Abstract
Blood contains microparticles (MPs) derived from a variety of cell types, including platelets, monocytes and endothelial cells. In addition, tumors release MPs into the circulation. MPs are formed from membrane blebs that are released from the cell surface by proteolytic cleavage of the cytoskeleton. All MPs are procoagulant because they provide a membrane surface for the assembly of components of the coagulation protease cascade. Importantly, the procoagulant activity is increased by the presence of anionic phospholipids, particularly phosphatidylserine (PS), and the procoagulant protein tissue factor (TF), which is the major cellular activator of the clotting cascade. High levels of platelet-derived PS+ MPs are present in healthy individuals, whereas the number of TF+,PS+ MPs is undetectable or very low. However, levels of PS+, TF+ MPs are readily detected in a variety of diseases and monocytes appear to be the primary cellular source. In cancer, PS+, TF+ are derived from tumors and may serve as a useful biomarker to identify patients at risk for venous thrombosis. This review will summarize our current knowledge on the role of procoagulant MPs in hemostasis and thrombosis.
Keywords: Microparticles, procoagulant, tissue factor, hemostasis, thrombosis
1. Introduction
Microparticles (MPs) are present in the blood of healthy individuals and are increased in various diseases, including cardiovascular disease, diabetes, sepsis and cancer.1-3 They are small membrane vesicles derived from activated cells and apoptotic cells and are commonly referred to as microparticles (MPs). MPs range in size from 100-1000 nm in diameter, but are typically are 200 nm in size. They mediate cell-cell communication by transferring a cargo of cell surface receptors, mRNAs, and microRNAs from the cell of origin to target cells.4 In addition, MPs may bind and fuse with the plasma membrane of the target cell or be engulfed by the target cell.4 Importantly, MPs have been proposed to play roles in thrombosis, inflammation and angiogenesis.1-3
Platelet-derived MPs are strongly procoagulant because they contain the anionic phospholipid PS. An early study by Chargaff and West in 1946 showed that the clotting time of recalcified normal human plasma was prolonged by the removal of MPs using high speed centrifugation.5 In 1967, Wolf reported that activation of platelets resulted in the generation of “platelet dust” and that these platelet-derived MPs supported thrombin generation in platelet poor plasma.6
MPs containing both PS and the procoagulant protein TF have the highest level of procoagulant activity. TF is the primary cellular activator of the clotting cascade.7 Early studies suggested blood did not contain significant levels of TF and that all TF was expressed by extravascular cells in healthy individuals where it formed a “hemostatic envelope” around blood vessels.8 However, in 1999, Giesen and colleagues discovered that blood of healthy individuals contained very low levels of functional TF (so-called blood-borne TF). They showed that blood-borne TF contributed to thrombus formation in ex vivo models.9 TF+ MPs were observed near the surface of platelets in the thrombus.
Despite these provocative studies, whether or not blood of healthy individuals contains significant levels of functional TF remains highly controversial. Some investigators believe that there is no functional TF in unstimulated blood of healthy individuals.10 Similarly, others have failed to detect measureable TF activity in plasma or associated with MPs isolated from unstimulated whole blood.11 One study found that isolated MPs from healthy controls generated thrombin is a TF-independent manner.12 However, other groups have reported very low levels of TF activity in blood in the form of MPs in healthy individuals, although these levels are close to the detection limit of the assays.13-15. Recently, it was reported that 95% of TF activity in blood was present on peripheral blood mononuclear cells and only 5% was present on MPs.16 Importantly, TF+ MPs can be easily isolated from a small volume of plasma. Another important consideration is that TF+ MPs in blood may be recruited to sites of vascular injury in vivo. In fact, circulating TF was found to accumulate in thrombi formed in the saphenous vein of mice but not in hemostatic clots.17 Furthermore, elegant studies by the Furie group have shown that TF+ MPs are recruited to thrombi formed in a laser injured mouse cremaster arteriole (see below).18
2. Procoagulant properties of MPs
a. Role of PS
The plasma membrane of normal cells has an asymmetrical distribution of lipids in the inner and outer membranes. Anionic phospholipids, such as PS, are located almost exclusively in the inner monolayer. During the formation of MPs there is loss of membrane asymmetry with ionic phospholipids being transferred to the outer membrane of the MP.19 Importantly, the presence of PS significantly increases the procoagulant activity of MPs because it facilitates the assembly of components of the clotting cascade. This is due to an electrostatic interaction between positively charged γ-carboxyglutamic acid (GLA) domains in the clotting proteins and PS on the membrane. Clotting proteins that contain a GLA domain include Factors VII (FVII), IX and X, and prothrombin (Figure 1). PS on the surface of MPs can be detected using flow cytometry. Activated platelets generate PS+ MPs, although one study found both PS+ and PS- MPs.20
Figure 1. Assembly of coagulation complexes on a PS+ phospholipid membrane.
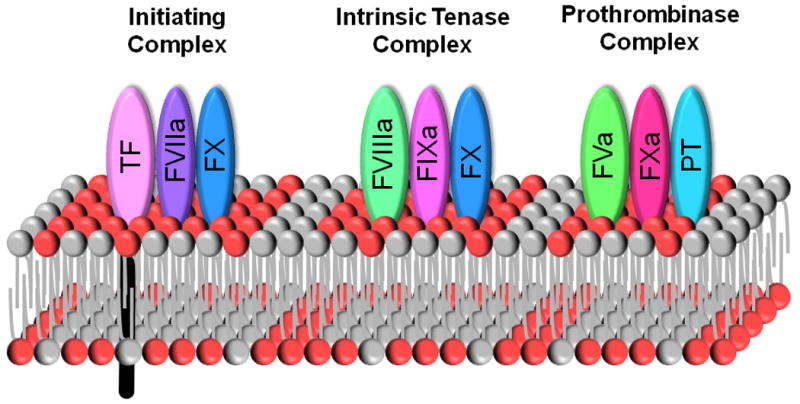
Coagulation complexes (TF:FVIIa; FVIIIa:FIXa; and FVa:FXa) assemble on a membrane surface. The presence of anionic phospholipids, such as PS (red), facilitate the binding of FVIIa, FIXa, FXa, and prothrombin (PT) by interaction with the Gla domains within the proteins. Phospholipids originating from the outer membrane before disruption of membrane asymmetry are shown in grey.
b. Role of TF
TF is a receptor for FVII/VIIa (Figure 1). The TF:FVIIa complex activates both FX and FIX to initiate blood coagulation.7 The presence of TF on MPs dramatically increases their procoagulant activity. TF has a high affinity for FVII/FVIIa and therefore TF+ MPs in blood will readily bind FVII/FVIIa. The TF:FVIIa complex is regulated by tissue factor pathway inhibitor (TFPI).21 This Kunitz-type inhibitor is primarily synthesized by endothelial cells and circulates in blood to prevent inappropriate activation of the coagulation cascade.21 It inhibits the TF:FVIIa complex in a FXa-dependent manner. Therefore, it is likely that some of the TF:FVIIa complexes present on MPs in blood will be inhibited by TFPI.
Posttranslational modifications of TF, such as glycosylation, may also affect TF activity.22-24 This means that TF+ MPs from different cellular sources may have different procoagulant activities. In addition, the TF:FVIIa complex can be found in low (also called encrypted) and high activity states, which is thought to be due to differences in the conformation of TF, reviewed previously.25 The different states were discovered because disruption of TF+ cells increased TF activity without a change in TF antigen.26 This increase in TF activity was associated with an increase in PS, which led some investigators to propose that PS may induce a conformational change in TF that increases it specific activity.25 Other mechanisms of TF activation have been proposed and it is possible that there are different mechanisms that regulate TF:FVIIa activity on MPs derived from different cell types.27
Some investigators have proposed that MPs in blood contain TF in a low activity state to prevent inadvertent activation of the coagulation cascade. By analogy with cells, this could be due to the fact that levels of PS on the MPs are below the optimal level for full TF activity. If this notion is correct freezing the MPs would increase PS levels on the outer membrane and also increase TF activity. However, TF activity of MPs isolated from plasma of patients undergoing total knee arthroplasty was not increased by ionomycin treatment or freezing to increase PS exposure.16 Another group also found no difference in TF activity of fresh MPs versus frozen MPs isolated from healthy individuals and cancer patients.15 In addition, freezing MPs did not increase the level of PS (F. Dignat-George, pers. com.). We prepared monocyte-derived MPs from human whole blood stimulated with bacterial lipopolysaccharide (LPS) as a model system. Consistent with other studies, frozen MPs had the same TF activity as fresh MPs (Lee et al. submitted). These results indicated that the TF is fully active on washed MPs and that PS levels are not limiting.
3. Measurement of the levels of MPs using functional assays
There are many ways to measure MPs. Flow cytometry can be used to determine the cellular origin of the different MPs, although there are concerns about the detection limit of this approach.28 Electron microscopy, atomic force microscopy, and dynamic light scattering all can be used to determine the size of MPs but do not provide information on the biological properties of the MPs.29 Measurement of TF antigen levels on MPs have been recently reviewed.30-31 We will focus on functional assays that measure the procoagulant activity of isolated MPs. Advantages of functional assays include their high sensitivity, simplicity and the use of well-defined reagents. For instance, we found that pancreatic cancer patients had higher levels of MP TF activity than healthy controls whereas a TF antigen assay failed to detect a difference.14 However, functional assays do not provide any information on the cellular source of the MPs or their physical properties. Ideally, a combination of methods should be used to characterize MPs.
a. PS-dependent MP assays
As mentioned above, the presence of PS on the surface of MPs allows assembly of the different coagulation protease complexes on the MPs. One commercial assay, called the Zymuphen MP Activity assay (Hyphen BioMed), quantifies the level of PS in the MP population.32 Briefly, PS+ MPs are captured on an ELISA plate coated with annexin V-streptavidin and incubated with FV, FX and prothrombin to from the prothrombinase complex that cleaves prothrombin to thrombin. A chromogenic substrate for thrombin is added to assess the levels of thrombin. The values are expresses as PS equivalents. Another assay, called procoagulant phospholipid (Proag PPL) (Stago), measures the procoagulant activity of MPs added to phospholipid-free porcine plasma.33 Equal volumes of test plasma and phospholipid-free plasma are mixed before the addition of FXa and the clotting time is measured. The level of phospholipid in the sample in the form of MPs is calculated using a standard curve prepared with synthetic phospholipids.
b. TF-dependent MP assays
Two strategies have been employed to measure TF activity of MPs isolated from plasma by capture or centrifugation. Aras and colleagues used a monoclonal antibody (1B10) to capture MPs from a variety of cell types, including monocytes.13 The level of TF activity of captured MPs is measured by adding FVIIa and FX in the presence or absence of an anti-TF antibody. Another study captures TF+ MPs using a biotinylated anti-TF antibody and then measures their TF activity by adding FVIIa and FX.34 A commercial assay called the Zymuphen MP TF assay (Hyphen BioMed) is available and captures PS+ MPs in the same way as the Zymuphen MP activity assay. However, there is no data in the literature using this assay.
