Abstract
Background
It has recently been suggested that overexpression of palladin in sporadic pancreatic cancer may contribute to pancreatic cancer’s invasive and migratory abilities. This hypothesis was based on reverse transcriptase-polymerase chain reaction analyses of bulk pancreatic tissue, yet pancreatic cancer is a complex admixture of neoplastic epithelial cells and desmoplastic stroma.
Design
Immunohistochemical labeling of tissue microarrays was used to define the patterns of palladin protein expression in 177 ductal adenocarcinomas of the pancreas. Western blot analysis was used to determine the epitope(s) of palladin recognized by the antibody as well as the relative levels of palladin expression in short-term cultures of stromal fibroblasts, non-neoplastic ductal cells and pancreatic cancer cell lines.
Results
Immunolabeling revealed that the palladin protein was strongly overexpressed in non-neoplastic stromal cells in 171 (96.6%) of the 177 evaluable pancreatic cancers. By contrast, the overexpression of palladin protein by the neoplastic epithelial cells relative to normal pancreatic epithelium was observed in only 22 (12.4%) of the 177 cancers. Western blot analysis confirmed that the antibody recognizes the ~90 kDa isoform of palladin, and demonstrated that fibroblast cell lines had higher expression of palladin than pancreatic cancer cell lines.
Conclusions
The overexpression of palladin relative to normal pancreas in the majority of pancreatic cancers is limited to non-neoplastic stromal cells. This observation highlights the limitations of relying on bulk tissues when analyzing gene expression. Since palladin is not overexpressed in most pancreatic cancer cells, the overexpression of palladin is not likely to be responsible for pancreatic cancer cells invasive and migratory abilities.
Keywords: pancreas, pancreatic cancer, palladin, immunohistochemistry
Introduction
Linkage analysis performed on a large kindred with multiple pancreatic cancers suggested that a familial pancreatic cancer gene may be present on chromosome 4q32-34.1 Pogue-Geile et al. used a two-pronged approach to discover this gene. First, they hypothesized that abnormalities in the expression of genes within the area of linkage on 4q32-34 may help localize the putative familial pancreatic cancer gene.2 They therefore created a custom built mRNA expression microarray with clones representative of the genes in the area of linkage on 4q32-34.2 They then utilized these arrays to evaluate gene expression levels in bulk normal pancreas, in bulk pancreatic cancer tissue, and in pancreatic intraepithelial neoplasia (PanIN) lesions.2 The second approach they took was to sequence multiple candidate genes within the area of linkage on 4q32-34.2
Pogue-Geile et al. found a sequence alteration in the PALLD gene that tracked with the disease phenotype in the kindred with linkage.2 This base pair change caused a proline to serine amino acid change in palladin, the protein coded for by the PALLD gene, suggesting that the sequence alteration may have functional consequences. Interestingly, the amino acid change discovered by Pogue-Geile et al. is modeled to be in the protein region that specifically interacts with alpha-actinin.2,3 They functionally demonstrated that when mutant palladin was expressed in Hela cells, it acted in a dominant negative fashion by disrupting normal stress fiber organization, although it is unclear exactly how disruption of microfilaments could explain a putative contribution to carcinogenesis.2 Remarkably, the gene expression studies conducted by Pogue-Geile et al. also revealed abnormalities in PALLD mRNA expression.2 Utilizing reverse transcriptase-polymerase chain reaction (RT-PCR), PALLD mRNA expression was found to be increased in bulk samples of sporadic pancreatic cancer relative to bulk samples of normal pancreas. The non-neoplastic www.landesbioscience.com Cancer Biology & Therapy 325 pancreatic parenchyma adjacent to infiltrating cancer and microdissected PanIN lesions also showed elevated levels of PALLD mRNA expression.2
