Abstract
Hematopoietic cell-transplantation (HCT) is a highly specialized and resource-intense medical procedure that can be associated with disparities in access to transplantation. Barriers to access to HCT are multifactorial, complex and interrelated. Our current knowledge of specific barriers that prevent access to HCT is very limited. As the utilization of HCT increases, it is imperative that underserved populations receive the benefit of this life-saving procedure. We review the prevailing literature on access to HCT and describe research priorities for eliminating disparities in transplantation. Better understanding of these complex barriers will minimize inequities, inform health policy, guide development of interventions targeted to eliminate disparities and continue the expansion of HCT in the future.
Keywords: Hematopoietic-cell transplantation, autologous, allogeneic, access, underserved populations
INTRODUCTION
Hematopoietic-cell transplantation (HCT) is curative therapy for a variety of malignant and non-malignant hematologic disorders. The utilization of HCT has progressively increased over the last four decades since reports of first successful transplantations in 1968 and an estimated 50,000 transplants are performed worldwide every year, including 20,000 in the United States.1 With emerging indications, improvements in technology and supportive care, and increasing availability of alternative graft sources and reduced intensity conditioning regimens, use of HCT can be expected to increase further in the future. However, HCT is a highly specialized, technologically sophisticated, resource-intense and expensive procedure that can be associated with health-care associated disparities. These health care disparities have clinical, ethical and policy implications. We review the available literature on access to HCT and describe barriers that need to be addressed to ensure equitable access to HCT for all populations.
Access to Cancer Care
Access to health care has been defined as “the timely use of affordable personal health services to achieve the best possible health outcomes”.2,3 Mandelblatt et al have very elegantly summarized the complexities of access to health care; according to them, “the process of gaining access to care represents dynamic interactions of diverse individuals in their social context interfacing with health care providers, who, in turn, are operating in a variety of changing and often constrained medical care structures and environments”.3 Disparities exist in the health care of minority populations in the United States and minorities do not have the same access to health care and as a result do not receive the same quality of health care and have poorer overall health status than non-minorities.4–6 The Institute of Medicine in its report, Unequal Treatment: Confronting Racial and Ethnic Disparities in Healthcare, further concluded that even when other healthcare access-related factors, such as ability to pay for care are the same, racial and ethnic minorities receive lower quality healthcare than Whites.5 The report recommended a comprehensive, multi-level strategy to eliminate these disparities, including increasing awareness among providers, patients, payors, health plan purchasers and the society at large, enhancing training and education and conducting research on interventions.
Cancer care is also associated with disparities in detection, treatment and outcomes for specific high-risk populations. These high-risk populations include elderly patients, women, patients of Black or Hispanic race/ethnicity, the under- or un-insured, patients from the lower socioeconomic strata, patients with lower levels of health literacy and education, and patients with rural residence.3,7–11 The origins of these inequities are multifactorial and complex. Patient barriers include demographics, language, acculturation, attitudes and family and cultural contexts.3 In addition, health care providers play an important role in ensuring access to cancer care. Physician and provider specific barriers that have been reported in the literature include age or race biases, biases and beliefs about screening and treatment efficacy, deficient knowledge and training, lack of confidence, lack of culturally sensitive resources, lack of time, concerns about patient acceptance, cost concerns, and logistic or organizational barriers.3,12–15 Finally, health care system barriers such as organizational and structural factors and reimbursement and financial forces can facilitate or hinder access to optimal cancer care.3 Factors can also be closely interrelated; for instance, racial and ethnic minorities are more likely to be uninsured compared to Whites.16
Access to Hematopoietic-cell Transplantation
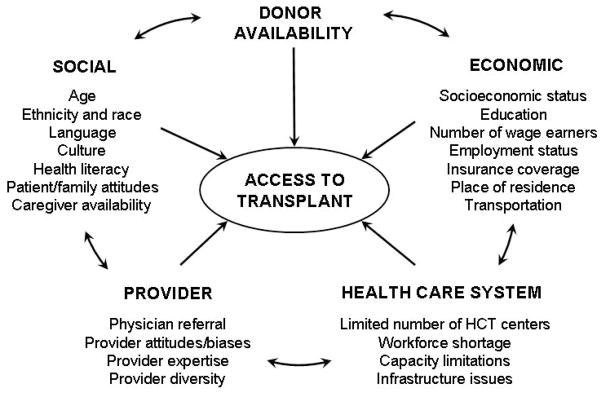
HCT is most commonly performed for malignant hematologic disorders and barriers that prevent access to cancer care may also be relevant for HCT. However, additional barriers may also have a role since HCT is a high-cost and sophisticated medical procedure that involves complex interactions between patient, provider and health-care system factors (Figure 1).