Tesselaar and colleagues used centrifugation to isolate MPs from healthy individuals and cancer patients and measure their TF activity.15 MPs are incubated with FVII, FX and a chromogenic substrate for FXa for 90 minutes. Synthetic phospholipids are also added to the assay to provide an excess of phospholipid. Importantly, assays are performed in the presence or absence of an anti-human TF antibody to distinguish TF-dependent and TF-independent FXa generation. Levels of MP TF activity in healthy individuals was very low (132 fM Xa min-1). In a subsequent paper, a lower level of MP TF activity was reported (4.1 fM Xa min-1) because some of the MPs were removed by an additional centrifugation step.35 In parallel to the Osanto group, we developed a functional MP TF activity assay.14 MPs were pelleted from platelet poor plasma by centrifugation at 20,200 g for 15 minutes and washed two times before with FVIIa and FX for 2 hours. Finally, a chromogenic substrate for FXa was added for 15 minutes. We also performed the assay in the presence of either a control antibody or an anti-TF antibody to measure TF-dependent and TF-independent FXa activity. We found low levels of MP TF activity (0.21 pg/mL) in healthy individuals. We also found that plasma preparation significantly affected the levels of MP TF activity. MP TF activity of platelet free plasma was 64% lower than the activity of MPs prepared from platelet poor plasma (Lee et al submitted). An important difference in the two MP TF activity assays is that we do not added exogenous phosphoplipid. In fact, we found that addition of exogenous synthetic phospholipids increases total FXa generation in a TF-independent manner (Manly D. and Mackman, unpublished data). These results indicate that pre-analytical variables, such as plasma preparation, have a major impact on the level of TF activity of the MPs. In addition, the specific activity of the recombinant TF used to prepared standard curves can vary significantly making it difficult to compare levels of MP TF activity in different studies.
4. Cellular sources of procoagulant MPs in blood
a. PS+ MPs
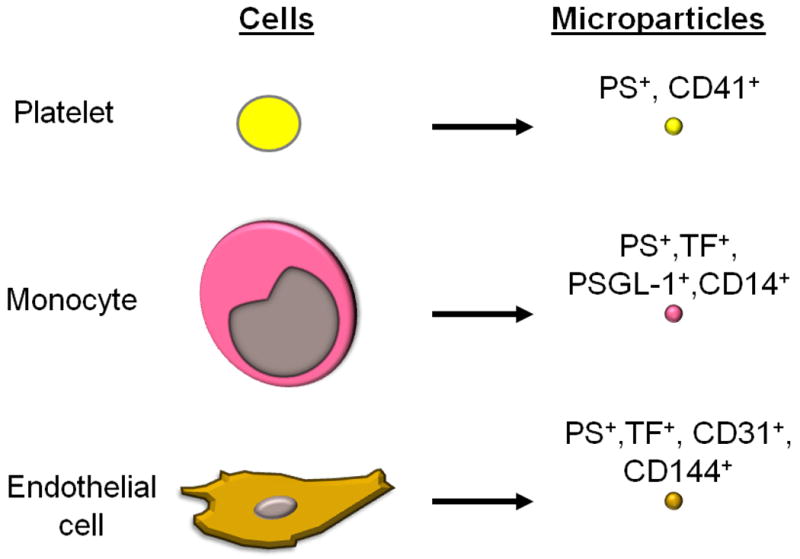
The cellular source of MPs is defined primarily on the basis of their cell surface antigens. CD41 is used to identify MP derived from platelets (Figure 2). Several studies indicate that platelets are the major source of PS+ MPs in blood and represent 70-90% of all circulating MPs.12, 36-38 As noted above activated platelets release PS+ MPs.6 The conclusion that CD41+ MPs are all derived from platelets was recently challenged because cultured megakaryocytes were found to also shed CD41+ MPs.39 In fact, one study analyzed the origin of CD41+ MPs in blood using markers specific for platelets or megakaryocytes and concluded that the majority of these MPs are derived from megakaryocytes.39 At present the source of the increased numbers of CD41+ MPs in different diseases is not known.
Figure 2. Surface markers of MPs released by different vascular cells.
b. TF+ MPs
i. Monocytes
One study concluded that unstimulated monocytes do not express TF.10 However, two other studies found TF expression in subset of unstimulated leukocytes and monocytes from healthy individuals.9 Furthermore, LPS stimulation of monocytes increases TF expression and the release of TF+ MPs.8, 10, 40-41 One study found that MPs from monocytic THP-1 cells expressed CD15 that mediated binding to P-selectin on activated platelets.42 Another study reported that THP-1 cell-derived MPs were enriched in TF and P-selectin glycoprotein 1 (PSGL-1) (Figure 2), which would allow docking onto activated platelets and endothelial cells by binding to P-selectin.43 Interestingly, a recent study found that MPs derived from LPS stimulated monocytes expressed low levels of TFPI.44 Importantly, plasma from patients with meningococcal sepsis contained MPs that expressed TF and the monocyte marker CD14 (Figure 2).45 More recently, Aras and colleagues reported a transient increase in MPs that express both TF+ and CD14+ in a human endotoxemia model.13 Monocyte-derived TF+ MPs are also elevated in sickle cell disease.46 These studies indicate that monocytes are likely to be the major source of TF+ MPs in health and disease.
ii. Neutrophils
Neutrophils have also been reported to express TF in response to complement C5a.47-49 However, a recent study found that monocyte-derived TF+ MPs can readily bind to neutrophils, which may explain some of the reports of neutrophil TF expression.50 We found that deletion of TF in myeloid cells reduced fetal loss in a mouse model of antiphospholipid antibody syndrome49, but it was not possible to distinguish between a role for TF expression by neutrophils versus monocytes.
iii. Endothelial cells
Cultured endothelial cells express TF in response to a variety of agonists, including cytokines and LPS.51 However, there is limited evidence that endothelial cells express TF in vivo. Studies with animal models of endotoxemia and sepsis have reported TF expression in endothelial cells in the splenic vasculature and at branch points in the aorta.52-53 As stated above, it is possible that part or all of this TF staining is due to the binding of monocyte-derived TF+ MPs that are known to be present in septic animals. Indeed, TF staining of endothelial cells was restricted to granular structures that also contained the leukocyte marker PSGL-1.53 Moreover, we found that a selective deletion of the TF gene in endothelial cells did not reduce the activation of coagulation in a mouse endotoxemia model.54 However, in sickle cell mice TF expression was observed on endothelial cells of the pulmonary veins.55 In addition, endothelial cell-derived MPs were observed in sickle cells patients in crisis and expressed both TF and CD144 (Figure 2).46 Interestingly, the TF activity of MPs derived from activated endothelial cells was markedly increased by inhibition of TFPI whereas there was only a modest change using MPs from stimulated monocytes, suggesting that these TF+ MPs from different cellular sources have different TF activity.56-57 These studies indicate that endothelial cells may release TF+ MPs in certain diseases.
iv. Platelets
Platelets have been reported to express TF.58-61 However, other investigators failed to detect any TF in resting or activated platelets.10, 62-63 There are several explanations for these conflicting results. Some of the studies did not use inhibitory anti-TF antibodies to demonstrate that the procoagulant activity of the platelets is indeed due to TF. This is important because high concentrations of FVIIa can activate FX in a TF-independent manner.64 In addition, the presence of TF on platelets may be due, in part, to the binding of monocyte-derived TF+ MPs to activated platelets. In the study by Zillman and colleagues, treatment of whole blood with collagen increased platelet TF expression.61 However, this study did not exclude the possibility that that collagen activation of the platelets exposed P-selectin on the cell surface and allowed binding of monocyte-derived TF+ MPs in the whole blood. It is more difficult to explain the reports of TF pre-mRNA and mRNA expression in platelets and do-novo synthesis of TF protein by platelets, although monocyte contamination of the platelet preparations is always a concern.59-60 In conclusion, platelets may express very low levels of TF but it seems unlikely that they provide a major contribution to the pool of TF+ MPs present in healthy individual and patients.
5. Clearance of MPs
MPs have a relatively short half-life in the circulation. One study examined the role of the PS binding protein lactadherin in the clearance of platelet-derived PS+ MPs.65 They found that the number of CD42+ MPs was significantly higher in lactadherin-deficient mice compared with wild-type (WT) littermates. In addition, splenectomized WT mice had more circulating CD42+ MPs than control mice suggesting that the spleen was involved in the clearance of these MPs. Another study found elevated levels of PS+ MPs in lactadherin-deficient mice.66 Clearance of tumor-derived human TF+ MPs was also examined in control and splenectomized mice.67 In control mice peak levels of TF+ MPs were observed at 30 minutes and none were detected at 120 minutes, whereas in the splenectomized mice significant levels of TF+ MPs were observed at 120 minutes. Furthermore, human TF antigen was detected in the spleen. These results support the conclusion that the spleen is the major site for the clearance of PS+ MPs with or without TF.
6. Role of MPs in hemostasis
a. PS + MPs
Platelets mediate primary hemostasis. One of the key events in platelet activation is the exposure of PS on the cell surface. By analogy, PS+ MPs derived from platelets can be viewed as a smaller version of activated platelet and express receptors for both collagen and von Willebrand factor (Figure 3). Therefore, it has been proposed that these MPs may play a role in hemostasis (Figure 3).68 However, it is very difficult to separate the roles of platelets and platelet-derived MPs in hemostasis because one cannot selectively remove the MPs. This is also true for megakaryocyte-derived MPs.39 Patients with Castaman's defect and Scott syndrome have a bleeding tendency that appears to be due to a defect in the ability of activated platelets to translocate PS to the surface of the cells.69-70 Platelets from these patients also have a defect in the generation of PS+ MP in vitro, which has been used by some to argue that platelet MPs are important for hemostasis. However, further studies are needed before it can be concluded that platelet-derived MPs are required for hemostasis.
Figure 3. Proposed roles of platelet-derived and monocyte-derived MPs in hemostasis and thrombosis.
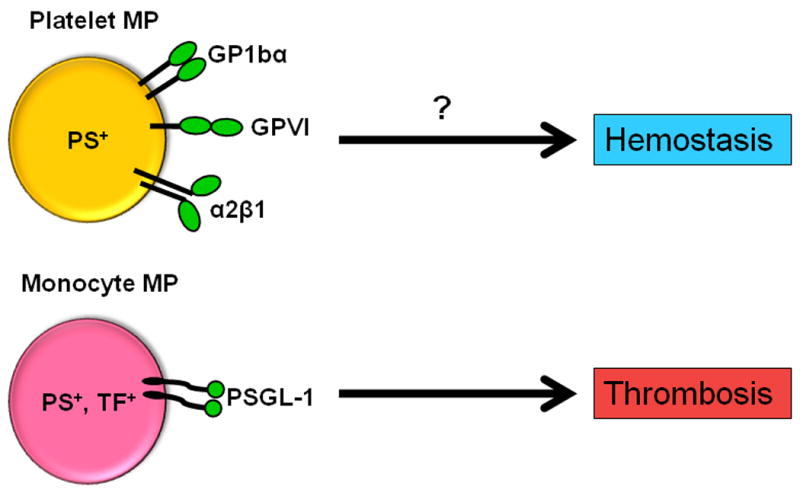
PS+ MPs derived from platelets may play a role in hemostasis and may enhance thrombosis in certain diseases. Conversely, PS+, TF+ MP derived from monocytes may contribute to thrombosis and have a minor role in hemostasis.
b. TF+ MPs
After vessel injury extravascular TF comes into contact with blood and the clotting cascade is activated to form a hemostatic plug. Mice lacking either TF or FVII do not survive indicating that the TF:FVIIa complex is essential for hemostasis.71 The clotting cascade can be divided into two phases: initiation and propagation.72 This is analogous to a stick of dynamite where the fuse represents the initiation phase and the dynamite represents the propagation phase. The TF:FVIIa complex of the extrinsic pathway in the major trigger of the clotting cascade and generates small amounts of thrombin. In contrast, the intrinsic pathway (FXI, IX, VIIIa) is required for the burst of thrombin generation. These two phases can be easily separated in vitro but clotting in vivo is more complex due to flow.