These findings led Pogue-Geile et al. to propose that PALLD gene mutations cause familial pancreatic cancer, and that the overexpression of palladin, the protein product of the PALLD gene, may “be responsible for or contribute to the tumor’s strong invasive and migratory abilities.2”
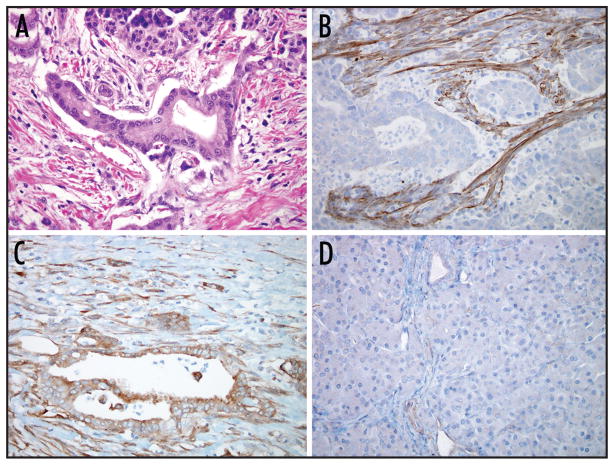
These experiments, however, relied on bulk pancreatic tissues and a method (RT-PCR) that doesn’t provide topographical information. The tumor formed by pancreatic ductal adenocarcinoma is a complex admixture of entrapped normal pancreas, non-neoplastic fibroblasts, vessels, inflammatory cells and neoplastic epithelial cells.4 Only a minority of the cells in a pancreatic cancer are actual neoplastic cells (Fig. 1A). Studies of gene expression patterns in pancreatic cancer should be based on a firm understanding of this pathology, and should utilize methods that allow the investigator to correlate expression with cell morphology. For example, apolipoprotein C-1, apolipoprotein D, SPARC and MMP11 are significantly overexpressed in microarray analysis of “bulk” cancer specimens, but when examined utilizing methods that localize gene expression to specific cell types, the expression of these genes can be seen to be exclusively localized to the non-neoplastic stromal elements.5–7 In fact, studies by Iacobuzio-Donahue et al. have defined distinct compartments of aberrant gene expression in the peritumoral stroma, including the angioendothelium, stromal cells immediately adjacent to the invasive neoplastic epithelium, and stromal cells distant from the invasive neoplastic epithelium, underscoring the critical importance of topographical tissue validation for novel gene markers.7
Figure 1.
Hematoxylin and eosin stained section of ductal adenocarcinoma of the pancreas (A). Note that the islet of Langerhans above, and stromal fibroblasts on either side of the neoplastic gland in the center. Immunolabeling for palladin (B–D). Overexpression by non-neoplastic stromal cells with no detectable expression in the neoplastic cells was the most common pattern of labeling observed (B). The neoplastic epithelial cells overexpressed palladin in 12% of the cases (C), while the normal pancreatic parenchyma showed weak labeling of small vessels (D).
We therefore examined palladin protein expression in a large series of well-characterized infiltrating ductal adenocarcinomas of the pancreas using immunohistochemical labeling.
Methods
Patients
The 177 patients represented in the tissue microarrays employed in this study underwent pancreaticoduodenectomy at Johns Hopkins Hospital between 1998 and 2003. This study was approved by the Institutional Review Board of The Johns Hopkins Medical Institutions (JHMI).
Immunohistochemical labeling
The expression of palladin protein was examined utilizing immunohistochemical labeling of tissue microarrays (TMAs). Ten tissue microarrays containing a total of 177 different surgically resected infiltrating ductal adenocarcinomas of the pancreas and a variety of normal tissues were constructed as previously described.8 These 10 TMAs were selected for analysis from the 17 TMAs available from patients from this time period. Each carcinoma and adjacent non-neoplastic tissue was represented four times in each tissue microarray to account for potential tumor heterogeneity. Unstained four-micron sections of each tissue microarray were stained on a Benchmark XT autostainer (Ventana Medical Systems, Inc, Tucson, AZ). The staining protocol included routine deparaffinization, standard antigen retrieval with conditioner 1 (CC1), sixteen minutes of primary antibody incubation (rabbit- anti-Palladin, Proteintech Group, Inc. Chicago, IL.; 1:100 at room temperature), followed by amplification (Amplifier A and B, 8 minutes each) and blocking (Blocker A and B, 4 minutes each). Labeling was detected using I-view detection kit (Ventana Medical Systems, Inc, Tucson, AZ). All sections were counterstained with hematoxylin. The primary antibody was omitted in controls.
