SUMMARY
The class IA phosphatidylinsositol 3-kinases (PI3Ks) form a critical node in the insulin metabolic pathway; however, the precise roles of the different isoforms of this enzyme remain elusive. Using tissue-specific gene inactivation, we demonstrate that p110α catalytic subunit of PI3K is a key mediator of insulin metabolic actions in the liver. Thus, deletion of p110α in liver results in markedly blunted insulin signaling with decreased generation of PIP3 and loss of insulin activation of Akt, defects that could not be rescued by overexpression of p110β. As a result, mice with hepatic knockout of p110α display reduced insulin sensitivity, impaired glucose tolerance, and increased gluconeogenesis, hypolipidemia, and hyperleptinemia. The diabetic syndrome induced by loss of p110α in liver did not respond to metformin treatment. Together, these data indicate that the p110α isoform of PI3K plays a fundamental role in insulin signaling and control of hepatic glucose and lipid metabolism.
INTRODUCTION
The class I phosphatidylinsositol 3-kinases (PI3Ks) comprise a family of phospholipid kinases activated by both receptor tyrosine kinases (class IA PI3Ks) and G protein-coupled receptors (class IB PI3Ks). Upon activation, these enzymes rapidly catalyze the formation of phosphatidylinositol(3,4,5)-trisphosphate (PIP3 for short), which acts as an intracellular second messenger by binding with high affinity to pleckstrin homology (PH) domains in target molecules. Activation of class IA PI3K by insulin and IGF receptors results in stimulation of downstream pathways, leading to glucose uptake, glycogen synthesis, protein synthesis, proliferation, cell growth, and survival (Katso et al., 2001; Cheatham et al., 1994; Taniguchi et al., 2006a, 2006b; Zhao et al., 2006). These metabolic growth effects depend on activation of downstream kinases such as Akt/PKB and the atypical isoforms of protein kinase C (PKC) (Taniguchi et al., 2006a; Farese et al., 2005; Vanhaesebroeck et al., 1997; Fruman et al., 1998; Geering et al., 2007; Engelman et al., 2006).
Class IA PI3Ks are heterodimers consisting of a regulatory subunit, usually designated p85, and a catalytic subunit, designated p110. Both subunits of class IA PI3K, however, exist as several isoforms. The catalytic subunit isoforms p110α, p110β, and p110δ are encoded by three genes, pik3ca, pik3cb, and pik3cd, respectively. The p110α and p110β isoforms are ubiquitously expressed, whereas p110δ is predominantly expressed in leukocytes (reviewed in Engelman et al., 2006). The precise roles and relative contributions of individual catalytic subunits of class IA PI3K in the multiple effects of PI3K in metabolism and cell growth are still poorly understood. Mice with homozygous germline deletion of p110α or p110β die early during embryonic development (Bi et al., 1999, 2002), indicating specific roles for each isoform during embryogenesis, but limiting the information that can be gained about the roles of these isoforms in adult tissues. Heterozygous deletion of either p110α or p110β has no effect on insulin signaling, but mice with heterozygous loss of both isoforms show an impaired insulin response (Brachmann et al., 2005), suggesting that the two isoforms might play complementary roles in insulin action. We have recently shown that liver-specific ablation of p110β had little effect on insulin stimulation of Akt/PKB and showed only very mildly impaired insulin sensitivity and glucose tolerance (Jia et al., 2008). Similarly, Hirsch and colleagues showed that mice with germline knockin of kinase-dead (KD) p110β (p110βK805R) developed only mild insulin resistance even at 6 months of age (Ciraolo et al., 2008).
The role p110α in insulin action is less clear. Mice with a heterozygous germline knockin of a KD allele of p110α (p110αD933A) develop insulin resistance and glucose intolerance as well as hyperphagia and increased adiposity; however, this mutant p110α can compete with both wild-type p110α and p110β for binding to regulatory subunits (Foukas et al., 2006). Chemical inhibitors of p110α, but not inhibitors of p110β or p110δ, have also been shown to block insulin-stimulated phosphorylation of Akt/PKB in 3T3-L1 adipocytes and L6 myotubes and to decrease glucose transport and insulin sensitivity in animals (Knight et al., 2006), but their sites of tissue action are probably multiple and remain unclear. To circumvent the limitations encountered in these global inactivation and inhibition strategies in the present study, we have addressed the role of the p110α isoform of PI3K in hepatic insulin action by creating mice with acute or long-term liver-specific deletion of p110α utilizing conditional inactivation of the pik3ca gene (Zhao et al., 2006). Our data demonstrate directly that liver p110α plays a critical role in the metabolic actions of insulin and that disruption of this isoform in liver results in impaired insulin action and glucose homeostasis that cannot be rescued by p110β or ameliorated by metformin. In addition, deletion of p110α in liver creates a metabolic crosstalk that affects other insulin-sensitive tissues, including skeletal muscle and fat.
RESULTS
The liver plays a central role in mediating insulin’s metabolic actions and glucose homeostasis. To investigate the impact of p110α loss on hepatic insulin action, we created two mouse models of deletion of p110α in liver (L-p110α): one acute, by injection of adenoviruses expressing the Cre recombinase (Ade-Cre) into liver of 8- to 10-week-old mice carrying homozygous floxed p110α alleles (p110αloxp/loxp) (Zhao et al., 2006), and one chronic, with early onset by breeding the floxed mice with transgenic mice carrying the Cre recombinase driven by the albumin promoter (albumin-Cre) (Taniguchi et al., 2006a). In both models, there was >90% reduction of p110α in the liver, while p110α expression remained unchanged in livers of control mice, as measured by western blot analysis (Figures S1A and S1B). In both types of L-p110α knockout (KO) mice, we observed no alterations of p110α levels in other tissues as compared to that of controls (Figures S1A and S1B; data not shown). p110β protein levels remained unaltered when p110α was ablated in the liver (Figure S1B).
