SUMMARY
Malignant gliomas are aggressive brain tumors with limited therapeutic options, and improvements in treatment require a deeper molecular understanding of this disease. As in other cancers, recent studies have identified highly tumorigenic subpopulations within malignant gliomas, known generally as cancer stem cells. Here we demonstrate that glioma stem cells (GSCs) produce nitric oxide via elevated nitric oxide synthase-2 (NOS2) expression. GSCs depend on NOS2 activity for growth and tumorigenicity, distinguishing them from non-GSCs and normal neural progenitors. Gene expression profiling identified many NOS2-regulated genes, including the cell cycle inhibitor cell division autoantigen-1 (CDA1). Further, high NOS2 expression correlates with decreased survival in human glioma patients, and NOS2 inhibition slows glioma growth in a murine intracranial model. These data provide insight into how GSCs are mechanistically distinct from their less tumorigenic counterparts, and suggest that NOS2 inhibition may be an efficacious approach to treating this devastating disease.
INTRODUCTION
Malignant gliomas are highly lethal brain tumors that portend a dismal prognosis for patients. Despite modern surgical and medical treatments, the median survival for glioblastoma patients (WHO grade IV astrocytoma) remains only 14.6 months (Stupp et al., 2005), emphasizing a need for improved therapies. The identification of highly tumorigenic subpopulations within gliomas has fueled enthusiasm for development of novel anti-glioma therapeutics. Due to their high tumorigenic potential and stem cell-like behavior, these cells have earned a variety of names, including tumor propagating cells or cancer stem cells (CSCs). Unlike the bulk tumor mass, CSCs exhibit sustained self-renewal and produce secondary tumors that recapitulate the parent tumor’s features and cellular diversity (Bonnet and Dick, 1997; Galli et al., 2004; Lapidot et al., 1994; Singh et al., 2003; Yuan et al., 2004). The concept of CSCs provides a rational hierarchical explanation for cellular heterogeneity observed within tumors (Reya et al., 2001), which is complementary to stochastic mutations with clonal outgrowths (Shackleton et al., 2009). Regardless of the etiology for tumor heterogeneity, the potent tumor-propagation capacity of CSCs suggests a utility for glioma stem cell (GSC)-directed therapies.
As their name suggests, CSCs share features with non-neoplastic stem cells. Gene expression profiles of GSCs resemble those of embryonic stem cells (Ben-Porath et al., 2008) and non-malignant neural stem cells (Taylor et al., 2005). Disruption of several stem cell-specific pathways (Bar et al., 2007; Clement et al., 2007; Fan et al., 2006) abrogates CSC proliferation and tumorigenesis, though canonical stem cell signals (e.g., Hedgehog, Notch, Wnt) are clearly critical to normal stem cell physiology as well (Androutsellis-Theotokis et al., 2006; Reya et al., 2003; Wechsler-Reya and Scott, 1999). Development of strategies that target CSCs while sparing normal stem cell function is therefore necessary to attain a CSC-selective therapeutic index, a notion that has been supported by leukemic versus hematopoietic stem cells (Yilmaz et al., 2006). In contrast, this concept is relatively unexplored in GSCs versus neural stem cells.
Endogenous nitric oxide (NO) exhibits pleotropic roles within cancer cells and tumors, and studies employing inhibition or genetic deletion of endogenous NO synthases (NOSs) support a tumor-promoting role for NO (Fukumura et al., 2006; Williams and Djamgoz, 2005). Downstream effects of endogenous NO in cancer include: chemotherapeutic resistance (Fetz et al., 2009; Yang et al., 2002), evasion of apoptosis (Engels et al., 2008; Levesque et al., 2003) and enhanced proliferation (Lim et al., 2008). Nitric oxide synthase isoforms exhibit heterogeneous expression patterns within glioma cell populations (Bakshi et al., 1998; Cobbs et al., 1995). This heterogeneity may reflect a NOS expression pattern that is restricted to specific glioma subpopulations. This raises the possibility that NOS activity could be unique to GSC subpopulations, as one determinant of glioma heterogeneity relates to the existence of GSCs. Along these lines, studies have suggested a pro-tumorigenic role for NO in gliomas (Charles et al., 2010; Yamaguchi et al., 2002). Endothelial NOS3 localizes near neoplastic cells displaying stem cell markers, and exogenous NO donors support stem cell signaling pathways in murine glioma cells (Charles et al., 2010). However, the therapeutic possibilities of targeting NOS3 in glioma are limited, as previous human trials of inhibitors with anti-NOS3 activity resulted in adverse outcomes and increased mortality (Alexander et al., 2007; Avontuur et al., 1998; Lopez et al., 2004).
The possibility of GSC-specific endogenous NO synthesis remain unevaluated, and the contribution of other more targetable NOS isoforms to GSCs remains unexamined. Given the precedence that NO can support tumor growth, and the aforementioned studies suggesting a pro-GSC effect for NO, we hypothesized that endogenous NO production might be augmented within GSCs relative to non-stem glioma cells (non-GSCs), thus promoting the established tumorigenic phenotype of GSCs.
RESULTS
Endogenous NO contributes to growth of GSCs, which is abrogated by heterologous expression of the bacterial NO-consuming enzyme flavohemoglobin
Employing techniques described in the Dirks group’s original report first validating CD133 as a GSC cell surface marker (Singh et al., 2003), we characterized a variety of human tumor specimens and xenografts in which positive selection for CD133 segregates GSC-enriched populations from non-GSCs, as demonstrated by measures of self-renewal, stem cell marker expression, and tumor propagation potential (Bao et al., 2006a; Li et al., 2007). When CD133-based selection is utilized and stem-cell permissive culture conditions employed (Lee et al., 2006), CD133 marker expression is maintained (Figure S1A and S1B).
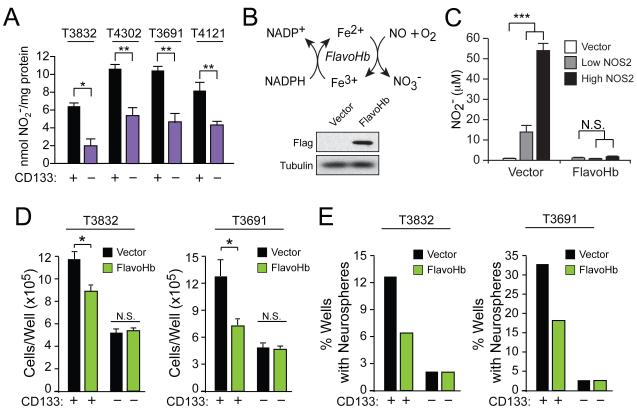
Using this CD133-based selection system, we compared the NO production capacity of CD133+ glioma cells (GSCs) with CD133− glioma cells (non-GSCs). We measured nitrite (NO2−), a stable byproduct of NO, in the culture medium using matched cultures from xenografted patient specimens. GSCs produced more NO2− than matched non-GSCs (Figure 1A), suggesting that elevated NO synthesis may be a distinctive feature of GSCs.
Figure 1. GSCs synthesize NO, and flavohemoglobin-mediated NO depletion decreases GSC growth.
(A) Nitrite (NO2−) was quantified in the conditioned media of glioma xenografts (T3832, T4302, T3691, T4121) sorted into CD133+ (GSC) and CD133− (non-GSC) populations, and normalized to cellular protein. (B) Microbial flavohemoglobin (FlavoHb) catalyzes the reaction of NO with oxygen to form inert nitrate (NO3−). A Western blot verified Flag-tagged FlavoHb expression in GSCs. (C) FlavoHb expression in NOS2-transfected HEK293 cells decreased the total NO2− measured in media, reflecting conversion of NO to NO3−. FlavoHb decreased (D) growth of xenograft-derived GSCs as measured by trypan blue exclusion and (E) GSC neurosphere formation capacity as measured 10 d after single cells were individually sorted into wells; scale bar = 50 μm. N.S., not significant; *, p<0.05; **, p<0.01; ***, p<0.001. See also Figure S1.
To examine the function of endogenous NO in GSCs, we designed and biochemically validated a novel strategy to deplete NO in mammalian cells (Forrester et al., 2011). While not conserved in mammals, bacteria and fungi employ flavohemoglobin (FlavoHb) – a potent NO-consuming enzyme that converts NO to nitrate (NO3−) (Figure 1B) – to protect from nitrosative stress (Gardner et al., 1998; Hausladen et al., 2001; Hausladen et al., 1998). Within GSCs and non-GSCs, we employed lentiviral-based expression of the E. coli FlavoHb . Efficient NO-consumption by this approach was confirmed in HEK293 cells transfected with a CMV-driven NOS2, which results in supraphysiologic levels of NO (Figure 1C). Expression of FlavoHb impaired GSC growth (Figure 1D and Figure S1C) and neurosphere formation (Figure 1E and Figure S1D), though these effects were absent in CD133− non-GSCs and did not impact HEK293 cells that lack NO-dependence (Figure S1E). Consumption of NO in GSCs via FlavoHb abrogated critical GSCs properties in vitro, suggesting a pro-growth role for endogenous NO synthesis in GSCs.
