression has been shown to be dependent on JNK activation, the phosphorylation of JNK in lung cells following LPS stimulation was also examined. LPS induced JNK activation in WT lung cells and this response was further enhanced in ARTg lung cells (Fig. 4A).
FIGURE 4.
The role of human AR expression in regulating lung cell responses to LPS (A) and neutrophil responses to fMLP (B). A, Lung cells were isolated from untreated WT or ARTg mice and stimulated with vehicle or LPS for 15 min or 3 h. IL-6 and TNF-α production was measured using ELISA, and activation of JNK was measured using immunoblot. Data represent results from three individual mice in each group. *, p < 0.05 when compared with no-LPS-treated controls; #, p < 0.05 when compared with WT lung cells and ARTg lung cells in the corresponding group. B, Bone marrow neutrophils from WT or ARTg mice were stimulated with fMLP in suspension, and up-regulation of surface CD11b and changes in mean FSC values were evaluated using flow cytometry. Data represent results from three individual mice in each group. *, p < 0.05 when compared with no-fMLP-treated controls; #, p < 0.05 when compared with WT neutrophils and ARTg neutrophils in the corresponding group.
In addition, the effect of human AR expression on the neutrophil response to chemoattractants was examined. fMLP-induced CD11b up-regulation on bone marrow-derived neutrophils was similar in WT and ARTg mice (Fig. 4B). Interestingly, neutrophil shape changes, as evaluated by changes in forward scatter (FSC) values, were significantly enhanced in ARTg neutrophils compared with WT neutrophils (Fig. 4B).
These in vivo data indicated that human AR expression resulted in enhanced inflammatory responses in the early phase of CLP-induced injury in mice: more inflammatory mediators were produced; neutrophil accumulation in the lungs was enhanced, and the activation of Rho kinase in lung ECs was augmented. Ex vivo studies provided evidence that human AR expression and activity augmented LPS-induced JNK activation and IL-6 expression in lung cells and enhanced neutrophil shape changes induced by fMLP. The following studies were thus performed to determine the role of AR activation in regulating EC responses to inflammatory cytokines, neutrophil responses to chemoattractants, and neutrophil-lung EC interactions using well-characterized in vitro models. Primary human pulmonary microvascular ECs and neutrophils isolated from healthy volunteers were used for these studies.
AR activity is required for TNF-α-induced JNK activation in human lung ECs
Production of inflammatory cytokines such as TNF-α during CLP plays important roles in regulating the progression of inflammatory responses by targeting pulmonary microvascular endothelium to induce expression of adhesion molecules, chemokines, and cytokines. To address whether AR activity in lung ECs is required for TNF-α-induced signaling pathways, the effect of an AR inhibitor, zopolrestat, was examined. ECs were pretreated with a control vehicle or zopolrestat in a dose-dependent manner before they were stimulated with 20 ng/ml TNF-α for 15 min (peak of JNK and p38 activation) or 3 h (plateau phase of JNK and p38 activation). In a dose-dependent manner, zopolrestat inhibited TNF-α-induced JNK activation (Fig. 5, A and B). Activation of MKK4, one of the MKKs that phosphorylate and activate JNK, was also inhibited (Fig. 5, A and B). One of the upstream signaling pathways regulating TNF-α-induced MKK4/JNK activation in lung ECs is the Rho kinase pathway (12). Pretreatment with zopolrestat inhibited TNF-α-induced Rho kinase activation, as evaluated by phosphorylation of the myosin light chain, one of the major downstream Rho kinase effectors (Fig. 5, A and B). Activation of p38, however, was not significantly inhibited by pretreatment with zopolrestat (Fig. 5, A and B). The effect of another class of AR inhibitors, sorbinil, on TNF-α-induced JNK activation was also examined. Sorbinil dose-dependently inhibited TNF-α-induced JNK activation at both 15 min and 3 h (Fig. 5C). Activation of JNK leads to AP-1-dependent transcription of inflammatory genes such as IL-6 (12, 16). Pretreatment with zopolrestat prevented TNF-α-induced IL-6 production (Fig. 5D). By contrast, TNF-α-induced ICAM-1 expression on EC surface was not inhibited by treatment with zopolrestat (Fig. 5E). These data indicate that AR activity is required for TNF-α-induced JNK activation, but not p38 activation or ICAM-1 expression.
FIGURE 5.