Figure 1.
Potential barriers to access to hematopoietic-cell transplantation
The available literature on access to HCT is very limited (Table 2). Studies have generally had insufficient statistical power to detect differences among access indicators and/or have frequently utilized databases with limited applicability to understanding access issues, particularly their cause. Inpatient hospital discharge databases have been frequently utilized in these studies. However, such databases may not be truly appropriate to health disparities research as they may not capture the universe of patients with hematologic malignancies or who received transplants in a population cohort. Also, disease and procedure codes are used to identify patients from these datasets; medical codes lack precision and important prognostic factors such as disease stage and remission status which may affect decision making regarding use of HCT could not be considered in these analyses. Furthermore, contemporaneous studies are lacking and the majority of studies addressed barriers in an era when present day transplantation techniques were not widely available.
Possible limitations in study design notwithstanding, previously conducted studies have increased our characterization of barriers that limit access to transplantation. For instance, current literature highlights substantial geographic variation in access to HCT. Mitchell et al, in a study that utilized hospital discharge data from four states (California, Massachusetts, Maryland and New York) for the years 1988 and 1991, showed state wise differences in access to HCT by insurance coverage and race.17 Compared to patients with private insurance, Medicaid beneficiaries in California, New York and Maryland/Massachusetts were 78%, 64% and 61% as likely to receive a transplant for leukemia and 69%, 56% and 32% as likely to receive a transplant for lymphoma, respectively. Similarly, compared to Whites, Hispanics were significantly less likely to receive a transplant for lymphoma in New York but had similar rates of transplantation for lymphoma in California and for leukemia in all four states.
Specific disparities to access to HCT that have been identified in the literature include:
Age
Studies that have investigated age as a predictor of access to HCT indicate that younger patients are more likely to receive a transplant compared to older patients. Mitchell et al showed that each 10-year increase in age was associated with 10% to 18% (variation by state) lower odds of receiving HCT for leukemia or lymphoma.17 In another study that used inpatient discharge data for the state of Texas from 1999, Hwang et al reported that elderly (age ≥65 years) patients had a significantly lower likelihood of receiving a transplant for leukemia with each year increase in age; however, no age effect was noted among pediatric (age <18 years) and adult (age 18–64 years) recipients.18 In a study using Arizona hospital discharge data from 1997–2003, Cho has also reported that increasing age reduces the probability of receiving a transplant for leukemia; they did not detect any effect of age on the likelihood of transplantation for lymphoma.19 These previous studies pre-date the advent of reduced-intensity and non-myeloablative preparative regimens which are associated with lower risks of morbidity and mortality and now allow transplantation as a viable treatment option for older patients. Additional factors may impact utilization of HCT in older patients. Older patients may decline or their providers may not recommend aggressive therapies such as HCT. Also, some differences in transplant utilization among older patients may be appropriate since older patients may have comorbidities and disease characteristics which may make them ineligible for transplantation; previous studies have not been able to sufficiently account for these important variables.
Gender
Mitchell et al and Cho found no impact of gender on the likelihood of transplantation for leukemia or lymphoma.17,19 Hwang et al reported that elderly men were more likely to receive a transplant for leukemia than elderly women, but they found no effect of gender on pediatric and adult HCT recipients. In a study that specifically tried to address the issue of gender and access to HCT, Mehta et al used data for 1989–1999 from the International Bone Marrow Transplant Registry and Surveillance Epidemiology and End Results (SEER) database.20 They concluded that there was no significant bias towards the use of HCT in males compared to females. However, in a more recent and large study that used data from the Center for International Blood and Marrow Transplant Research (CIBMTR) and SEER, Joshua et al showed that men were more likely to receive HCT than women and this difference was primarily seen in autologous HCT for lymphoma or myeloma, but not in allogeneic HCT.21 The reasons for gender specific disparities, if any, are unclear and need to be explored in future studies.