Expression of NOS2 within GSCs is responsible for their distinctive NO synthesis
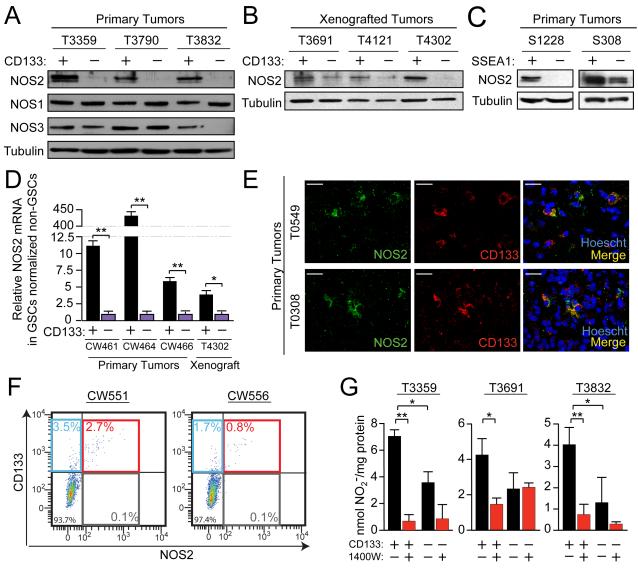
While FlavoHb blocked NO availability and decreased GSC growth, the source of GSC-derived NO remained unclear. Glioma stem cells from primary patient specimens (Figure 2A) and human glioma xenografts (Figure 2B) displayed higher levels of NOS2 protein than matched non-GSCs, while no consistent expression pattern for NOS1 or NOS3 was observed. These data suggest that NOS2 expression in GSCs might contribute to their malignant properties, as: 1) NOS2 is the most highly-productive NOS, 2) NOS2 is regulated largely at the level of transcription, and 3) GSCs demonstrated elevated endogenous NO production that contributed to GSC growth.
Figure 2. GSCs selectively express the NOS2 isoform, which is primarily responsible for elevated NO synthesis.
Western analysis of NOS2 expression in CD133+ cells (GSCs) vs. CD133− cells (non-GSCs) in (A) primary human specimens and (B) xenografted tumors. (C) NOS2 expression levels were compared using Western analysis in GSCs versus non-GSCs isolated via SSEA1-based sorting from S1228 and S308 tumors, for which SSEA1 has been previously validated as a functional marker of GSCs. (D) GSCs and non-GSCs immediately isolated by FACS from fresh primary human brain tumors were analyzed for NOS2 mRNA by qRT-PCR. (E) Immunofluorescence of primary human tumor tissue sections revealed co-expression of CD133 and NOS2; scale bars = 25 μm. (F) Human primary malignant gliomas co-expressed NOS2 and CD133 via flow analysis immediately following isolation from fresh tissue. (G) Media from xenograft-isolated cells with or without daily treatment with 100 μM 1400W was evaluated for NO2− levels, expressed as quantities normalized to total cellular protein. *, p<0.05; **, p<0.01. See also Figure S2.
Although CD133 is useful for identifying GSCs (Bao et al., 2006a; Galli et al., 2004; Singh et al., 2003), it is not the only marker that may enrich for GSC phenotypes. The optimal method for defining GSC marker effectiveness likely depends on individual tumor characteristics and is a topic of active investigation. The marker Stage Specific Embryonic Antigen-1 (SSEA1; CD15) has been reported to effectively isolate GSCs from some tumors with low CD133 expression (Son et al., 2009). Cell lysates from two of these previously-reported tumors revealed elevated NOS2 expression in SSEA1+ GSCs relative to SSEA1− non-GSCs (Figure 2C). Positive selection for SSEA1 segregated for tumorigenic GSCs (as measured in transplantation assays) in these tumors from which we acquired SSEA1+ and SSEA1− protein lysates (Son et al., 2009). These data demonstrate that NOS2 co-segregates with GSC phenotypes in gliomas where CD133 or SSEA1 are useful for enriching GSCs.
To examine whether differential NOS2 expression is inadvertently driven by cell culture conditions, qRT-PCR was utilized to quantify NOS2 mRNA isolated from CD133+ and CD133− populations isolated by fluorescence activated cell sorting (FACS) from fresh dissociated human gliomas without any intervening culture. Levels of NOS2 mRNA were higher in GSCs relative to non-GSCs from three different primary human gliomas and a xenograft (Figure 2D). Previously described minor NOS2 splice variants (Eissa et al., 1996; Eissa et al., 1998; Tiscornia et al., 2004) were not detected in GSCs or non-GSCs (Figure S2A). Although these data indicate that full-length NOS2 transcripts are elevated in GSCs in vivo, we further evaluated NOS2 protein and GSCs in human tissue. Immunofluorescent staining demonstrated co-expression of CD133 and NOS2 protein in human glioma tissue sections (Figure 2E and Figure S2B). Flow cytometry analysis of dissociated primary human glioma specimens showed that greater than 80% of NOS2 positive cells also express CD133 (Figure 2F). These findings collectively support the notion that NOS2 expression is elevated in GSCs.
To determine if NOS2 is critical for GSC NO production, cells were treated with the highly selective NOS2 inhibitor 1400W (Garvey et al., 1997). Synthesis of NO was markedly attenuated in 1400W-treated GSCs, which had elevated NO production at baseline versus non-GSCs (Figure 2G; Figure S2C). Though in some tumors 1400W qualitatively decreased NO production in non-GSCs, this effect was less pronounced and not statistically significant in the setting of low overall NO production and minimal NOS2 expression in non-GSCs (Figure 2G).
Genetic or pharmacologic blockade of NOS2 inhibits GSC growth and proliferation
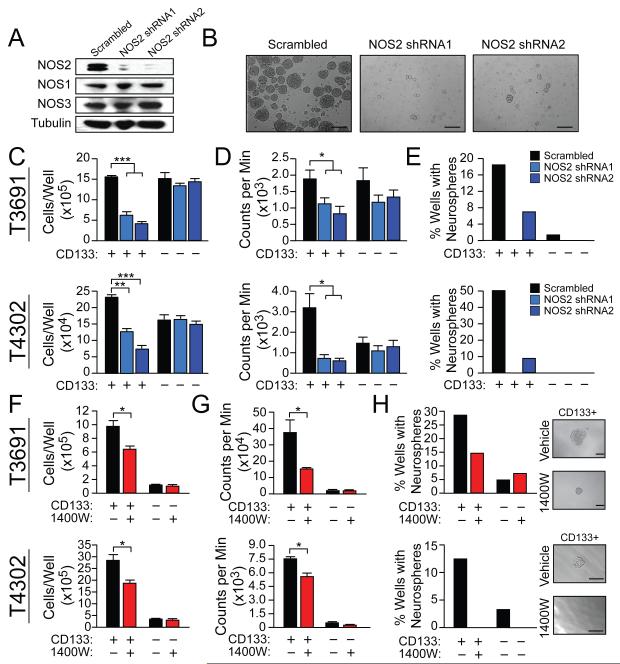
Consumption of NO by FlavoHb blocked GSC growth and neurosphere formation (Figures 1D and 1E and Figures S1C and S1D) and NO production in GSCs was largely NOS2-dependent (Figure 2G). We therefore hypothesized that NOS2 activity in GSCs contributes to their known malignant properties. Short hairpin RNA (shRNA)-mediated knockdown of NOS2 (Figure 3A) resulted in decreased GSC growth and proliferation (Figures 3B-D and Figures S3A-E), but had minimal effect on non-GSCs (Figures 3B-D, and Figures S3A-B, S3D-E). Further, NOS2-directed shRNA decreased neurosphere formation in xenograft-derived GSCs (Figure 3E and Figure S3C). Neurospheres surviving NOS2-directed shRNA still expressed NOS2 (assessed by qRT-PCR; data not shown) and thus were likely derived from cells that did not undergo NOS2-knockdown or silenced NOS2-directed shRNA expression. Though FlavoHb does not affect the expression of NOS2 (Figure S3F), the anti-growth effect of NOS2-directed shRNA was comparable to results with FlavoHb-mediated NO consumption in GSCs (Figure S3G). Consistent with these results, the NOS2 inhibitor 1400W decreased GSC survival, proliferation, and neurosphere formation (Figures 3F-H and Figures S3H-J), as did several other less-potent or less-selective NOS2 inhibitors (Figure S3K).
Figure 3. Knockdown or inhibition of NOS2 decreases GSC growth and neurosphere formation.