Effects of Acute Ablation of p110α in the Liver
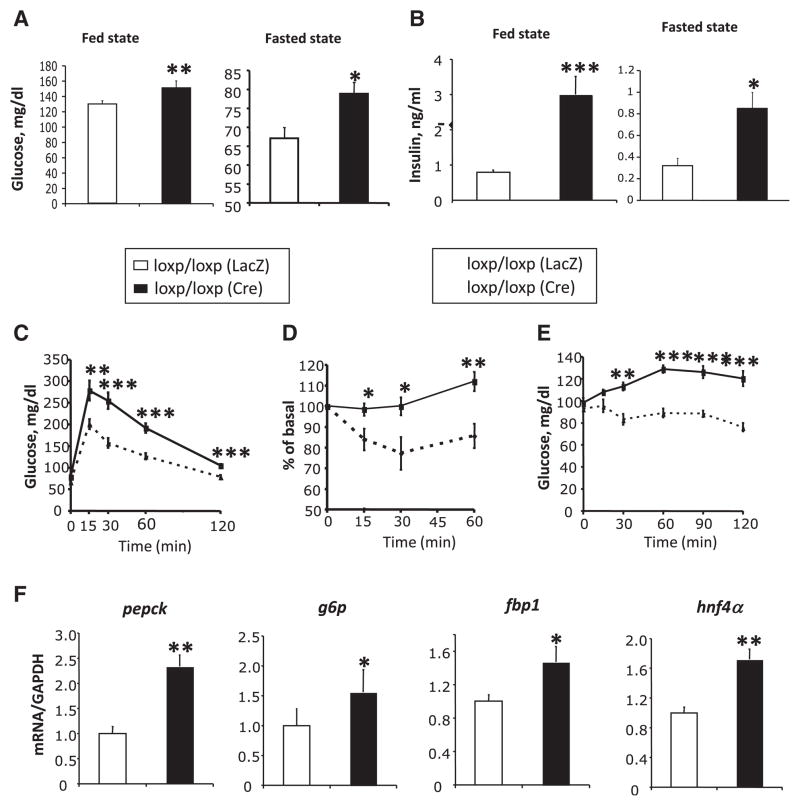
Ade-Cre-mediated ablation of p110α in the liver of adult mice resulted in a marked reduction of Akt/PKB phosphorylation in response to insulin stimulation (Figure S1A). This acute liver-specific loss of p110α resulted in 15%–20% increases in glucose levels in both the fed and fasted state (Figure 1A) and a 4-fold increase in fed and fasted serum insulin levels (Figure 1B). Mice with acute L-p110α ablation also displayed impaired glucose tolerance (Figure 1C) with no impairment in insulin secretion (Figure S2A) and had reduced insulin sensitivity upon challenge with an intraperitoneal (i.p.) insulin tolerance test (ITT) (Figure 1D), all indicating insulin resistance secondary to acute deletion of L-p110α.
Figure 1. Metabolic Phenotype of Mice with Acute Hepatic Loss of p110α.
(A) Fed and fasted blood glucose levels in mice of the indicated genotypes were measured 12 days after adenovirus injection (n = 8–10).
(B) Serum insulin levels in the fasted and random fed states were measured 2 weeks after adenovirus injection (n = 8–10).
(C) Glucose tolerance tests (GTT) on mice of indicated genotypes 12 days after adenovirus injection (n = 9).
(D) Insulin tolerance tests (ITT) on mice of indicated genotypes 2 weeks after adenovirus injection. Results represent blood glucose concentration as a percentage of starting value at zero time (n = 7–9).
(E) Pyruvate challenge on mice of indicated genotypes 16 days after adenovirus injection (n = 7–8).
(F) Quantitative RT-PCR analysis of mRNA levels of phosphoenolpyruvate carboxykinase (pck1), glucose 6-phosphatase (g6pc), fructose 1,6-bisphosphatase (fbp1), and hepatic nuclear factor-4α (hnf4α) from the fasting mouse livers 3 weeks after adenovirus injection.
Increased gluconeogenesis is one of the hallmarks of hepatic insulin resistance in type 2 diabetes. To determine whether the reduced insulin sensitivity and glucose tolerance in mice with KO of p110α in liver were due to increased hepatic glucose output, we subjected these animals to a challenge with pyruvate, a major gluconeogenic substrate. Administration of pyruvate had no effect on blood glucose levels in control mice, but produced an ~25% increase in glucose in the L-p110α KO mice at 60 min after administration (Figure 1E). This was associated with 1.5- to 2.5-fold increases in the expression of a number of enzymes and transcription factors involved in gluconeogenesis, including phosphoenolpyruvate carboxykinase (pck1), glucose 6-phosphatase (g6pc), hepatic nuclear factor-4α (hnf4α), and fructose 1,6-bisphosphatase (fbp1) in the L-p110α-deficient mice (Figure 1F). Mice with acute ablation of p110α in liver also showed a 34% reduction in serum free fatty acids, a 28% reduction in total serum cholesterol, and a 44% reduction in serum triglycerides compared with control animals, as well as decreased lipogenic gene expression and hepatic triglyceride content (Figures S3A–S3C), all of which were statistically significant. In addition, serum leptin levels and the expression of leptin receptor in p110α-deficient livers increased approximately 8-fold relative to control (Figure S2B). This increase in circulating leptin and hepatic leptin receptor expression is similar to our previous finding in mice with severe hepatic insulin resistance due to ablation of the insulin receptor gene in liver (Cohen et al., 2007).
Effects of Chronic Loss of p110α in Liver
Insulin Signaling Is Impaired in p110α-Deficient Hepatocytes
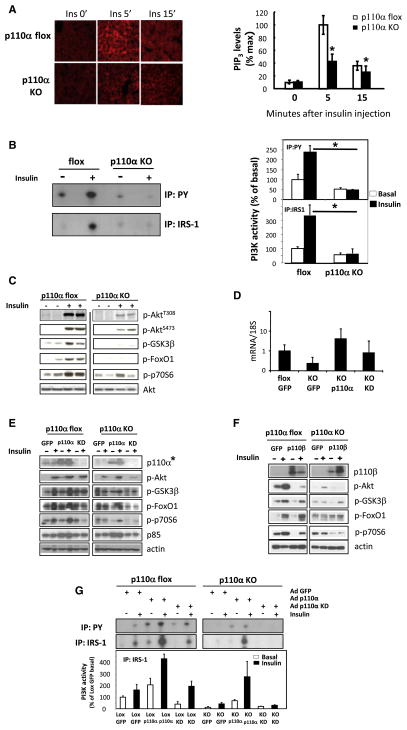
To determine effects of chronic deletion of p110α in liver, we created a model of liver-specific inactivation by crossing mice homozygous for the floxed allele of pik3ca with mice carrying the albumin-Cre transgene (Taniguchi et al., 2006a). Chronic loss of p110α in liver resulted in a dramatic reduction in the levels of PIP3 in hepatocytes following insulin stimulation as assessed by in situ immunofluorescence with anti-PIP3 antibody (Figure 2A). Thus, there was up to 10-fold elevation of PIP3 levels in hepatocytes of control animals 5 min after insulin stimulation, which declined over the next 10 min, whereas the induction of PIP3 in response to insulin in L-p110α KO hepatocytes was reduced by >60% (Figure 2A). This was associated with an almost complete loss of IRS-1- and phosphotyrosine (PY)-associated PI3K activity in livers of these mice in response to insulin (Figure 2B) and a marked reduction in phosphorylation of Akt/PKB, GSK3β, FoxO1, and p70S6K (Figure 2C). p110β-associated PI3K activity was almost undetectable in both the control and L-p110α KO and was not changed at the basal or insulin-stimulated state (Figure S1C), supporting recent reports that p110β plays little role in insulin signaling (Jia et al., 2008; Ciraolo et al., 2008; Knight et al., 2006). Taken together, these data indicate that p110α is the major PI3K catalytic subunit involved in the hepatic insulin signaling.
Figure 2. Hepatic Insulin Signaling in Mice with Chronic Deletion of p110α.