So how do we fit TF+ MPs into this scheme of the clotting system and hemostasis? The low level of TF expression in a subset of unstimulated monocytes together with circulating TF+ MP may play a role in hemostasis by maintaining the idling of the clotting cascade (a low level activation that is dependent on the TF:FVIIa complex).73 In addition, it has also been proposed that TF+ MP play a role in clot growth. It has been proposed that after injury vessel wall TF will trigger clotting but will then be covered by platelets and the clot, thus blocking it from further participation in clotting.74 Circulating TF+ MP may provide an alternative source of TF that would be recruited to the growing thrombus that could re-initiate clotting and thus contribute to its growth.
There are arguments for and against this notion. Those investigators against the idea argue that levels of TF+ MP in healthy individuals are too low to contribute to thrombin generation in the presence of an intact intrinsic pathway. Support for this view was provided by in vitro experiments using whole blood showing that resupply of TF to an ongoing TF-initiated clotting reaction did not enhance thrombin generation.75 Similarly, we found that the presence of TF+ MP in plasma shortened the lag time but did not change the total thrombin generation in a calibrated automated thrombogram assay, consistent with a role in initiation but not propagation.76 Those in favor of the idea that TF+ MPs contribute to clot growth argue that flow is required to deliver the TF+ MP to the clot. One study showed that increasing the shear rate of the blood to 650 s-1 produced the maximal amount of TF-dependent FXa generation in ex-vivo thrombi.77 Furthermore, leukocyte-derived TF+ MPs have been shown to contribute to thrombus growth in an injured mouse cremaster arteriole (see below).78-79 One study found that increasing the number of circulating MPs, some of which expressed TF, restored hemostasis in a mouse model of hemophilia A.80
An added complexity to analyzing the role of TF+ MPs in hemostasis is that larger vessels contain more TF in the vessel wall than smaller vessels. In larger vessels it has been estimated that there is a ratio of 1000:1 in vessel wall TF to circulating TF+ MPs in healthy individuals and mice.10, 81 This suggests that circulating TF+ MPs are more likely to contribute to hemostasis in small vessels than larger vessels, and also organs that express low levels of TF, such as the liver and skeletal muscle.71 However, at present it is unclear if the low levels of circulating TF+ MPs in healthy individuals are required for hemostasis (Figure 3). A recent study proposed that TF+ MPs present in plasma may contribute to hemostasis of superficial wounds.82
7. Role of platelet-derived MPs in thrombosis
There are few studies that have investigated the role of platelet-derived MPs in thrombosis, although these are elevated in a number of diseases. Heparin-induced thrombocytopenia (HIT) is associated with heparin therapy and is associated with decreased platelet counts but paradoxically with thrombosis. One study found that incubation of platelets with heparin and immunoglobulin induced the generation of MPs, which led to the notion that these MPs may trigger thrombosis.83 However, a more recent study showed a role for monocytes in thrombosis in HIT.84 Moreover, we found that a heparin-PF4 antibody complex induced monocyte TF expression and the release of TF+ MPs.85 These data suggest that monocyte-derived TF+ MPs rather than platelet-derived MPs may initiate thrombosis in HIT.
8. TF+ MPs and activation of coagulation in mice and in animal models of thrombosis
Previous studies have shown that the vessel wall is the major source of TF that contributes to thrombosis in a mouse model of carotid artery injury.81, 86-87 However, it should be noted that these experiments were performed on healthy mice that have very low levels of circulating TF+ MPs. In fact, Reinhardt and colleagues found that mice injected with human monocyte-derived TF+ MPs had increased fibrin accumulation in a carotid artery ligation model.88 This result indicates that TF+ MPs can contribute to thrombosis in this model. However, larger numbers of MPs were used that likely far exceed the levels of circulating MPs observed in disease states. In this review, we will focus on studies of that analyze TF+ MPs in models of activation of coagulation and thrombosis.
a. Role of hematopoietic cell-derived TF+ MPs in a mouse model of microvascular thrombosis induced by laser injury
The laser injury model of arteriole thrombosis is the best system to examine the role of MPs in thrombus formation because there is minimal injury to the vessel wall.89 This model utilizes a focused laser beam to damage the endothelium and induce thrombosis. It utilizes the microcirculation of living mice and analyzes the thrombus formation by confocal microscopy. Arterioles of the cremaster muscle are typically used in this model.
Falati and colleagues observed the rapid accumulation of TF and fibrin upstream of the thrombus, consistent with recruitment of circulating TF+ MPs to the thrombus.90 However, the highest concentrations of TF were found at the thrombus-vessel wall interface, suggesting that vessel wall TF also contributed to thrombus formation. In a subsequent study, they demonstrated accumulation of ex vivo generated TF+ MPs in the developing thrombus.18 Next, they used PSGL-1 and P-selectin null mice to demonstrate that the recruitment of MP was largely dependent upon the interaction of MP PSGL-1 with platelet P-selectin (Figure 4). Gross and colleagues examined the kinetics of TF incorporation into the thrombus.79 Accumulation of TF+ MPs occurred rapidly in a developing thrombus and peaked 60 seconds after the initiation of injury. By comparison, the appearance of TF+ leukocytes first appeared 3 minutes after the initiation of injury, which demonstrates that MPs accumulate before monocytes.
Figure 4. Role of TF+ MPs in microvascular and venous thrombosis.
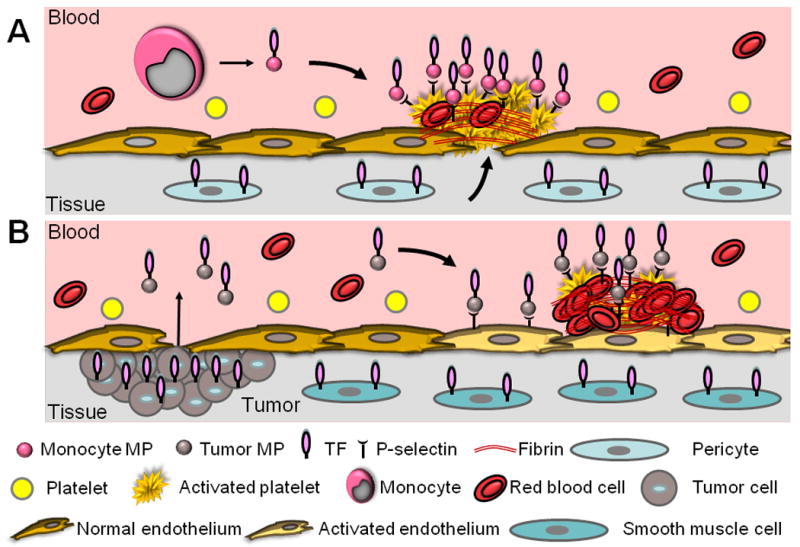
A. Microvascular thrombosis in healthy mice. Leukocyte-derived TF+ MPs and vessel wall TF both appear to contribute to thrombosis induced by laser injury to cremaster arterioles. B. Cancer associated venous thrombosis. Tumors release TF+ MPs into the circulation where they are proposed to bind to activated endothelium and trigger venous thrombosis.
To examine the cellular source of TF that contributed to thrombosis in the laser injury model, we utilized low TF mice that only express 1% levels of human TF compared to WT control mice.78 Low TF mice were found to develop small platelet-rich thrombi with reduced levels of both TF and fibrin in the laser injury model. We performed a reciprocal bone marrow transplantation to determine the role of hematopoietic cell-derived TF+ MPs in thrombus formation.78 Low TF mice containing bone marrow from WT donors had larger thrombi with increased levels of fibrin than thrombi of low TF mice. Conversely, WT mice containing low TF bone marrow had smaller thrombi with less fibrin that thrombi in WT mice. These data indicated that both hematopoietic cell-derived TF+ MPs and vessel wall TF both contributed to thrombus formation in this model (Figure 4) Experiments are currently being performed using mice with a deficiency of TF in myeloid cells, endothelial cells or smooth muscle cells (SMCs) to more precisely determine the cellular sources of TF that contributes to thrombus formation in this model. Clearly, the interaction between PSGL-1 and P-selectin likely plays a key role in the recruitment of monocyte-derived TF+ MPs to the site of thrombosis in different diseases.
b. TF+ MPs and animal models of venous thrombosis
The role of leukocytes and TF+ MPs in venous thrombosis has been analyzed in several animal models. For example, Himber and colleagues used a model in which a collagen-coated thread is inserted in the jugular vein of rabbits and found TF-dependent fibrin accumulation and thrombus propagation.91 TF+ leukocytes accumulated in the thrombus and it was assumed that TF+ MPs also contributed to fibrin formation in this model.
The most commonly utilized model of venous thrombosis is the inferior vena cava (IVC) ligation model (reviewed previously).92 This model is not the best model for studying the role of circulating TF+ MPs in thrombosis because delivery of MPs to the site of injury is impeded by the ligation and there is injury to the vessel wall. Ramacciotti and colleagues examined MPs in a mouse IVC ligation model.93 They found that thrombus weight correlated negatively with leukocyte-derived MPs, suggesting that these MPs were consumed by the thrombus. Moreover, injection of MPs into mice after IVC ligation increased the thrombus weight at the earlier time-points. Zhou and colleagues used a rat model of IVC ligation and found a rapid accumulation of TF+ leukocytes within the thrombus in conjunction with P-selectin expression in endothelial granules.94 They further demonstrated focal areas of denuded endothelium. Biro and colleagues demonstrated that MPs, obtained from human pericardial blood following cardiac surgery, increased thrombus formation in a rat model of IVC ligation in a TF-dependent manner.95 Similar to the laser injury model, mice deficient in either PSGL-1 or P-selectin have smaller thrombi in the IVC ligation model.96 We found that low TF mice had smaller thrombi compared with control mice in the IVC ligation model.81 However, chimeric bone marrow transplantation demonstrated that hematopoietic cell-derived TF did not play a role in thrombosis. This result indicates that there are roles of PSGL-1 and P-selectin beyond mediating the docking of TF+ MPs to the growing thrombus.
Other models have been developed that are more suitable for the analysis of TF+ MPs in venous thrombosis because they maintain blood flow across the injury site that would permit delivery of circulating MPs to the thrombus.97-98 The role of hematopoietic cell-derived TF+ MPs in these models is being evaluated.
c. TF+ MPs and activation of coagulation in a mouse model of endotoxemia
Recently, our group demonstrated that MP TF activity was increased in a mouse model of endotoxemia.99 Further, we observed a linear correlation between MP TF activity and levels of thrombin-antithrombin (TAT) complex, a marker of the activation of coagulation. These results suggest that TF+ MPs may contribute to the activation of coagulation in this model. However, further studies are needed to ascertain the different cellular sources of TF+ MPs and their role in the activation of coagulation.