(A) Western analysis was employed to compare the selectivity of NOS2-directed shRNAs to NOS2 relative to NOS1 or NOS3 in T3691 CD133+ cells (GSCs). (B) Representative images of neurospheres from (A); scale bar = 50 μm. Following NOS2-directed shRNA treatment of GSCs and CD133− cells (non-GSCs), (C) the number of viable cells were measured by trypan blue exclusion and (D) proliferation was measured by 3H thymidine incorporation. (E) Neurosphere formation following NOS2-directed shRNA treatment of GSCs was measured 10 d after single infected cells were individually sorted into wells. Following inhibition of NOS2 with daily administration of 100 μM 1400W to GSCs and non-GSCs, the following were measured: (F) viability by trypan blue exclusion, (G) proliferation by 3H thymidine incorporation, and (H) neurosphere formation capacity. Representative images of neurospheres assessed in (H) are displayed; scale bar = 50 μm. *, p<0.05; **, p<0.01 ***, p<0.001. See also Figure S3.
Targeting NOS2 decreases cell cycle rate and increases expression of cell division autoantigen 1 (CDA1)
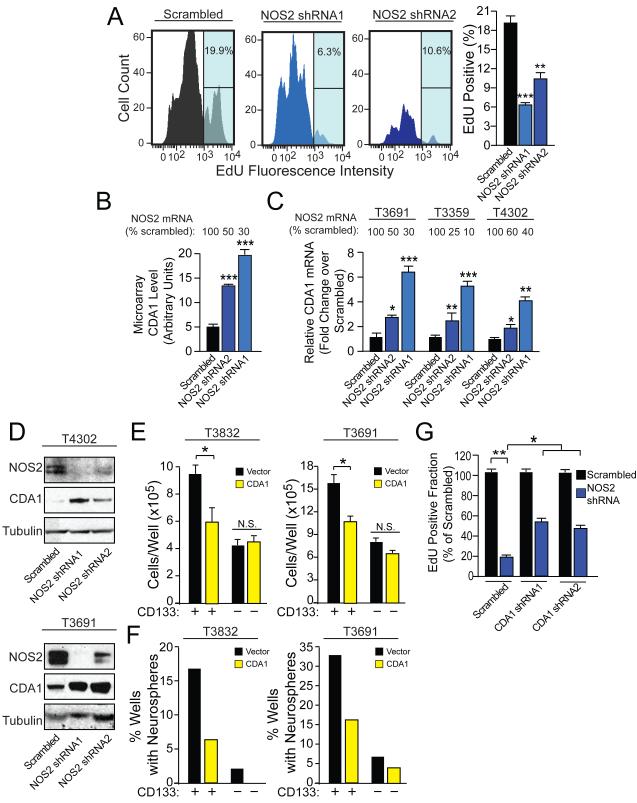
Due to the decreased growth of GSC populations after NOS2-directed interventions, we interrogated the rate of cell cycle transit within individual GSCs using a 5-ethynyl-2′-deoxyuridine (EdU) incorporation assay. Both NOS2-directed shRNA and NOS2 inhibitor treatment decreased the rate of cell cycle transit in GSCs (Figure 4A and Figures S4A and S4B), suggesting that endogenous NOS2 activity effects a pro-proliferative phenotype in GSCs.
Figure 4. GSC cell cycle flux is supported by NOS2 activity, which modulates gene expression including the cell cycle inhibitor CDA1.
(A) 5-ethynyl-2′-deoxyuridine (EdU) incorporation assay was employed to evaluate the effect of NOS2-directed shRNA on S-phase transit in CD133+ glioma cells (GSCs). (B) Microarray analysis demonstrated that NOS2-directed shRNA increased transcript expression of the cell cycle inhibitor, CDA1 (2 xenografts in duplicate). NOS2-dependent suppression of CDA1 in GSCs was validated by (C) qRT-PCR and (D) Western analysis. (E) Viability of GSCs and CD133− cells (non-GSCs) was evaluated after treatment with vector or CDA1 expressing lentivirus. F) Neurosphere formation capacity was evaluated 10 d after single vector or CDA1-overexpressing cells were sorted into wells. (G) The decreased EdU incorporation from NOS2-directed shRNAs was partially blocked by concurrent expression of CDA1-directed shRNA. *, p<0.05; **, p < 0.01; ***, p < 0.001, N.S., not significant. See also Figure S4 and Table S1.
We next screened for potential downstream molecular effectors of the pro-proliferative effects of GSC-specific NOS2 expression. We performed a microarray analysis of gene expression changes associated with NOS2-directed knockdown in human cells with endogenous NOS2 expression. Comparison of GSCs with or without NOS2 knockdown revealed a variety of gene expression patterns altered by NOS2-directed shRNA treatment, and NOS2 was one of the differentially-represented genes, verifying successful knockdown (Table S1). Gene expression changes were further investigated by Ingenuity Pathway Analysis (Figures S4C and S4D).
Of the genes altered by NOS2-directed shRNA, we wanted to identify genes that were associated with human survival and with NOS2 expression in the REpository for Molecular BRAin Neoplasia DaTa (REMBRANDT) database (NCI, 2005). The REMBRANDT database contains microarray-derived gene expression data from biopsies from 577 human patients with malignant glioma for which clinical outcome is known. The REMBRANDT database permits the retrospective analysis of each microarray-analyzed gene in the context of patient survival. Using this database, 49 of the NOS2-dependent probes identified in the microarray correlated with patient survival and 35 correlated with NOS2 levels in the direction predicted by the microarray (Table S1). Only 11 probes satisfied both of these criteria, of which three were associated with the cell cycle inhibitor CDA1 (gene tspyl2). The only gene in the list with a known role in cell cycle or proliferation was CDA1. In the microarray studies, targeting of NOS2 with directed shRNAs resulted in increased CDA1 levels (Figure 4B). Although this is the first report of NO-dependent repression of CDA1, this protein has previously been reported to be a pan-cell cycle inhibitor and tumor suppressor, likely working through inhibition of multiple cyclin dependent kinases (Chai et al., 2001; Kandalaft et al., 2008; Tu et al., 2007). NOS2-dependent suppression of CDA1 was confirmed in several glioma xenografts by qRT-PCR (Figure 4C) and Western blotting (Figure 4D). Exposure of HEK293 cells to physiologic levels of the NO donor diethylenetriamine NONOate (DETA-NO) or transfection with NOS2 also inhibited CDA1 expression (Figure S4E). However, NO did not substantially affect CDA1 protein (Figure S4F) or transcript (Figure S4G) stability. These data support the notion that CDA1 is transcriptionally repressed by NOS2-dependent NO production in a pathway that is not restricted to GSCs.
As predicted by this model, CDA1 over-expression mimics the effects of NOS2 shRNAs in GSCs. Increasing CDA1 expression preferentially decreased GSC numbers (Figure 4E) and neurosphere formation (Figure 4F) with minimal effects on non-GSCs. In converse experiments, CDA1-directed shRNA also partially rescued the anti-growth effect of NOS2-directed shRNA in CD133+ GSCs (Figure 4G and Figures S4H and S4I). Thus, NOS2-dependent repression of CDA1 contributes, at least in part, to the pro-proliferative effect of endogenous NOS2 expression in GSCs.
Elevated NOS2 expression is associated with poor prognosis in humans with malignant glioma and is inversely correlated with CDA1 levels
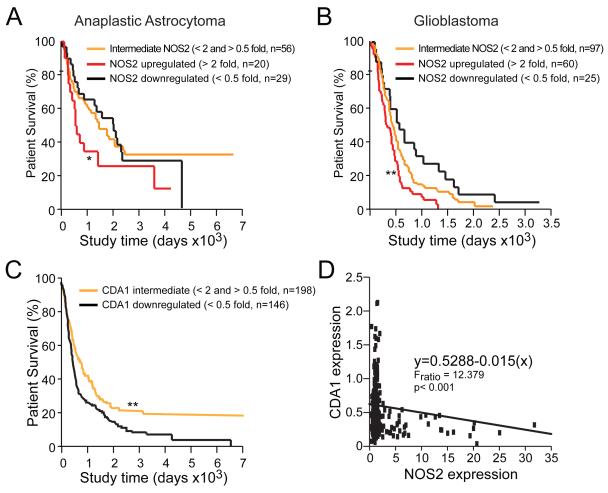
The pro-proliferative effect of NOS2 in human GSCs in vitro compelled us to evaluate whether NOS2 expression in gliomas correlated with patient survival. Evaluation of data contained in the REMBRANDT database revealed that high NOS2 expression in human gliomas, irrespective of grade, is inversely correlated with patient survival. Survival of astrocytoma and GBM patients with elevated NOS2 mRNA is reduced (Figures 5A and 5B). Although these retrospective data cannot determine if NOS2 (or any gene) is an independent predictor of survival, these data do suggest that expression of NOS2 is a negative prognostic factor for human glioma patients.