AR activity is required for TNF-α-induced activation of Rho kinase, MKK4, and JNK and production of IL-6 in human pulmonary microvascular ECs. A, ECs were pretreated with vehicle or zopolrestat at the indicated doses before they were stimulated with 20 ng/ml TNF-α for 15 min or 3 h. Activation of JNK, MKK4, Rho kinase, or p38 was examined. A representative experiment is shown. B, Densitometric analysis of multiple experiments shown in A. Data represent mean ± SEM (n = 4 independent samples for each data point). #, p < 0.05 when compared with samples treated with control vehicle in the corresponding group. C, The effect of another class of AR inhibitors, sorbinil, on TNF-α-induced JNK activation. ECs were pretreated with vehicle or sorbinil at the indicated doses before they were stimulated with 20 ng/ml TNF-α for 15 min or 3 h. Activation of JNK was examined. Data represent mean ± SEM (n = 4 for each data point). #, p < 0.05 when compared with samples treated with control vehicle in the corresponding group. D, Pretreatment with zopolrestat inhibited TNF-α-induced IL-6 production by ECs as evaluated using ELISA. Data represent mean ± SEM (n = 4 for each data point). *, p < 0.05 when compared with no-TNF-treated controls; #, p < 0.05 when compared with samples treated with control vehicle in the corresponding group. E, Pretreatment with zopolrestat had no effect on TNF-α-induced ICAM-1 expression as examined by using flow cytometry. Data represent mean ± SEM (n = 4 for each data point). *, p < 0.05 when compared with no- TNF-α-treated samples and no significance was observed between samples treated with control vehicle and samples treated with zopolrestat (p < 0.05).
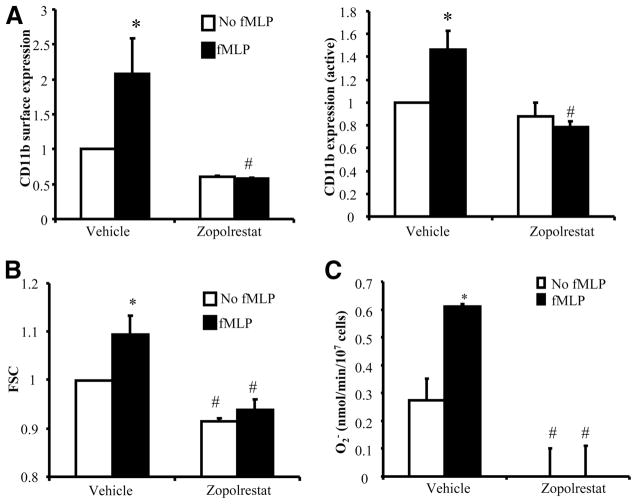
AR activity is required for fMLP-induced CD11b up-regulation and superoxide generation in human neutrophils
The role of AR in regulating human neutrophil responses to a chemoattractant was also evaluated using zopolrestat. Stimulation of human neutrophils isolated from venous blood with fMLP caused an up-regulation of total CD11b on neutrophils as well as the active CD11b epitope in the I domain recognized by the CBRM1/5 Ab (Ref. 26 and Fig. 6A). Both responses were completely inhibited when neutrophils were pretreated with zopolrestat (Fig. 6A). In addition, neutrophils stimulated with fMLP in suspension showed changes in cell shape that could be detected as changes in mean values in FSC using flow cytometry. In control neutrophils, fMLP stimulation caused an increase in mean FSC (Fig. 6B). This response was completely inhibited by pre-treatment of neutrophils with zopolrestat (Fig. 6B). Respiratory burst was evaluated using the cytochrome c reduction assay. fMLP induced superoxide production in human neutrophils (Fig. 6C). Pretreatment with zopolrestat reduced the baseline as well as fMLP-induced superoxide production (Fig. 6C).
FIGURE 6.
AR activity is required for CD11b up-regulation and superoxide generation induced by fMLP in human neutrophils. Purified human neutrophils were pretreated with 200 μM zopolrestat for 30 min before they were stimulated with control buffer or 1 μM fMLP. A, Up-regulation of cell surface total CD11b or the active CD11b recognized by CBRM1/5 Ab was evaluated using flow cytometry. B, Changes in mean FSC were evaluated using flow cytometry. C, Superoxide release was evaluated using the cytochrome c reduction assay. Data represent mean ± SEM from three individual healthy donors. #, p < 0.05 when compared with samples treated with control vehicle in the corresponding group.