(A) Livers from control flox mice and mice with chronic deletion of p110α were fixated, and liver sections were stained with antibodies for PIP3 lipids at 0′, 5′, and 15′ after vena cava insulin or saline injection. Data represent mean ± SEM (n = 4), *p < 0.05.
(B) PI3K activity from liver lysates immunoprecipitated with phosphotyrosine (PY) or IRS-1 of fasted mice of indicated genotypes (n = 4–5), *p < 0.05.
(C) Western blot of liver protein lysates from p110α flox and L-p110α KO mice treated with saline or insulin for 5′ through inferior vena cava injection.
(D) Quantitative RT-PCR of p110α (pik3ca) gene expression in liver from mice injected with adenovirus expressing GFP, a wild-type p110α, or a kinase-dead (KD) mutant of p110α lacking the kinase domain.
(E) Western blot of liver lysates prepared from p110α flox and L-p110α KO mice injected with control adenovirus (GFP) or adenovirus expressing a wild-type or a KD version of p110α. Mice were treated with either saline or insulin for 5′ through inferior vena cava injection. (*The p110α antibody does not recognize the truncated KD p110α.)
(F) Western blot of liver protein lysates prepared from control flox and L-p110α KO mice subjected to tail vein injection with control adenovirus expressing GFP or adenovirus expressing p110β and treated with saline or insulin for 5′ through inferior vena cava injection. Data are representative of three separate experiments.
(G) PI3K activity from liver lysates immunoprecipitated with phosphotyrosine (PY) or IRS-1 of fasted mice of indicated genotypes injected with adenovirus expressing GFP, a wild-type, or a KD version of p110α (n = 2–6).
Reconstitution of p110α Null Liver with p110α versus p110β
To further investigate the importance of the PI3K p110α isoform in insulin signaling in liver, we reconstituted the liver of L-p110α KO mice with p110α, p110β, or a p110α KD mutant lacking its kinase domain by tail vein injection of an adenoviral vector directing expression of either of these genes. Expression of wild-type p110α and p110β were confirmed by immunoblot analysis using antibodies specific to the kinase domain (Figures 2E and 2F). Expression of KD p110α was confirmed by quantitative RT-PCR (Figure 2D). Reconstitution of L-p110α KO livers with wild-type p110α resulted in a restoration of insulin-stimulated IRS-1- and PY-associated PI3K activity, as well as phosphorylation of Akt/PKB, GSK3β, p70S6 kinase, and FoxO1 to normal or near-normal levels, whereas reconstitution with KD p110α had no effect (Figures 2E and 2G). More importantly, when the L-p110α KO mice were reconstituted by injection with adenovirus expressing p110β, insulin-stimulated phosphorylation of Akt/PKB and other downstream components was not restored (Figure 2F). In fact, increasing expression of p110β impaired insulin signaling in control animals with reduced phosphorylation of Akt/PKB, GSK3β, and p70S6 kinase. p85 protein levels were not different between control and L-p110α KO mice and were increased following p110α reconstitution (Figure 2E). This was likely due to an overall increase in p110 concentration (~4-fold above normal by qRT-PCR), consistent with the known ability of p110 to stabilize the regulatory subunit of PI3K (Yu et al., 1998; Zhao et al., 2006). Thus, liver p110α is crucial for proper insulin signaling in this tissue, and the effect of p110α loss on liver insulin signaling cannot be compensated for by overexpression of p110β.
Physiological Characteristics of L-p110α KO Mice
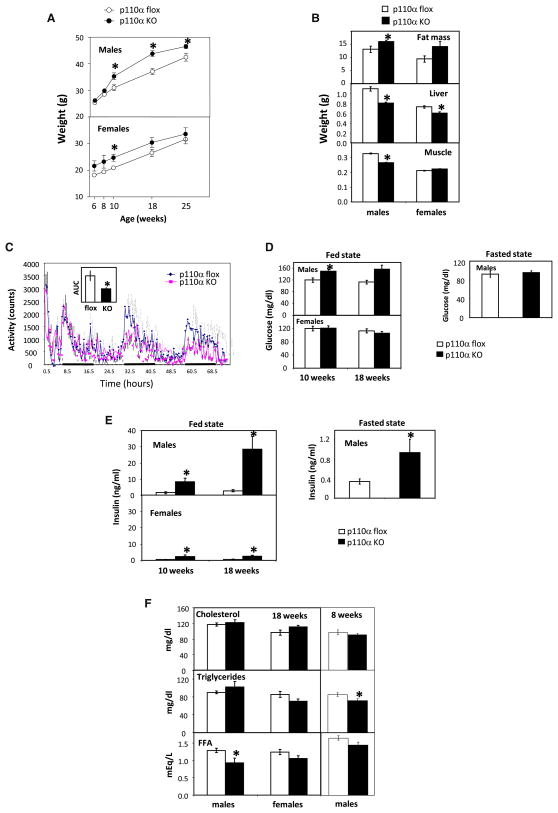
L-p110α KO mice displayed mild adult-onset obesity. Starting at 10 weeks of age, male L-p110α KO mice showed more rapid weight gain and by age 25 weeks weighed 9.4% more than controls (46.5 ± 0.8 g versus 42.5 ± 1.5 g, p < 0.05) (Figure 3A). Furthermore, male L-p110α KO mice had a 22.8% increase in fat mass compared to controls, as measured by DEXA scanning (p < 0.05) (Figure 3B). Female L-p110α KO mice exhibited increased weight at 6 weeks of age and by 25 weeks weighed 6.3% more than controls (33.6 ± 2.3 g versus 31.6 ± 1.7 g, p < 0.05). DEXA scanning in females also indicated a trend toward increased adipose mass (Figure 3B, p = 0.06). The same was true when the data were expressed as percent body fat: 31% ± 1.5% in control males versus 35.7% ± 1.2% in L-p110α KO males (p < 0.05); 31.9% ± 2.9% in control females versus 39.7% ± 1.9% in L-p110α KO females (p = 0.06). Assessment of energy expenditure, food intake, and activity in the male L-p110α KO and control mice for 72 hr using CLAMS metabolic chambers revealed no differences in O2 consumption, CO2 production, heat production, respiratory exchange ratio, or food intake (data not shown). However, male L-p110α KO mice did have a significantly lower activity level than wild-type control mice (Figure 3C).
Figure 3. Physiological Characteristics of Mice with Chronic Hepatic Deletion of p110α.
(A) Whole-body weight. Open circles, p110α flox control mice; filled circles, L-p110α KO mice. Data represent mean ± SEM (n = 8–10), *p < 0.05.
(B) Tissue weight. Data represent mean ± SEM (n = 8–10), *p < 0.05.
(C) Activity level of male flox controls and L-p110α KO mice during 72 hr in metabolic chambers. The inset shows the area under the curve (AUC) for both genotypes.
(D) Serum glucose levels of p110α flox and L-p110α KO mice at 10 and 18 weeks, random fed and fasted state.