Figure 5. Human glioma patient survival is correlated with characteristic NOS2 and CDA1 mRNA expression patterns.
NOS2 mRNA expression inversely correlated with survival when glioma patient specimens are segregated via tumor grade to anaplastic astrocytoma (A) or GBM (B) using the REMBRANDT database; *, p<0.05; **, p<0.01 relative to all other groups. (C) Downregulation of CDA1 correlated with poor patient survival in REMBRANDT; **, p < 0.01 for decreased patient survival with downregulated CDA1 expression relative to biopsies with intermediate NOS2 expression. (D) Inverse correlation of tumor-specific NOS2 and CDA1 expression in REMBRANDT was determined using Jump8 software.
Assessment of REMBRANDT data also revealed that patients with tumors demonstrating low CDA1 expression have worse clinical outcomes than patients with intermediate levels of CDA1 expression (Figure 5C). These results are consistent with the known tumor suppressor and anti-proliferative effects of CDA1 (Chai et al., 2001; Kandalaft et al., 2008; Tu et al., 2007), and the ability for NOS2-derived NO to suppress CDA1 expression in glioma cells. Further supporting NOS2-dependent repression of CDA1, expression of CDA1 in human gliomas is inversely correlated with NOS2 expression (Figure 5D). Again, we cannot exclude the possibility that other factors contribute to this correlation because we are unable to use continuous multivariate models to evaluate independent predictive power using this database. Further, the retrospective nature of this analysis precludes our ability to assess whether NOS2 or CDA1 levels are absolutely prognostic. However, it is compelling that both NOS2 and CDA1 are associated with survival, as this observation serves to support not only the importance of NOS2 in glioma biology, but also that CDA1 may represent a critical molecular effector of the pro-GSC role for NOS2.
Normal neural progenitor cells express low levels of NOS2 and exhibit minimal NOS2 growth-dependence
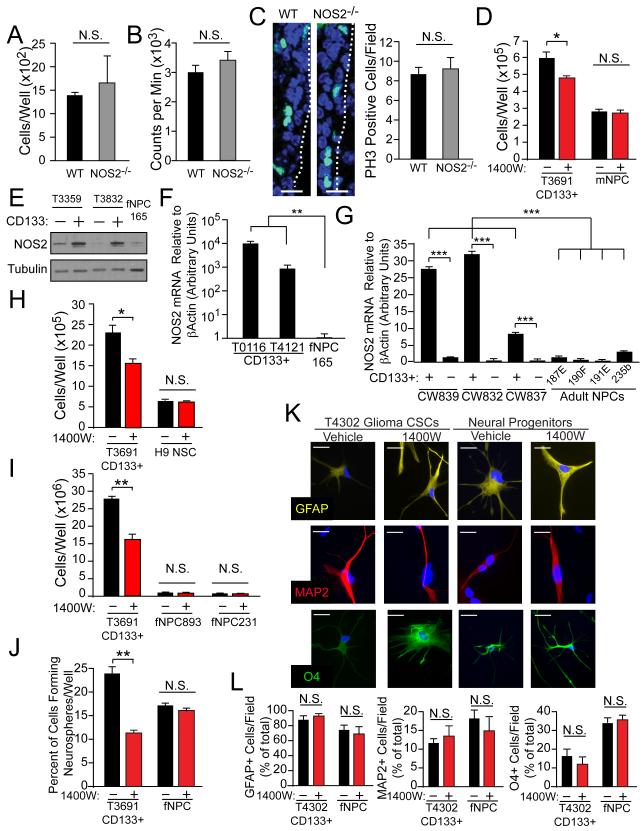
Interventions directed against NOS2 decreased the proliferation of GSCs, and high NOS2 expression within human gliomas is associated with negative patient prognosis. While these data suggest a potential anti-tumor effect for NOS2-directed treatments in vivo, GSC-directed therapies could have toxic effects on normal stem cells due to shared molecular characteristics. We therefore evaluated the expression and functional importance of NOS2 within normal neural progenitor cells (NPCs) to assess the therapeutic margin for NOS2-directed treatment strategies in vivo. Although NOS2 inhibition or knockdown abrogates the proliferation of GSCs, NOS2 knockout (NOS2−/−) mice appear to undergo normal neural development (MacMicking et al., 1995; Wei et al., 1995), suggesting that NOS2 is not essential for normal neural progenitor (NPC) function. Further, NOS2 expression in brain tissue has a variety of reported roles, both positive and negative, in the regulation of neurogenesis in mice after ischemia (Iadecola et al., 1997; Luo et al., 2007; Zhao et al., 2000). To more fully examine the effects of NOS2 inhibitors in NPCs, we evaluated NPCs from mouse models as well as human fetal and adult NPCs (Figure 6). Comparison of NPCs derived from wild type (WT) and NOS2−/− mice revealed similar levels of cell growth and viability (Figures 6A and 6B). Similar levels of the proliferation marker phospho-histone H3 (PH3) were observed in the NPC-rich subependymal zone of WT and NOS2−/− adult mouse brains (Figure 6C). Since knockout mice may develop mechanisms by which they are able to compensate for the deletion of any given gene, we evaluated growth of WT mouse NPCs in response to acute administration of NOS2 inhibitor and observed no impact on growth (Figure 6D).
Figure 6. Normal mouse and human neural progenitor cells exhibit minimal NOS2-independence.
(A) Viability measured by trypan blue exclusion and (B) proliferation measured by 3H thymidine incorporation was evaluated in adult wild type (WT) versus NOS2−/− mouse neural progenitor cells. (C) Immunofluorescence was used to measure phospho-histone H3 (PH3; green)-positive cells per field in the peri-ventricular region of WT and NOS2−/− littermates; dashed line - ventricular border, scale bar = 50 μm. (D) Viability by trypan blue exclusion was measured in CD133+ cells (GSCs) and mouse neural progenitors with control or daily 100 μM 1400W treatment. (E) Western analysis compared NOS2 expression in normal fetal NPCs (fNPCs) versus CD133+ GSCs and CD133− non-GSCs. NOS2 mRNA levels were determined by qRT-PCR in (F) fNPCs versus GSCs, and in (G) human adult NPCs versus GSCs and non-GSCs from fresh primary gliomas immediately post-FACS isolation. CD133-mediated enrichment for the functional properties of GSCs in these tumors was validated using neurosphere formation. The effects of daily 1400W treatment on the viability of GSCs versus (H) embryonic stem cell-derived NPCs and (I) two preparations of fNPCs. (J) Neurosphere formation of GSCs versus fNPCs was quantified after 10 d of daily 1400W treatment. Multilineage differentiation capacity with vehicle or daily 1400W treatment in GSCs and fNPCs was evaluated by staining for astrocytic (GFAP), neuronal (Tuj1), and oligodendrocytic (O4) markers, shown as (K) representative high power immunofluorescence images and (L) percent of marker positive cells per low power field; scale bar = 10 μm. *, p<0.05; **, p<0.01, ***, p<0.001, N.S., not significant. See also Figure S5.
The potential role of NOS2 in normal NPCs was next analyzed in human cells. Normal human fetal NPCs (fNPCs) expressed markedly less NOS2 than GSCs (Figures 6E and 6F). Adult human NPCs isolated from normal human brain specimens also expressed less NOS2 mRNA than xenograft-derived CD133+ GSCs (Figure S5A), as did CD133+ cells from primary human tumor specimens from which mRNA was harvested immediately after FACS sorting (i.e., without the influence of cell culture; Figure 6G). Inhibition of NOS2 had no growth impact on embryonic stem cell-derived NPCs (Figure 6H), nor did it affect the growth of two different fNPC preparations (Figure 6I). Unlike effects on GSCs, treatment with NOS2 inhibitor did not decrease neurosphere formation in fNPCs (Figure 6J). Human fNPCs and GSCs also have the capacity for multilineage differentiation, with GSCs being capable of aberrant differentiation (i.e., the expression of markers from multiple lineages in a single cell), also a known characteristic of GBM (Martinez-Diaz et al., 2003; Perry et al., 2009). However, NOS2 inhibition did not affect multilineage differentiation patterns in fNPCs or GSCs (Figures 6K and 6L and Figure S5B), suggesting: 1) NOS2 inhibition does not affect the differentiation potential of NPCs, and 2) the anti-GSC effect of NOS2 inhibition does not relate to a promotion of differentiation. Together, these data suggest that NOS2-directed treatments would likely spare normal NPC growth and function, thus providing a favorable therapeutic margin for treating gliomas.