The relative intensity of labeling (from 0 to 3+) was scored by two observers at a multi-headed microscope. The observers were unaware of the patients’ clinical characteristics, and a consensus was reached for each case. The relative intensity of labeling of the non-neoplastic stroma was evaluated as was the relative intensity of labeling of the neoplastic cells. A score of 0 was assigned if there was no appreciable labeling. A score of 1+ was used for labeling equal to that observed in the non-neoplastic acinar and ductal cells from the same case. A score of 2+ was given for tissues labeling discernibly more intensely than the adjacent non-neoplastic acinar and ductal cells, but less intensely than the most intensely labeling cells on the slide. A score of 3+ was reserved for compartments that strongly and diffusely labeled with the antibody. In the statistical analyses, labeling was considered positive if the cells labeled at an intensity of 2 or 3+; that is if they labeled more intensely than the normal non-neoplastic acinar and ductal cells from the same case. Labeling of 0 or 1+ was considered negative for overexpression.
PALLD mRNA expression
Levels of palladin RNA in pancreatic cancer tissues and cell lines were examined in a set of Affymetrix U133A oligonucleotide microarray profiles that we have previously generated9–11 and in the case of hTERT-HPNE cells and cancer associated fibroblast lines using U133 2.0 microarrays (unpublished data).
Western blot analysis
Three major isoforms of palladin have been described, with molecular weights of ~200 kDa, 140 kDa, and 90 kDa.2,3,12 The ~90 kDa isoform is the isoform that was reported to be overexpressed in pancreatic cancer.2 The product description provided by the manufacturer of the antibody we employed (Protein Tech Group, Inc, Chicago, Illinois), includes an image of a Western blot demonstrating that the antibody recognizes an ~90 kDa isoform and an ~50kDa isoform of palladin. In order to confirm that the antibody we employed recognized the ~90 kDa isoform of palladin, we performed a Western blot analysis using lysates from two immortalized cultures established from normal pancreas (HPDE [human pancreatic ductal epithelium] from Tsao et al13 and hTERT-HPNE cells14,15), three pancreatic cancer cell lines (BxPc3, Capan1, and Panc1), and two primary cultures of pancreatic cancer associated fibroblasts (CAF1 and CAF3). Equal amounts of protein (30 μg per lane) were separated by 4–12% gradient SDS gel electrophoresis, transferred to nitrocellulose membranes, and incubated in blocking solution (5% milk in TBS with 0.1% Tween 20) for 30 minutes. Membranes were incubated overnight with polyclonal or monoclonal antibodies directed against palladin (Protein Tech Group, Inc.) at 1:100 dilution or against GAPDH (glyceraldehyde-3-phosphate dehydrogenase) at 1:5000 dilution. Secondary HRP-linked antibodies (Santa Cruz Biotechnology, Amersham Biosciences) were applied at 1:2000 dilution and proteins were detected using an ECL kit (Amersham Biosciences).
Statistical data analysis
Simple descriptive statistics were used to compare the clinicopathological variables according to immunohistochemical palladin expression. Significance was set at p < 0.05.
Results
Patients
The 177 patients represented in the tissue microarrays included 93 males, 84 females (mean age 65 years). Two patients were stage 1A, four patients were stage 1B, 19 patients were stage stage 2A, 149 patients were stage 2B, and 3 patients were stage 3. Four patients had well-differentiated, 97 patients had moderately differentiated and 76 patients poorly differentiated pancreatic adenocarcinomas. Median survival for the 177 patients was 15 months. The average tumor size was approximately 3.2 cm. Of the 177 patients, 127 (72%) are deceased. These patient demographics are representative of the patients treated surgically at The Johns Hopkins Hospital for pancreatic cancer.16
Western blotting
To confirm that the antibody we used in this study for immunohistochemistry is specific for Palladin, we performed Western blotting in immortalized cultures derived from normal pancreatic ducts (HPDE, hTERT-HPNE), two pancreatic cancer cell lines (BxPc3 and Panc1), and two primary cultures of pancreatic cancer associated fibroblasts (CAF1 and CAF3). In both the immortalized cultures and the pancreatic cancer cell lines, the antibody detected a single band at approximately 90 kD. Levels of the 90 kD Palladin isoform were similar in pancreatic cancer cell lines (Fig. 2, lanes 3 and 4) relative to cells immortalized from ducts of the normal pancreas (Fig. 2, lanes 5 and 6). The antibody also recognized high expression levels of a smaller Palladin isoform in the cancer associated fibroblasts that was not seen in cells derived from pancreatic ducts (Fig. 2, lanes 1 and 2). This smaller isoform is also noted in the Western blot included in the package insert for the antibody. Five additional pancreatic cancer cell lines (AsPc1, Capan1, Hs766T, MiaPaca, and Su8686) also expressed the 90kD Palladin isoform at levels similar to those of HPDE and hTERT-HPNE (data not shown). These results, taken together with our immunohistochemistry data, suggest that Palladin is primarily expressed by stromal cells in the pancreas but was not overexpressed at the protein level in any of the 7 pancreatic ductal adenocarcinoma lines tested.