(E) Serum insulin levels of p110α flox and L-p110α KO mice at 10 and 18 weeks, random fed and fasted state.
(F) Serum lipid levels of p110α flox and L-p110α KO mice at 8 and 18 weeks, random fed state.
Despite the increase in body weight, both female and male L-p110α KO mice had an ~20% decrease in liver weight compared to control animals (Figure 3B). In addition, male KO mice had an ~20% decrease in muscle weight (Figure 3B). Neither male nor female KO mice differed in nose-tail length compared to control animals (data not shown). Hepatic triglyceride content in L-p110α KO mice was also similar to that in the control animals (data not shown), and oil red O staining revealed no alteration in accumulation of fat in the liver of L-p110α mice (Figure S3D). Surprisingly, expression levels of several lipogenic genes were decreased in the fed state in L-p110α KO mice compared to control animals (Figure S3E). While gluconeogenesis is mainly mediated via PIP3 activation of Akt, hepatic lipid synthesis has been shown to be preferentially mediated by atypical PKC (aPKC) downstream of PI3K (Taniguchi et al., 2006a; Matsumoto et al., 2003; Standaert et al., 2004; Sajan et al., 2009). Interestingly, activation of aPKC in response to insulin stimulation was almost completely ablated in L-p110α KO animals (Figure S3F), indicating that p110α is required for insulin activation of this downstream serine kinase. Taken together, L-p110α KO mice have smaller livers but normal hepatic morphology and lipid content despite impaired lipogenesis.
L-p110α KO Mice Are Glucose and Insulin Intolerant
As predicted, chronic inactivation of liver p110α resulted in insulin resistance on glucose metabolism. Male L-p110α KO mice had only slightly elevated fed and normal fasting glucose levels (Figure 3D), and female L-p110α KO mice showed no difference in glucose levels compared to controls (Figure 3D). However, both female and male L-p110α KO animals had marked elevated circulating insulin levels that were 5- and 10-fold higher than normal at 10 weeks of age, respectively, and this hyperinsulinemia became more pronounced in male L-p110α KO mice at 18 weeks of age (Figure 3E). Male L-p110α KO mice also had markedly increased insulin levels in the fasted state (Figure 3E). As seen upon acute deletion of p110α, mice with developmental ablation of p110α also showed dramatically increased serum leptin levels and leptin receptor expression levels in the liver, both at 8 weeks (data not shown) and at 18 weeks (Figure S2C). In contrast to the mice with acute deletion of p110α, however, there were no dramatic differences in serum lipid levels between KO animals and controls (Figure 3F).
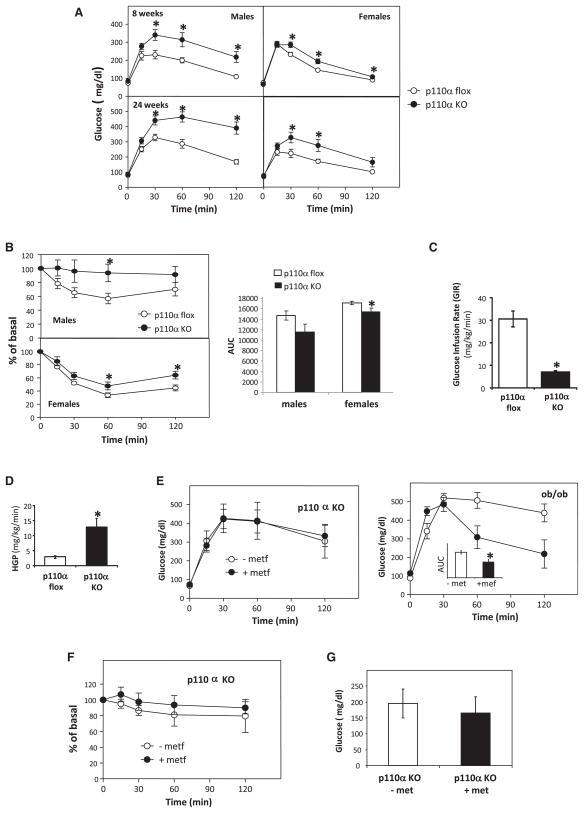
Both L-p110α KO males and females exhibited significantly impaired glucose tolerance upon glucose tolerance testing (GTT) by 8 weeks of age, and this became more severe by 24 weeks of age (Figure 4A). ITTs indicated that these L-p110α mice were also resistant to the effects of i.p. administrated insulin on reducing blood glucose levels (Figure 4B). In contrast to the acute mouse model of p110α deletion, mice with chronic deletion of p110α displayed no difference in gluconeogenic gene expression compared to flox control mice in either the fed or fasted state (data not shown).
Figure 4. Metabolic Phenotype of Mice with Chronic Deletion of p110α.
(A) Mice were given an i.p. injection of 2 g dextrose/kg BW after an overnight fast.
(B) Mice were given an i.p. injection of 1.25 U/kg BW insulin. Results represent blood glucose concentration as a percentage of starting value atzerotime. Areaunderthe curve (AUC) was calculated with baseline = 100% of basal. Open circles, p110α flox; filled circles, L-p110α KO. Data represent mean ± SEM (n = 8–10), *p < 0.05.
(C and D) Insulin sensitivity quantified as glucose infusion rate (GIR) (C) and hepatic glucose production (HGP) (D) in response to insulin were measured by hyperinsulinemic-euglycemic clamp (n = 3–4), *p < 0.05.
(E) Mice were treated with metformin for 3 weeks, and GTT was performed by i.p. injection of 2 g dextrose/kg BW after an overnight fast. Ob/ob mice were used as controls. The inset shows the AUC for untreated or metformin-treated ob/ob mice.
(F) ITT was performed by i.p. injection of 1.25 U/kg BW insulin. Open circles, L-p110α KO or ob/ob mice treated without metformin; filled circles, L-p110α KO or ob/ob mice treated with 300 mg metformin/kg BW/day for 3 weeks. Data represent mean ± SEM (n = 3–4), *p < 0.05.
(G) Serum glucose levels. Data represent mean ± SEM (n = 4).
To obtain a more accurate estimation of hepatic glucose production (HGP) in vivo, we performed hyperinsulinemic-euglycemic clamps with D-[3-3H]glucose and [14C]deoxyglucose infusions. L-p110α KO animals had a significantly impaired peripheral glucose uptake, with a glucose infusion rate (GIR) that was approximately 20% of that of the flox control (Figure 4C). More importantly, while basal HGP in the chronic L-p110α KO mice was not different from the flox controls (data not shown), KO mice showed a 4.4-fold increase in HGP during the insulin clamp (p < 0.05) (Figure 4D). Thus, chronic L-p110α KO mice are markedly insulin resistant and have impaired glucose tolerance as well as impaired suppression of HGP in response to insulin.