AR activity regulates the interaction between TNF-α-activated lung ECs and neutrophils
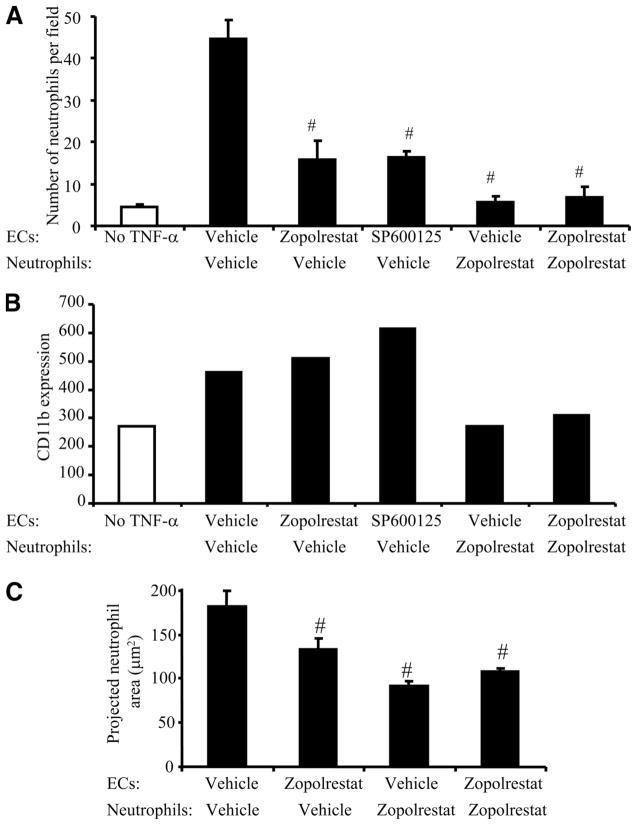
Whether AR activity regulates the adhesion between TNF-α-stimulated ECs and neutrophils was examined in the following experiments. TNF-α stimulation of lung ECs induces the expression of chemoattractants by ECs, which are sufficient to prime neutrophils in the absence of exogenous neutrophil activators (27). Indeed, few neutrophils adhered to ECs when ECs were not stimulated with TNF-α (Fig. 7A). Neutrophil adhesion to TNF-α-stimulated ECs was inhibited when ECs were treated with zopolrestat, indicating that AR activity in ECs is required for neutrophil adhesion. Since AR activation was required for TNF-α-induced JNK activation in lung ECs, the effect of inhibiting JNK in EC was also examined using a JNK inhibitor, SP600125. Treatment of lung ECs with SP600125 also inhibited neutrophil-EC adhesion, suggesting that JNK-dependent mechanisms are required and that AR in ECs regulates neutrophil adhesion likely through a JNK-dependent mechanism (Fig. 7A). Pretreatment of neutrophils with zopolrestat also inhibited neutrophil adhesion to ECs, consistent with the observations that activation of AR in neutrophils is required for CD11b up-regulation on neutrophils following stimulation with a chemoattractant.
FIGURE 7.
AR activity in both neutrophils and pulmonary microvascular ECs is required for neutrophil adhesion to TNF-α-stimulated ECs. A, Neutrophil adhesion to TNF-α-treated ECs required AR activity in both neutrophils and ECs. Data represent mean ± SEM (n = 3 independent experiments). #, p < 0.05 when compared with samples treated with control vehicle in the corresponding group. B and C, AR activity in ECs was required for neutrophil spreading on ECs, but not CD11b up-regulation, during neutrophil adhesion, while AR activity in neutrophils was required for neutrophil spreading and CD11b up-regulation. B, CD11b expression was evaluated using flow cytometry and the data are representative of two independent experiments. C, The neutrophil area was evaluated using confocal microscopy. Five to nine different fields from each slide were averaged and the data shown are representative of three independent experiments.
Efficient neutrophil adhesion to TNF-α-stimulated ECs requires coordinated changes in neutrophil cell shape and activation of neutrophil adhesion molecules such as CD11b. CD11b expression was increased on neutrophils adherent to ECs and this increase was inhibited when neutrophils were pretreated with zopolrestat, consistent with the critical role of AR in regulating neutrophil responses to chemoattractants (Fig. 7B). Treatment of ECs with zopolrestat alone did not inhibit CD11b up-regulation (Fig. 7B). Interestingly, the measurement of the neutrophil area indicated that neutrophils were less spread when ECs or neutrophils were pretreated with zopolrestat (Fig. 7C). These results suggest that at least two mechanisms underlie the activation of neutrophils by TNF-α-stimulated ECs: an AR-dependent mechanism for neutrophil spreading and a JNK- and AR-independent mechanism for CD11b up-regulation.
Discussion
This study presented evidence that AR plays an important role in regulating the inflammatory response. Acute inflammatory responses induced by CLP are enhanced in ARTg mice. In both neutrophils and lung ECs, activity of AR is required for their responses to soluble inflammatory mediators and regulates neutrophil-EC adhesion.
