Abstract
Background: Breast cancer mortality is declining in many Western countries. If mammography screening contributed to decreases in mortality, then decreases in advanced breast cancer incidence should also be noticeable.
Patients and methods: We assessed incidence trends of advanced breast cancer in areas where mammography screening is practiced for at least 7 years with 60% minimum participation and where population-based registration of advanced breast cancer existed. Through a systematic Medline search, we identified relevant published data for Australia, Italy, Norway, Switzerland, The Netherlands, UK and the USA. Data from cancer registries in Northern Ireland, Scotland, the USA (Surveillance, Epidemiology and End Results (SEER), and Connecticut), and Tasmania (Australia) were available for the study. Criterion for advanced cancer was the tumour size, and if not available, spread to regional/distant sites.
Results: Age-adjusted annual percent changes (APCs) were stable or increasing in ten areas (APCs of −0.5% to 1.7%). In four areas (Firenze, the Netherlands, SEER and Connecticut) there were transient downward trends followed by increases back to pre-screening rates.
Conclusions: In areas with widespread sustained mammographic screening, trends in advanced breast cancer incidence do not support a substantial role for screening in the decrease in mortality.
Keywords: breast cancer, cancer registry, incidence, screening, stage
introduction
Since 1990, breast cancer mortality in women has fallen in many countries with populations of mainly European ancestry [1–3]. This improvement in mortality could be related to earlier detection of cancer with screening and the efficacy of treatments. Studies done so far have not been able to assess with precision how much of the mortality decrease was due to screening or to adjuvant therapy. For instance, a study that developed seven independent statistical models found that the contribution of screening to decreases in breast cancer mortality in the United States could range from 28% to 65% [4].
Mammographic screening aims to detect cancer at an earlier stage that would be less life threatening and easier to cure than if detected clinically. Diagnosis procedures are not influenced by subsequent treatments. Hence, a reduction of advanced cancer incidence should reflect the impact of screening activities alone, thus providing an estimate of the contribution of screening to the observed mortality decrease. Longstanding broad consensus exists for considering a decrease in advanced breast cancer incidence as the best early indicator of the impact of screening [5–11]. Following this logic, in a previous work, we found that when all randomised trials of mammographic screening were considered, breast cancer mortality reductions were directly proportional to the fall in the incidence of advanced breast cancer [12].
In the United States, nationwide mammographic screening has been introduced in 1983 and screening coverage of women ≥40 years reached 50% in 1990 and 71% in 1997 [13]. In a recent report, using data from the Surveillance, Epidemiology and End Results (SEER) cancer registries, Esserman et al. [14] concluded that breast screening may increase the burden of low-risk cancers without significantly reducing the incidence of more aggressively growing tumours. In view of these unexpected conclusions, we performed a systematic review of published data on incidence trends of advanced breast cancer incidence in areas where mammographic screening was in operation for ≥7 years and where participation to screening was high. We could also make use of relevant data made available by several population-based cancer registries established in areas with high mammography screening coverage.
patients and methods
criteria for data or study selection
Data or studies were selected if they reported time trend of advanced invasive breast cancer incidence in the general population, outside the context of randomised trials or cohort studies. Further inclusion criteria were details on cancer size or on stage; incidence reported for at least 7 years after commencement of breast cancer screening and screening attendance in target group of ≥60% per biennium (triennium in the UK) during at least the last 3–4 years of observation.
We excluded studies that reported size or stage as a percentage of total number of cancer cases, as this type of metric varies with the number of smaller tumours detected [14]. We also excluded studies that obtained results via application of mathematical models to cancer registry data.
search for published data on size- or stage-specific breast cancer incidence
The literature review was performed using Medline from January 1990 to June 2009. Algorithms incorporating keywords: ‘breast cancer’, ‘size’, ‘stage’, and ‘trends’ were used. We searched for keywords in the title and abstract, without language restrictions.
In addition, we performed a manual search of references cited in the selected articles, in related articles according to Medline and in International Agency for Research on Cancer library resources, e.g. the articles used for the Handbook on Breast Cancer Screening [7]. We also performed searches for publicly available reports from bodies in charge of screening programmes.
Data from peer-reviewed articles was supplemented with data from several population-based cancer registries whose data were published in Cancer in Five Continents volumes VIII and IX (www-dep.iarc.fr).
definition of advanced breast cancer
Breast cancer invasive component size was used as an indicator for advanced disease. The size for distinguishing early and advanced cancers was 20 mm as this threshold also distinguishes tumour classes T1 and T2 or above in both the tumour–node–metastasis (TNM) and American Joint Committee on Cancer manuals [15, 16].
If size was not available, data were examined by stage at diagnosis. Stage I cancers were considered as early, and stages II–IV as advanced since stage I breast cancers are equal or <20 mm and are without lymph node involvement or distant metastases. Several registries reported cancer as localised (cancer confined to the breast, including nipple and areola), regional (cancer invading surrounding tissues or involving axillary or internal mammary lymph nodes) or distant (cancer involving infraclavicular, supraclavicular, cervical or other distant lymph nodes or systemic metastases) according to the Surveillance, Epidemiology and End Results (SEER) classification method [17]. We used the term ‘non-local’ cancer when we talked of both regional and distant cancers.
data extraction and analysis
This study concentrates on invasive breast cancer. When data were not reported in tables, we extracted incidence rates by digitising figures using the two-dimensional coordinate tool of the Acrobat software (Adobe Acrobat, version 7.1).
Statistical analyses were performed on age groups targeted for screening. However, incidence rates reported in publications sometimes covered larger age groups. For the nine oldest SEER areas and Connecticut, we took women aged 40–69 years, as screening in the United States is most frequent in this age range [18]. Studies often used different reference populations for age adjustment of incidence rates. Two studies on women aged 50–69 years in Firenze (Italy) [19] and in Norway [20] did not report age adjustment, but because of the narrow age range studied, this is unlikely to have changed overall trends.