Loss of p110α in the Liver-Induced Hyperglycemia Cannot Be Ameliorated by Metformin
Metformin is the most widely used drug for the treatment of type 2 diabetes and is known to exert its effects primarily by inhibiting hepatic gluconeogenesis (Hundal et al., 2000; Mithieux et al., 2002). To investigate if metformin could reverse the insulin resistance and hyperglycemia seen in the L-p110α KO mice, animals were treated with 300 mg/kg BW/day for 3 weeks. Ob/ob mice, a genetic model of obesity and type 2 diabetes, were included as a positive control and showed improved GTT in response to the metformin treatment (Figure 4E). In contrast, metformin treatment did not lower serum glucose levels upon GTT or ITT in L-p110α KO mice (Figures 4E and 4F), nor did metformin treatment lower random fed serum glucose levels (Figure 4G). While the mechanism of metformin in suppressing gluconeogenesis is not clear, part of its activity is believed to be achieved by activating AMP-activated protein kinase (AMPK) in liver (Zhou et al., 2001; Shaw et al., 2005). Consistent with the lack of glucose effect, we did not observe AMPK phosphorylation or the phosphorylation of its downstream target, ACC, following metformin treatment in hepatocytes of L-p110α KO mice (data not shown). Thus, p110α in the liver is required for the metformin’s effect on insulin sensitivity.
DISCUSSION
In the present study, we have defined the exact roles of p110α in liver in insulin metabolic action and whole-body glucose homeostasis using two mouse models of liver-specific p110α inactivation. We found that ablation of p110α in liver by either approach markedly impaired insulin signaling, as evidenced by diminished phosphorylation levels of Akt/PKB and other downstream components in response to insulin stimulation. This defective insulin responsiveness led to hyperinsulinemia and hyperglycemia and, in the chronic L-p110α KO mice, reduced liver weight and mild obesity. While PI3K has been known to be central to insulin’s metabolic action (Katso et al., 2001; Cheatham et al., 1994; Taniguchi et al., 2006a, 2006b; Zhao et al., 2006), our data demonstrate that it is the p110α catalytic subunit that is the primary PI3K required for the metabolic actions following insulin receptor activation in the liver. Consistent with this, germ-line knockin of a KD allele of p110β or acute liver-specific ablation of p110β has little effect on the ability of insulin to activate Akt/PKB in hepatocytes and only mildly impaired their insulin sensitivity and glucose homeostasis (Jia et al., 2008; Ciraolo et al., 2008), consistent with a prior pharmacologic study showing that p110β plays little role in insulin-stimulated Akt/PKB activation (Knight et al., 2006).
Lack of liver p110α resulted in an impaired insulin signal with markedly decreased activation of downstream molecules, including Akt/PKB, GSK3β, and p70S6 kinase. While it was not surprising that this defect in signaling was restored by reintroducing p110α in the liver, it was somewhat surprising that increasing expression of p110β had no effect to rescue the p110α deletion. Indeed, overexpression of p110β seemed to further impair insulin signaling at the level of Akt/PKB, GSK3β, and p70S6, suggesting very distinct roles of the two p110 isoforms in liver insulin signaling. This is consistent with growing evidence that the two isoforms have markedly different roles in cellular signaling, metabolism, and oncogenic transformation despite their similar structure and substrate specificity (Jia et al., 2008; Ciraolo et al., 2008; Funaki et al., 2000; Wymann and Pirola, 1998). The lipid kinase activity of p110β is significantly lower than that of p110α (Beeton et al., 2000). Thus, the paradoxical negative effect of p110β overexpression may result from differences in catalytic activity, subcellular localization, or a loss in signal strength as the less active p110β isoform replaces p110α in p85 complexes responding to limiting numbers of activated insulin receptor. While mice heterozygous for either p110α or p110β have no defect in glucose metabolism, mice heterozygous for loss of both p110α and p110β exhibited hyperinsulinemia and glucose intolerance, suggesting some complementary role of these two isoforms (Brachmann et al., 2005). Interestingly, this occurs with no measurable reduction in insulin-stimulated Akt/PKB phosphorylation.
We have previously reported that LIRKO mice with liver-specific disruption of insulin receptor and absent insulin signaling in hepatocytes show impaired insulin sensitivity and glucose tolerance, as well as reduced liver and muscle weight (Michael et al., 2000a). Our data presented here indicate that p110α is the primary insulin-responsive PI3K isoform responsible for this phenotype, and thus, not surprisingly, L-p110α KO mice share many of the features with the LIRKO mice, further supporting the importance of p110α in liver insulin action. The cause of the reduced muscle weight in mice with liver-specific alterations is not clear but may relate to the marked hyperinsulinemia resulting in a secondary desensitization of insulin and/or IGF-1 signaling in muscle. Indeed, in LIRKO mice, there is a 15%–20% decrease in expression of insulin receptor in muscle (Michael et al., 2000b). Alternatively, the hepatic insulin resistance in the L-p110α KO and LIRKO mice leads to alterations in IGF-1 or IGF-1 binding proteins or changes in other signaling pathways important for muscle growth.
Even though both liver and muscle weight are decreased in L-p110α KO male mice, total body weight is increased due to increased fat mass. L-p110α KO mice show no difference in CO2 production, O2 consumption, RER, or food intake; however, L-p110α KO mice do have a decreased activity level compared to flox controls. Whether this leads to the increased body weight or is a response to the increase in weight is unclear (Tou and Wade, 2002). Interestingly, L-p110α KO mice have significantly decreased peripheral (muscle) glucose uptake, as indicated by the markedly decreased GIR during the hyperinsulinemic-euglycemic clamp, despite intact insulin signaling in muscle (data not shown). Some of this decrease could be due to the decrease in muscle mass. In any case, this marked decreased response in muscle glucose uptake coupled with the impaired insulin action of the liver could lead to a redistribution of substrates like glucose to white adipose tissue, where they could be taken up and stored as fat, explaining the increased fat mass observed in these mice despite no apparent effect on food intake.
The increased fat mass observed in the L-p110α KO mice was accompanied by increased circulating levels of leptin and leptin receptor. Mice with liver-specific loss of the insulin receptor also show increased leptin levels with increased expression of both membrane-associated and -soluble forms of the leptin receptor in liver and increased circulating leptin receptor in serum (Cohen et al., 2007). Thus, alterations in insulin signaling in liver can lead to increased circulating leptin for a variety of reasons.
By employing both acute somatic and chronic developmental ablation of p110α in the liver, we were able to identify any potential compensatory responses for p110α inactivation that might occur in the development. Of note, we were also interested in acute ablation, since it may mimic the clinical setting in cancer patients treated with PI3K inhibitors, as in clinical trials currently underway. In general, our findings in the acute and chronic models of p110α deletion show many similarities, but also a few interesting differences. Acute loss of p110α in liver resulted in alterations in circulating lipids with decreased serum levels of free fatty acids, cholesterol, and triglycerides, indicating a role of the PI3K pathway in whole-body lipid homeostasis in the short term. In the long-term KO mice, however, with the exception of mild changes in triglycerides at young ages, the changes in these lipids were not significant, suggesting that compensation by other tissues, such as muscle and adipose tissue, might have occurred. The decreased serum free fatty acid levels seen in the older L-p110α KO mice may be due in part to a suppression of lipolysis in the adipose tissue by the increased circulating insulin levels. It is also possible that compensatory changes in the liver itself have occurred, since the chronic KO mice show normal levels of hepatic lipid content despite decreased aPKC activity in response to insulin and decreased expression of many of the genes involved in lipogenesis.