Race
The role of race in access to HCT has to be interpreted with caution. Race is a complex social, cultural and political construct and not a biological concept and accordingly, the definition of race has changed and evolved over time. Self-reported race is most accurate, but race is frequently assigned by centers or providers for a number of databases. Among the earliest studies of access to HCT, Mitchell et al showed that Blacks were less likely than Whites to undergo a transplant for leukemia or lymphoma in each of the four states investigated.17 Joshua et al have also shown that the likelihood of undergoing HCT for leukemia, lymphoma and multiple myeloma is significantly lower for Blacks compared to Whites and that these differences existed for autologous, matched sibling donor and unrelated donor HCT.21 On the other hand, Hwang et al and Cho did not find any impact of race of transplantation rates.18,19
Insurance Status
HCT is a costly procedure,22,23 and patients with no or limited health insurance coverage may have a lower likelihood of receiving HCT. Three previous studies have attempted to address the impact of insurance coverage on access to HCT. Mitchell et al showed that patients enrolled with Medicaid, self-paid (uninsured) patients and Health Maintenance Organization (HMO) enrollees with leukemia or lymphoma were significantly less likely to undergo HCT compared with patients with private insurance.17 The authors suggested that the lower probability of getting a transplant among HMO enrollees could be related to coverage restrictions or delay in approval for costly medical procedures such as HCT. Cho also reported that patients with less generous insurance coverage were less likely to receive HCT for leukemia and lymphoma.19 However, Hwang et al did not find any association between payor status (commercial insurance, HMO, Medicare, Medicaid, self-pay and other payor) and receipt of HCT for leukemia.18 Their findings were specific to the state of Texas and authors commented that the lack of relationship between payor status and HCT use could be due to equitable access to HCT or lack of statistical power to identify significant associations. The interaction of insurance status with other sociodemographic factors, albeit complex, has not been explored. For example, children may have greater access to HCT compared to adults due to the presence of hospital, state or federal programs that may provide coverage for HCT.
Other barriers
Education status, estimated by Zip Code of residence, was not identified as a major factor associated with transplant access by Mitchell et al.17 Cho examined the association between hospital characteristics and likelihood of transplantation for leukemia in Arizona.19 Patients with leukemia and lymphoma admitted to a minor teaching hospitals (vs. major teaching hospitals) and small or medium sized hospitals (vs. large hospitals, >250 beds) were less likely to receive a transplant. Patients admitted to for-profit hospitals and government-owned hospitals had comparable probabilities of transplantation.
Areas for Further Research
Research is needed to further characterize disparities in access to HCT, identify causes for any disparities deemed inappropriate and to investigate interventions to mitigate those barriers. However, health disparities research in HCT can be very challenging. Typically, a very large population cohort has to be assembled for studies of access to health-care. A reliable estimate of the ‘numerator’ (e.g., number of transplants for a disease) and ‘denominator’ (e.g., total number of patients with that disease for whom a transplant is appropriate) for a cohort over a given period of time is also needed. Finally, comprehensive information about the factors that may be associated with disparate access (e.g., race and ethnicity, education status, socioeconomic status, cultural attitudes, insurance/payment status) is required. Large administrative databases such as the SEER-Medicare dataset are frequently used to identify disparities in access to cancer care. Since these large databases cannot robustly collect information about access indicators, surrogate variables are used to evaluate barriers that may impact access to treatment (e.g., Zip Code to assign socioeconomic status, education status and place of residence). The majority of federal and claims databases do not have robust information about hematologic malignancies and HCT and details of important prognostic factors (e.g. cytogenetic risk) that predict which patients should be considered for HCT are generally not available. However, good quality studies using administrative and claims databases may be possible in the near future due to enhanced data collection efforts by multiple groups. For example, SEER has collected data on MDS and chronic myeloproliferative disorders since 2001. Since 2007, all allogeneic transplants in the United States should be reported to the CIBMTR under the requirements of the Stem Cell Therapeutic and Research Act of 2005.
Database studies can be useful for identification of some aspects of health-care disparities. However, they are not optimal for evaluation of individual patient and provider level barriers or to identify the causes of disparate access. Additional health services research methodologies (e.g. patients and physician surveys, qualitative research methods such as focus groups) will be needed to better understand obstacles to universal access to HCT and must also account for inherent inequities in access to HCT due to biological and medical factors. For instance, ethnic and racial minorities have a lower probability of finding a suitable donor and have a higher prevalence of comorbidities that may make them ineligible for transplantation.24–26 Once etiologies of disparate access have been characterized, studies evaluating targeted interventions to address barriers to access are also needed.