9. Role of TF+ MPs in the activation of coagulation in tumor-bearing mice and venous thromboembolism cancer patients
Venous thromboembolism (VTE) is a term used to describe both deep-vein thrombosis and pulmonary embolism. Venous thrombi occur as a result of changes in blood flow, activation of the endothelium, and/or changes in the blood itself. This is known as Virchow's triad.100 The association between cancer and thrombosis has been known since the mid-19th century. Malignant tumors were found to release procoagulant plasma membrane vesicles (know referred to as MPs) both in vitro and in vivo.101 Subsequent studies showed that the procoagulant activity of the MPs derived from tumors cells was due to TF.67, 102-103 Importantly, circulating tumor cell-derived TF+ MPs may trigger the formation of venous thrombi in the absence of vessel injury (Figure 4).
a. Tumor-derived TF+ MPs and activation of coagulation in mice
Mouse studies have been used to analyze tumor-derived TF+ MPs and the activation of coagulation. For example, Yu and colleagues found that human TF antigen was released into the blood from human colorectal tumors or epithelial carcinoma cells grown subcutaneously in mice.104-105 Further, the level of circulating TF was proportional to the size of the tumor and tumors expressing higher levels of TF resulted in higher levels of circulating TF.104 Davila and colleagues also detected an increase in circulating tumor-derived TF+ MP procoagulant activity proportional to tumor size in a pancreatic cancer orthotopic mouse model.67 Recently, we found that the elevated levels of TAT in mice with pancreatic tumors were reduced by inhibition of TF with a monoclonal anti-mouse TF antibody (Wang and Mackman, unpublished data). Thomas and colleagues utilized a model of ferric chloride-induced injury of mesenteric arterioles and demonstrated a decreased time to occlusion in tumor-bearing mice and it WT mice receiving tumor cell-derived MPs.106 They also found that pancreatic tumors expressing enhanced green fluorescent protein released labeled MPs that became incorporated into thrombi. Interestingly, the tumor cell-derived MPs expressed PSGL-1 and bound to the thrombus in a P-selectin dependent manner. However, this study did not establish a role for tumor cell-derived TF+ MPs in thrombus formation. These results indicate that tumors produce procoagulant TF+ MPs, which enter the bloodstream via the leaky vasculature of the tumor and likely trigger venous thrombosis (Figure 4). Further experiments with different tumor lines are needed to analyze how tumor-derived TF+ MPs initiate thrombosis in mouse tumor models.
b. Clinical studies on TF+ MPs and cancer patients
We will focus on studies that measured levels of TF+ MPs using flow cytometry, impedance or activity assays because measurement of plasma TF antigen and plasma TF activity is associated with technical problems.30, 107 Recent clinical studies have demonstrated an increase in TF+ MPs in cancer patients (Table). For example, Hron and colleagues showed that patients with advanced colorectal cancer had a 2-fold increase in circulating TF+ MPs compared to healthy controls.38 Zwicker and colleagues found an association between TF+ MPs and cancer-associated VTE.108 Another study reported that cancer patients with VTE had higher levels of TF+ MPs compared to cancer patients without VTE, both of which were elevated over healthy controls.109 Finally, a patient with severe Trousseau's syndrome had extremely high levels of TF antigen in the plasma associated primarily with MPs.110 Together these results indicate that increased levels of MP TF antigen can be predictive of VTE in cancer patients.
Table 1. Circulating TF in Clinical Studies.
| Disease State | Specific Conditon | TF+ Flow Cytometry | MP TF Activity | Major Finding of Study | Reference |
|---|---|---|---|---|---|
| Cancer | Colorectal | Yes | No | Increased TF+ MPs in cancer versus healthy controls, correlated with D-dimer | 38 |
| Cancer | Pancreatic and Breast | Yes | Yes | Increased TF+MPs and increase in MP TF activity in cancer vs controls Increased MP TF activity associated with decreased survival |
15 |
| Cancer | Multiple Forms | No | Yes | Incresed MP TF activity in metastatic cancer patients vs healthy controls | 136 |
| Cancer | Pancreatic | No | Yes | Increasing MP TF activity and TF antigen predictive of VTE | 14 |
| Cancer | Prostate | Yes | Yes** | MP TF activity is increased in cancer vs controls and correlated with D-dimer | 112 |
| Cancer | Multiple Myeloma | No | Yes | MP TF activity is increased in cancer vs controls and reduced with chemotherapy | 35 |
| Cancer | Multiple Forms | No | Yes | Cancer patients with VTE had higher MP TF activity vs controls. Increased MP-TF activity resulted in decreased survival vs patients with low MP TF activity | 111 |
| Cancer | Multiple Forms | Yes* | No | Increased TF+ MPs in cancer patients vs controls and are predictive of VTE | 108 |
| Cancer | Pancreatobiliary | No | Yes | Increased MP TF activity in cancer patients and correlated to VTE | 114 |
| Cancer | Multiple Forms | No | Yes | Increased MP TF activity in patients with cancer and VTE versus cancer w/o VTE | 113 |
| Cancer | Multiple Forms | Yes | No | Increased TF+ MPs in cancer patients w/ and w/o VTE versus healthy controls. | 109 |
| ACS | MI | Yes | Yes*** | Decreased TFPI+ MPs and increased MP TF activity after thrombolysis | 132 |
| ACS | MI | No | Yes | MP TF activity increased in patients with persistent occlusion vs controls | 133 |
| ACS | MI | No | Yes | MP TF activity was increase in failed vs successful thrombolysis in patients | 134 |
| ACS | MI | No | Yes*** | MP TF activity increased in lesion blood versus post angioplasty | 131 |
| Diabetes | Type II Diabetes | Yes | No | 2× increased TF+ MPs in patients with type II diabetes versus healthy controls | 37 |
| Diabetes | Type II Diabetes | Yes | No | 3× increased TF+ MPs in patients with type II diabetes versus healthy controls | 135 |
| Sepsis | Meningococcal | Yes | No | Increased TF+ MPs in patients with meningococcal sepsis | 45 |
| Endotoxemia | LPS administration | Yes | Yes | Increased TF+ MPs and MP TF activity in healthy volunteers given endotoxin | 13 |
| Sickle Cell | Sickle Cell Disease | Yes | Yes** | Increased TF+ MPs and MP TF activity in sickle cell patients versus controls | 46 |
Abbreviations: ACS (acute coronary syndrome), MI (myocardial infarction), TF (tissue factor), MP (microparticles), VTE (venous thromboembolism), TFPI (tissue factor pathway inhibitor).
Microparticles assesses by flow impedence.
MP TF activity assessed by 1-stage clotting reaction with inclusion of anti-TF antibody.
Assay is considered controversial and may not be indicative of specific MP TF activity.
Similar to the TF antigen studies, several groups have demonstrated an increase in MP TF activity in cancer patients with VTE in retrospective studies (Table). For example, Tesselaar and colleagues found increased levels of MP TF activity in patients with disseminated breast and pancreatic cancer compared with healthy controls.15 In a subsequent study, they found higher levels of MP TF activity in unselected cancer patients presenting with acute VTE compared with patients without VTE.111 Surprisingly, the majority of cancer patients presenting with acute VTE during chemotherapy had low levels of MP TF activity, suggesting that chemotherapy did not increase circulating MP TF activity. However, it may be necessary to collect multiple blood samples after chemotherapy to detect a transient increase in MP TF activity. Another study reported that patients with early stage prostate cancer had significantly higher levels of MP TF activity compared with healthy controls.112 Recently, we observed that cancer patients with VTE had significantly higher MP TF activity than cancer patients without VTE.113 Similarly, we demonstrated that MP TF activity was elevated in patients with pancreatic or biliary cancers and significantly correlated with VTE.114
We analyzed MP TF activity and plasma TF antigen levels prospectively in 11 advanced or metastatic pancreatic cancer patients that had repeat blood draws over a 20 week period.14 Nine of 11 patients had no significant change in MP TF activity over time but had elevated levels of MP TF activity compared with controls. However, 1 patient started with a low level of MP TF activity level that increased in each of the subsequent blood draws and was at the highest level prior to a VTE event. Another patient started with an elevated level of MP TF activity and increased over time before a VTE. We also observed similar changes in TF antigen levels in the plasma in the two patients using an in-house ELISA assay. Similarly, Zwicker and colleagues found a 7-fold increased risk of thrombosis in VTE-free cancer patients with elevated levels of TF+ MPs versus cancer patients negative for TF+ MPs in a 2 year follow up.108 Interestingly, we found that cancer patient with elevated levels of MP TF activity but not PS+ MPs had a higher risk of VTE (van Doormaal et al. unpublished data). These data suggest that elevated levels of MP TF activity may be predictive of VTE.
Similar to the mouse studies, the major source of circulating TF+ MPs in cancer patients is the tumor. Zwicker and colleagues found a decrease in circulating TF+ MPs shortly after cancer resection.108 Similarly, Haubold and colleagues observed a decrease in MP TF activity after prostatectomy.112 Finally, levels of MP activity measured using the Zymuphen assay were decreased post resection in cancer patients with glioblastoma.115
Several studies have demonstrated an association between levels of TF+ MPs and mortality. Tesselaar and colleagues reported that high levels of MP TF activity were associated with a decrease in overall survival in patients with disseminated breast and pancreatic cancer.15 In addition, cancer patients with VTE presenting with the highest MP TF activity had a lower survival versus patients with low MP TF activity.111 Finally, we found a median survival time of only 98.5 days in a high MP TF activity group compared to 231 days for a low MP TF activity group in a cohort of 117 pancreatic and biliary cancer patients.114
Taken together, these studies strongly suggest that TF+ MPs and MP TF activity may have prognostic value in identifying cancer patients with increased of VTE. It should be noted that pancreatic cancer patients have higher levels of circulating TF+ MPs than patients with other types of cancer, suggesting that TF+ MPs may not be a useful biomarker for thrombosis risk in all types of cancer. Indeed, cancer type was one factor that was used in a risk assessment score to predict thrombosis in cancer patients.116 Interestingly, a new clinical trial (Microparticle Thromboprophylaxis with Enoxaparin in Cancer or MicroTEC; http://clinicaltrials.gov) will evaluate the benefits of prophylaxis with low molecular weight heparin in cancer patients with high levels of TF+ MPs in the prevention of cancer-induced thrombosis.117
10. Analysis of TF+ MPs in hyperlipidemic mice and in patients with cardiovascular disease
Plaque disruption and subsequent arterial thrombosis is a major complication of atherosclerosis (termed atherothrombosis). This results in acute vascular syndromes, such as myocardial infarction and stroke.118 Large amounts of TF are present in atherosclerotic plaques.119 Further, TF expression increases with the progression of atherosclerotic plaques and higher TF activity is observed in plaques with thrombi.120 Importantly, much of this TF is speculated to be in the form of TF+ MPs.2 The following sections will review studies on TF+ MPs in animal models of arterial thrombosis and in human atherothrombosis.
a. Role of hematopoietic cell-derived TF+ MPs in the activation of coagulation in hyperlipidemic mice
Hyperlipidemia results in the formation of oxidized low density lipoproteins (oxLDLs). Indeed, patients with high levels of oxLDL autoantibodies have elevated levels of TF+ MPs derived from monocytes.121 We found that oxLDL induces TF expression in monocytic cells and the release of TF+ MPs. (Owens and Mackman, unpublished data). Furthermore, hyperlipidemic mice have elevated levels of MP TF activity and TAT.122 Hyperlipidemic mice containing bone marrow from low TF mice had decreased levels of MP TF activity, indicating that hematopoietic cells were the source of the TF+ MPs. These results suggest that a TF+ MPs may enhance arterial thrombosis, for instance after rupture of an atherosclerotic plaque. Indeed, previous studies have shown that hyperlipidemic mice had shorter occlusion times in the carotid artery model when compared with control mice.81, 123-124 However, it is difficult to determine the contribution of elevated levels of TF+ MPs in models of arterial thrombosis due to the large amounts of TF in the vessel wall. Interestingly, we have found that hyperlipidemic mice have increased levels of fibrin accumulation compared to controls in a laser injury model of cremaster arterioles (Owens et al, unpublished data). Further experiments are required to show that this increase in fibrin is dependent on the elevated levels of TF+ MPs in the hyperlipidemic mice.
b. Clinical studies
Leroyer and colleagues demonstrated that atherosclerotic plaques had 200-fold higher concentrations of leukocyte, erythrocyte, SMC, and endothelial cell-derived MPs compared with the blood of the patients, which mainly consisted of platelet MPs.125 Importantly, more than 50% of the MPs isolated from the plaques were TF+ MPs. Another study found that 97% of the total MP procoagulant activity extracted from atherosclerotic plaques was due to TF.126 Moreover, the MPs isolated from the plaques were highly thrombogenic compared to the MPs isolated from the blood of the same patients. In a subsequent proteomics analysis of atherosclerotic plaque MPs, Mayr and colleagues demonstrated 90% of the plaque-derived MPs were CD14+ indicating monocyte/macrophage origin.127 Finally, Bonderman and colleagues demonstrated increased TF activity associated with MPs when analyzing the scrapings from ex vivo endarterectomy samples.128 It is speculated that during plaque rupture, these TF+ MPs initiate thrombosis.