Metformin, a lipophilic biguanide, lowers blood glucose, inhibits adipose tissue lipolysis, and improves whole-body insulin sensitivity primarily by reducing hepatic glucose output (Inzucchi et al., 1998; Stumvoll et al., 1998; Zhou et al., 2001). Metformin is the most commonly used drug for type 2 diabetes and would be a plausible drug to ameliorate the metabolic side effects of chronic use of PI3K inhibitors in the treatment of cancer patients. Unfortunately, metformin treatment neither reduced the blood glucose levels in GTT nor improved the insulin sensitivity in the L-p110α KO mice, suggesting that hepatic p110α or one of its downstream targets is crucial for metformin action. These data suggest that metformin is unlikely to ameliorate the metabolic side effects of PI3K inhibitors in cancer patients and indicate that caution should be taken in treating cancer patients who are diabetic or at high risk for developing glucose intolerance.
In summary, our study demonstrates that p110α plays a critical role in hepatic insulin/PI3K signaling and is required for normal glucose and lipid homeostasis. Deletion of p110α in liver, either developmentally or acutely, results in a marked reduction in insulin-stimulated PI3K activity and altered whole-body glucose homeostasis and insulin sensitivity. Since the PI3K pathway is the major site of insulin resistance in type 2 diabetes (Cusi et al., 2000 and others), it is possible that activators of this pathway may be useful in treatment of type 2 diabetes. On the other hand, our data raise concern that prolonged use of PI3K inhibitors, especially those targeted against p110α, for the treatment of cancer (Ward et al., 2003; Ward and Finan, 2003) may induce a variety of metabolic disorders, including overt diabetes.
EXPERIMENTAL PROCEDURES
Animals
To create the chronic liver KO mice, p110α lox-lox mice were crossed with mice hemizygous for the albumin-Cre recombinase transgene (Taniguchi et al., 2006a). Mice were housed on a 12 hr light cycle and fed a standard rodent chow (Lab Diet; Brentwood, MO) and water ad libitum. Metformin (Sigma-Aldrich, St. Louis), was mixed with regular chow to deliver 300 mg metformin/kg BW/day for 3 weeks. Mice with an acute deletion of liver p110α were created by tail vein injection of 2 × 109 pfu per mouse of adeno-Cre virus (University of Iowa Gene Transfer Vector Core). All protocols for animal use and euthanasia were approved by the Animal Care Use Committee of the Joslin Diabetes Center, Dana-Farber Cancer Institute, and Harvard Medical School in accordance with NIH guidelines.
Metabolic and Physiological Methods
Insulin was measured with ELISA (Crystal Chem Inc.; Downers Grove, IL). Cholesterol, triglycerides (Stanbio Laboratory; Boerne, TX), and free fatty acids (Wako USA; Richmond, VA) were measured with the colorimetric and enzymatic methods, respectively. GTTs were performed by i.p. injection of 2 g dextrose/kg BW after an overnight fast. ITTs were performed by i.p. injection of 1.25 U/kg BW insulin (NovoLog, Novo Nordisk Inc.; Copenhagen, Denmark). The pyruvate challenge was performed by injecting 2 g/kg of pyruvate (Sigma-Aldrich) i.p. after an 18 hr fast. Fat and lean mass were measured by dual-energy X-ray absorptiometry (DEXA) scanning. CLAMS metabolic cage studies were performed over a 3 day period following 1 day of acclimation.
Hyperinsulinemic-Euglycemic Clamp
The hyperinsulinemic-euglycemic clamp technique was performed as described previously (Norris et al., 2003) with an insulin infusion of 3.5 mU/kg/min. Blood glucose was maintained at 130 mg/dl during the clamp. HGP was assessed by subtraction of the GIR from whole-body glucose turnover as measured with D-[3-3H]glucose (Finegood et al., 1988).
In Vivo Insulin Signaling
Animals were fasted overnight and anesthetized with 2-2-2 Tribromoethanol (Sigma; St. Louis), followed by injection of 5 U of insulin (NovoLog, Novo Nordisk Inc.) or saline via the inferior vena cava. Five minutes after the injection, liver and muscle were excised, weighed, and snap-frozen in liquid nitrogen.
Adenoviral Injection
Adenoviruses for p110α and p110β were provided by T. Asano, Hiroshima University, Hiroshima, Japan (Asano et al., 2000). The p110α KD mutant adenovirus was created in the lab from a pcDNA3-p110α mutant vector (gift of Lewis Cantley) that was derived from nucleotides 1–1731 of mouse pik3ca cDNA (Klippel et al., 1994). The p110α KD adenovirus had an additional small truncation of 31 bases at the 5′ end of the pik3ca gene. Animals were injected with 200 μl containing 10 × 1010 viral particles and sacrificed 4 days later following vena cava injection with insulin or saline.
PIP3 Quantification and Liver Morphology Analysis
Following vena cava injection of insulin or saline, mice were perfused via the heart with 10% neutral buffered formalin. PIP3 levels were measured by immunofluorescence with a monoclonal antibody (IgM, Echelon Biosciences; Salt Lake City, UT) as described (Kitamura et al., 2004). Sectioned liver samples (10 μm) from four different mice were used for quantification. The fluorescence intensity of 16 fields per slide was measured and analyzed with VH-H1A5 analyzer software (KEYENCE; Osaka, Japan). Liver morphology was analyzed on livers from fixed mice by H&E staining.
In Vitro Kinase Assays
Livers were extracted into tissue homogenization buffer and subjected to immunoprecipitation with p110α, p110β, IRS-1, or PY for PI3K assays as previously described (Ueki et al., 2000). Assays of aPKC activity were performed by immunoprecipitation from liver lysates with a rabbit polyclonal antiserum (Santa Cruz Biotechnology, Inc.; Santa Cruz, CA) that recognizes the C termini of both PKC-λ and PKC-ζ, collected on Sepharose-AG beads (Santa Cruz Biotechnology) (Sajan et al., 2004).
Determination of Hepatic Triglyceride Content
Liver triglyceride content was measured as described previously (Wang et al., 2009) using an enzymatic method (Serum Triglyceride Determination Kit, TR0100; Sigma) and expressed relative to liver weight.
Antibodies
Actin antibody was from Santa Cruz Biotechnology; p85 antibody was from Upstate-Millipore (Billerica, MA); and anti-vinculin antibody was from Sigma. All other antibodies were from Cell Signaling Technology, Inc. (Danvers, MA).