The CLP model used in this study induced a mild injury and did not result in any mortality by 24 h. This model allows us to focus on the early events that occur before the development of severe systemic inflammatory responses characterized by multiorgan dysfunction including ARDS/ALI. The presence of systemic inflammatory responses was confirmed by elevated IL-6 and TNF-α levels in plasma and by the release of neutrophils from bone marrow. Lung ECs showed activation of Rho kinase, a signaling molecule that is important for regulating EC permeability increases and JNK activation induced by inflammatory cytokines (12). However, there was no detectable neutrophil infiltration into the lungs of WT mice. Measurement of AR activity in the lungs of WT mice showed that although there is low expression/activity of AR in the WT lungs, no significant increase in AR activity following CLP was noted. In contrast, in the lungs of ARTg mice, a significant increase in AR activity was induced following CLP. It is interesting to note that AR activity does not directly correlate with the amount of AR protein in the untreated mice. This may be due to the fact that AR has low intrinsic affinity for its substrate and that AR activity can be modulated through posttranslational modification such as oxidation (28). The production of IL-6 and TNF-α was also increased, indicating an exaggerated systemic inflammatory response. Circulating IL-6 increases in septic patients and in mice during CLP-induced sepsis and high IL-6 levels correlate with high mortality in septic patients as well as in CLP-induced sepsis in mice (29–31). Moreover, Rho kinase activation in lung ECs and neutrophil accumulation into the lungs were enhanced in ARTg mice. This enhanced inflammatory response in the lungs of ARTg mice was accompanied by an increase in AR activity in the lungs of ARTg mice following CLP. These enhanced inflammatory responses observed in ARTg mice during CLP may be due to a direct role for AR in regulating signaling pathways leading to enhanced inflammatory cytokine/chemokine production and/or due to a role for AR in regulating cell responses to inflammatory mediators. Our in vivo, ex vivo, and in vitro studies have suggested that both mechanisms exist. Lung cells from ARTg mice showed more robust JNK activation and IL-6 production in response to LPS stimulation, supporting a role for AR in regulating cytokine production in the lung. The production of TNF-α by WT or ARTg lung cells in response to LPS, however, was not different. This is in contrast to increased serum TNF-α levels during CLP in ARTg mice. This difference is interesting and may be attributed to a different role played by AR in regulating TNF-α production in different tissues during inflammation. Besides playing a role in regulating cytokine production, AR clearly regulates specific signaling pathways in both lung ECs and neutrophils in response to inflammatory mediators.
During inflammation, circulating neutrophils are recruited to the site of inflammation and this transient neutropenia is accompanied by the release of neutrophils from the bone marrow into the circulation. This neutrophil release response may be induced by numerous inflammatory mediators and TLR agonists (21). Both WT and ARTg mice efficiently release their bone marrow neutrophils by 24 h, indicating that the enhanced neutrophil recruitment into the lungs of ARTg mice may not be due to a difference in the neutrophil bone marrow release response. Interestingly, the increase in neutrophil infiltration into the lungs of ARTg mice was totally accounted for by the increase in the immature, low GR1-expressing neutrophils, consistent with the notion that the newly released and immature neutrophils have longer transit time through the pulmonary circulation than mature neutrophils (32). These data also suggest that neutrophil release from the bone marrow into the circulation alone is not sufficient to induce neutrophil sequestration into the lungs and that lung parenchymal cell such as the lung ECs have to be activated and coordinate neutrophil accumulation.
Neutrophil adhesion to lung ECs is a prerequisite for neutrophil recruitment from blood into the lungs during inflammation. Cytokine activation of lung ECs plays an important role in regulating neutrophil adhesion by inducing the expression of chemoattractants on ECs, thereby activating neutrophils to express adhesion molecules such as CD11b (27). The data presented in this study indicate that AR activity in both lung ECs and neutrophils is required for neutrophil adhesion. On the EC side, AR activity is required for TNF-α-stimulated ECs to induce neutrophil adhesion in a JNK-dependent manner, while on the neutrophil side, AR activity is required for neutrophils to up-regulate CD11b expression and to release superoxide. As a result, when AR is blocked in either ECs or neutrophils, neutrophil adhesion to TNF-α-activated ECs is impaired.
Activation of lung ECs by cytokines such as TNF-α plays an important role in promoting neutrophil adhesion and activation and, therefore, mediates the progression of an inflammatory response in the lung. In this context, TNF-α induces ICAM-1 expression on lung ECs and production of mediators that promote neutrophil adhesion and activation such as platelet-activating factor, IL-8, and GM-CSF (27, 33, 34). AR activity in ECs is required for TNF-α-induced Rho kinase and JNK activation, but not p38 activation or ICAM-1 expression. Interestingly, activation of the Rho kinase and JNK pathways play essential roles in regulating neutrophil infiltration into the lungs (35, 36). These data suggest that AR modulates lung inflammation, at least in part, by regulating these important acute inflammatory signaling pathways. Since TNF-α-induced JNK activation requires reactive oxygen species production (13–15), these data led us to hypothesize that AR flux regulates JNK activation by altering the balance of oxidant/antioxidants. AR inhibitors have been reported to blunt both hyperglycemia-induced superoxide production by cultured ECs and hyperglycemia-induced endothelium-dependent superoxide production by aortic rings, as well as to protect reduced glutathione tissue levels, and to normalize markers of oxidative stress in diabetic tissue, such as malondialdehyde (17, 37–39). Excess flux through AR has been linked to 1) excess oxidation of NADPH, essential for glutathione reduction, as well as 2) SDH-mediated excess production of NADH (10), a potential substrate for NADH oxidase and for mitochondrial metabolism. Examination of neutrophil shape and CD11b expression demonstrates that neutrophils adherent to TNF-α-activated ECs are elongated and express higher levels of CD11b than neutrophils added to untreated ECs. These results indicate that neutrophil activators produced by TNF-α-activated lung ECs are in close proximity to the EC surface. Interestingly, the mediators that regulate neutrophil shape changes and CD11b expression can be differentiated based on the requirement for AR activity: the mediators that regulate neutrophil shape changes require AR activity, while those that regulate neutrophil CD11b up-regulation do not. These data support previous observations that multiple EC-associated neutrophil activators are responsible for neutrophil priming and activation during neutrophil adhesion to cytokine-activated ECs and indicate a role for AR in lung ECs in regulating neutrophil-EC interactions.