Log-linear regressions were fitted to estimate changes in age-adjusted incidence over time, from which we derived annual percent changes (APC) and 95% confidence intervals (95% CI) [21]. When incidence data were reported for years preceding screening, APC calculations included the 3 years before the presumed start year, but not earlier data. Rates for New South Wales were reported for 4 years only, 1986, 1988, 1992, and 1995 [22]. Therefore, before APC calculation, rates of a given year were interpolated to directly preceding and following years. In years where rates were still missing, the average of two closest years was computed. In Geneva (Switzerland), because rates by 5-year age blocks were reported, we took APCs computed by authors [23]. A trend was labelled significant if the 95% CI did not include 0.
results
study selection
We retrieved 590 articles published in peer-reviewed journals. Application of the selection criteria resulted in the retention of 35 studies. Twenty-seven were excluded for the following reasons:
1 They were earlier reports of SEER data USA [24–31], of the Connecticut Tumour Registry [32, 33], of Firenze (Italy) cancer registry [34, 35], and of pilot screening project in Norway [36];
2 Areas where screening was operational for <7 years or where reported data mainly concerned the prevalent screening round [37, 38];
3 Studies not based on population-based cancer registries or conducted in selected sub-groups (e.g. membership of a health insurance plan) or without appropriate denominator [39–49].
4 Studies with erratic variations in stage over time most probably reflecting changes in practice (e.g. sentinel node biopsy) [50].
Data of eight studies published in peer-reviewed journals displayed in Table 1 were used [8,19–23, 51, 58, 59], related to areas in Australia, Italy, The Netherlands, Norway, Switzerland, and the United States. For The Netherlands, we also retrieved a report and a congress abstract issued by the Dutch National Evaluation Team for Breast Cancer Screening that reported incidence rates of advanced cancer for more recent years [53, 60]. Data collected by the Tasmania Cancer Registry [52], the Northern Ireland Cancer Registry [54], the Scottish Cancer Registry [55], the nine oldest SEER areas [56], the National Cancer Institute's Historical Connecticut Tumour Registry (part of the nine oldest SEER registries) [57] satisfied the eligibility criteria and were available for this study.
Table 1.
Temporal trends in incidence of early and of advanced BC
| Country and area | Data source [reference] | Cancer registry | Cancer categories for advanced BC | Study period | Population source | Studied age groups | % Women with missing data |
Female population in studied age groups | Incidence of |
||
| APC (%) | 95% CI | ||||||||||
| Australia | |||||||||||
| New South Wales | Kricker et al. [22] | New South Wales Central Cancer registry | ≥20 mm | 1986, 1989, 1992, 1995 | New South Wales Central Cancer registry | 50–69 | 7 | 550 000 | 1.6 | 0.7–2.5 | |
| ≥30 mm | −1.5 | −2.4 to−0.5 | |||||||||
| Victoria | Harmer et al. [51] | Victoria Cancer Registry | >15 mm | 1988–1996 | Victoria Cancer Registry | ≥25 | 23 | 1 519 824 | 1.0 | −0.1 to 2.0 | |
| Tasmania | Tasmania Cancer Registry [52] | Tasmania Cancer Registry | ≥20 mm | 1997–2007 | Tasmania Cancer Registry | All ages | NA | 250 000 | 1.5 | −0.3 to 3.4 | |
| ≥50 mm | −0.2 | −3.7 to 3.5 | |||||||||
| Italy | |||||||||||
| City of Firenze | Pacci et al. [19] | Tuscany Cancer Registry | ≥20 mm | 1990–1999 | Resident female population invited to screening until 1996a | 50–69 | NA | 54.495 | −1.6 | −4.4 to 1.3 | |
| The Netherlands | |||||||||||
| All the country | Frachebound et al.; NTBEC [8, 53] | The Netherlands Cancer Registry | >20 mm | 1989–2000 | The Netherlands Cancer Registry | 50–69 | <5 | 1 591 200 | −2.0 | −2.7 to −1.2 | |
| >20 mm and metastasis in node or in distant organs | −1.6 | −2.3 to −0.9 | |||||||||
| Northern Ireland | |||||||||||
| All the country | Northern Ireland Cancer Registry [54] | Northern Ireland Cancer Registry | ≥20 mm | 1993–2003 | Northern Ireland Cancer Register | 50–64 | 14 | 131 479 | 0.6 | −0.4 to 1.6 | |
| ≥30 mm | 1.6 | 0.1–3.2 | |||||||||
| Norway | |||||||||||
| Four countiesb | Hofvind et al. [20] | Cancer Register of Norway | Non-localc | 1993–2003 | NordCan databased | 50–69 | 24 | 181 400 | 1.7 | b1.2–2.2 | |
| Scotland | |||||||||||
| All the country | Scottish Cancer Registry [55] | Scottish Cancer Registry | ≥20 mm | 1993–2007 | Scottish Cancer Registry | 50–64 | 9 | 490 613 | 0.4 | −0.6 to 1.6 | |
| ≥30 mm | 0.5 | −0.7 to 1.8 | |||||||||
| Switzerland | |||||||||||
| Geneva | Verkooijen et al. [23] | Geneva Cancer Registry | Non-localc | 1985–1999 | Verkooijen et al. [23] | All ages | 1 | 200 000 | 0.12g | P = NS | |
| United States of America | |||||||||||
| Nine oldest SEER areas | 9 oldest SEER registries [56] | 9 oldest SEER registriese | Non-localc | 1980–2007 | SEERf | 40–69 | 4 | 6 134 000 | −0.4 | −0.6 to 0.1 | |
| Distant | 0.1 | −0.2 to 0.3 | |||||||||
| Connecticut | Connecticut Tumour Registry (part of nine oldest SEER areas) [57] | Connecticut Tumour Registry (part of nine oldest SEER areas) | Non-localc | 1980–2006 | Connecticut Tumour Registry | 40–69 | 4 | 621 000 | −0.5 | −0.8 to 0.2 | |
| Distant | −1.0 | −1.6 to −0.5 | |||||||||
| New Mexico | Escobedo et al. [58] | New Mexico Tumour Registry | Non-localc | 1980–1997 | SEER (f) | All ages | NA | 901 033 | −0.3 | −0.7 to 0.1 | |
| Rhode Island | Coburn et al. [59] | Non-localc | 1995–2001h | Coburn et al. [59] | ≥40 | NAi | 250 00 | −0.1 | NA | ||
APC, annual percents change in %; BC, breast cancer; 95% CI, 95% confidence interval; NA, not reported or data not available for making calculations; NS, statistically non significant.