RNA Extraction and Gene Expression Analysis
RNA was extracted by crushing frozen tissue in liquid nitrogen followed by extraction using the RNeasy kit (QIAGEN; Valencia, CA). cDNA was prepared using the High-Capacity cDNA Archive Kit (Applied Biosystems; Foster City, CA) with random hexamer primers. Gene expression was analyzed by real-time RT-PCR and ABI PRISM Sequence Detection System (Applied Biosystems). Samples were normalized to 18S or GAPDH. Primer sequences are available on request.
Statistics
All data are presented as mean ± SEM. The Student’s t test was used for statistical analysis between two unpaired groups.
Supplementary Material
Acknowledgments
This work was supported by NIH R01 grants DK33201 and DK55545 to C.R.K. and CA134502 and CA089393-08S1 to J.J.Z. Additional support came from the V Foundation (J.J.Z.), the Claudia Barr Program (J.J.Z.), the Swedish Research Council (V.R.S.), the NIH-funded Joslin Diabetes and Endocrinology Center (grant DK34834), and the Mary K. Iacocca Professorship fund. In compliance with Harvard Medical School guidelines, we disclose the consulting relationships: Novartis Pharmaceuticals, Inc. (J.J.Z.) and Sirtris/GSK Pharmaceuticals (C.R.K.).
Footnotes
Supplemental Information includes three figures and can be found with this article online at doi:10.1016/j.cmet.2010.02.002.
References
- Asano T, Kanda A, Katagiri H, Nawano M, Ogihara T, Inukai K, Anai M, Fukushima Y, Yazaki Y, Kikuchi M, et al. p110beta is up-regulated during differentiation of 3T3-L1 cells and contributes to the highly insulin-responsive glucose transport activity. J Biol Chem. 2000;275:17671–17676. doi: 10.1074/jbc.M910391199. [DOI] [PubMed] [Google Scholar]
- Beeton CA, Chance EM, Foukas LC, Shepherd PR. Comparison of the kinetic properties of the lipid- and protein-kinase activities of the p110alpha and p110beta catalytic subunits of class-Ia phosphoinositide 3-kinases. Biochem J. 2000;350:353–359. [PMC free article] [PubMed] [Google Scholar]
- Bi L, Okabe I, Bernard DJ, Wynshaw-Boris A, Nussbaum RL. Proliferative defect and embryonic lethality in mice homozygous for a deletion in the p110alpha subunit of phosphoinositide 3-kinase. J Biol Chem. 1999;274:10963–10968. doi: 10.1074/jbc.274.16.10963. [DOI] [PubMed] [Google Scholar]
- Bi L, Okabe I, Bernard DJ, Nussbaum RL. Early embryonic lethality in mice deficient in the p110beta catalytic subunit of PI 3-kinase. Mamm Genome. 2002;13:169–172. doi: 10.1007/BF02684023. [DOI] [PubMed] [Google Scholar]
- Brachmann SM, Ueki K, Engelman JA, Kahn RC, Cantley LC. Phosphoinositide 3-kinase catalytic subunit deletion and regulatory subunit deletion have opposite effects on insulin sensitivity in mice. Mol Cell Biol. 2005;25:1596–1607. doi: 10.1128/MCB.25.5.1596-1607.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheatham B, Vlahos CJ, Cheatham L, Wang L, Blenis J, Kahn CR. Phosphatidylinositol 3-kinase activation is required for insulin stimulation of pp70 S6 kinase, DNA synthesis, and glucose transporter translocation. Mol Cell Biol. 1994;14:4902–4911. doi: 10.1128/mcb.14.7.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciraolo E, Iezzi M, Marone R, Marengo S, Curcio C, Costa C, Azzolino O, Gonella C, Rubinetto C, Wu H, et al. Phosphoinositide 3-kinase p110beta activity: key role in metabolism and mammary gland cancer but not development. Sci Signal. 2008;1:ra3. doi: 10.1126/scisignal.1161577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SE, Kokkotou E, Biddinger SB, Kondo T, Gebhardt R, Kratzsch J, Mantzoros CS, Kahn CR. High circulating leptin receptors with normal leptin sensitivity in liver-specific insulin receptor knock-out (LIRKO) mice. J Biol Chem. 2007;282:23672–23678. doi: 10.1074/jbc.M704053200. [DOI] [PubMed] [Google Scholar]
- Cusi K, Maezono K, Osman A, Pendergrass M, Patti ME, Pratipanawatr T, DeFronzo RA, Kahn CR, Mandarino LJ. Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J Clin Invest. 2000;105:311–320. doi: 10.1172/JCI7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- Farese RV, Sajan MP, Standaert ML. Atypical protein kinase C in insulin action and insulin resistance. Biochem Soc Trans. 2005;33:350–353. doi: 10.1042/BST0330350. [DOI] [PubMed] [Google Scholar]
- Finegood DT, Bergman RN, Vranic M. Modeling error and apparent isotope discrimination confound estimation of endogenous glucose production during euglycemic glucose clamps. Diabetes. 1988;37:1025–1034. doi: 10.2337/diab.37.8.1025. [DOI] [PubMed] [Google Scholar]
- Foukas LC, Claret M, Pearce W, Okkenhaug K, Meek S, Peskett E, Sancho S, Smith AJ, Withers DJ, Vanhaesebroeck B. Critical role for the p110alpha phosphoinositide-3-OH kinase in growth and metabolic regulation. Nature. 2006;441:366–370. doi: 10.1038/nature04694. [DOI] [PubMed] [Google Scholar]
- Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- Funaki M, Katagiri H, Inukai K, Kikuchi M, Asano T. Structure and function of phosphatidylinositol-3,4 kinase. Cell Signal. 2000;12:135–142. doi: 10.1016/s0898-6568(99)00086-8. [DOI] [PubMed] [Google Scholar]
- Geering B, Cutillas PR, Nock G, Gharbi SI, Vanhaesebroeck B. Class IA phosphoinositide 3-kinases are obligate p85-p110 heterodimers. Proc Natl Acad Sci USA. 2007;104:7809–7814. doi: 10.1073/pnas.0700373104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundal RS, Krssak M, Dufour S, Laurent D, Lebon V, Chandramouli V, Inzucchi SE, Schumann WC, Petersen KF, Landau BR, Shulman GI. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000;49:2063–2069. doi: 10.2337/diabetes.49.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzucchi SE, Maggs DG, Spollett GR, Page SL, Rife FS, Walton V, Shulman GI. Efficacy and metabolic effects of metformin and troglitazone in type II diabetes mellitus. N Engl J Med. 1998;338:867–872. doi: 10.1056/NEJM199803263381303. [DOI] [PubMed] [Google Scholar]