Figure 2.
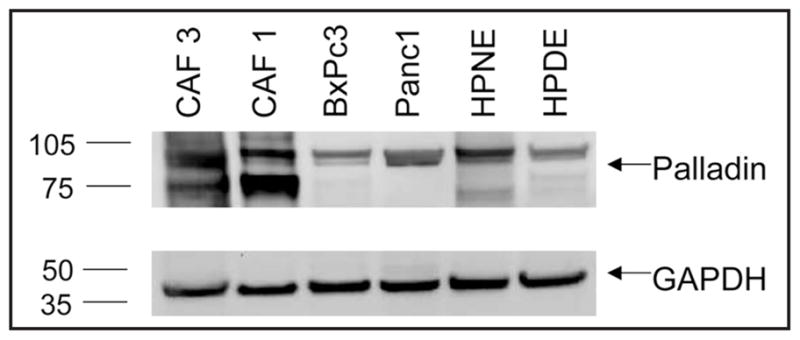
Western blot analysis of palladin expression using the same antibody used in the immunolabeling studies. Primary cultures of pancreatic cancer associated fibroblasts, CAF1 and CAF2, were loaded in lanes 1 and 2; the pancreatic cancer cell lines BxPc3 and Panc1 in lanes 3 and 4; and the normal pancreatic cell lines HPDE and hTERT-HPNE were loaded in lanes 5 and 6.
Palladin RNA expression
We also examined Palladin RNA expression in a panel of pancreatic tissues that we have previously subjected to global gene expression analysis using Affymetrix oligonucleotide microarrays10,11 (and unpublished data). The pancreas samples examined included the HPDE and hTERT-HPNE immortalized cell lines, five pancreatic cancer cell lines (AsPc1, CFPac, HS766T, MiaPaca, and Panc1), and the two pancreatic cancer associated fibroblast cultures (CAF1 and CAF2). Signal intensities of Palladin RNA (corresponding to the probeset 200897s, which represents the 90kD isoform) were as follows: HPDE; 206.6, hTERT-HPNE; 3019.5, AsPc1; 482.6, CFPac; 1038.3, HS766T; 748.1, MiaPaca2; 50.9, Panc1; 560.3, CAF1; 4980.6, and CAF2; 5594.6. The levels of GAPDH across these cell lines were similar such that palladin/GAPDH signal intensities provided similar information. Although palladin RNA levels were variable in different samples, and four of the five pancreatic cancer cell lines had modestly higher mRNA levels than the HPDE cell line, none of the cancer cell lines had higher mRNA levels than the non-neoplastic HPNE cell line. Furthermore, both of the cancer associated fibroblast samples had much higher RNA levels than all of the non-stromal pancreatic tissues examined. These results agree with our protein expression data, in which Palladin expression levels are comparable between pancreatic cancer cells and non-neoplastic pancreatic cells, with high expression levels predominantly seen in stromal fibroblasts.
Immunohistochemical labeling of normal pancreas
Immunolabeling with antibodies directed against palladin only weakly labeled the normal pancreas (Fig. 1B). Most acinar and ductal cells were either completely negative or showed only a “blush” of labeling. The walls of small blood vessels and scattered stromal spindle shaped cells labeled with mild to moderate intensity.
Immunohistochemical labeling of neoplastic pancreas
Palladin was strongly overexpressed in non-neoplastic stromal cells relative to normal pancreatic epithelium in 171 (96.6%) of the 177 evaluable pancreatic cancers (Fig. 1C). By contrast, the overexpression (2+ and 3+) of palladin by the neoplastic epithelial cells was observed in only 22 (12.4%) of 177 of the evaluable cancers (Fig. 1D). To determine if the pancreatic cancers with increased levels of palladin protein expression had different clinicopathological features, we examined demographic and pathological data for the 177 patients and their cancers. The mean age of the 22 patients whose neoplastic epithelial cells overexpressed palladin was 64 years and 10 (45%) of the 22 patients were male. All 22 patients had stage 2 disease (two patients were stage 2A, and 20 were stage 2B). The average tumor size of these 22 patients was 3.3 cm. Sixteen of these 22 patients had moderately differentiated and six patients had poorly differentiated carcinomas. The median survival for these 22 patients was 16 months. These clinicopathological features were not significantly different from the 155 patients whose pancreatic cancers did not overexpress palladin (p > 0.05).