Data from Paci et al 2002 [35] for female population until 1996, and estimation until from 1996 to 1999 obtained using projection based on linear regression.
Counties of Akerhus, Hordaland, Oslo, Rogaland.
Local, regional, or distant SEER stage (Shambough et al. [17]): regional and distant extension were grouped as non-local.
Engholm et al. [61].
Connecticut, Hawaai, Iowa, New Mexico, Utah, Atlanta, Detroit, San Francisco-Oakland, Seattle-Puget Sound.
Incidence data reported by 5-year blocks; APC as reported by Verkooijen et al. [23].
Incidence data for 1995-97 and 1999-2002 (see Figure 1).
Missing data on stage was reported as “low” after 1993, without other precision.
Screening coverage (proportion of the woman population for which screening is available) and participation (women regularly attending screening) have gradually increased in all areas, included in this study. In all areas, the percentage of eligible women participating in mammography screening was high, ranging from 60% in Firenze (Italy) to 88% in Rhode Island (USA). Details on screening activities in selected can be found in the additional material, available at Annals of Oncology online. For the SEER and Connecticut Tumor Registry, before 1998, size was missing for 20%–42% of invasive cancers and thus, we used the local/regional/distant classification for which there were 3.6% unstaged cancers per year on average.
advanced breast cancer incidence
Temporal trends in size-specific breast cancer in the 11 main studies are reported as APC in Table 1. Figures 1–3 display temporal trends in advanced breast cancer incidence. The differences in incidence rates between areas were partly due to differences in the age groups considered and to the use of different reference populations for standardisation of rates. Such differences do not affect time trends.
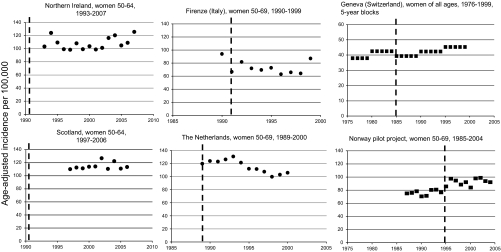
Figure 1.
Age-adjusted incidence rates of advanced breast cancer in Northern Ireland, Scotland, Firenze (Italy), The Netherlands, Geneva (Switzerland), and Norway. The interrupted vertical line indicates the year of screening start. Criterion for advanced cancer is a tumour size > or ≥20 mm (dots) or non-local cancer (squares). Rates were age adjusted using the World standard population (for Northern Ireland and Scotland) and the European standard population (for The Netherlands and Geneva). No information on age-adjustment was provided for the Norway pilot project and for Firenze (Italy).
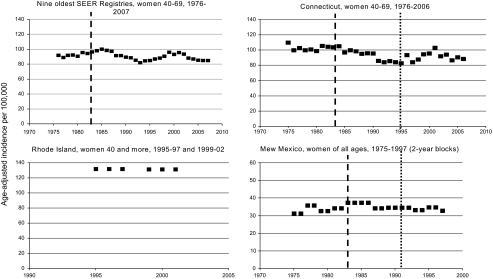
Figure 2.
Age-adjusted incidence rates of advanced breast cancer in the United States. The interrupted vertical line indicates the year of screening start (not displayed in Rhode Island where it started in 1983). The doted line is the first year of the Breast and Cervical Cancer Early Detection Program. Criterion for advanced cancer is a tumour size >20 mm (dots) or non-local cancer (squares), or (for Rhode Island) stages II–IV (squares). Rates were age adjusted using the 2000 US standard population but for New Mexico where the 1970 US standard population was used.
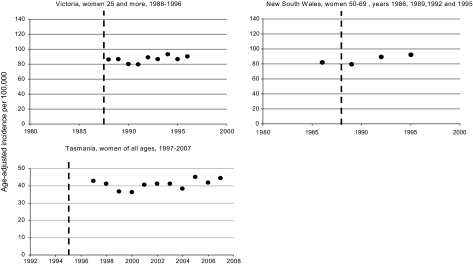
Figure 3.
Age-adjusted incidence rates of advanced breast cancer in Victoria and New South Wales (Australia). The interrupted vertical line indicates the year of screening start. Criterion for advanced cancer is a tumour size >15 mm in Victoria and ≥20 mm in New South Wales and Tasmania. Rates were age adjusted using the World standard population for Victoria and Tasmania and the 1991 Australia standard population for New South Wales.
In Firenze (Figure 1), the incidence of advanced cancers was reported for a single year (1990) before the screening programme started. The rate decreased abruptly in 1991 and then stabilised until 1998, and in 1999, it came back to the 1990 level. The APC of −1.6% is essentially due to the high 1990 rate at the beginning.