Soejima and colleagues were the first to describe increased levels of plasma TF antigen patients with unstable angina.129 Further, Mallat and colleagues found an increase in circulating procoagulant MPs in patients presenting with acute coronary syndrome (unstable angina and myocardial infarction) versus those with stable angina and non-coronary patients (Table).130 Moreover, Morel and associates demonstrated an increase in procoagulant monocyte and endothelial cell-derived MPs in patients undergoing angioplasty.131 Steppich and colleagues examined patients with acute myocardial infarction (AMI) randomized to either intravenous thrombolysis or coronary stenting.132 The numbers of TF+ MPs were similar in both groups of patients, although the thrombolysis group had a decrease in the number of TF+ MPs that co-stained for TFPI. Huisse and colleagues prospectively enrolled 123 patients with AMI and demonstrated that MP TF activity was increased in the patients with persistent occlusion versus healthy controls.133 In a follow-up study, they found that patients who failed to achieve thrombolysis had significantly elevated MP TF activity.134 These results suggest the failure to resolve thrombi may be due to resupply of TF+ MPs from the circulation to the thrombus. Morel and colleagues demonstrated an increase in MP TF activity in blood collected at the site of thrombus and at the site of thrombus post angioplasty, both of which were significantly increase over levels collected in the femoral blood.131
These results indicate that levels of TF+ MPs, most likely derived from activated monocytes and macrophages, are elevated in patients with cardiovascular disease. However, it is unclear if levels of TF+ MPs will have a value in predicting future thrombotic events because the major source of TF in these cases is the plaque itself. Nevertheless, further studies are needed to determine if acute coronary syndrome patients with elevated levels of TF+ MPs have a worse prognosis.
11. Other diseases with elevated levels of TF+ MPs
Elevated levels of circulating TF+ MPs are observed in a variety of disease states, including sepsis, diabetes, and sickle cell disease (Table).34, 37, 46, 131, 135-136 It is speculated that increased levels of TF+ MPs are likely to contribute to thrombosis. As an example, Shet and colleagues found an increase in monocyte and endothelial cell-derived TF+ MPs in sickle cell patients compared with healthy controls. These MPs had functional TF activity that was associated with enhanced activation of coagulation in sickle cell patients with steady state disease or undergoing crisis. One study found that patients presenting with acute VTE in the absence of malignant cancer did not have elevated levels of TF+ MPs, whereas another study demonstrated patients with unprovoked VTE had higher TF+ MPs that control patients.109, 117 Cumulatively, these data demonstrate an increase in TF+ MPs and MP TF activity in a variety of disease states. It is also suggestive that TF+ MPs may serve as prognostic indicators for the risk of thrombotic events and potentially survival. Further prospective trials are needed to demonstrate that TF+ MPs are a valuable biomarker in diseases associated with thrombosis.
12. Conclusion
There are numerous reports analyzing levels of PS+ and TF+ MPs in health and disease. However, methods of MP analysis are not optimal and this has led to a lot of variation between studies. Development of machines that can more accurately quantify levels of PS+ and TF+ MPs in plasma and standardization of functional clotting assays will help to advance the field. At present, it is unclear if PS+ MPs or TF+ MPs play a role in hemostasis. In contrast, several studies have shown an association between tumor-derived TF+ MPs and VTE in cancer patients, suggesting that these TF+ MP trigger venous thrombosis. Therefore, TF+ MPs may be a useful biomarker to identify cancer patients, and possibly other patients, that have an increased risk of venous thrombosis. The elevated levels of monocyte-derived TF+ MPs observed in hyperlipidemia patients may also contribute to arterial thrombosis after rupture of atherosclerotic plaques. Further studies are needed to examine if and how different forms of MPs play a role in hemostasis and thrombosis.
Acknowledgments
Sources of funding: Nigel Mackman is currently supported by a National Institutes of Health grant (R01-HL095096). A. Phillip Owens III was supported by an American Heart Association Mid-Atlantic Postdoctoral Fellowship (09POST2250515) and currently by an NIH F32 NRSA Postdoctoral Fellowship (1F32-HL099175).
Non-standard Abbreviations and Acronyms
- AMI
acute myocardial infarction
- FVII
factor VII
- FX
factor X
- GLA
γ-carboxyglutamic acid
- HIT
heparin-induced thrombocytopenia
- IVC
inferior vena cava
- LPS
lipopolysaccharide
- MP
microparticle
- oxLDL
oxidized low density lipoprotein
- PS
phosphatidylserine
- PSGL-1
P-selectin glycoprotein ligand 1
- PT
prothrombin
- SMC
smooth muscle cells
- TAT
thrombin-antithrombin
- TF
tissue factor
- TFPI
tissue factor pathway inhibitor
- VTE
venous thromboembolism
Footnotes
Disclosures: None.
References
- 1.Burnier L, Fontana P, Kwak BR, Angelillo-Scherrer A. Cell-derived microparticles in haemostasis and vascular medicine. Thromb Haemost. 2009;101:439–451. [PubMed] [Google Scholar]
- 2.Morel O, Toti F, Hugel B, Bakouboula B, Camoin-Jau L, Dignat-George F, Freyssinet JM. Procoagulant microparticles: Disrupting the vascular homeostasis equation? Arterioscler Thromb Vasc Biol. 2006;26:2594–2604. doi: 10.1161/01.ATV.0000246775.14471.26. [DOI] [PubMed] [Google Scholar]
- 3.Nieuwland R, Sturk A. Why do cells release vesicles? Thromb Res. 2010;125 1:S49–51. doi: 10.1016/j.thromres.2010.01.037. [DOI] [PubMed] [Google Scholar]
- 4.Mause SF, Weber C. Microparticles: Protagonists of a novel communication network for intercellular information exchange. Circ Res. 2010;107:1047–1057. doi: 10.1161/CIRCRESAHA.110.226456. [DOI] [PubMed] [Google Scholar]
- 5.Chargaff E, West R. The biological significance of the thromboplastic protein of blood. J Biol Chem. 1946;166:189–197. [PubMed] [Google Scholar]
- 6.Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13:269–288. doi: 10.1111/j.1365-2141.1967.tb08741.x. [DOI] [PubMed] [Google Scholar]
- 7.Mackman N, Tilley RE, Key NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:1687–1693. doi: 10.1161/ATVBAHA.107.141911. [DOI] [PubMed] [Google Scholar]
- 8.Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. Am J Pathol. 1989;134:1087–1097. [PMC free article] [PubMed] [Google Scholar]
- 9.Giesen PL, Rauch U, Bohrmann B, Kling D, Roque M, Fallon JT, Badimon JJ, Himber J, Riederer MA, Nemerson Y. Blood-borne tissue factor: Another view of thrombosis. Proc Natl Acad Sci U S A. 1999;96:2311–2315. doi: 10.1073/pnas.96.5.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butenas S, Bouchard BA, Brummel-Ziedins KE, Parhami-Seren B, Mann KG. Tissue factor activity in whole blood. Blood. 2005;105:2764–2770. doi: 10.1182/blood-2004-09-3567. [DOI] [PubMed] [Google Scholar]
- 11.Osterud B, Breimo ES, Olsen JO. Blood borne tissue factor revisited. Thromb Res. 2008;122:432–434. doi: 10.1016/j.thromres.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Berckmans RJ, Nieuwland R, Boing AN, Romijn FP, Hack CE, Sturk A. Cell-derived microparticles circulate in healthy humans and support low grade thrombin generation. Thromb Haemost. 2001;85:639–646. [PubMed] [Google Scholar]
- 13.Aras O, Shet A, Bach RR, Hysjulien JL, Slungaard A, Hebbel RP, Escolar G, Jilma B, Key NS. Induction of microparticle- and cell-associated intravascular tissue factor in human endotoxemia. Blood. 2004;103:4545–4553. doi: 10.1182/blood-2003-03-0713. [DOI] [PubMed] [Google Scholar]
- 14.Khorana AA, Francis CW, Menzies KE, Wang JG, Hyrien O, Hathcock J, Mackman N, Taubman MB. Plasma tissue factor may be predictive of venous thromboembolism in pancreatic cancer. J Thromb Haemost. 2008;6:1983–1985. doi: 10.1111/j.1538-7836.2008.03156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tesselaar ME, Romijn FP, Van Der Linden IK, Prins FA, Bertina RM, Osanto S. Microparticle-associated tissue factor activity: A link between cancer and thrombosis? J Thromb Haemost. 2007;5:520–527. doi: 10.1111/j.1538-7836.2007.02369.x. [DOI] [PubMed] [Google Scholar]
- 16.Johnson GJ, Leis LA, Bach RR. Tissue factor activity of blood mononuclear cells is increased after total knee arthroplasty. Thromb Haemost. 2009;102:728–734. doi: 10.1160/TH09-04-0261. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman M, Whinna HC, Monroe DM. Circulating tissue factor accumulates in thrombi, but not in hemostatic plugs. J Thromb Haemost. 2006;4:2092–2093. doi: 10.1111/j.1538-7836.2006.02085.x. [DOI] [PubMed] [Google Scholar]
- 18.Falati S, Liu Q, Gross P, Merrill-Skoloff G, Chou J, Vandendries E, Celi A, Croce K, Furie BC, Furie B. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle p-selectin glycoprotein ligand 1 and platelet p-selectin. J Exp Med. 2003;197:1585–1598. doi: 10.1084/jem.20021868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morel O, Jesel L, Freyssinet JM, Toti F. Cellular mechanisms underlying the formation of circulating microparticles. Arterioscler Thromb Vasc Biol. 2011;31:15–26. doi: 10.1161/ATVBAHA.109.200956. [DOI] [PubMed] [Google Scholar]
- 20.Perez-Pujol S, Marker PH, Key NS. Platelet microparticles are heterogeneous and highly dependent on the activation mechanism: Studies using a new digital flow cytometer. Cytometry A. 2007;71:38–45. doi: 10.1002/cyto.a.20354. [DOI] [PubMed] [Google Scholar]
- 21.Broze GJ., Jr Tissue factor pathway inhibitor. Thromb Haemost. 1995;74:90–93. [PubMed] [Google Scholar]