Discussion
It has recently been suggested that the overexpression of the protein palladin plays a significant role in the pathogenesis of pancreatic cancer.2 We used a commercially available antibody to the 90 kDa isoform of palladin overexpressed in bulk pancreatic cancer tissues and found that the antibody labeled the non-neoplastic stromal cells in 96.6% of 177 pancreatic cancers, and only 12.4% of the neoplastic epithelial cells in 177 evaluable pancreatic cancers. We confirmed PALLD mRNA expression in fibroblasts using gene expression array analysis of short term cultures of fibroblasts from pancreatic cancer. These results underscore the critical importance of tissue validation for novel gene markers, and suggest that the overexpression of palladin by neoplastic epithelial cells is not likely to be responsible for pancreatic cancer’s invasive and migratory abilities.
The expression of palladin protein by fibroblasts in the desmoplastic stroma associated with an invasive cancer would be expected based on the known function of the protein.3,12,17–20 Palladin is a well-documented microfilament associated protein, where it is co-localizes with alpha-actinin in a “beads on a string” pattern along f-actin stress fibers.21,22 The palladin protein binds a number of actin-binding proteins including profillin, alpha-actinin, ezrin and vasodilator-stimulated phosphoprotein [VASP].2,3,12,17–20 Palladin is therefore believed to play an important role in recruiting these other proteins to sites of actin dynamics, thereby functioning in cytoskeletal formation, cell movement and cell-extracellular matrix interactions.2,3,12,17–20 It should therefore not be surprising that actin filaments, and therefore palladin proteins, are important in fibroblast function and are overexpressed in fibroblasts in desmoplastic stroma.17 The expression of palladin in non-neoplastic fibroblasts not only explains the expression of palladin in bulk samples of cancer, but it would also explain the observation made by Pogue-Geile et al. that palladin is also overexpressed in the non-neoplastic pancreatic parenchyma adjacent to a cancer.2 Pancreatic cancers obstruct the local pancreatic ducts producing chronic pancreatitis and therefore scarring in the adjacent pancreatic parenchyma.4
If palladin is predominantly overexpressed in stromal fibroblasts and not in neoplastic epithelial cells in surgically resected pancreatic cancers, how can we explain the observation that palladin mRNA levels were reported to be increased in pancreatic cancer cell lines2? First, the mRNA levels reported by Pogue-Geile et al. in pancreatic cancer cell lines were only higher relative to a single non-neoplastic cancer cell line, HPDE.2 In the present study we found that the mRNA levels in several pancreatic cancer cell lines are lower than the mRNA levels in another non-neoplastic cell line, hTERT-HPNE. Second, it is likely that a number of genes are aberrantly induced in human cancer cell lines simply by their having been passaged in vitro, particularly under conditions of growth serum supplementation. For example, in our own experience, we have demonstrated >3-fold overexpression of the cell adhesion molecule MelCAM/MUC18 in microarray analysis of biliary cancer cell lines, yet found complete lack of expression of the corresponding protein in tissue sections of resected biliary cancers.23 Given the unequivocal evidence that human cell lines accrue progressive genetic and epigenetic alterations in culture,24 the cell line- tissue disconcert for palladin expression should not be entirely unexpected, and highlights the critical importance of validating gene expression results in primary biosamples such as surgically resected cancers.
Further studies are clearly needed to define the role of palladin in the development of pancreatic cancer. For example, the present study utilized a single commercially available antibody. Further studies utilizing additional antibodies as they become available should incorporate an understanding of the complex pathology of pancreatic cancer; a cancer in which the majority of cells in bulk biosamples are non-neoplastic cells.4
Acknowledgments
The Sol Goldman Pancreatic Cancer Research Center, the Michael Rolfe Foundation, and the NIH SPORE (Specialized Programs of Research Excellence) in Gastrointestinal Cancer Grant CA62924, CA90709.