AR activity is essential for neutrophil responses induced by cytokine-stimulated ECs and by exogenous chemoattractants such as fMLP. fMLP-induced CD11b up-regulation as well as superoxide generation are completely prevented by AR inhibition. In addition, upon adhesion to TNF-α-activated ECs, inhibition of AR in neutrophils is sufficient to inhibit CD11b up-regulation, neutrophil shape changes, and neutrophil adhesion to ECs. These data indicate that AR is required for neutrophils to respond to chemoattractants produced by TNF-α-stimulated ECs, thereby modulating neutrophil adhesion. The assembly and activation of NADPH oxidase, which uses NADPH to convert oxygen to superoxide, mediates respiratory burst-induced superoxide generation (40). Neutrophils deficient in glucose metabolism have impaired respiratory burst, chemotaxis, and are unable to accumulate at the site of inflammation (41– 43). Conversely, an acute increase in extracellular glucose concentration increases cytoplasmic NADPH and promotes neutrophil superoxide production (44, 45). The increase in the NADPH level interestingly correlates with a concomitant decrease in glycolysis, which led to the hypothesis that the pathway leading to NADPH production competes with glycolysis for the shared substrate, glucose-6-phosphate (43). Excess flux via AR results in a decrease in the cofactors necessary for glycolysis (10). These data raised the hypothesis that in response to chemoattractants, excess flux via AR favors NADPH and NADH accumulation for superoxide generation.
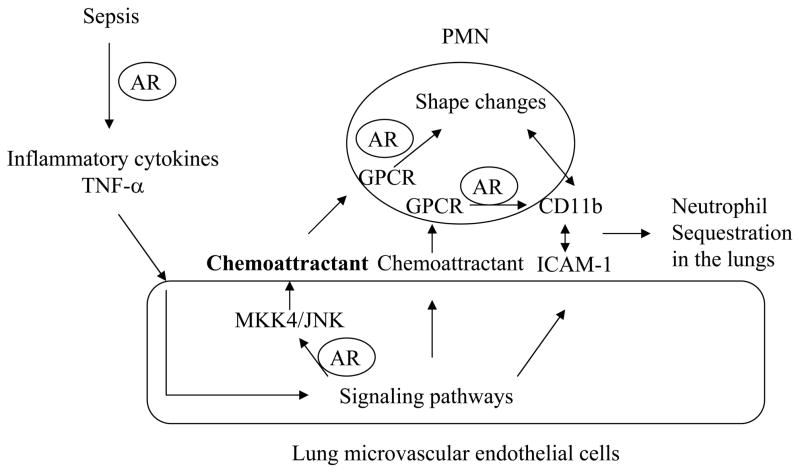
These data led us to propose the following working hypothesis through which AR regulates neutrophil-EC interactions in the lungs during early inflammatory responses in sepsis (Fig. 8). AR regulates the production of inflammatory cytokines such as TNF-α. Moreover, AR activity in both neutrophils and cytokine-stimulated ECs is required for neutrophil adhesion to ECs. In ECs, AR activity regulates neutrophil shape changes through a MKK4/ JNK-dependent mechanism, while in neutrophils, AR activity is required for neutrophils to respond to EC-associated chemoattractants and CD11b up-regulation. The coordinated CD11b/ICAM-1-mediated adhesion and neutrophil shape changes modulate neutrophil sequestration in the lungs, an early event in the development of lung injury in sepsis. These data raise hypotheses for future studies to test how AR-catalyzed pathways regulate inflammatory signaling in both ECs and neutrophils leading to neutrophil-EC adhesion in the lung.
FIGURE 8.
Working hypothesis through which AR regulates neutrophil-lung EC interactions during sepsis. The steps that are regulated by AR are indicated in the model. GPCR, G protein-coupled receptor; PMN, polymorphonuclear neutrophil.