- Jia S, Liu Z, Zhang S, Liu P, Zhang L, Lee SH, Zhang J, Signoretti S, Loda M, Roberts TM, Zhao JJ. Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature. 2008;454:776–779. doi: 10.1038/nature07091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, Waterfield MD. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2001;17:615–675. doi: 10.1146/annurev.cellbio.17.1.615. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Kitamura Y, Nakae J, Giordano A, Cinti S, Kahn CR, Efstratiadis A, Accili D. Mosaic analysis of insulin receptor function. J Clin Invest. 2004;113:209–219. doi: 10.1172/JCI17810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klippel A, Escobedo JA, Hirano M, Williams LT. The interaction of small domains between the subunits of phosphatidylinositol 3-kinase determines enzyme activity. Mol Cell Biol. 1994;14:2675–2685. doi: 10.1128/mcb.14.4.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight ZA, Gonzalez B, Feldman ME, Zunder ER, Goldenberg DD, Williams O, Loewith R, Stokoe D, Balla A, Toth B, et al. A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell. 2006;125:733–747. doi: 10.1016/j.cell.2006.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Ogawa W, Akimoto K, Inoue H, Miyake K, Furukawa K, Hayashi Y, Iguchi H, Matsuki Y, Hiramatsu R, et al. PKClambda in liver mediates insulin-induced SREBP-1c expression and determines both hepatic lipid content and overall insulin sensitivity. J Clin Invest. 2003;112:935–944. doi: 10.1172/JCI18816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000a;6:87–97. [PubMed] [Google Scholar]
- Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000b;6:87–97. [PubMed] [Google Scholar]
- Mithieux G, Guignot L, Bordet JC, Wiernsperger N. Intrahepatic mechanisms underlying the effect of metformin in decreasing basal glucose production in rats fed a high-fat diet. Diabetes. 2002;51:139–143. doi: 10.2337/diabetes.51.1.139. [DOI] [PubMed] [Google Scholar]
- Norris AW, Chen L, Fisher SJ, Szanto I, Ristow M, Jozsi AC, Hirshman MF, Rosen ED, Goodyear LJ, Gonzalez FJ, et al. Muscle-specific PPARgamma-deficient mice develop increased adiposity and insulin resistance but respond to thiazolidinediones. J Clin Invest. 2003;112:608–618. doi: 10.1172/JCI17305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajan MP, Standaert ML, Miura A, Kahn CR, Farese RV. Tissue-specific differences in activation of atypical protein kinase C and protein kinase B in muscle, liver, and adipocytes of insulin receptor substrate-1 knockout mice. Mol Endocrinol. 2004;18:2513–2521. doi: 10.1210/me.2004-0045. [DOI] [PubMed] [Google Scholar]
- Sajan MP, Standaert ML, Nimal S, Varanasi U, Pastoor T, Mastorides S, Braun U, Leitges M, Farese RV. The critical role of atypical protein kinase C in activating hepatic SREBP-1c and NFkappaB in obesity. J Lipid Res. 2009;50:1133–1145. doi: 10.1194/jlr.M800520-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standaert ML, Sajan MP, Miura A, Kanoh Y, Chen HC, Farese RV, Jr, Farese RV. Insulin-induced activation of atypical protein kinase C, but not protein kinase B, is maintained in diabetic (ob/ob and Goto-Kakazaki) liver. Contrasting insulin signaling patterns in liver versus muscle define phenotypes of type 2 diabetic and high fat-induced insulin-resistant states. J Biol Chem. 2004;279:24929–24934. doi: 10.1074/jbc.M402440200. [DOI] [PubMed] [Google Scholar]
- Stumvoll M, Nurjhan N, Perriello G, Dailey G, Gerich JE. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N Engl J Med. 1998;333:550–554. doi: 10.1056/NEJM199508313330903. [DOI] [PubMed] [Google Scholar]
- Taniguchi CM, Kondo T, Sajan M, Luo J, Bronson R, Asano T, Farese R, Cantley LC, Kahn CR. Divergent regulation of hepatic glucose and lipid metabolism by phosphoinositide 3-kinase via Akt and PKClambda/zeta. Cell Metab. 2006a;3:343–353. doi: 10.1016/j.cmet.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Taniguchi CM, Tran TT, Kondo T, Luo J, Ueki K, Cantley LC, Kahn CR. Phosphoinositide 3-kinase regulatory subunit p85alpha suppresses insulin action via positive regulation of PTEN. Proc Natl Acad Sci USA. 2006b;103:12093–12097. doi: 10.1073/pnas.0604628103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tou JC, Wade CE. Determinants affecting physical activity levels in animal models. Exp Biol Med (Maywood) 2002;227:587–600. doi: 10.1177/153537020222700806. [DOI] [PubMed] [Google Scholar]
- Ueki K, Algenstaedt P, Mauvais-Jarvis F, Kahn CR. Positive and negative regulation of phosphoinositide 3-kinase-dependent signaling pathways by three different gene products of the p85alpha regulatory subunit. Mol Cell Biol. 2000;20:8035–8046. doi: 10.1128/mcb.20.21.8035-8046.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Leevers SJ, Panayotou G, Waterfield MD. Phosphoinositide 3-kinases: a conserved family of signal transducers. Trends Biochem Sci. 1997;22:267–272. doi: 10.1016/s0968-0004(97)01061-x. [DOI] [PubMed] [Google Scholar]
- Wang XL, Suzuki R, Lee K, Tran T, Gunton JE, Saha AK, Patti ME, Goldfine A, Ruderman NB, Gonzalez FJ, Kahn CR. Ablation of ARNT/HIF1beta in liver alters gluconeogenesis, lipogenic gene expression, and serum ketones. Cell Metab. 2009;9:428–439. doi: 10.1016/j.cmet.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SG, Finan P. Isoform-specific phosphoinositide 3-kinase inhibitors as therapeutic agents. Curr Opin Pharmacol. 2003;3:426–434. doi: 10.1016/s1471-4892(03)00078-x. [DOI] [PubMed] [Google Scholar]
- Ward S, Sotsios Y, Dowden J, Bruce I, Finan P. Therapeutic potential of phosphoinositide 3-kinase inhibitors. Chem Biol. 2003;10:207–213. doi: 10.1016/s1074-5521(03)00048-6. [DOI] [PubMed] [Google Scholar]
- Wymann MP, Pirola L. Structure and function of phosphoinositide 3-kinases. Biochim Biophys Acta. 1998;1436:127–150. doi: 10.1016/s0005-2760(98)00139-8. [DOI] [PubMed] [Google Scholar]
- Yu J, Zhang Y, McIlroy J, Rordorf-Nikolic T, Orr GA, Backer JM. Regulation of the p85/p110 phosphatidylinositol 3′-kinase: stabilization and inhibition of the p110α catalytic subunit by the p85 regulatory subunit. Mol Cell Biol. 1998;18:1379–1387. doi: 10.1128/mcb.18.3.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JJ, Cheng H, Jia S, Wang L, Gjoerup OV, Mikami A, Roberts TM. The p110alpha isoform of PI3K is essential for proper growth factor signaling and oncogenic transformation. Proc Natl Acad Sci USA. 2006;103:16296–16300. doi: 10.1073/pnas.0607899103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.