References
- 1.Eberle MA, Pfutzer R, Pogue-Geile KL, Bronner MP, Crispin DA, Kimmey MB, Duerr RH, Kruglyak L, Whitcomb DC, Brentnall TA. A new susceptibility locus for autosomal dominant pancreatic cancer maps to chromosome 4q32-34. Am J Med Genet. 2002;70:1044–8. doi: 10.1086/339692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pogue-Geile KL, Chen R, Bronner MP, Crnogorac-Jurcevic T, Moyes KW, Dowen S, Otey C, Crispin DA, Whitcomb DC, Brentnall TA. Palladin mutation causes familial pancreatic cancer and suggests a new cancer mechanism. PLoS Medicine. 2006;3:e516. doi: 10.1371/journal.pmed.0030516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Otey CA, Rachlin A, Moza M, Arneman D, Carpen O. The palladin/myotilin/myopalladin family of actin-associated scaffolds. Int Rev Cytol. 2005;246:31–58. doi: 10.1016/S0074-7696(05)46002-7. [DOI] [PubMed] [Google Scholar]
- 4.Hruban RH, Klimstra DS, Pitman MB. Atlas of tumor pathology: Tumors of the pancreas. IV. Washington, DC: Armed Forces Institute of Pathology; 2006. [Google Scholar]
- 5.Sato N, Fukushima N, Maehara N, Matsubayashi H, Koopmann J, Su GH, Hruban RH, Goggins M. SPARC/osteonectin is a frequent target for aberrant methylation in pancreatic adenocarcinoma and a mediator of tumor-stromal interactions. Oncogene. 2003;22:5021–30. doi: 10.1038/sj.onc.1206807. [DOI] [PubMed] [Google Scholar]
- 6.Infante MH, Sato N, Tonascia J, Klein AP, Riall TS, Yeo CJ, Iacobuzio-Donahue CA, Goggins M. Peritumoral fibroblast SPARC expression and patient outcome with resectable pancreatic adenocarcinoma. J Clin Oncol. 2007;25:319–25. doi: 10.1200/JCO.2006.07.8824. [DOI] [PubMed] [Google Scholar]
- 7.Iacobuzio-Donahue CA, Ryu B, Hruban RH, Kern SE. Exploring the host desmoplastic response to pancreatic carcinoma: Gene expression of stromal and neoplastic cells at the site of primary invasion. Am J Pathol. 2002;160:91–9. doi: 10.1016/S0002-9440(10)64353-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koopmann J, Fedarko NS, Jain A, Maitra A, Iacobuzio-Donahue CA, Rahman A, Hruban RH, Yeo CJ, Goggins M. Evaluation of osteopontin as biomarker for pancreatic adenocarcinoma. Cancer Epidemiol Biomarkers Prev. 2004;13:487–91. [PubMed] [Google Scholar]
- 9.Fukushima N, Koopmann J, Sato N, Prasad N, Carvalho R, Leach SD, Hruban RH, Goggins M. Gene expression alterations in the non-neoplastic parenchyma adjacent to infiltrating pancreatic ductal adenocarcinoma. Mod Pathol. 2005;18:779–87. doi: 10.1038/modpathol.3800337. [DOI] [PubMed] [Google Scholar]
- 10.Sato N, Fukushima N, Maitra A, Matsubayashi H, Yeo CJ, Cameron JL, Hruban RH, Goggins M. Discovery of novel targets for aberrant methylation in pancreatic carcinoma using high-throughput microarrays. Cancer Res. 2003;63:3735–42. [PubMed] [Google Scholar]
- 11.Sato N, Fukushima N, Maitra A, Iacobuzio-Donahue CA, van Heek NT, Cameron JL, Yeo CJ, Hruban RH, Goggins M. Gene expression profiling identifies genes associated with invasive intraductal papillary mucinous neoplasms of the pancreas. Am J Pathol. 2004;164:903–14. doi: 10.1016/S0002-9440(10)63178-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rachlin AS, Otey CA. Identification of palladin isoforms and characterization of an isoform-specific interaction between Lasp-1 and palladin. J Cell Sci. 2006;119:995–1004. doi: 10.1242/jcs.02825. [DOI] [PubMed] [Google Scholar]