Footnotes
This work was supported by National Institutes of Health Grants HL 070009 (to Q.W.), HL 60901 (to A.M.S.), and HL 61783 (to R.R.).
Abbreviations used in this paper: ARDS, acute respiratory distress syndrome; ALI, acute lung injury; AR, aldose reductase; SDH, sorbitol dehydrogenase; EC, endothelial cell; CLP, cecal ligation and puncture; WT, wild type; FSC, forward scatter.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Hotchkiss RS, I, Karl E. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 2.Matthay MA, Zimmerman GA. Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am J Respir Cell Mol Biol. 2005;33:319–327. doi: 10.1165/rcmb.F305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrison DH, Bohren KM, Ringe D, Petsko GA, Gabbay KH. An anion binding site in human aldose reductase: mechanistic implications for the binding of citrate, cacodylate, and glucose 6-phosphate. Biochemistry. 1994;33:2011–2020. doi: 10.1021/bi00174a006. [DOI] [PubMed] [Google Scholar]
- 4.Grimshaw CE, Bohren KM, Lai CJ, Gabbay KH. Human aldose reductase: pK of tyrosine 48 reveals the preferred ionization state for catalysis and inhibition. Biochemistry. 1995;34:14374–14384. doi: 10.1021/bi00044a014. [DOI] [PubMed] [Google Scholar]
- 5.Cumbie BC, Hermayer KL. Current concepts in targeted therapies for the pathophysiology of diabetic microvascular complications. Vasc Health Risk Manag. 2007;3:823–832. [PMC free article] [PubMed] [Google Scholar]
- 6.Ananthakrishnan R, Kaneko M, Hwang YC, Gomes T, Caspersen C, Ramasamy R. Aldose reductase mediates myocardial ischemia-reperfusion injury in part by opening mitochondrial permeability transition pore. Am J Physiol. 2009;296:H333–H341. doi: 10.1152/ajpheart.01012.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ho EC, Lam KS, Chen YS, Yip JC, Arvindakshan M, Yamagishi S, Yagihashi S, Oates PJ, Ellery CA, Chung SS, Chung SK. Aldose reductase-deficient mice are protected from delayed motor nerve conduction velocity, increased c-Jun NH2-terminal kinase activation, depletion of reduced glutathione, increased superoxide accumulation, and DNA damage. Diabetes. 2006;55:1946–1953. doi: 10.2337/db05-1497. [DOI] [PubMed] [Google Scholar]
- 8.Kaneko M, Bucciarelli L, Hwang YC, Lee L, Yan SF, Schmidt AM, Ramasamy R. Aldose reductase and AGE-RAGE pathways: key players in myocardial ischemic injury. Ann NY Acad Sci. 2005;1043:702–709. doi: 10.1196/annals.1333.081. [DOI] [PubMed] [Google Scholar]
- 9.Vikramadithyan RK, Hu Y, Noh HL, Liang CP, Hallam K, Tall AR, Ramasamy R, Goldberg IJ. Human aldose reductase expression accelerates diabetic atherosclerosis in transgenic mice. J Clin Invest. 2005;115:2434–2443. doi: 10.1172/JCI24819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang YC, Kaneko M, Bakr S, Liao H, Lu Y, Lewis ER, Yan S, Ii S, Itakura M, Rui L, et al. Central role for aldose reductase pathway in myocardial ischemic injury. FASEB J. 2004;18:1192–1199. doi: 10.1096/fj.03-1400com. [DOI] [PubMed] [Google Scholar]
- 11.Ramana KV, Willis MS, White MD, Horton JW, DiMaio JM, Srivastava D, Bhatnagar A, Srivastava SK. Endotoxin-induced cardiomyopathy and systemic inflammation in mice is prevented by aldose reductase inhibition. Circulation. 2006;114:1838–1846. doi: 10.1161/CIRCULATIONAHA.106.630830. [DOI] [PubMed] [Google Scholar]
- 12.Mong PY, Petrulio C, Kaufman HL, Wang Q. Activation of Rho kinase by TNF-α is required for JNK activation in human pulmonary microvascular endothelial cells. J Immunol. 2008;180:550–558. doi: 10.4049/jimmunol.180.1.550. [DOI] [PubMed] [Google Scholar]