NOS2-directed interventions decrease glioma growth in vivo
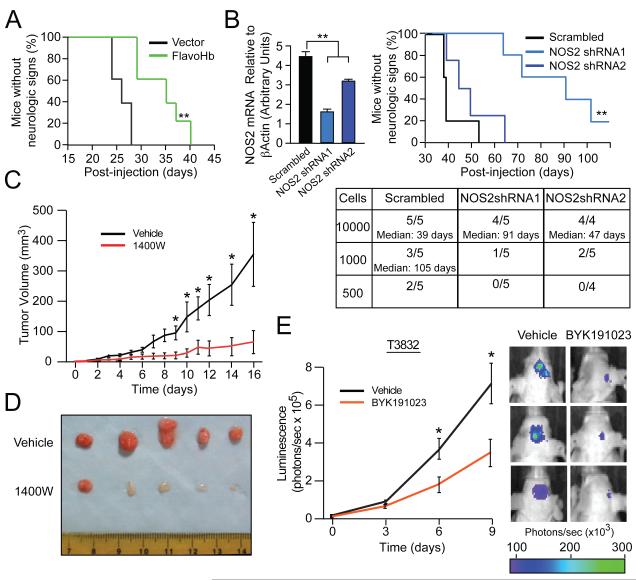
Next we sought to evaluate the potential anti-tumor effects of NOS2-directed interventions in vivo. First, we explored the relationship between cellular NO availability and tumor growth by implanting FlavoHb-expressing CD133+ GSCs into the brains of athymic mice, noting that consumption of cellular NO extended animal survival (Figure 7A). Next, to specifically evaluate the role of NOS2 expression within GSCs and tumorigenicity, we intracranially implanted NOS2-shRNA expressing GSCs into the forebrains of athymic mice. Consistent with results obtained with GSCs expressing the NO-consuming FlavoHb (Figure 7A), the time to development of neurological signs correlated with the extent of NOS2 knockdown (Figure 7B), suggesting that synthesis of NO by NOS2 contributes to the tumorigenicity of GSCs.
Figure 7. The tumor initiation and maintenance potential of GSCs is reduced by NO depletion and NOS2 knockdown/inhibition.
(A) Survival of athymic mice was tracked following intracranial implantation of 5000 CD133+ GSCs expressing either vector or FlavoHb. (B) An intracranial in vivo limiting dilution survival assay (employing 10000, 1000, 500 cells per mouse) was performed using T3691 CD133+ cells, with qRT-PCR-verified NOS2 knockdown. The table displays number of mice developing tumors and median time to neurologic signs. The survival curve displayed depicts mice injected with 10000 GSCs. (C) Tumor volumes and (D) images of GSC derived subcutaneous xenografts treated with daily intraperitoneal vehicle (n=6) or 1400W (n=6). (E) T3832 luciferase-expressing GSC-derived intracranial xenografts treated with intraperitoneal vehicle or BYK191023 (n=17/group) after engraftment and tracked by bioluminescence. Real-time images from median three animals on day 9 are shown (right). *, p<0.05; **, p<0.01. See also Figure S6.
To translate our findings into a clinically relevant approach, we studied the anti-tumor activity of small molecule inhibitors against NOS2 in vivo. First, we examined the effects of the NOS2 inhibitor 1400W in mice bearing subcutaneous human glioma xenografts. Mice receiving 50 mg/kg of 1400W daily for 2 weeks had reduced tumor volumes compared to vehicle controls (Figure 7C). When tumors were excised after 17 days, overall tumor burden was markedly decreased by 1400W (Figure 7D), suggesting that NOS2 inhibitors have anti-glioma effects in vivo. In these same tumors, there were indications of grossly decreased tumor angiogenesis as indicated by a decrease in vascularity (Figure 7C).
To confirm the efficacy of systemically administered NOS2 inhibitors using a more anatomically-relevant model, NOS2 inhibitors were used against intracranial glioma xenografts. The blood brain barrier (BBB), however, provides a formidable pharmacokinetic obstacle for charged or polar compounds such as the NOS2 inhibitor 1400W. Penetration of the BBB by 1400W is limited (Rebello et al., 2002), thus prompting the selection of alternative NOS2 inhibitors for BBB penetration (Figure S6A). The more lipophilic NOS2 inhibitor, BYK191023 (Strub et al., 2006) exhibited similar efficacy as 1400W for inhibition of CD133+ GSC growth in vitro (Figure S6B). However, BYK191023 possesses more suitable characteristics for BBB penetration, so it was therefore considered ideal for application in the intracranial glioma model. Luciferase-expressing glioma xenografts were implanted into the brains of athymic mice. After an engraftment period, tumor-bearing animals were randomly assigned to treatment groups and mice treated with 60 mg/kg BYK191023 twice daily demonstrated significantly decreased tumor growth as measured by luminescence using two different patient-derived glioma xenografts in separate experiments(Figure 7E and Figure S6C). To assess whether these effects of NOS2 inhibition were related to GSCs, control and BYK191023 treated tumors were dissociated and cells evaluated for functional characteristics of GSCs. In vivo NOS2 inhibitor treatment decreased the ability of cells to form neurospheres (Figure S6D) and initiate secondary tumors in re-transplantation assays (Figure S6E). Inhibitor-treated tumors therefore had fewer GSCs or GSCs with diminished self-renewal and tumorigenic capacity.
To more readily distinguish between requirements for NOS2 in tumor initiation or maintenance, an inducible NOS2 knockdown construct was used to knockdown NOS2 at implantation and then shRNA induction was released to allow tumor growth with normal NOS2 expression levels. Transient knockdown during tumor engraftment did not affect overall tumor growth kinetics in vivo (Figure S6F). These data suggest that the effect of NOS2 activity in GSCs relates primarily to the maintenance, rather than the engraftment/initiation, of glioma. No toxicities were observed in the mice treated with BYK191023, and BYK191023 was without effect on the slow-cycling sub-ependymal neural progenitors in non-tumor bearing mice (Figures S6G and S6H). Collectively, these data suggest that NOS2-directed therapeutics may represent a non-toxic and effective anti-GSC strategy with an effect primarily linked to tumor maintenance.
DISCUSSION
Here we demonstrate that NO synthesis, secondary to high NOS2 expression, is a distinctive feature of GSCs relative to non-GSCs and normal neural progenitors. Blockade of cellular NO availability with FlavoHb-based consumption or NOS2 inhibition/knockdown resulted in decreased GSC growth and tumorigenic capacity, suggesting an integral role for NO and endogenous NOS2 activity in the biology of GSCs. These findings are consistent with the wealth of reports showing that endogenously produced NO is generally cytoprotective (Rai et al., 1998; Sinz et al., 1999). In addition, systemic NOS2 inhibitors have been shown to block tumor growth (Thomsen et al., 1997), and NOS2 has an established cytoprotective role in chronic lymphocytic leukemia (Levesque et al., 2003; Zhao et al., 1998). Until our study, a role for CSC-synthesized NO has remained unexplored.
The molecular mechanisms by which NOS2 facilitates GSC proliferation and tumor growth are likely broad, as NO regulates a wide range of signaling pathways. The microarray analysis of GSCs treated with NOS2-knockdown suggests that NOS2 plays a role in regulating gene transcription of a variety of targets (Table S1) including suppression of the cell cycle inhibitor CDA1, which has not previously been identified as an NO-regulated gene. Initiated by our microarray studies, we were able to determine that CDA1 repression mediates, at least in part, NO-mediated proliferation in GSCs. We provide evidence that NO likely represses overall transcription rates of CDA1, versus effects on CDA1 mRNA or protein stability (Figures S4F, S4G). Further, it has previously been demonstrated that GSCs support tumor-mediated angiogenesis (Bao et al., 2006b), and our studies suggest a role for NOS2 as a pro-angiogenic factor in GSCs, as NOS2 inhibitor-treated tumors exhibited a gross decrease in blood vessels (Figure 7D). However, the contribution of NOS2 toward glioma angiogenesis remains a question for future study, while many other downstream targets for CSC-derived NO remain to be analyzed in the context of GSCs.
Previous studies have reported a role for NO in facilitating glioma cell growth (Lam-Himlin et al., 2006; Yamaguchi et al., 2002), and it has recently been suggested that NO synthesized by NOS3 (in the endothelium) or NOS1 (in glioma cells) may represent mechanisms by which the vasculature and neoplastic cells interact with each other to affect glioma growth and response to therapy (Charles et al., 2010; Kashiwagi et al., 2008). In particular, a recent publication (Charles et al., 2010) proposes a role for endothelium in promoting stem-like phenotypes in glioma cells in a PDGF-driven mouse model of glioma. Through in vitro administration of exogenous NO donors, they propose a role for NO (purportedly derived from the NOS3 activity in the vasculature in vivo) in the maintenance of GSC stem cell signaling. However, while their data do suggest that exogenous NO can promote certain CSC phenotypes, the source of this NO in vivo was never conclusively demonstrated beyond correlative staining. Further, even if endothelial NOS3 activity does play a role in sustaining GSCs, it is unlikely that NOS3-directed therapies will find clinical utility due to the negative impacts of inhibiting NOS3 in humans (Alexander et al., 2007; Avontuur et al., 1998; Lopez et al., 2004). Finally, the side scatter-based method for isolating GSCs has recently been called into question as a valid technique for GSC isolation (Broadley et al., 2010).