- 22.Butenas S, Orfeo T, Mann KG. Tissue factor in coagulation: Which? Where? When? Arterioscler Thromb Vasc Biol. 2009;29:1989–1996. doi: 10.1161/ATVBAHA.108.177402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Egorina EM, Sovershaev MA, Osterud B. Regulation of tissue factor procoagulant activity by post-translational modifications. Thromb Res. 2008;122:831–837. doi: 10.1016/j.thromres.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Krudysz-Amblo J, Jennings ME, 2nd, Mann KG, Butenas S. Carbohydrates and activity of natural and recombinant tissue factor. J Biol Chem. 2010;285:3371–3382. doi: 10.1074/jbc.M109.055178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bach RR. Tissue factor encryption. Arterioscler Thromb Vasc Biol. 2006;26:456–461. doi: 10.1161/01.ATV.0000202656.53964.04. [DOI] [PubMed] [Google Scholar]
- 26.Bach RR, Moldow CF. Mechanism of tissue factor activation on hl-60 cells. Blood. 1997;89:3270–3276. [PubMed] [Google Scholar]
- 27.Chen VM, Ahamed J, Versteeg HH, Berndt MC, Ruf W, Hogg PJ. Evidence for activation of tissue factor by an allosteric disulfide bond. Biochemistry. 2006;45:12020–12028. doi: 10.1021/bi061271a. [DOI] [PubMed] [Google Scholar]
- 28.Lacroix R, Robert S, Poncelet P, Dignat-George F. Overcoming limitations of microparticle measurement by flow cytometry. Semin Thromb Hemost. 2010;36:807–818. doi: 10.1055/s-0030-1267034. [DOI] [PubMed] [Google Scholar]
- 29.Williams JC, Mackman N. Mps or ics? Blood. 2011;117:1101–1102. doi: 10.1182/blood-2010-11-318691. [DOI] [PubMed] [Google Scholar]
- 30.Key NS, Mackman N. Tissue factor and its measurement in whole blood, plasma, and microparticles. Semin Thromb Hemost. 2010;36:865–875. doi: 10.1055/s-0030-1267040. [DOI] [PubMed] [Google Scholar]
- 31.Zwicker JI, Trenor CC, 3rd, Furie BC, Furie B. Tissue factor-bearing microparticles and thrombus formation. Arterioscler Thromb Vasc Biol. 2011 doi: 10.1161/ATVBAHA.109.200964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aupeix K, Hugel B, Martin T, Bischoff P, Lill H, Pasquali JL, Freyssinet JM. The significance of shed membrane particles during programmed cell death in vitro, and in vivo, in hiv-1 infection. J Clin Invest. 1997;99:1546–1554. doi: 10.1172/JCI119317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Dreden P, Rousseau A, Savoure A, Lenormand B, Fontaine S, Vasse M. Plasma thrombomodulin activity, tissue factor activity and high levels of circulating procoagulant phospholipid as prognostic factors for acute myocardial infarction. Blood Coagul Fibrinolysis. 2009;20:635–641. doi: 10.1097/MBC.0b013e32832e05dd. [DOI] [PubMed] [Google Scholar]
- 34.Bakouboula B, Morel O, Faure A, Zobairi F, Jesel L, Trinh A, Zupan M, Canuet M, Grunebaum L, Brunette A, Desprez D, Chabot F, Weitzenblum E, Freyssinet JM, Chaouat A, Toti F. Procoagulant membrane microparticles correlate with the severity of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;177:536–543. doi: 10.1164/rccm.200706-840OC. [DOI] [PubMed] [Google Scholar]
- 35.Auwerda JJ, Yuana Y, Osanto S, de Maat MP, Sonneveld P, Bertina RM, Leebeek FW. Microparticle-associated tissue factor activity and venous thrombosis in multiple myeloma. Thromb Haemost. 2011;105:14–20. doi: 10.1160/TH10-03-0187. [DOI] [PubMed] [Google Scholar]
- 36.Horstman LL, Ahn YS. Platelet microparticles: A wide-angle perspective. Crit Rev Oncol Hematol. 1999;30:111–142. doi: 10.1016/s1040-8428(98)00044-4. [DOI] [PubMed] [Google Scholar]
- 37.Diamant M, Nieuwland R, Pablo RF, Sturk A, Smit JW, Radder JK. Elevated numbers of tissue-factor exposing microparticles correlate with components of the metabolic syndrome in uncomplicated type 2 diabetes mellitus. Circulation. 2002;106:2442–2447. doi: 10.1161/01.cir.0000036596.59665.c6. [DOI] [PubMed] [Google Scholar]
- 38.Hron G, Kollars M, Weber H, Sagaster V, Quehenberger P, Eichinger S, Kyrle PA, Weltermann A. Tissue factor-positive microparticles: Cellular origin and association with coagulation activation in patients with colorectal cancer. Thromb Haemost. 2007;97:119–123. [PubMed] [Google Scholar]
- 39.Flaumenhaft R, Mairuhu AT, Italiano JE. Platelet- and megakaryocyte-derived microparticles. Semin Thromb Hemost. 2010;36:881–887. doi: 10.1055/s-0030-1267042. [DOI] [PubMed] [Google Scholar]
- 40.Egorina EM, Sovershaev MA, Bjorkoy G, Gruber FX, Olsen JO, Parhami-Seren B, Mann KG, Osterud B. Intracellular and surface distribution of monocyte tissue factor: Application to intersubject variability. Arterioscler Thromb Vasc Biol. 2005;25:1493–1498. doi: 10.1161/01.ATV.0000168413.29874.d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Satta N, Toti F, Feugeas O, Bohbot A, Dachary-Prigent J, Eschwege V, Hedman H, Freyssinet JM. Monocyte vesiculation is a possible mechanism for dissemination of membrane-associated procoagulant activities and adhesion molecules after stimulation by lipopolysaccharide. J Immunol. 1994;153:3245–3255. [PubMed] [Google Scholar]
- 42.Rauch U, Bonderman D, Bohrmann B, Badimon JJ, Himber J, Riederer MA, Nemerson Y. Transfer of tissue factor from leukocytes to platelets is mediated by cd15 and tissue factor. Blood. 2000;96:170–175. [PubMed] [Google Scholar]
- 43.Del Conde I, Shrimpton CN, Thiagarajan P, Lopez JA. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106:1604–1611. doi: 10.1182/blood-2004-03-1095. [DOI] [PubMed] [Google Scholar]
- 44.Bajaj MS, Ghosh M, Bajaj SP. Fibronectin-adherent monocytes express tissue factor and tissue factor pathway inhibitor whereas endotoxin-stimulated monocytes primarily express tissue factor: Physiologic and pathologic implications. J Thromb Haemost. 2007;5:1493–1499. doi: 10.1111/j.1538-7836.2007.02604.x. [DOI] [PubMed] [Google Scholar]
- 45.Nieuwland R, Berckmans RJ, McGregor S, Boing AN, Romijn FP, Westendorp RG, Hack CE, Sturk A. Cellular origin and procoagulant properties of microparticles in meningococcal sepsis. Blood. 2000;95:930–935. [PubMed] [Google Scholar]
- 46.Shet AS, Aras O, Gupta K, Hass MJ, Rausch DJ, Saba N, Koopmeiners L, Key NS, Hebbel RP. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood. 2003;102:2678–2683. doi: 10.1182/blood-2003-03-0693. [DOI] [PubMed] [Google Scholar]
- 47.Kambas K, Markiewski MM, Pneumatikos IA, Rafail SS, Theodorou V, Konstantonis D, Kourtzelis I, Doumas MN, Magotti P, Deangelis RA, Lambris JD, Ritis KD. C5a and tnf-alpha up-regulate the expression of tissue factor in intra-alveolar neutrophils of patients with the acute respiratory distress syndrome. J Immunol. 2008;180:7368–7375. doi: 10.4049/jimmunol.180.11.7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ritis K, Doumas M, Mastellos D, Micheli A, Giaglis S, Magotti P, Rafail S, Kartalis G, Sideras P, Lambris JD. A novel c5a receptor-tissue factor cross-talk in neutrophils links innate immunity to coagulation pathways. J Immunol. 2006;177:4794–4802. doi: 10.4049/jimmunol.177.7.4794. [DOI] [PubMed] [Google Scholar]
- 49.Redecha P, Tilley R, Tencati M, Salmon JE, Kirchhofer D, Mackman N, Girardi G. Tissue factor: A link between c5a and neutrophil activation in antiphospholipid antibody induced fetal injury. Blood. 2007;110:2423–2431. doi: 10.1182/blood-2007-01-070631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Egorina EM, Sovershaev MA, Olsen JO, Osterud B. Granulocytes do not express but acquire monocyte-derived tissue factor in whole blood: Evidence for a direct transfer. Blood. 2008;111:1208–1216. doi: 10.1182/blood-2007-08-107698. [DOI] [PubMed] [Google Scholar]
- 51.Parry GC, Mackman N. Transcriptional regulation of tissue factor expression in human endothelial cells. Arterioscler Thromb Vasc Biol. 1995;15:612–621. doi: 10.1161/01.atv.15.5.612. [DOI] [PubMed] [Google Scholar]
- 52.Drake TA, Cheng J, Chang A, Taylor FB., Jr Expression of tissue factor, thrombomodulin, and e-selectin in baboons with lethal escherichia coli sepsis. Am J Pathol. 1993;142:1458–1470. [PMC free article] [PubMed] [Google Scholar]
- 53.Lupu C, Westmuckett AD, Peer G, Ivanciu L, Zhu H, Taylor FB, Jr, Lupu F. Tissue factor-dependent coagulation is preferentially up-regulated within arterial branching areas in a baboon model of escherichia coli sepsis. Am J Pathol. 2005;167:1161–1172. doi: 10.1016/S0002-9440(10)61204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pawlinski R, Wang JG, Owens AP, 3rd, Williams J, Antoniak S, Tencati M, Luther T, Rowley JW, Low EN, Weyrich AS, Mackman N. Hematopoietic and nonhematopoietic cell tissue factor activates the coagulation cascade in endotoxemic mice. Blood. 2010;116:806–814. doi: 10.1182/blood-2009-12-259267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Solovey A, Kollander R, Shet A, Milbauer LC, Choong S, Panoskaltsis-Mortari A, Blazar BR, Kelm RJ, Jr, Hebbel RP. Endothelial cell expression of tissue factor in sickle mice is augmented by hypoxia/reoxygenation and inhibited by lovastatin. Blood. 2004;104:840–846. doi: 10.1182/blood-2003-10-3719. [DOI] [PubMed] [Google Scholar]
- 56.Kushak RI, Nestoridi E, Lambert J, Selig MK, Ingelfinger JR, Grabowski EF. Detached endothelial cells and microparticles as sources of tissue factor activity. Thromb Res. 2005;116:409–419. doi: 10.1016/j.thromres.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 57.Bajaj MS, Bajaj SP. Tissue factor pathway inhibitor: Potential therapeutic applications. Thromb Haemost. 1997;78:471–477. [PubMed] [Google Scholar]
- 58.Camera M, Brambilla M, Toschi V, Tremoli E. Tissue factor expression on platelets is a dynamic event. Blood. 2010;116:5076–5077. doi: 10.1182/blood-2010-09-307306. [DOI] [PubMed] [Google Scholar]
- 59.Panes O, Matus V, Saez CG, Quiroga T, Pereira J, Mezzano D. Human platelets synthesize and express functional tissue factor. Blood. 2007;109:5242–5250. doi: 10.1182/blood-2006-06-030619. [DOI] [PubMed] [Google Scholar]
- 60.Schwertz H, Tolley ND, Foulks JM, Denis MM, Risenmay BW, Buerke M, Tilley RE, Rondina MT, Harris EM, Kraiss LW, Mackman N, Zimmerman GA, Weyrich AS. Signal-dependent splicing of tissue factor pre-mrna modulates the thrombogenicity of human platelets. J Exp Med. 2006;203:2433–2440. doi: 10.1084/jem.20061302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zillmann A, Luther T, Muller I, Kotzsch M, Spannagl M, Kauke T, Oelschlagel U, Zahler S, Engelmann B. Platelet-associated tissue factor contributes to the collagen-triggered activation of blood coagulation. Biochem Biophys Res Commun. 2001;281:603–609. doi: 10.1006/bbrc.2001.4399. [DOI] [PubMed] [Google Scholar]