- 13.Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFα-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 14.Pham CG, Bubici C, Zazzeroni F, Papa S, Jones J, Alvarez K, Jayawardena S, De Smaele E, Cong R, Beaumont C, et al. Ferritin heavy chain upregulation by NF-κB inhibits TNFα-induced apoptosis by suppressing reactive oxygen species. Cell. 2004;119:529–542. doi: 10.1016/j.cell.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 15.Sakon S, Xue X, Takekawa M, Sasazuki T, Okazaki T, Kojima Y, Piao JH, Yagita H, Okumura K, Doi T, Nakano H. NF-κB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. EMBO J. 2003;22:3898–3909. doi: 10.1093/emboj/cdg379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ventura JJ, Kennedy NJ, Lamb JA, Flavell RA, Davis RJ. c-Jun NH2-terminal kinase is essential for the regulation of AP-1 by tumor necrosis factor. Mol Cell Biol. 2003;23:2871–2882. doi: 10.1128/MCB.23.8.2871-2882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker CI, Gaines HO, Baue AE. Evaluation of factors affecting mortality rate after sepsis in a murine cecal ligation and puncture model. Surgery. 1983;94:331–335. [PubMed] [Google Scholar]
- 18.Mong PY, Wang Q. Activation of Rho kinase isoforms in lung endothelial cells during inflammation. J Immunol. 2009;182:2385–2394. doi: 10.4049/jimmunol.0802811. [DOI] [PubMed] [Google Scholar]
- 19.Goodman EB, Anderson DC, Tenner AJ. C1q triggers neutrophil superoxide production by a unique CD18-dependent mechanism. J Leukocyte Biol. 1995;58:168–176. doi: 10.1002/jlb.58.2.168. [DOI] [PubMed] [Google Scholar]
- 20.Heuer JG, Bailey DL, Sharma GR, Zhang T, Ding C, Ford A, Stephens EJ, Holmes KC, Grubbs RL, Fynboe KA, et al. Cecal ligation and puncture with total parenteral nutrition: a clinically relevant model of the metabolic, hormonal, and inflammatory dysfunction associated with critical illness. J Surg Res. 2004;121:178–186. doi: 10.1016/j.jss.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 21.Christopher MJ, Link DC. Regulation of neutrophil homeostasis. Curr Opin Hematol. 2007;14:3–8. doi: 10.1097/00062752-200701000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Ueda Y, Kondo M, Kelsoe G. Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J Exp Med. 2005;201:1771–1780. doi: 10.1084/jem.20041419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doerschuk CM. Mechanisms of leukocyte sequestration in inflamed lungs. Microcirculation. 2001;8:71–88. [PubMed] [Google Scholar]
- 24.Tasaka S, Koh H, Yamada W, Shimizu M, Ogawa Y, Hasegawa N, Yamaguchi K, Ishii Y, Richer SE, Doerschuk CM, Ishizaka A. Attenuation of endotoxin-induced acute lung injury by the Rho-associated kinase inhibitor, Y-27632. Am J Respir Cell Mol Biol. 2005;32:504–510. doi: 10.1165/rcmb.2004-0009OC. [DOI] [PubMed] [Google Scholar]
- 25.Loirand G, Guerin P, Pacaud P. Rho kinases in cardiovascular physiology and pathophysiology. Circ Res. 2006;98:322–334. doi: 10.1161/01.RES.0000201960.04223.3c. [DOI] [PubMed] [Google Scholar]
- 26.Pluskota E, Woody NM, Szpak D, Ballantyne CM, Soloviev DA, Simon DI, Plow EF. Expression, activation, and function of integrin αMβ2 (Mac-1) on neutrophil-derived microparticles. Blood. 2008;112:2327–2335. doi: 10.1182/blood-2007-12-127183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi T, Hato F, Yamane T, Fukumasu H, Suzuki K, Ogita S, Nishizawa Y, Kitagawa S. Activation of human neutrophil by cytokine-activated endothelial cells. Circ Res. 2001;88:422–429. doi: 10.1161/01.res.88.4.422. [DOI] [PubMed] [Google Scholar]
- 28.Kaiserova K, Tang XL, Srivastava S, Bhatnagar A. Role of nitric oxide in regulating aldose reductase activation in the ischemic heart. J Biol Chem. 2008;283:9101–9112. doi: 10.1074/jbc.M709671200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riedemann NC, Neff TA, Guo RF, Bernacki KD, Laudes IJ, Sarma JV, Lambris JD, Ward PA. Protective effects of IL-6 blockade in sepsis are linked to reduced C5a receptor expression. J Immunol. 2003;170:503–507. doi: 10.4049/jimmunol.170.1.503. [DOI] [PubMed] [Google Scholar]
- 30.Calandra T, Gerain J, Heumann D, Baumgartner JD, Glauser MP. High circulating levels of interleukin-6 in patients with septic shock: evolution during sepsis, prognostic value, and interplay with other cytokines: the Swiss-Dutch J5 Immunoglobulin Study Group. Am J Med. 1991;91:23–33. doi: 10.1016/0002-9343(91)90069-a. [DOI] [PubMed] [Google Scholar]