- 13.Liu N, Furukawa T, Kobari M, Tsao MS. Comparative phenotypic studies of duct epithelial cell lines derived from normal human pancreas and pancreatic carcinoma. Am J Pathol. 1998;153:263–9. doi: 10.1016/S0002-9440(10)65567-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee KM, Yasuda H, Hollingsworth MA, Ouellette MM. Notch 2-positive progenitors with the intrinsic ability to give rise to pancreatic ductal cells. Lab Invest. 2005;85:1003–1012. doi: 10.1038/labinvest.3700298. [DOI] [PubMed] [Google Scholar]
- 15.Lee KM, Nguyen C, Ulrich AB, Pour PM, Ouellette MM. Immortalization with telomerase of the Nestin-positive cells of the human pancreas. Biochem Biophys Res Commun. 2003;301:1038–44. doi: 10.1016/s0006-291x(03)00086-x. [DOI] [PubMed] [Google Scholar]
- 16.Winter JM, Cameron JL, Campbell KA, Arnold M, Chang DC, Coleman J, Hodgin MB, Sauter PK, Hruban RH, Riall TS, Schulick RD, Choti MA, Lillemoe KD, Yeo CJ. 1423 pancreaticoduodenectomies for pancreatic cancer: A single institution experience. J Gastrointest Surg. 2006;10:1199–211. doi: 10.1016/j.gassur.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 17.Liu XS, Luo HJ, Yang H, Wang L, Kong H, Jin YE, Wang F, Gu MM, Chen Z, Lu ZY, Wang ZG. Palladin regulates cell and extracellular matrix interaction through maintaining normal actin cytoskeleton architecture and stabilizing Beta1-integrin. J Cell Biochem. 2006:1288–300. doi: 10.1002/jcb.21126. [DOI] [PubMed] [Google Scholar]
- 18.Goicoechea S, Arneman D, Disanza A, Garcia-Mata R, Scita G, Otey CA. Palladin binds to Eps8 and enhances the formation of dorsal ruffles and podosomes in vascular smooth muscle cells. J Cell Sci. 2006;119:3316–24. doi: 10.1242/jcs.03076. [DOI] [PubMed] [Google Scholar]
- 19.Boukhelifa M, Moza M, Johansson T, Rachlin A, Parast M, Huttelmaier S, Roy P, Jockusch BM, Carpen O, Karlsson R, Otey CA. The proline-rich protein palladin is a binding partner for profilin. FEBS J. 2006;273:26–33. doi: 10.1111/j.1742-4658.2005.05036.x. [DOI] [PubMed] [Google Scholar]
- 20.Ronty M, Taivainen A, Moza M, Otey CA, Carpen O. Molecular analysis of the interaction between palladin and alpha-actinin. FEBS Lett. 2004;566:30–4. doi: 10.1016/j.febslet.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Parast MM, Otey CA. Characterization of palladin, a novel protein localized to stress fibers and cell adhesions. J Cell Biol. 2000;150:643–56. doi: 10.1083/jcb.150.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mykkanen OM, Gronholm M, Ronty M, Lalowski M, Salmikangas P, Suila H, Carpen O. Characterization of human palladin, a microfilament-associated protein. Mol Biol Cell. 2001;12:3060–73. doi: 10.1091/mbc.12.10.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansel DE, Rahman A, Hidalgo M, Thuluvath PJ, Lillemoe KD, Shulick R, Ku JL, Park JG, Miyazaki K, Ashfaq R, Wistuba II, Varma R, Hawthorne L, Geradts J, Argani P, Maitra A. Identification of novel cellular targets in biliary tract cancers using global gene expression technology. Am J Pathol. 2003;163:217–29. doi: 10.1016/S0002-9440(10)63645-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maitra A, Arking DE, Shivapurkar N, Ikeda M, Stastny V, Kassauei K, Sui G, Cutler DJ, Liu Y, Brimble SN, Noaksson K, Hyllner J, Schulz TC, Zeng X, Freed WJ, Crook J, Abraham S, Colman A, Sartipy P, Matsui S, Carpenter M, Gazdar AF, Rao M, Chakravarti A. Genomic alterations in cultured human embryonic stem cells. Nat Genet. 2005;37:1099–03. doi: 10.1038/ng1631. [DOI] [PubMed] [Google Scholar]