- 62.Bouchard BA, Mann KG, Butenas S. No evidence for tissue factor on platelets. Blood. 2010;116:854–855. doi: 10.1182/blood-2010-05-285627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Osterud B. Tissue factor expression in blood cells. Thromb Res. 2010;125 1:S31–34. doi: 10.1016/j.thromres.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 64.Bom VJ, Bertina RM. The contributions of ca2+, phospholipids and tissue-factor apoprotein to the activation of human blood-coagulation factor x by activated factor vii. Biochem J. 1990;265:327–336. doi: 10.1042/bj2650327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dasgupta SK, Abdel-Monem H, Niravath P, Le A, Bellera RV, Langlois K, Nagata S, Rumbaut RE, Thiagarajan P. Lactadherin and clearance of platelet-derived microvesicles. Blood. 2009;113:1332–1339. doi: 10.1182/blood-2008-07-167148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ait-Oufella H, Kinugawa K, Zoll J, Simon T, Boddaert J, Heeneman S, Blanc-Brude O, Barateau V, Potteaux S, Merval R, Esposito B, Teissier E, Daemen MJ, Leseche G, Boulanger C, Tedgui A, Mallat Z. Lactadherin deficiency leads to apoptotic cell accumulation and accelerated atherosclerosis in mice. Circulation. 2007;115:2168–2177. doi: 10.1161/CIRCULATIONAHA.106.662080. [DOI] [PubMed] [Google Scholar]
- 67.Davila M, Amirkhosravi A, Coll E, Desai H, Robles L, Colon J, Baker CH, Francis JL. Tissue factor-bearing microparticles derived from tumor cells: Impact on coagulation activation. J Thromb Haemost. 2008;6:1517–1524. doi: 10.1111/j.1538-7836.2008.02987.x. [DOI] [PubMed] [Google Scholar]
- 68.Pasquet JM, Toti F, Nurden AT, Dachary-Prigent J. Procoagulant activity and active calpain in platelet-derived microparticles. Thromb Res. 1996;82:509–522. doi: 10.1016/0049-3848(96)00101-6. [DOI] [PubMed] [Google Scholar]
- 69.Castaman G, Yu-Feng L, Rodeghiero F. A bleeding disorder characterised by isolated deficiency of platelet microvesicle generation. Lancet. 1996;347:700–701. doi: 10.1016/s0140-6736(96)91259-3. [DOI] [PubMed] [Google Scholar]
- 70.Weiss HJ. Scott syndrome: A disorder of platelet coagulant activity. Semin Hematol. 1994;31:312–319. [PubMed] [Google Scholar]
- 71.Mackman N. Tissue-specific hemostasis in mice. Arterioscler Thromb Vasc Biol. 2005;25:2273–2281. doi: 10.1161/01.ATV.0000183884.06371.52. [DOI] [PubMed] [Google Scholar]
- 72.Mann KG, Orfeo T, Butenas S, Undas A, Brummel-Ziedins K. Blood coagulation dynamics in haemostasis. Hamostaseologie. 2009;29:7–16. [PMC free article] [PubMed] [Google Scholar]
- 73.Mackman N. The role of tissue factor and factor viia in hemostasis. Anesth Analg. 2009;108:1447–1452. doi: 10.1213/ane.0b013e31819bceb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hathcock JJ, Nemerson Y. Platelet deposition inhibits tissue factor activity: In vitro clots are impermeable to factor xa. Blood. 2004;104:123–127. doi: 10.1182/blood-2003-12-4352. [DOI] [PubMed] [Google Scholar]
- 75.Orfeo T, Butenas S, Brummel-Ziedins KE, Mann KG. The tissue factor requirement in blood coagulation. J Biol Chem. 2005;280:42887–42896. doi: 10.1074/jbc.M505506200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ollivier V, Wang J, Manly D, Machlus KR, Wolberg AS, Jandrot-Perrus M, Mackman N. Detection of endogenous tissue factor levels in plasma using the calibrated automated thrombogram assay. Thromb Res. 2010;125:90–96. doi: 10.1016/j.thromres.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Balasubramanian V, Grabowski E, Bini A, Nemerson Y. Platelets, circulating tissue factor, and fibrin colocalize in ex vivo thrombi: Real-time fluorescence images of thrombus formation and propagation under defined flow conditions. Blood. 2002;100:2787–2792. doi: 10.1182/blood-2002-03-0902. [DOI] [PubMed] [Google Scholar]
- 78.Chou J, Mackman N, Merrill-Skoloff G, Pedersen B, Furie BC, Furie B. Hematopoietic cell-derived microparticle tissue factor contributes to fibrin formation during thrombus propagation. Blood. 2004;104:3190–3197. doi: 10.1182/blood-2004-03-0935. [DOI] [PubMed] [Google Scholar]
- 79.Gross PL, Furie BC, Merrill-Skoloff G, Chou J, Furie B. Leukocyte-versus microparticle-mediated tissue factor transfer during arteriolar thrombus development. J Leukoc Biol. 2005;78:1318–1326. doi: 10.1189/jlb.0405193. [DOI] [PubMed] [Google Scholar]
- 80.Hrachovinova I, Cambien B, Hafezi-Moghadam A, Kappelmayer J, Camphausen RT, Widom A, Xia L, Kazazian HH, Jr, Schaub RG, McEver RP, Wagner DD. Interaction of p-selectin and psgl-1 generates microparticles that correct hemostasis in a mouse model of hemophilia a. Nat Med. 2003;9:1020–1025. doi: 10.1038/nm899. [DOI] [PubMed] [Google Scholar]
- 81.Day SM, Reeve JL, Pedersen B, Farris DM, Myers DD, Im M, Wakefield TW, Mackman N, Fay WP. Macrovascular thrombosis is driven by tissue factor derived primarily from the blood vessel wall. Blood. 2005;105:192–198. doi: 10.1182/blood-2004-06-2225. [DOI] [PubMed] [Google Scholar]
- 82.Berckmans RJ, Sturk A, Schaap MC, Nieuwland R. Cell-derived vesicles exposing coagulant tissue factor in saliva. Blood. 2011 doi: 10.1182/blood-2010-06-290460. [DOI] [PubMed] [Google Scholar]
- 83.Warkentin TE, Hayward CP, Boshkov LK, Santos AV, Sheppard JA, Bode AP, Kelton JG. Sera from patients with heparin-induced thrombocytopenia generate platelet-derived microparticles with procoagulant activity: An explanation for the thrombotic complications of heparin-induced thrombocytopenia. Blood. 1994;84:3691–3699. [PubMed] [Google Scholar]
- 84.Rauova L, Hirsch JD, Greene TK, Zhai L, Hayes VM, Kowalska MA, Cines DB, Poncz M. Monocyte-bound pf4 in the pathogenesis of heparin-induced thrombocytopenia. Blood. 2010;116:5021–5031. doi: 10.1182/blood-2010-03-276964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Glover S, Key NS, Arepally GM, Mackman N, Kasthuri RS. Characterization of receptors involved in heparin antibody complex mediated induction of tissue factor expression in monocytes. ASH Meeting December 2009. 2009 Abstract 224. [Google Scholar]
- 86.Kretz CA, Vaezzadeh N, Gross PL. Tissue factor and thrombosis models. Arterioscler Thromb Vasc Biol. 2010;30:900–908. doi: 10.1161/ATVBAHA.108.177477. [DOI] [PubMed] [Google Scholar]
- 87.Wang L, Miller C, Swarthout RF, Rao M, Mackman N, Taubman MB. Vascular smooth muscle-derived tissue factor is critical for arterial thrombosis after ferric chloride-induced injury. Blood. 2009;113:705–713. doi: 10.1182/blood-2007-05-090944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reinhardt C, von Bruhl ML, Manukyan D, Grahl L, Lorenz M, Altmann B, Dlugai S, Hess S, Konrad I, Orschiedt L, Mackman N, Ruddock L, Massberg S, Engelmann B. Protein disulfide isomerase acts as an injury response signal that enhances fibrin generation via tissue factor activation. J Clin Invest. 2008;118:1110–1122. doi: 10.1172/JCI32376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rosen ED, Raymond S, Zollman A, Noria F, Sandoval-Cooper M, Shulman A, Merz JL, Castellino FJ. Laser-induced noninvasive vascular injury models in mice generate platelet- and coagulation-dependent thrombi. Am J Pathol. 2001;158:1613–1622. doi: 10.1016/S0002-9440(10)64117-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Falati S, Gross P, Merrill-Skoloff G, Furie BC, Furie B. Real-time in vivo imaging of platelets, tissue factor and fibrin during arterial thrombus formation in the mouse. Nat Med. 2002;8:1175–1181. doi: 10.1038/nm782. [DOI] [PubMed] [Google Scholar]
- 91.Himber J, Wohlgensinger C, Roux S, Damico LA, Fallon JT, Kirchhofer D, Nemerson Y, Riederer MA. Inhibition of tissue factor limits the growth of venous thrombus in the rabbit. J Thromb Haemost. 2003;1:889–895. doi: 10.1046/j.1538-7836.2003.00110.x. [DOI] [PubMed] [Google Scholar]
- 92.Day SM, Reeve JL, Myers DD, Fay WP. Murine thrombosis models. Thromb Haemost. 2004;92:486–494. [PubMed] [Google Scholar]
- 93.Ramacciotti E, Hawley AE, Farris DM, Ballard NE, Wrobleski SK, Myers DD, Jr, Henke PK, Wakefield TW. Leukocyte- and platelet-derived microparticles correlate with thrombus weight and tissue factor activity in an experimental mouse model of venous thrombosis. Thromb Haemost. 2009;101:748–754. [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou J, May L, Liao P, Gross PL, Weitz JI. Inferior vena cava ligation rapidly induces tissue factor expression and venous thrombosis in rats. Arterioscler Thromb Vasc Biol. 2009;29:863–869. doi: 10.1161/ATVBAHA.109.185678. [DOI] [PubMed] [Google Scholar]
- 95.Biro E, Sturk-Maquelin KN, Vogel GM, Meuleman DG, Smit MJ, Hack CE, Sturk A, Nieuwland R. Human cell-derived microparticles promote thrombus formation in vivo in a tissue factor-dependent manner. J Thromb Haemost. 2003;1:2561–2568. doi: 10.1046/j.1538-7836.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- 96.Myers DD, Hawley AE, Farris DM, Wrobleski SK, Thanaporn P, Schaub RG, Wagner DD, Kumar A, Wakefield TW. P-selectin and leukocyte microparticles are associated with venous thrombogenesis. J Vasc Surg. 2003;38:1075–1089. doi: 10.1016/s0741-5214(03)01033-4. [DOI] [PubMed] [Google Scholar]
- 97.Cooley BC, Szema L, Chen CY, Schwab JP, Schmeling G. A murine model of deep vein thrombosis: Characterization and validation in transgenic mice. Thromb Haemost. 2005;94:498–503. doi: 10.1160/TH05-03-0170. [DOI] [PubMed] [Google Scholar]
- 98.Diaz JA, Hawley AE, Alvarado CM, Berguer AM, Baker NK, Wrobleski SK, Wakefield TW, Lucchesi BR, Myers DD., Jr Thrombogenesis with continuous blood flow in the inferior vena cava. A novel mouse model. Thromb Haemost. 2010;104:366–375. doi: 10.1160/TH09-09-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang JG, Manly D, Kirchhofer D, Pawlinski R, Mackman N. Levels of microparticle tissue factor activity correlate with coagulation activation in endotoxemic mice. J Thromb Haemost. 2009;7:1092–1098. doi: 10.1111/j.1538-7836.2009.03448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Manly DA, Boles J, Mackman N. Role of tissue factor in venous thrombosis. Annu Rev Physiol. 2010 doi: 10.1146/annurev-physiol-042210-121137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dvorak HF, Quay SC, Orenstein NS, Dvorak AM, Hahn P, Bitzer AM, Carvalho AC. Tumor shedding and coagulation. Science. 1981;212:923–924. doi: 10.1126/science.7195067. [DOI] [PubMed] [Google Scholar]