In The Netherlands, the National Evaluation Team for Breast Cancer Screening published size-specific incidence from 1989 until 1997 [8]. Incidences until 2000 were displayed in the 11th Evaluation Report [53]. In 2008, incidence rates by TNM stage until 2003 were reported during the International Union Against Cancer (UICC) World Cancer Congress in Geneva, Switzerland [60]. The incidence of cancers over 20 mm in women 50–69 first decreased by 12.2% from 1989 to 1997 with the lowest rate observed in 1998 (Figure 1). However, rates increased again in 1999 and 2000. The report made during the 2008 UICC World Cancer Congress in Geneva (Switzerland) concluded that ‘the initially observed decrease of advanced breast cancers after the implementation of mammography screening did not continue after 1997, but, was followed by an increase to the initial level’ [60]. Of note, in this report, ‘advanced’ equals cancers >20 mm in size with positive lymph nodes and/or distant metastases (M1) which represented ∼60% of all cancers >20 mm.
In Northern Ireland and in Scotland, trends in cancer ≥20 mm remained stable (Figure 1).
No change in incidence of advanced cancers occurred in the four Norwegian counties in the years following implementation of the pilot screening programmes in 1996.
In Geneva, Switzerland, trends in incidence rates of localised and non-localised (SEER definition) were reported separately for ductal and lobular invasive carcinoma. The latter type of breast cancer is more difficult to detect by mammography and we retained only data for ductal carcinoma.
No change in the incidence of advanced ductal cancers was evident in Geneva.
In the nine oldest SEER registries (USA), during a 16-year period, the incidence trends of non-local cancers were erratic with periods of decrease alternating with periods of increase (Figure 2). In 2001, rates had returned to levels before screening started. After 2000, the incidence of advanced cancers decreased, but similar decreases were noticeable for early cancer (<20 mm size of local cancers, data not displayed) [62]. The population in Connecticut represent 14% of the population in the nine oldest SEER areas. From 1975 to 2006, the incidence of non-local cancer fluctuated like the nine oldest SEER areas. In 1995, the Breast and Cervical Cancer Early Detection Program was launched. The incidence of advanced cancer peaked again in 2000 when participation to screening had reached 84%. Alike the nine oldest SEER areas, the incidence of advanced cancers decreased after 2000, but larger decreases were noticeable for early cancer (<20 mm size of local cancers, data not displayed).
In New Mexico, the incidence of advanced cancers increased slightly from 32.3 per 100 000 women of all ages in 1979–80 to 36.9 in 1983–84. It remained the same in 1985–86 and slightly decreased to 34.2 in 1987–88. After 1987–88, the incidence remained stable until 1997 and was not affected by the Breast and Cervical Cancer Early Detection Program launched in 1991.
In Rhode Island, the annual incidence of stages II–IV breast cancers in women 40 years of age and more was unchanged at 132.0 per 100 000 for the period 1995–97 and 131.3 for the period 1999–2001 (incidence rates for 1998 were not reported).
In Victoria, New South Wales, and Tasmania, trends in advanced cancer remained constant over time (Figure 3).
Incidence of large cancers or of cancer that already had disseminated in distant organ at diagnosis was available in some areas (Table 1). Cancer ≥30 mm size represented 50% and 46% of all cancer ≥20 mm in Northern Ireland and in Scotland, respectively. Upward trends of cancer ≥30 mm were noticeable.
In the nine oldest SEER registries (USA), distant cancers represented ∼15% of all non-local cancers. Their incidence remained stable in the nine old SEER registries, while in Connecticut significant downward trends were noticeable with APC of −1.0% from 1980 to 2006. However, the decrease in the incidence of advanced breast cancer observed until 1995 started in years preceding screening start and well before participation to screening was substantial (APC 1975–2006: −1.1%; 95% CI −1.6 to −0.6).
In New South Wales, while there was a significant decrease in the incidence of cancers ≥30 mm (APC = −1.5; 95% CI −2.4 to −0.5), there was a concomitant strong increase in the incidence of cancers 20–29 mm (APC = 4.1%; 95% CI 3.5% to 4.7%). In Tasmania, the incidence of cancers >49 mm remained stable over the 11-year period of observation.
Missing data on size or stage were variable, from 1% in Geneva (Switzerland) to 24% in Norway. No association between amounts of missing and time trends were noticed (data not shown).
discussion
In areas included in this study, breast cancer mortality fell dramatically after 1990 with APCs ranging from −1.7% to −2.5% [1–3]. Often, mortality decreases started before screening started or was offered to the majority of women. In examining trends of breast cancer by size or by stage, we expected to observe decreases in the incidence rate of advanced breast cancer. In contrast, this study found that in general, incidence rates of advanced breast cancer did not change much despite 7–15 years of good participation in mammographic screening. There were variations in some areas, with transient downward trends. These, however, were followed by increases back to pre-screening rates. For instance, in The Netherlands, trends in the falling incidence of advanced cancer for the first 7 years of screening were compatible with the results of most trials. However, incidence rates in 2003 were close to initial values in 1989 just before the launch of the national breast screening programme. Likewise, the rates of advanced breast cancer in the USA in 2000 were nearly back to levels before screening started. The fall of rates in the USA after 2001 was linked to massive drops in the use of hormone replacement treatment (HRT) after publication of the Women's Health Initiative trial, and falls of rates have affected breast cancer of all stages and of all sizes [31, 62]. Similar rate decreases of early and advanced breast cancer after massive cessation of HRT use by postmenopausal women have been observed in other countries [63, 64].
Comparison of breast screening activities showed that the numbers of large screen-detected cancers >20 mm were very similar in the USA and the UK in spite of considerable differences in the way screening is implemented [65, 66]. These elements indicate that the lower than expected decrease in advanced cancer incidence seems not attributable to age at screening, to screening frequency or to the way screening is implemented.