Our report of NOS2 expression and activity within GSCs, however, is distinct from these previously identified roles for NO in glioma. This is the first study to identify a cell-autonomous, GSC-specific source of NO, and definitively highlights the expression and biological effects of the NOS2 isoform within GSCs using loss-of-function, multiple inhibitors, and NO-consumption. Moreover, our loss-of-function studies identify many genetic targets for NOS2 in GSCs (including the cell cycle inhibitor protein CDA1), providing novel mechanistic information distinct from previous studies using exogenous NO donors. Finally, NOS2 inhibition may represent an anti-glioma treatment option with an acceptably low toxicity profile, as demonstrated by the negligible toxicity of NOS2 inhibitor administration to humans (Brindicci et al., 2009; Dover et al., 2006; Singh et al., 2007).
We evaluated the toxicity of NOS2 inhibition on normal NPCs, and our results suggest nominal expression of NOS2 in normal NPCs as well as minimal role for NOS2 in NPC growth. Our ability to fully assess the role of NOS2 in normal adult human NPCs was restricted by the lack of functionally-validated established cell surface markers for human adult NPCs, so we could not assess NOS2 in NPCs acutely sorted from fresh human brain tissue. Thus, we utilized a strategy by which human adult neural stem cells are isolated by nestin-driven GFP via viral infection, which requires several days of cell culture. Cell culture has the capacity to select for cell populations or induce genetic or epigenetic changes in cells, and thus our studies of adult human NPCs are limited by the necessity of cell culture with these previously validated techniques (Keyoung et al., 2001).
Though one required characteristic of CSCs relates to their ability to generate tumors in transplantation assays, it is critical to realize that the molecular characteristics supporting the malignant characteristics of GSCs are not necessarily the same molecular alterations that permit transformation and thus generation of tumors de novo (Visvader, 2011). The anti-tumor effect of NOS2 inhibitors against engrafted tumors implies a role for NOS2 in the GSC-supported tumor maintenance. However, this does not mean that NOS2 is essential for the initial transformative event in tumors. In fact, our data suggest that the role of NOS2 relates primarily to tumor maintenance and not engraftment capacity (Figure S6F).
We observed anti-tumor effects for 1400W and BYK191023 against glioma xenografts. Although 1400W effectively decreased subcutaneous tumor growth, it displayed limited efficacy against intracranial tumors (data not shown), likely due to the poor pharmacokinetic parameters of 1400W for intracranial delivery and BBB penetration (i.e., LogP = 0.71, cationic at neutral pH). The more lipophilic NOS2-selective inhibitor BYK191023, however, demonstrated effective anti-tumor activity against intracranial xenografts (Figure 7E). Though neither drug abolished tumor growth completely, both delayed tumor growth to a significant extent and may be useful when combined in multi-modal treatment regimens that also target the tumor bulk.
BYK191023 could be a strong candidate for clinical evaluation as it possesses the following desirable characteristics: 1) exhibits at least 1000 fold selectivity for NOS2 over NOS1 and NOS3 (MacMicking et al., 1995; Strub et al., 2006), 2) adheres to “Lipinski’s rules of five” for optimal pharmacokinetics and bioavailability (Lipinski et al., 2001), 3) is sufficiently lipophilic for BBB penetration (i.e., LogP 1.84), and 4) decreases GSC growth and survival in vitro and in vivo. In combination with the minimal toxic potential for NOS2 inhibition in humans and the effective anti-GSC activity demonstrated in our investigations, the data provided here will hopefully serve as an impetus for evaluation of NOS2-directed therapies as a component of multimodal treatment regimens for human glioma.
EXPERIMENTAL PROCEDURES
(Extended Experimental Procedures provided in Supplementary Information)
Isolation and culture of GSCs and non-GSCs from xenografts and primary human specimens
GSCs and non-GSCs were isolated from tumor tissue or xenografts (Table S2) as previously described (Bao et al., 2006a; Li et al., 2009).
Nitrite level determination by 4,5-diaminofluorescein (DAF-2)
For determination of cellular nitrite production, conditioned supernatants or nitrite standards were added to 4,5-diaminofluorescein (Kojima et al., 1998; Nakatsubo et al., 1998) (DAF-2; Cayman Chemical). Samples were analyzed post-acidification and neutralization for fluorescence measured with λex = 488 nm and λem = 525 nm and results normalized to cellular protein content (measured by Bradford assay; Bio-Rad).
Neurosphere formation assay
Neurosphere formation assays were performed similar to our prior report (Li et al., 2009) with propidium iodide negative cells sorted by FACS to a single cells per well of 96 well plates. For NOS2 inhibitor studies, vehicle or 100 μM 1400W was added daily. Neurosphere formation was measured as the percent of wells with neurospheres after 10 days.
Assessment of in vivo (intracranial) glioma progression following treatment with NOS2 inhibitors, NOS-knockdown and flavohemoglobin
For bioluminescence imaging, GSCs stably expressing firefly luciferase were intracranially injected into the right forebrains of 4-6 week old athymic nude mice and the Xenogen system used for imaging every three days. Survival studies were performed as in our prior report (Li et al., 2009) with intracranial injection of lentivirally infected CD133+ cells. Mice were monitored daily until the development of neurological or constitutional signs (e.g., ataxia, lethargy, seizures).
Retrospective analysis of NOS2 and CDA1 gene expression in human gliomas
The National Cancer Institute’s Repository for Molecular Brain Neoplasia Data (REMBRANDT, http://rembrandt.nci.nih.gov, accessed 6/25/10) was evaluated for correlations between clinical outcome/survival and gene expression in malignant glioma biopsies. For REMBRANDT, “upregulated” is defined as expression > 2.0 fold relative to mean values in normal tissue, whereas “downregulated” is defined as expression < 0.5 fold relative to mean values in normal tissue. “Intermediate” expression is the range between “upregulated” and “downregulated” (i.e., between 0.5 and 2 fold relative to mean values in normal tissue).