In order for underserved populations to obtain the benefit of a life-saving procedure such as HCT, it is imperative that the medical community work to reduce inappropriate disparities and ensure equitable access to transplantation. Our current knowledge about specific barriers that prohibit access to HCT is very limited. While waiting for better information, the medical community, including payors, policy makers and healthcare providers must use the current awareness of disparate access as a call to action to examine their own practices and work to eliminate inappropriate disparities. Better understanding of these complex barriers will minimize inequities, inform health policy, guide development of interventions targeted to eliminate disparities and continue the expansion of HCT in the future.
Table 1.
Access to hematopoietic cell transplantation: summary of published studies
| Reference | Access focus | Data sources | Study design | N | Population characteristics | Results |
|---|---|---|---|---|---|---|
| Mitchell et al (1997)17 | Age Gender Race Education Insurance |
Inpatient hospital discharge data for California, Maryland, Massachusetts and New York | ICD-9 codes used to identify inpatients with leukemia or lymphoma and recipients of auto or allo HCT in 1988 and 1991 |
|
|
|
| Mehta et al (2003)20 | Gender | SEER, IBMTR | SEER incidence rates and data from IBMTR used to estimate rates of allo HCT for AML, ALL and CML from 1989–1999* |
|
|
|
| Hwang et al (2004)18 | Age Gender Race Insurance Comorbidity |
Texas inpatient hospital discharge data | ICD-9 codes used to identify inpatients with acute or chronic leukemia’s and recipients of auto or allo HCT in 1999 |
|
|
|
| Cho (2006)19 † | Age Gender Race Insurance Center factors Comorbidities |
Arizona inpatient hospital discharge data | ICD-9 codes used to identify inpatients with leukemia or lymphoma and recipients of auto or allo HCT from 1997–2003 |
|
|
|
| Joshua et al (2007)21 | Gender Race |
SEER, US Census Bureau and CIBMTR | SEER incidence rates and data from CIBMTR used to estimate rates of auto and allo HCT for leukemia, lymphoma and myeloma from 1997–2002 |
|
|
|
ICD – international classification of diseases; auto – autologous; allo – allogeneic; HCT – hematopoietic cell transplantation; International Classification of Diseases; SEER – Surveillance Epidemiology and End Results; IBMTR – International Bone Marrow Transplant Registry (now CIBMTR); CIBMTR – Center for International Blood and Marrow Transplant Research; AML – acute myeloid leukemia; ALL – acute lymphoblastic leukemia; CML – chronic myeloid leukemia; CLL – chronic lymphocytic leukemia; NHL – non-Hodgkin’s lymphoma; MM – multiple myeloma; HMO – Health Maintenance Organization
HCT reported to IBMTR from 1989–1992 and SEER incidence estimates from 1992–1999 were used for this analysis
Non-peer reviewed manuscript
References
- 1.Pasquini MC, Wang Z, Schneider L. Current use and outcome of hematopoietic stem cell transplantation: part I-CIBMTR Summary Slides, 2007. [Accessed 08/01/09];CIBMTR Newsletter. 2007 13(2):5–9. Available at: http://www.cibmtr.org/PUBLICATIONS/Newsletter/index.html.
- 2.Millman M. Access to health care in America. Washingon, DC: National Academy Press; 1993. [PubMed] [Google Scholar]
- 3.Mandelblatt JS, Yabroff KR, Kerner JF. Equitable access to cancer services: A review of barriers to quality care. Cancer. 1999;86:2378–2390. [PubMed] [Google Scholar]
- 4.Groman R, Ginsburg J. Racial and ethnic disparities in health care: a position paper of the American College of Physicians. Ann Intern Med. 2004;141:226–232. doi: 10.7326/0003-4819-141-3-200408030-00015. [DOI] [PubMed] [Google Scholar]
- 5.Institute of Medicine. Unequal Treatment: Confronting Racial and Ethnic Disparities in Healthcare. Washington, DC: The National Academies Press; 2003. [PubMed] [Google Scholar]
- 6.Agency for Healthcare Research and Quality. 2008 National Healthcare Quality Report. Rockville, MD: U.S. Department of Health and Human Services, Agency for Healthcare Research and Quality; 2009. [Google Scholar]