- 102.Dvorak HF, Van DeWater L, Bitzer AM, Dvorak AM, Anderson D, Harvey VS, Bach R, Davis GL, DeWolf W, Carvalho AC. Procoagulant activity associated with plasma membrane vesicles shed by cultured tumor cells. Cancer Res. 1983;43:4434–4442. [PubMed] [Google Scholar]
- 103.Yu JL, Rak JW. Shedding of tissue factor (tf)-containing microparticles rather than alternatively spliced tf is the main source of tf activity released from human cancer cells. J Thromb Haemost. 2004;2:2065–2067. doi: 10.1111/j.1538-7836.2004.00972.x. [DOI] [PubMed] [Google Scholar]
- 104.Yu JL, May L, Lhotak V, Shahrzad S, Shirasawa S, Weitz JI, Coomber BL, Mackman N, Rak JW. Oncogenic events regulate tissue factor expression in colorectal cancer cells: Implications for tumor progression and angiogenesis. Blood. 2005;105:1734–1741. doi: 10.1182/blood-2004-05-2042. [DOI] [PubMed] [Google Scholar]
- 105.Yu J, May L, Milsom C, Anderson GM, Weitz JI, Luyendyk JP, Broze G, Mackman N, Rak J. Contribution of host-derived tissue factor to tumor neovascularization. Arterioscler Thromb Vasc Biol. 2008;28:1975–1981. doi: 10.1161/ATVBAHA.108.175083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Thomas GM, Panicot-Dubois L, Lacroix R, Dignat-George F, Lombardo D, Dubois C. Cancer cell-derived microparticles bearing p-selectin glycoprotein ligand 1 accelerate thrombus formation in vivo. J Exp Med. 2009;206:1913–1927. doi: 10.1084/jem.20082297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bogdanov VY, Cimmino G, Tardos JG, Tunstead JR, Badimon JJ. Assessment of plasma tissue factor activity in patients presenting with coronary artery disease: Limitations of a commercial assay. J Thromb Haemost. 2009;7:894–897. doi: 10.1111/j.1538-7836.2009.03315.x. [DOI] [PubMed] [Google Scholar]
- 108.Zwicker JI, Liebman HA, Neuberg D, Lacroix R, Bauer KA, Furie BC, Furie B. Tumor-derived tissue factor-bearing microparticles are associated with venous thromboembolic events in malignancy. Clin Cancer Res. 2009;15:6830–6840. doi: 10.1158/1078-0432.CCR-09-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Campello E, Spiezia L, Radu CM, Bulato C, Castelli M, Gavasso S, Simioni P. Endothelial, platelet, and tissue factor-bearing microparticles in cancer patients with and without venous thromboembolism. Thromb Res. 2011 doi: 10.1016/j.thromres.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 110.Del Conde I, Bharwani LD, Dietzen DJ, Pendurthi U, Thiagarajan P, Lopez JA. Microvesicle-associated tissue factor and trousseau's syndrome. J Thromb Haemost. 2007;5:70–74. doi: 10.1111/j.1538-7836.2006.02301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tesselaar ME, Romijn FP, van der Linden IK, Bertina RM, Osanto S. Microparticle-associated tissue factor activity in cancer patients with and without thrombosis. J Thromb Haemost. 2009;7:1421–1423. doi: 10.1111/j.1538-7836.2009.03504.x. [DOI] [PubMed] [Google Scholar]
- 112.Haubold K, Rink M, Spath B, Friedrich M, Chun FK, Marx G, Amirkhosravi A, Francis JL, Bokemeyer C, Eifrig B, Langer F. Tissue factor procoagulant activity of plasma microparticles is increased in patients with early-stage prostate cancer. Thromb Haemost. 2009;101:1147–1155. [PubMed] [Google Scholar]
- 113.Manly DA, Wang J, Glover SL, Kasthuri R, Liebman HA, Key NS, Mackman N. Increased microparticle tissue factor activity in cancer patients with venous thromboembolism. Thromb Res. 2010;125:511–512. doi: 10.1016/j.thromres.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bharthuar A, Khorana AA, Hutson A, Wang J, Mackman N, Iyer RV. Association of elevated tissue factor (tf) with survival and thromboembolism (te) in pancreaticobiliary cancers (pbc) J Clin Oncol. 2010;28(suppl) abstr 4126. [Google Scholar]
- 115.Sartori MT, Della Puppa A, Ballin A, Saggiorato G, Bernardi D, Padoan A, Scienza R, d'Avella D, Cella G. Prothrombotic state in glioblastoma multiforme: An evaluation of the procoagulant activity of circulating microparticles. J Neurooncol. 2010 doi: 10.1007/s11060-010-0462-8. [DOI] [PubMed] [Google Scholar]
- 116.Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902–4907. doi: 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zwicker JI. Predictive value of tissue factor bearing microparticles in cancer associated thrombosis. Thromb Res. 2010;125 2:S89–91. doi: 10.1016/S0049-3848(10)70022-0. [DOI] [PubMed] [Google Scholar]
- 118.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 119.Wilcox JN, Smith KM, Schwartz SM, Gordon D. Localization of tissue factor in the normal vessel wall and in the atherosclerotic plaque. Proc Natl Acad Sci U S A. 1989;86:2839–2843. doi: 10.1073/pnas.86.8.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Marmur JD, Thiruvikraman SV, Fyfe BS, Guha A, Sharma SK, Ambrose JA, Fallon JT, Nemerson Y, Taubman MB. Identification of active tissue factor in human coronary atheroma. Circulation. 1996;94:1226–1232. doi: 10.1161/01.cir.94.6.1226. [DOI] [PubMed] [Google Scholar]
- 121.Matsumoto N, Nomura S, Kamihata H, Kimura Y, Iwasaka T. Increased level of oxidized ldl-dependent monocyte-derived microparticles in acute coronary syndrome. Thromb Haemost. 2004;91:146–154. doi: 10.1160/TH03-04-0247. [DOI] [PubMed] [Google Scholar]
- 122.Owens AP, III, Temel RE, Barcel AD, Marshall SM, McDaniel AL, Mackman N. Hyperlipidemia increases microparticle tissue factor and the activation of coagulation: A tale of mice and monkeys. Circulation. 2010;122 Abstract 20151. [Google Scholar]
- 123.Eitzman DT, Westrick RJ, Xu Z, Tyson J, Ginsburg D. Hyperlipidemia promotes thrombosis after injury to atherosclerotic vessels in apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol. 2000;20:1831–1834. doi: 10.1161/01.atv.20.7.1831. [DOI] [PubMed] [Google Scholar]
- 124.Westrick RJ, Bodary PF, Xu Z, Shen YC, Broze GJ, Eitzman DT. Deficiency of tissue factor pathway inhibitor promotes atherosclerosis and thrombosis in mice. Circulation. 2001;103:3044–3046. doi: 10.1161/hc2501.092492. [DOI] [PubMed] [Google Scholar]
- 125.Leroyer AS, Isobe H, Leseche G, Castier Y, Wassef M, Mallat Z, Binder BR, Tedgui A, Boulanger CM. Cellular origins and thrombogenic activity of microparticles isolated from human atherosclerotic plaques. J Am Coll Cardiol. 2007;49:772–777. doi: 10.1016/j.jacc.2006.10.053. [DOI] [PubMed] [Google Scholar]
- 126.Mallat Z, Hugel B, Ohan J, Leseche G, Freyssinet JM, Tedgui A. Shed membrane microparticles with procoagulant potential in human atherosclerotic plaques: A role for apoptosis in plaque thrombogenicity. Circulation. 1999;99:348–353. doi: 10.1161/01.cir.99.3.348. [DOI] [PubMed] [Google Scholar]
- 127.Mayr M, Grainger D, Mayr U, Leroyer AS, Leseche G, Sidibe A, Herbin O, Yin X, Gomes A, Madhu B, Griffiths JR, Xu Q, Tedgui A, Boulanger CM. Proteomics, metabolomics, and immunomics on microparticles derived from human atherosclerotic plaques. Circ Cardiovasc Genet. 2009;2:379–388. doi: 10.1161/CIRCGENETICS.108.842849. [DOI] [PubMed] [Google Scholar]
- 128.Bonderman D, Teml A, Jakowitsch J, Adlbrecht C, Gyongyosi M, Sperker W, Lass H, Mosgoeller W, Glogar DH, Probst P, Maurer G, Nemerson Y, Lang IM. Coronary no-reflow is caused by shedding of active tissue factor from dissected atherosclerotic plaque. Blood. 2002;99:2794–2800. doi: 10.1182/blood.v99.8.2794. [DOI] [PubMed] [Google Scholar]
- 129.Soejima H, Ogawa H, Yasue H, Kaikita K, Nishiyama K, Misumi K, Takazoe K, Miyao Y, Yoshimura M, Kugiyama K, Nakamura S, Tsuji I, Kumeda K. Heightened tissue factor associated with tissue factor pathway inhibitor and prognosis in patients with unstable angina. Circulation. 1999;99:2908–2913. doi: 10.1161/01.cir.99.22.2908. [DOI] [PubMed] [Google Scholar]
- 130.Mallat Z, Benamer H, Hugel B, Benessiano J, Steg PG, Freyssinet JM, Tedgui A. Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation. 2000;101:841–843. doi: 10.1161/01.cir.101.8.841. [DOI] [PubMed] [Google Scholar]
- 131.Morel O, Pereira B, Averous G, Faure A, Jesel L, Germain P, Grunebaum L, Ohlmann P, Freyssinet JM, Bareiss P, Toti F. Increased levels of procoagulant tissue factor-bearing microparticles within the occluded coronary artery of patients with st-segment elevation myocardial infarction: Role of endothelial damage and leukocyte activation. Atherosclerosis. 2009;204:636–641. doi: 10.1016/j.atherosclerosis.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 132.Steppich B, Mattisek C, Sobczyk D, Kastrati A, Schomig A, Ott I. Tissue factor pathway inhibitor on circulating microparticles in acute myocardial infarction. Thromb Haemost. 2005;93:35–39. doi: 10.1160/TH04-06-0393. [DOI] [PubMed] [Google Scholar]
- 133.Huisse MG, Lanoy E, Tcheche D, Feldman LJ, Bezeaud A, Angles-Cano E, Mary-Krause M, de Prost D, Guillin MC, Steg PG. Prothrombotic markers and early spontaneous recanalization in st-segment elevation myocardial infarction. Thromb Haemost. 2007;98:420–426. [PMC free article] [PubMed] [Google Scholar]
- 134.Huisse MG, Ajzenberg N, Feldman L, Guillin MC, Steg PG. Microparticle-linked tissue factor activity and increased thrombin activity play a potential role in fibrinolysis failure in st-segment elevation myocardial infarction. Thromb Haemost. 2009;101:734–740. [PubMed] [Google Scholar]
- 135.Sommeijer DW, Hansen HR, van Oerle R, Hamulyak K, van Zanten AP, Meesters E, Spronk HM, ten Cate H. Soluble tissue factor is a candidate marker for progression of microvascular disease in patients with type 2 diabetes. J Thromb Haemost. 2006;4:574–580. doi: 10.1111/j.1538-7836.2005.01763.x. [DOI] [PubMed] [Google Scholar]
- 136.Tilley RE, Holscher T, Belani R, Nieva J, Mackman N. Tissue factor activity is increased in a combined platelet and microparticle sample from cancer patients. Thromb Res. 2008;122:604–609. doi: 10.1016/j.thromres.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]