Randomised trials of breast cancer screening and their reviews concluded that mammographic screening of women aged 50–69 years decreases breast cancer mortality by 20% to 25% [8, 67]. In a previous work, we showed that in these trials, breast cancer mortality reductions were directly proportional to the fall in the incidence of advanced breast cancer [12]. Results of this study do not concur with expectations from mammography randomised trials despite the fact that women living in areas included in this study were at least as compliant to screening and had more screening rounds than women had in the intervention groups of most randomised trials. For instance, in the Swedish Two-County trial, the cumulative incidence of advanced breast cancer steadily decreased by 4.4% per year in women allocated to mammography compared with the control group [5, 6]. Two to four screening rounds at 24–33 month-interval lead to a 31% reduction in advanced breast cancer incidence and 32% in breast cancer mortality [12]. In contrast, until 2002 (when the massive discontinuation of hormone replacement treatment took place), no or only little change occurred in the incidence of advanced breast cancer in the USA, despite that over >10–15 years, most US women 40–69 years had three to five times more mammography screenings than in the Two-County Trial.
Other cancers subject to screening have shown remarkable decreases in both the incidence of advanced cancer and of mortality. For instance, in the USA, similarly to breast cancer mortality, colorectal cancer mortality has decreased by 1.8% per year from 1990 to 2006 [68]. A substantial contribution of earlier detection to the reduction of mortality is supported by steady decreases in the incidence of regional (−2.5% per year) and of distant (−1.2% per year) colorectal cancer in the SEER data [69]. In Iceland, age-adjusted annual mortality rate from cervical cancer decreased from 5.0 per 100 000 in 1974–78 to 2.1 in 1999–2003 [61]. At the same time, incidence of stages II–IV cervical cancer decreased by a factor two to three, depending on age [70].
What are the possible reasons for modest or no reduction in the incidence of advanced breast cancer?
First, in the absence of screening, would advanced breast cancer incidence have increased as the incidence of earlier breast cancer? This is very unlikely since generalisation of mammographic screening has been itself at the origin of sharp increases in the incidence of small breast cancers, many of which are deemed to be of low malignant potential [71]. In The Netherlands, no increase in proportions of advanced cancers in women not attending screening was observed [53], and there were no time changes in the incidence of advanced breast cancer in women <50 and >69 years of age in which screening is rare [8]. In Victoria (Australia), the incidence of advanced breast cancer in non-attending women was similar to that observed among screened women [51].
Second, reassurance of women with negative screening could have led to greater numbers of interval cancer >20 mm. This is unlikely to be the case. A study in The Netherlands concluded that this factor played only a minor role in breast cancer screening [72].
Mammographic screening aims to detect tumours in the breast, rather than lymph node or distant metastases. Therefore, size of invasive cancer was preferred to other indicators of cancer progression. Furthermore, contrary to axillary node status, cancer size measurement has remained stable over time [12]. In this respect, some of the fluctuations in rates of cancers classified as ‘regional or distant’ in the USA may have been due to changes over time in lymph node status assessment and improvements in the diagnosis of disseminated cancer. Changes in lymph node status assessment have also been invoked for explaining the absence of persistent downward trend of the advanced breast cancer incidence in The Netherlands [60, 73]. But the stable incidence in large size cancer we have observed cannot be explained by changes in lymph node status assessment.
Our study has several limitations. In Victoria, New Mexico, and Switzerland, incidence rates were reported for wider age groups than those targeted by screening. However, ∼96% of breast cancers occur after age 39 [68] and any decrease in advanced breast cancer occurrence due to screening before age 70 is expected to persist after that age. In the USA, screening after 69 years of age is common and nationwide surveys showed that in 1997–98, 57% of US women aged ≥70 received screening within 2 years [18].
We did not find much published data on breast cancer incidence by cancer size or stage. This was often due to the absence of a population-based cancer registry or lack of recording of data on disease advancement by registries. Some registries reported high levels of missing stage or size data [10]. In many countries, co-existence of centrally organised screening programmes and non-centrally organised screening activities (see additional material, available at Annals of Oncology online) made it difficult to obtain the same quality statistics related to both types of screening.
In conclusion, there are unexpected differences between the results of randomised trials and screening with mammography as applied in general populations which require further investigation. Cancer registries should as a matter of routine collect size of breast cancers and the screening status to enable further monitoring of the impact of mammographic breast cancer screening.
disclosure
The authors declare no conflict of interest.
Acknowledgments
We wish to thank Dr David Brewster for access to the Scottish Cancer Registry data. Parts of this article are based on work done by PA and MB when they were staff members of the IARC, Lyon, France. The IARC, however, has no responsibility for any of the results, discussion, and conclusions of the present article.