- 31.Remick DG, Bolgos G, Copeland S, Siddiqui J. Role of interleukin-6 in mortality from and physiologic response to sepsis. Infect Immun. 2005;73:2751–2757. doi: 10.1128/IAI.73.5.2751-2757.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Eeden SF, Kitagawa Y, Klut ME, Lawrence E, Hogg JC. Polymorphonuclear leukocytes released from the bone marrow preferentially sequester in lung microvessels. Microcirculation. 1997;4:369–380. doi: 10.3109/10739689709146801. [DOI] [PubMed] [Google Scholar]
- 33.Prescott SM, Zimmerman GA, Mclntyre TM. Human endothelial cells in culture produce platelet-activating factor (1-alkyl-2-acetyl-sn-glycero-3-phosphocholine) when stimulated with thrombin. Proc Natl Acad Sci USA. 1984;81:3534–3538. doi: 10.1073/pnas.81.11.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bussolino F, Camussi G, Baglioni C. Synthesis and release of platelet-activating factor by human vascular endothelial cells treated with tumor necrosis factor or interleukin 1a. J Biol Chem. 1988;263:11856–11861. [PubMed] [Google Scholar]
- 35.Fahmy RG, Waldman A, Zhang G, Mitchell A, Tedla N, Cai H, Geczy CR, Chesterman CN, Perry M, Khachigian LM. Suppression of vascular permeability and inflammation by targeting of the transcription factor c-Jun. Nat Biotechnol. 2006;24:856–863. doi: 10.1038/nbt1225. [DOI] [PubMed] [Google Scholar]
- 36.Arndt PG, Young SK, Lieber JG, Fessler MB, Nick JA, Worthen GS. Inhibition of c-Jun N-terminal kinase limits lipopolysaccharide-induced pulmonary neutrophil influx. Am J Respir Crit Care Med. 2005;171:978–986. doi: 10.1164/rccm.200406-712OC. [DOI] [PubMed] [Google Scholar]
- 37.El-Remessy AB, Abou-Mohamed G, Caldwell RW, Caldwell RB. High glucose-induced tyrosine nitration in endothelial cells: role of eNOS uncoupling and aldose reductase activation. Invest Ophthalmol Vis Sci. 2003;44:3135–3143. doi: 10.1167/iovs.02-1022. [DOI] [PubMed] [Google Scholar]
- 38.Toth E, Racz A, Toth J, Kaminski PM, Wolin MS, Bagi Z, Koller A. Contribution of polyol pathway to arteriolar dysfunction in hyperglycemia: role of oxidative stress, reduced NO, and enhanced PGH2/TXA2 mediation. Am J Physiol. 2007;293:H3096–H3104. doi: 10.1152/ajpheart.01335.2006. [DOI] [PubMed] [Google Scholar]
- 39.Tang WH, Wu S, Wong TM, Chung SK, Chung SS. Polyol pathway mediates iron-induced oxidative injury in ischemic-reperfused rat heart. Free Radical Biol Med. 2008;45:602–610. doi: 10.1016/j.freeradbiomed.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 40.Dale DC, Boxer L, Liles WC. The phagocytes: neutrophils and monocytes. Blood. 2008;112:935–945. doi: 10.1182/blood-2007-12-077917. [DOI] [PubMed] [Google Scholar]
- 41.Kim SY, Nguyen AD, Gao JL, Murphy PM, Mansfield BC, Chou JY. Bone marrow-derived cells require a functional glucose 6-phosphate transporter for normal myeloid functions. J Biol Chem. 2006;281:28794–28801. doi: 10.1074/jbc.M604964200. [DOI] [PubMed] [Google Scholar]
- 42.Kim SY, Jun HS, Mead PA, Mansfield BC, Chou JY. Neutrophil stress and apoptosis underlie myeloid dysfunction in glycogen storage disease type Ib. Blood. 2008;111:5704–5711. doi: 10.1182/blood-2007-12-129114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheung YY, Kim SY, Yiu WH, Pan CJ, Jun HS, Ruef RA, Lee EJ, Westphal H, Mansfield BC, Chou JY. Impaired neutrophil activity and increased susceptibility to bacterial infection in mice lacking glucose-6-phosphatase-β. J Clin Invest. 2007;117:784–893. doi: 10.1172/JCI30443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kummer U, Zobeley J, Brasen JC, Fahmy R, Kindzelskii AL, Petty AR, Clark AJ, Petty HR. Elevated glucose concentrations promote receptor-independent activation of adherent human neutrophils: an experimental and computational approach. Biophys J. 2007;92:2597–2607. doi: 10.1529/biophysj.106.086769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olsen LF, Kummer U, Kindzelskii AL, Petty HR. A model of the oscillatory metabolism of activated neutrophils. Biophys J. 2003;84:69–81. doi: 10.1016/S0006-3495(03)74833-4. [DOI] [PMC free article] [PubMed] [Google Scholar]