- 7.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94:334–357. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 8.Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 9.Albano JD, Ward E, Jemal A, et al. Cancer mortality in the United States by education level and race. J Natl Cancer Inst. 2007;99:1384–1394. doi: 10.1093/jnci/djm127. [DOI] [PubMed] [Google Scholar]
- 10.Clegg LX, Li FP, Hankey BF, Chu K, Edwards BK. Cancer survival among US whites and minorities: a SEER (Surveillance, Epidemiology, and End Results) Program population-based study. Arch Intern Med. 2002;162:1985–1993. doi: 10.1001/archinte.162.17.1985. [DOI] [PubMed] [Google Scholar]
- 11.Clegg LX, Reichman ME, Miller BA, et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: selected findings from the surveillance, epidemiology, and end results: National Longitudinal Mortality Study. Cancer Causes Control. 2009;20:417–435. doi: 10.1007/s10552-008-9256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haggerty J, Tudiver F, Brown JB, Herbert C, Ciampi A, Guibert R. Patients’ anxiety and expectations: how they influence family physicians’ decisions to order cancer screening tests. Can Fam Physician. 2005;51:1658–1659. [PMC free article] [PubMed] [Google Scholar]
- 13.Tudiver F, Guibert R, Haggerty J, et al. What influences family physicians’ cancer screening decisions when practice guidelines are unclear or conflicting? J Fam Pract. 2002;51:760. [PubMed] [Google Scholar]
- 14.Battista RN, Williams JI, MacFarlane LA. Determinants of primary medical practice in adult cancer prevention. Med Care. 1986;24:216–224. doi: 10.1097/00005650-198603000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Battista RN, Williams JI, MacFarlane LA. Determinants of preventive practices in fee-for-service primary care. Am J Prev Med. 1990;6:6–11. [PubMed] [Google Scholar]
- 16.Ward E, Halpern M, Schrag N, et al. Association of insurance with cancer care utilization and outcomes. CA Cancer J Clin. 2008;58:9–31. doi: 10.3322/CA.2007.0011. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell JM, Meehan KR, Kong J, Schulman KA. Access to bone marrow transplantation for leukemia and lymphoma: the role of sociodemographic factors. J Clin Oncol. 1997;15:2644–2651. doi: 10.1200/JCO.1997.15.7.2644. [DOI] [PubMed] [Google Scholar]
- 18.Hwang JP, Lam TP, Cohen DS, Donato ML, Geraci JM. Hematopoietic stem cell transplantation among patients with leukemia of all ages in Texas. Cancer. 2004;101:2230–2238. doi: 10.1002/cncr.20628. [DOI] [PubMed] [Google Scholar]
- 19.Cho C. [Accessed 08-01-2009];Factors affecting stem cell transplantation for leukemia and lymphoma. 2006 http://hdl.handle.net/1961/3595.
- 20.Mehta P, Pollock BH, Nugent M, Horowitz M, Wingard JR. Access to stem cell transplantation: do women fare as well as men? Am J Hematol. 2003;72:99–102. doi: 10.1002/ajh.10273. [DOI] [PubMed] [Google Scholar]
- 21.Joshua TV, Rizzo JD, Zhang MJ, Horowitz MM. Access to hematopoietic stem cell transplantation: Effect of race and gender. Biol Blood Marrow Transplant. 2007;13 (Suppl):22. [Google Scholar]
- 22.Majhail NS, Mothukuri JM, Brunstein CG, Weisdorf DJ. Costs of hematopoietic cell transplantation: comparison of umbilical cord blood and matched related donor transplantation and the impact of post-transplant complications. Biol Blood Marrow Transplant. 2009;15:564–573. doi: 10.1016/j.bbmt.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Saito AM, Cutler C, Zahrieh D, et al. Costs of allogeneic hematopoietic cell transplantation with high-dose regimens. Biol Blood Marrow Transplant. 2008;14:197–207. doi: 10.1016/j.bbmt.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonow RO, Grant AO, Jacobs AK. The cardiovascular state of the union: confronting healthcare disparities. Circulation. 2005;111:1205–1207. doi: 10.1161/01.CIR.0000160705.97642.92. [DOI] [PubMed] [Google Scholar]
- 25.Kollman C, Weis T, Switzer GE, et al. Non-HLA barriers to unrelated donor stem cell transplantation. Bone Marrow Transplant. 2001;27:581–587. doi: 10.1038/sj.bmt.1702845. [DOI] [PubMed] [Google Scholar]
- 26.Norris K, Nissenson AR. Race, gender, and socioeconomic disparities in CKD in the United States. J Am Soc Nephrol. 2008;19:1261–1270. doi: 10.1681/ASN.2008030276. [DOI] [PubMed] [Google Scholar]