References
- 1.Hery C, Ferlay J, Boniol M, Autier P. Quantification of changes in breast cancer incidence and mortality since 1990 in 35 countries with Caucasian-majority populations. Ann Oncol. 2008;19:1187–1194. doi: 10.1093/annonc/mdn025. [DOI] [PubMed] [Google Scholar]
- 2.Hery C, Ferlay J, Boniol M, Autier P. Changes in breast cancer incidence and mortality in middle-aged and elderly women in 28 countries with Caucasian majority populations. Ann Oncol. 2008;19:1009–1018. doi: 10.1093/annonc/mdm593. [DOI] [PubMed] [Google Scholar]
- 3.Autier P, Boniol M, LaVecchia C, et al. Disparities in breast cancer mortality trends between thirty European countries: retrospective trend analysis of WHO mortality database. BMJ. 2010;341:c3620. doi: 10.1136/bmj.c3620. doi: 10.1136/bmj.c3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 5.Tabar L, Fagerberg CJ, Gad A, et al. Reduction in mortality from breast cancer after mass screening with mammography. Randomised trial from the Breast Cancer Screening Working Group of the Swedish National Board of Health and Welfare. Lancet. 1985;1:829–832. doi: 10.1016/s0140-6736(85)92204-4. [DOI] [PubMed] [Google Scholar]
- 6.Day NE, Williams DR, Khaw KT. Breast cancer screening programmes: the development of a monitoring and evaluation system. Br J Cancer. 1989;59:954–958. doi: 10.1038/bjc.1989.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.IARC. International Agency for Research on Cancer. Breast Cancer Screening; Handbook. Lyon, France: IARC Press; 2002. [Google Scholar]
- 8.Fracheboud J, Otto SJ, van Dijck JA, et al. Decreased rates of advanced breast cancer due to mammography screening in The Netherlands. Br J Cancer. 2004;91:861–867. doi: 10.1038/sj.bjc.6602075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith RA, Duffy SW, Gabe R, et al. The randomized trials of breast cancer screening: what have we learned? Radiol Clin N Am. 2004;42:793–806. doi: 10.1016/j.rcl.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 10.Pisani P, Forman D. Declining mortality from breast cancer in Yorkshire, 1983–1998: extent and causes. Br J Cancer. 2004;90:652–656. doi: 10.1038/sj.bjc.6601614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duffy SW, Tabar L, Vitak B, et al. The relative contributions of screen-detected in situ and invasive breast carcinomas in reducing mortality from the disease. Eur J Cancer. 2003;39:1755–1760. doi: 10.1016/s0959-8049(03)00259-4. [DOI] [PubMed] [Google Scholar]
- 12.Autier P, Héry C, Haukka J, et al. Advanced breast cancer and breast cancer mortality in randomized controlled trials on mammography screening. J Clin Oncol. 2009;27:5919–5923. doi: 10.1200/JCO.2009.22.7041. [DOI] [PubMed] [Google Scholar]
- 13.Blackman DK, Bennett EM, Miller DS. Trends in self-reported use of mammograms (1989–1997) and papanicolaou tests (1991–1997) MMWR. 1999;48(SS-6):1–22. [PubMed] [Google Scholar]
- 14.Esserman L, Shieh Y, Thompson I. Rethinking screening for breast cancer and prostate cancer. JAMA. 2009;15:1685–1692. doi: 10.1001/jama.2009.1498. [DOI] [PubMed] [Google Scholar]
- 15.AJCC. American Joint Committee on Cancer: AJCC Cancer Staging Manual. 6th edition. New York, NY: Springer; 2002. pp. 171–180. [Google Scholar]
- 16.UICC. International Union Against Cancer (UICC), TNM Classification of Malignant Tumours. 6th edition. New York: Wiley-Liss; 2002. [Google Scholar]
- 17.Shambough E, Gloecker Ries L, Young J, et al. SEER Extent of Disease—1988 Instructions. Washington, DC: National Institutes of Health; 1992. Codes and Coding. [Google Scholar]
- 18.Caplan LS. To screen or not to screen: the issue of breast cancer screening in older women. Public Health Rev. 2001;29:231–240. [PubMed] [Google Scholar]
- 19.Paci E, Duffy S. Overdiagnosis and overtreatment in service screening. Breast Cancer Res. 2005;7:266–270. doi: 10.1186/bcr1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hofvind S, Sorum R, Thoresen S. Incidence tumor characteristics of breast cancer diagnosed before and after implementation of a population-based screening program. Acta Oncol. 2008;47:225–231. doi: 10.1080/02841860701518041. [DOI] [PubMed] [Google Scholar]
- 21.Kleinbaum DG, Kupper LL, Muller AL. Applied Regression Analysis and Other Multivariable Methods. 2nd edition. Boston, MA: PWS-Kent; 1988. [Google Scholar]
- 22.Kricker A, Farac K, Smith D, et al. Breast cancer in New South Wales in 1972–1995: tumor size and the impact of mammographic screening. Int J Cancer. 1999;81:877–880. doi: 10.1002/(sici)1097-0215(19990611)81:6<877::aid-ijc7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 23.Verkooijen HM, Fioretta G, Vlastos G, et al. Important increase of invasive lobular breast cancer incidence in Geneva, Switzerland. Int J Cancer. 2003;104:778–781. doi: 10.1002/ijc.11032. [DOI] [PubMed] [Google Scholar]
- 24.Miller BA, Feuer EJ, Hankey BF. Recent incidence trends for breast cancer in women and the relevance of early detection: an update. CA Cancer J Clin. 1993;43:27–41. doi: 10.3322/canjclin.43.1.27. [DOI] [PubMed] [Google Scholar]
- 25.Garfinkel L, Boring CC, Heath CW., Jr Changing trends. An overview of breast cancer incidence and mortality. Cancer. 1994;74(1 Suppl):222–227. doi: 10.1002/cncr.2820741304. [DOI] [PubMed] [Google Scholar]
- 26.Chu KC, Tarone RE, Kessler LG, et al. Recent trends in U.S. breast cancer incidence, survival, and mortality rates. J Natl Cancer Inst. 1996;88:1571–1579. doi: 10.1093/jnci/88.21.1571. [DOI] [PubMed] [Google Scholar]