Microarray expression analysis of NOS2-knockdown in CD133+ GSCs
CD133+ cells derived from two different xenografts (T3359 and T3691) were treated in duplicate for 72 h with either non-targeting scramble control shRNA or two distinct NOS2-directed shRNAs. Total RNA was harvested, reverse transcribed, labeled, and hybridized to Illumina BeadArrays. BeadStudio software was used to normalize scanned chip data and to subtract background signal, and chip effects removed by ANOVA.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the sources of our funding, including: the National Institute of Neurological Disorders and Stroke (F30NS063496; CEE), Pediatric Brain Tumor Foundation of the US (JNR), American Brain Tumor Society (JNR and ABH), Goldhirsh Foundation (JNR), Duke Comprehensive Cancer Center Stem Cell Initiative Grant (JNR), the Damon Runyon Cancer Research Foundation (JNR), National Institutes of Health (NS054276, CA112958, CA116659, CA154130 for JNR; CA151522 for ABH), American Brain Tumor Association (JDL), National Cancer Institute (CA101954, CA11625 for AES), the Ben & Catherine Ivy Foundation (AES), and the Pediatric Brain Tumor Foundation of the United States (REM). We appreciate critical manuscript review by H. Suh, animal support provided by T. Myshrall, Y. Parker, and D. Lindner, M. Cook, B. Harvat, C. Shemo, and S. O’Bryant for flow cytometry assistance, D. Satterfield, L. Ehinger, J. Funkhouser, and J. Faison for technical assistance, the tissue provided by the Cleveland Clinic Foundation Tissue Procurement Service and S. Staugatis, R. Weil, and M. McGraw, and microarray analysis completed by P. Faber and W. Jones.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alexander JH, Reynolds HR, Stebbins AL, Dzavik V, Harrington RA, Van de Werf F, Hochman JS. Effect of tilarginine acetate in patients with acute myocardial infarction and cardiogenic shock: the TRIUMPH randomized controlled trial. JAMA. 2007;297:1657–1666. doi: 10.1001/jama.297.15.joc70035. [DOI] [PubMed] [Google Scholar]
- Androutsellis-Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW, Rueger MA, Bae SK, Kittappa R, McKay RD. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442:823–826. doi: 10.1038/nature04940. [DOI] [PubMed] [Google Scholar]
- Avontuur JA, Nolthenius R.P. Tutein, van Bodegom JW, Bruining HA. Prolonged inhibition of nitric oxide synthesis in severe septic shock: a clinical study. Crit Care Med. 1998;26:660–667. doi: 10.1097/00003246-199804000-00012. [DOI] [PubMed] [Google Scholar]
- Bakshi A, Nag TC, Wadhwa S, Mahapatra AK, Sarkar C. The expression of nitric oxide synthases in human brain tumours and peritumoral areas. J Neurol Sci. 1998;155:196–203. doi: 10.1016/s0022-510x(97)00315-8. [DOI] [PubMed] [Google Scholar]
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006a;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, Shi Q, McLendon RE, Bigner DD, Rich JN. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006b;66:7843–7848. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- Bar EE, Chaudhry A, Lin A, Fan X, Schreck K, Matsui W, Piccirillo S, Vescovi AL, DiMeco F, Olivi A, et al. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells. 2007;25:2524–2533. doi: 10.1634/stemcells.2007-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, Weinberg RA. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40:499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- Brindicci C, Ito K, Torre O, Barnes PJ, Kharitonov SA. Effects of aminoguanidine, an inhibitor of inducible nitric oxide synthase, on nitric oxide production and its metabolites in healthy control subjects, healthy smokers, and COPD patients. Chest. 2009;135:353–367. doi: 10.1378/chest.08-0964. [DOI] [PubMed] [Google Scholar]
- Broadley KW, Hunn MK, Farrand KJ, Price KM, Grasso C, Miller RJ, Hermans IF, McConnell MJ. Side Population is not Necessary or Sufficient for a Cancer Stem Cell Phenotype in Glioblastoma Multiforme. Stem Cells. 2010 doi: 10.1002/stem.582. [DOI] [PubMed] [Google Scholar]
- Chai Z, Sarcevic B, Mawson A, Toh BH. SET-related cell division autoantigen-1 (CDA1) arrests cell growth. J Biol Chem. 2001;276:33665–33674. doi: 10.1074/jbc.M007681200. [DOI] [PubMed] [Google Scholar]
- Charles N, Ozawa T, Squatrito M, Bleau AM, Brennan CW, Hambardzumyan D, Holland EC. Perivascular nitric oxide activates notch signaling and promotes stem-like character in PDGF-induced glioma cells. Cell Stem Cell. 2010;6:141–152. doi: 10.1016/j.stem.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol. 2007;17:165–172. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbs CS, Brenman JE, Aldape KD, Bredt DS, Israel MA. Expression of nitric oxide synthase in human central nervous system tumors. Cancer Res. 1995;55:727–730. [PubMed] [Google Scholar]
- Dover AR, Chia S, Ferguson JW, Cruden NL, Megson IL, Fox KA, Newby DE. Inducible nitric oxide synthase activity does not contribute to the maintenance of peripheral vascular tone in patients with heart failure. Clin Sci (Lond) 2006;111:275–280. doi: 10.1042/CS20060104. [DOI] [PubMed] [Google Scholar]
- Eissa NT, Strauss AJ, Haggerty CM, Choo EK, Chu SC, Moss J. Alternative splicing of human inducible nitric-oxide synthase mRNA. tissue-specific regulation and induction by cytokines. J Biol Chem. 1996;271:27184–27187. doi: 10.1074/jbc.271.43.27184. [DOI] [PubMed] [Google Scholar]
- Eissa NT, Yuan JW, Haggerty CM, Choo EK, Palmer CD, Moss J. Cloning and characterization of human inducible nitric oxide synthase splice variants: a domain, encoded by exons 8 and 9, is critical for dimerization. Proc Natl Acad Sci U S A. 1998;95:7625–7630. doi: 10.1073/pnas.95.13.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels K, Knauer SK, Loibl S, Fetz V, Harter P, Schweitzer A, Fisseler-Eckhoff A, Kommoss F, Hanker L, Nekljudova V, et al. NO signaling confers cytoprotectivity through the survivin network in ovarian carcinomas. Cancer Res. 2008;68:5159–5166. doi: 10.1158/0008-5472.CAN-08-0406. [DOI] [PubMed] [Google Scholar]
- Fan X, Matsui W, Khaki L, Stearns D, Chun J, Li YM, Eberhart CG. Notch pathway inhibition depletes stem-like cells and blocks engraftment in embryonal brain tumors. Cancer Res. 2006;66:7445–7452. doi: 10.1158/0008-5472.CAN-06-0858. [DOI] [PubMed] [Google Scholar]
- Fetz V, Bier C, Habtemichael N, Schuon R, Schweitzer A, Kunkel M, Engels K, Kovacs AF, Schneider S, Mann W, et al. Inducible NO synthase confers chemoresistance in head and neck cancer by modulating survivin. Int J Cancer. 2009;124:2033–2041. doi: 10.1002/ijc.24182. [DOI] [PubMed] [Google Scholar]
- Forrester MT, Eyler CE, Rich JN. Bacterial flavohemoglobin: a molecular tool to probe mammalian nitric oxide biology. Biotechniques. 2011;50:41–45. doi: 10.2144/000113586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumura D, Kashiwagi S, Jain RK. The role of nitric oxide in tumour progression. Nat Rev Cancer. 2006;6:521–534. doi: 10.1038/nrc1910. [DOI] [PubMed] [Google Scholar]
- Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- Gardner PR, Gardner AM, Martin LA, Salzman AL. Nitric oxide dioxygenase: an enzymic function for flavohemoglobin. Proc Natl Acad Sci U S A. 1998;95:10378–10383. doi: 10.1073/pnas.95.18.10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey EP, Oplinger JA, Furfine ES, Kiff RJ, Laszlo F, Whittle BJ, Knowles RG. 1400W is a slow, tight binding, and highly selective inhibitor of inducible nitric-oxide synthase in vitro and in vivo. J Biol Chem. 1997;272:4959–4963. doi: 10.1074/jbc.272.8.4959. [DOI] [PubMed] [Google Scholar]
- Hausladen A, Gow A, Stamler JS. Flavohemoglobin denitrosylase catalyzes the reaction of a nitroxyl equivalent with molecular oxygen. Proc Natl Acad Sci U S A. 2001;98:10108–10112. doi: 10.1073/pnas.181199698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausladen A, Gow AJ, Stamler JS. Nitrosative stress: metabolic pathway involving the flavohemoglobin. Proc Natl Acad Sci U S A. 1998;95:14100–14105. doi: 10.1073/pnas.95.24.14100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Zhang F, Casey R, Nagayama M, Ross ME. Delayed reduction of ischemic brain injury and neurological deficits in mice lacking the inducible nitric oxide synthase gene. J Neurosci. 1997;17:9157–9164. doi: 10.1523/JNEUROSCI.17-23-09157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandalaft LE, Zudaire E, Portal-Nunez S, Cuttitta F, Jakowlew SB. Differentially expressed nucleolar transforming growth factor-beta1 target (DENTT) exhibits an inhibitory role on tumorigenesis. Carcinogenesis. 2008;29:1282–1289. doi: 10.1093/carcin/bgn087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi S, Tsukada K, Xu L, Miyazaki J, Kozin SV, Tyrrell JA, Sessa WC, Gerweck LE, Jain RK, Fukumura D. Perivascular nitric oxide gradients normalize tumor vasculature. Nat Med. 2008;14:255–257. doi: 10.1038/nm1730. [DOI] [PubMed] [Google Scholar]
- Keyoung HM, Roy NS, Benraiss A, Louissaint A, Suzuki A, Hashimoto M, Rashbaum WK, Okano H, Goldman SA. High-yield selection and extraction of two promoter-defined phenotypes of neural stem cells from the fetal human brain. Nat Biotechnol. 2001;19:843–850. doi: 10.1038/nbt0901-843. [DOI] [PubMed] [Google Scholar]
- Kojima H, Nakatsubo N, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, Hirata Y, Nagano T. Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal Chem. 1998;70:2446–2453. doi: 10.1021/ac9801723. [DOI] [PubMed] [Google Scholar]
- Lam-Himlin D, Espey MG, Perry G, Smith MA, Castellani RJ. Malignant glioma progression and nitric oxide. Neurochem Int. 2006;49:764–768. doi: 10.1016/j.neuint.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Levesque MC, Misukonis MA, O’Loughlin CW, Chen Y, Beasley BE, Wilson DL, Adams DJ, Silber R, Weinberg JB. IL-4 and interferon gamma regulate expression of inducible nitric oxide synthase in chronic lymphocytic leukemia cells. Leukemia. 2003;17:442–450. doi: 10.1038/sj.leu.2402783. [DOI] [PubMed] [Google Scholar]
- Li F, Sonveaux P, Rabbani ZN, Liu S, Yan B, Huang Q, Vujaskovic Z, Dewhirst MW, Li CY. Regulation of HIF-1alpha stability through S-nitrosylation. Mol Cell. 2007;26:63–74. doi: 10.1016/j.molcel.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S, Shi Q, Cao Y, Lathia J, McLendon RE, et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 2009;15:501–513. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KH, Ancrile BB, Kashatus DF, Counter CM. Tumour maintenance is mediated by eNOS. Nature. 2008;452:646–649. doi: 10.1038/nature06778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- Lopez A, Lorente JA, Steingrub J, Bakker J, McLuckie A, Willatts S, Brockway M, Anzueto A, Holzapfel L, Breen D, et al. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit Care Med. 2004;32:21–30. doi: 10.1097/01.CCM.0000105581.01815.C6. [DOI] [PubMed] [Google Scholar]
- Luo CX, Zhu XJ, Zhou QG, Wang B, Wang W, Cai HH, Sun YJ, Hu M, Jiang J, Hua Y, et al. Reduced neuronal nitric oxide synthase is involved in ischemia-induced hippocampal neurogenesis by up-regulating inducible nitric oxide synthase expression. J Neurochem. 2007;103:1872–1882. doi: 10.1111/j.1471-4159.2007.04915.x. [DOI] [PubMed] [Google Scholar]
- MacMicking JD, Nathan C, Hom G, Chartrain N, Fletcher DS, Trumbauer M, Stevens K, Xie QW, Sokol K, Hutchinson N, et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- Martinez-Diaz H, Kleinschmidt-DeMasters BK, Powell SZ, Yachnis AT. Giant cell glioblastoma and pleomorphic xanthoastrocytoma show different immunohistochemical profiles for neuronal antigens and p53 but share reactivity for class III beta-tubulin. Arch Pathol Lab Med. 2003;127:1187–1191. doi: 10.5858/2003-127-1187-GCGAPX. [DOI] [PubMed] [Google Scholar]
- Nakatsubo N, Kojima H, Kikuchi K, Nagoshi H, Hirata Y, Maeda D, Imai Y, Irimura T, Nagano T. Direct evidence of nitric oxide production from bovine aortic endothelial cells using new fluorescence indicators: diaminofluoresceins. FEBS Lett. 1998;427:263–266. doi: 10.1016/s0014-5793(98)00440-2. [DOI] [PubMed] [Google Scholar]
- NCI [accessed 2010 June 25];National Cancer Institute REMBRANDT home page. 2005 http://rembrandt.nci.nih.gov.