- 27.Ghafoor A, Jemal A, Ward E, et al. Trends in breast cancer by race and ethnicity. CA Cancer J Clin. 2003;53:342–355. doi: 10.3322/canjclin.53.6.342. [DOI] [PubMed] [Google Scholar]
- 28.Schootman M, Jeffe D, Reschke A, Aft R. The full potential of breast cancer screening use to reduce mortality has not yet been realized in the United States. Breast Cancer Res Treat. 2004;85:219–222. doi: 10.1023/B:BREA.0000025410.41220.67. [DOI] [PubMed] [Google Scholar]
- 29.Hausauer AK, Keegan TH, Chang ET, Clarke CA. Recent breast cancer trends among Asian/Pacific islander, Hispanic, and African-American women in the US: changes by tumor subtype. Breast Cancer Res. 2007;9:R90. doi: 10.1186/bcr1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smigal C, Jemal A, Ward E, et al. Trends in breast cancer by race and ethnicity: update 2006. CA Cancer J Clin. 2006;56:168–183. doi: 10.3322/canjclin.56.3.168. [DOI] [PubMed] [Google Scholar]
- 31.Jemal A, Elizabeth Ward E, Thun MJ. Recent trends in breast cancer incidence rates by age and tumor characteristics among U.S. women. Breast Cancer Res. 2007;9:R28. doi: 10.1186/bcr1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson WF, Jatoi I, Devesa SS. Assessing the impact of screening mammography: breast cancer incidence and mortality rates in Connecticut (1943–2002) Breast Cancer Res Treat. 2006;99:333–340. doi: 10.1007/s10549-006-9214-z. [DOI] [PubMed] [Google Scholar]
- 33.Polednack AP. Trends in late-stage breast and cervical cancer incidence rates in Connecticut (United States) Cancer Causes Control. 2003;14:361–365. doi: 10.1023/a:1023972826767. [DOI] [PubMed] [Google Scholar]
- 34.Barchielli A, Paci E. Trends in breast cancer mortality, incidence, and survival, and mammographic screening in Tuscany, Italy. Cancer Causes Control. 2001;12:249–255. doi: 10.1023/a:1011280204842. [DOI] [PubMed] [Google Scholar]
- 35.Paci E, Duffy SW, Giorgi D, et al. Quantification of the effect of mammographic screening on fatal breast cancers: The Florence Programme 1990–96. Br J Cancer. 2002;87:65–69. doi: 10.1038/sj.bjc.6600301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hofvind S, Sørum R, Haldorsen T, Langmark F. Incidence of breast cancer before and after implementation of population-based mammography screening. Tidsskr Nor Lægeforen. 2006;126:2935–2938. (Article in Norwegian) [PubMed] [Google Scholar]
- 37.McCann J, Stockton D, Day N. Breast cancer in East Anglia: the impact of the breast screening programme on stage at diagnosis. J Med Screen. 1998;5:42–48. doi: 10.1136/jms.5.1.42. [DOI] [PubMed] [Google Scholar]
- 38.Schouten LJ, de Rijke JM, Schlangen JT, Verbeek AL. Evaluation of the effect of breast cancer screening by record linkage with the cancer registry, The Netherlands. J Med Screen. 1998;5:37–41. doi: 10.1136/jms.5.1.37. [DOI] [PubMed] [Google Scholar]
- 39.Hiatt RA, Tekawa IS. Mammography utilization in relation to stage at diagnosis for breast cancer: the Kaiser Permanente experience in a large pre-paid health plan. Prog Clin Biol Res. 1990;339:227–238. [PubMed] [Google Scholar]
- 40.Simon MS, Lemanne D, Schwartz AG, et al. Recent trends in the incidence of in situ and invasive breast cancer in the Detroit metropolitan area (1975–1988) Cancer. 1993;71:769–774. doi: 10.1002/1097-0142(19930201)71:3<769::aid-cncr2820710320>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 41.Vutuc C, Haidinger G, Waldhoer T. Prevalence of self-reported screening mammography and impact on breast cancer mortality in Austria. Wien Klin Wochenschr. 1998;110(13–14):485–490. [PubMed] [Google Scholar]
- 42.Kotwall C, Covington D, Churchill P, et al. Breast conservation surgery for breast cancer at a regional medical center. Am J Surg. 1998;176(6):510–514. [PubMed] [Google Scholar]
- 43.Harvey JM, Sterrett GF, McEvoy S, et al. Pathology reporting of breast cancer: trends in 1989–1999, following the introduction of mammographic screening in Western Australia. Pathology. 2005;37(5):341–346. doi: 10.1080/00313020500253141. [DOI] [PubMed] [Google Scholar]
- 44.Jensen AR, Madsen AH, Overgaard J. Trends in breast cancer during three decades in Denmark: stage at diagnosis, surgical management and survival. Acta Oncol. 2008;47:537–544. doi: 10.1080/02841860801982758. [DOI] [PubMed] [Google Scholar]
- 45.Malmgren JA, Mary K, Atwood MK, Henry G, Kaplan HG. Increase in mammography detected breast cancer over time at a community based regional cancer center: a longitudinal cohort study 1990–2005. BMC Cancer. 2008;8:131. doi: 10.1186/1471-2407-8-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taplin SH, Ichikawa L, Buist DSM, et al. Evaluating organized breast cancer screening implementation: the prevention of late-stage disease? Cancer Epidemiol Biomarkers Prev. 2004;13:225–234. doi: 10.1158/1055-9965.epi-03-0206. [DOI] [PubMed] [Google Scholar]
- 47.Glass AG, Lacey JV, Jr, Carreon JD, Hoover RN. Breast cancer incidence, 1980–2006: combined roles of menopausal hormone therapy, screening mammography, and estrogen receptor status. J Natl Cancer Inst. 2007;99:1152–1161. doi: 10.1093/jnci/djm059. [DOI] [PubMed] [Google Scholar]
- 48.Prehn A, Clarke C, Topol B, et al. Increase in breast cancer incidence in middle-aged women during the 1990s. Ann Epidemiol. 2002;12:476–481. doi: 10.1016/s1047-2797(01)00315-5. [DOI] [PubMed] [Google Scholar]
- 49.Ward E, Jemal A, Thun M. Regarding “increase in breast cancer incidence in middle-aged women during the 1990s”. Ann Epidemiol. 2005;15:424–425. doi: 10.1016/j.annepidem.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 50.Louwman WJ, Voogd AC, van Dijck JAMM, et al. On the rising trends of incidence and prognosis for breast cancer patients diagnosed 1975–2004: a long-term population-based study in southeastern Netherlands. Cancer Causes Control. 2007;19:97–106. doi: 10.1007/s10552-007-9075-8. [DOI] [PubMed] [Google Scholar]
- 51.Harmer C, Staples M, Kavanagh AM. Evaluation of breast cancer incidence: is the increase due entirely to mammographic screening? Cancer Causes Control. 1999;10:333–337. doi: 10.1023/a:1008949819969. [DOI] [PubMed] [Google Scholar]
- 52.Tasmanian Cancer Registry. Cancer in Tasmania: Incidence and Mortality, 1997–2007 (11 Reports) Hobart, Australia: Menzies Research Institute; 2010. [Google Scholar]
- 53.NETBCS. National Evaluation Team for Breast Cancer Screening. National evaluation of breast cancer screening in the Netherlands; 2005. 11th Evaluation report, Rotterdam. [Google Scholar]
- 54.Northern Ireland Cancer Registry, Centre for Public Health, School of Medicine, Dentistry & Biomedical Sciences Queen's University Belfast, Belfast. http://www.qub.ac.uk/research-centres/nicr/ (26 August 2010, date last accessed) [Google Scholar]
- 55.Scottish Cancer Registry Scottish Cancer Registry, Information Services Division of NHS National Services Scotland (ISD Scotland), Edinburgh. http://showcc.nhsscotland.com/isd//3535.html (26 August 2010, date last accessed) [Google Scholar]
- 56.Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence—SEER 17 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2009 Sub (1973–2007 Varying) ( www.seer.cancer.gov), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2010; based on the November 2009; submission. [Google Scholar]
- 57.Connecticut Cancer registry. Connecticut department of Health. http://www.ct.gov/dph/cwp/view.asp?a=3129&q=389716 (26 August 2010, date last accessed) [Google Scholar]
- 58.Escobedo LG, Zhong Z, Key C. Breast and cervical cancer screening and disease incidence and stage in New Mexico. Cancer Causes Control. 2002;13:137–145. doi: 10.1023/a:1014302121094. [DOI] [PubMed] [Google Scholar]
- 59.Coburn NG, Chung MA, Fulton J, Cady B. Decreased breast cancer tumor size, stage, and mortality in Rhode Island: an example of a well-screened population. Cancer Control. 2004;11:222–230. doi: 10.1177/107327480401100403. [DOI] [PubMed] [Google Scholar]
- 60.Fracheboud J, Otto SJ, Holland R, et al. Is There a Real Increase of Advanced Breast Cancers in Spite of Mammography Screening? Geneva, Switzerland: UICC 08 World Cancer Congress, International Union Against Cancer 27-31; 2008. (Abstr FC55) [Google Scholar]
- 61.Engholm G, Ferlay J, Christensen N, et al. NORDCAN: Cancer Incidence, Mortality and Prevalence in the Nordic Countries, Version 3.2. Association of Nordic Cancer Registries. Copenhagen, Denmark: Danish Cancer Society; 2008. http://www.ancr.nu; http://www-dep.iarc.fr/nordcan.htm. [Google Scholar]
- 62.Ravdin PM, Cronin KA, Howlader N, et al. The decrease in breast-cancer incidence in 2003 in the United States. New Eng J Med. 2007;356:1870–1876. doi: 10.1056/NEJMsr070105. [DOI] [PubMed] [Google Scholar]
- 63.Renard F, Vankrunkelsven P, Van Eycken L, et al. Decline in breast cancer incidence in the Flemish region of Belgium after a decline in hormonal replacement therapy. Ann Oncol. 2010 doi: 10.1093/annonc/mdq240. May 3 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 64.Daubisse-Marliac L, Delafosse P, Boitard JB, et al. Breast cancer incidence and time trend in France from 1990 to 2007: a population-based study from two French cancer registries. Ann Oncol. 2010 doi: 10.1093/annonc/mdq396. August 2 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 65.Smith-Bindman R, Chu PW, Miglioretti DL. Comparison of screening mammography in the United States and the United Kingdom. JAMA. 2003;290:2129–2137. doi: 10.1001/jama.290.16.2129. [DOI] [PubMed] [Google Scholar]
- 66.Smith-Bindman R, Ballard-Barbash R, Miglioretti DL, et al. Comparing the performance of mammography screening in the USA and the UK. J Med Screen. 2005;12:50–54. doi: 10.1258/0969141053279130. [DOI] [PubMed] [Google Scholar]
- 67.Humphrey LL, Helfand M, Chan BK, Woolf SH. Breast cancer screening: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137(5 part 1):347–360. doi: 10.7326/0003-4819-137-5_part_1-200209030-00012. [DOI] [PubMed] [Google Scholar]
- 68.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 69.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sigurdsson K, Sigvaldason H. Effectiveness of cervical cancer screening in Iceland, 1964–2002: a study on trends in incidence and mortality and the effect of risk factors. Acta Obstet Gynecol Scand. 2006;85:343–349. doi: 10.1080/00016340500432457. [DOI] [PubMed] [Google Scholar]
- 71.Chiu C, Morrell S, Page A, et al. Population-based mammography screening and breast cancer incidence in New South Wales, Australia. Cancer Causes Control. 2006;17:153–160. doi: 10.1007/s10552-005-2368-x. [DOI] [PubMed] [Google Scholar]
- 72.De Gelder R, van As E, Tilanus-Linthorst MM, et al. Breast cancer screening: evidence for false reassurance? Int J Cancer. 2008;123:680–686. doi: 10.1002/ijc.23540. [DOI] [PubMed] [Google Scholar]
- 73.Maaskant AJG, van de Poll-Franse LV, Voogd AC, et al. Stage migration due to introduction of the sentinel node procedure: a population-based study. Breast Cancer Res Treat. 2009;113:173–179. doi: 10.1007/s10549-008-9913-8. [DOI] [PubMed] [Google Scholar]