- Perry A, Miller CR, Gujrati M, Scheithauer BW, Zambrano SC, Jost SC, Raghavan R, Qian J, Cochran EJ, Huse JT, et al. Malignant gliomas with primitive neuroectodermal tumor-like components: a clinicopathologic and genetic study of 53 cases. Brain Pathol. 2009;19:81–90. doi: 10.1111/j.1750-3639.2008.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai RM, Lee FY, Rosen A, Yang SQ, Lin HZ, Koteish A, Liew FY, Zaragoza C, Lowenstein C, Diehl AM. Impaired liver regeneration in inducible nitric oxide synthasedeficient mice. Proc Natl Acad Sci U S A. 1998;95:13829–13834. doi: 10.1073/pnas.95.23.13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebello SS, Zhu B, McMonagle-Strucko K, Pulicicchio C, Merrill J, Luo Y, Shen L, Wang J, Adler D, Natarajan C. Pharmacokinetic and pharmacodynamic evaluation of inhibitors of inducible nitric oxide synthase (iNOS) in mice. AAPS PharmSci. 2002 [Google Scholar]
- Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Shackleton M, Quintana E, Fearon ER, Morrison SJ. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell. 2009;138:822–829. doi: 10.1016/j.cell.2009.08.017. [DOI] [PubMed] [Google Scholar]
- Singh D, Richards D, Knowles RG, Schwartz S, Woodcock A, Langley S, O’Connor BJ. Selective inducible nitric oxide synthase inhibition has no effect on allergen challenge in asthma. Am J Respir Crit Care Med. 2007;176:988–993. doi: 10.1164/rccm.200704-588OC. [DOI] [PubMed] [Google Scholar]
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- Sinz EH, Kochanek PM, Dixon CE, Clark RS, Carcillo JA, Schiding JK, Chen M, Wisniewski SR, Carlos TM, Williams D, et al. Inducible nitric oxide synthase is an endogenous neuroprotectant after traumatic brain injury in rats and mice. J Clin Invest. 1999;104:647–656. doi: 10.1172/JCI6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son MJ, Woolard K, Nam DH, Lee J, Fine HA. SSEA-1 is an enrichment marker for tumor-initiating cells in human glioblastoma. Cell Stem Cell. 2009;4:440–452. doi: 10.1016/j.stem.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strub A, Ulrich WR, Hesslinger C, Eltze M, Fuchss T, Strassner J, Strand S, Lehner MD, Boer R. The novel imidazopyridine 2-[2-(4-methoxy-pyridin-2-yl)-ethyl]-3H-imidazo[4,5-b]pyridine (BYK191023) is a highly selective inhibitor of the inducible nitric-oxide synthase. Mol Pharmacol. 2006;69:328–337. doi: 10.1124/mol.105.017087. [DOI] [PubMed] [Google Scholar]
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- Taylor MD, Poppleton H, Fuller C, Su X, Liu Y, Jensen P, Magdaleno S, Dalton J, Calabrese C, Board J, et al. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell. 2005;8:323–335. doi: 10.1016/j.ccr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Thomsen LL, Scott JM, Topley P, Knowles RG, Keerie AJ, Frend AJ. Selective inhibition of inducible nitric oxide synthase inhibits tumor growth in vivo: studies with 1400W, a novel inhibitor. Cancer Res. 1997;57:3300–3304. [PubMed] [Google Scholar]
- Tiscornia AC, Cayota A, Landoni AI, Brito C, Oppezzo P, Vuillier F, Robello C, Dighiero G, Gabus R, Pritsch O. Post-transcriptional regulation of inducible nitric oxide synthase in chronic lymphocytic leukemia B cells in pro- and antiapoptotic culture conditions. Leukemia. 2004;18:48–56. doi: 10.1038/sj.leu.2403169. [DOI] [PubMed] [Google Scholar]
- Tu Y, Wu W, Wu T, Cao Z, Wilkins R, Toh BH, Cooper ME, Chai Z. Antiproliferative autoantigen CDA1 transcriptionally up-regulates p21(Waf1/Cip1) by activating p53 and MEK/ERK1/2 MAPK pathways. J Biol Chem. 2007;282:11722–11731. doi: 10.1074/jbc.M609623200. [DOI] [PubMed] [Google Scholar]
- Visvader JE. Cells of origin in cancer. Nature. 2011;469:314–322. doi: 10.1038/nature09781. [DOI] [PubMed] [Google Scholar]
- Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron. 1999;22:103–114. doi: 10.1016/s0896-6273(00)80682-0. [DOI] [PubMed] [Google Scholar]
- Wei XQ, Charles IG, Smith A, Ure J, Feng GJ, Huang FP, Xu D, Muller W, Moncada S, Liew FY. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375:408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- Williams EL, Djamgoz MB. Nitric oxide and metastatic cell behaviour. Bioessays. 2005;27:1228–1238. doi: 10.1002/bies.20324. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Bell HS, Shinoda J, Holmes MC, Wharton SB, Whittle IR. Glioma tumorigenicity is decreased by iNOS knockout: experimental studies using the C6 striatal implantation glioma model. Br J Neurosurg. 2002;16:567–572. [PubMed] [Google Scholar]
- Yang DI, Yin JH, Mishra S, Mishra R, Hsu CY. NO-mediated chemoresistance in C6 glioma cells. Ann N Y Acad Sci. 2002;962:8–17. doi: 10.1111/j.1749-6632.2002.tb04052.x. [DOI] [PubMed] [Google Scholar]
- Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H, Morrison SJ. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- Yuan X, Curtin J, Xiong Y, Liu G, Waschsmann-Hogiu S, Farkas DL, Black KL, Yu JS. Isolation of cancer stem cells from adult glioblastoma multiforme. Oncogene. 2004;23:9392–9400. doi: 10.1038/sj.onc.1208311. [DOI] [PubMed] [Google Scholar]
- Zhao H, Dugas N, Mathiot C, Delmer A, Dugas B, Sigaux F, Kolb JP. B-cell chronic lymphocytic leukemia cells express a functional inducible nitric oxide synthase displaying anti-apoptotic activity. Blood. 1998;92:1031–1043. [PubMed] [Google Scholar]
- Zhao X, Haensel C, Araki E, Ross ME, Iadecola C. Gene-dosing effect and persistence of reduction in ischemic brain injury in mice lacking inducible nitric oxide synthase. Brain Res. 2000;872:215–218. doi: 10.1016/s0006-8993(00)02459-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.