Abstract
Few studies have quantitatively evaluated the uptake and outcomes of BRCA1/2 genetic counseling and testing in men. We conducted a prospective longitudinal study to describe and compare uptake of and psychosocial outcomes following BRCA1/2 testing in a sample of men and women at high-risk for carrying a BRCA1/2 mutation. Men (n = 98) and women (n = 243) unaffected with cancer completed baseline assessments prior to genetic counseling and testing and then 6- and 12-months post-testing. Most men (n = 94; 95.9%) opted to have genetic testing, of whom 44 received positive BRCA1/2 genetic test results and 50 received true negative results. Among women, 93.4% had genetic testing, of whom 79 received positive results and 148 received negative results. In multivariate models, male BRCA1/2 carriers reported significantly higher genetic testing distress (6-months: Z = 4.48, P < 0.0001; 12-months: Z = 2.78, P < 0.01) than male non-carriers. After controlling for baseline levels of distress, no statistically significant differences emerged between male and female BRCA1/2 carriers in psychological distress at 12-months post-testing, although absolute differences were evident over time. Predictors of distress related to genetic testing among male carriers at 12-months included higher baseline cancer-specific distress (Z = 4.73, P < 0.0001) and being unmarried (Z = 2.18, P < 0.05). Similarly, baseline cancer-specific distress was independently associated with cancer-specific distress at 6- (Z = 3.66, P < 0.001) and 12-months (Z = 4.44, P < 0.0001) post-testing among male carriers. Clinically, our results suggest that pre-test assessment of distress and creation of educational materials specifically tailored to the needs and concerns of male carriers may be appropriate in this important but understudied high-risk group.
Keywords: BRCA1/2, Cancer risk, Genetic testing, Male female comparisons, Men, Psychosocial outcomes, Test uptake
Introduction
Clinical practice for assessing familial cancer risk now includes genetic testing for BRCA1 and BRCA2 (BRCA1/2) mutations. Despite the perception that BRCA1/2 genetic testing is relevant primarily for women, both men and women are equally likely to inherit a mutation [1, 2]. Women who inherit a BRCA1 or BRCA2 mutation face significantly elevated lifetime risks of breast and ovarian cancer (up to 65 and 40%, respectively), for which aggressive surveillance and surgical risk reduction options are recommended [3–5]. Although the cancer risks in female carriers are much higher relative to male carriers, the latter also have elevated cancer risks. Most significantly, male BRCA2 carriers appear to have at least a four-fold increase in the risk of prostate cancer; BRCA2 associated prostate cancers may be more aggressive and associated with higher mortality relative to sporadic cases [6, 7]. Male BRCA1 carriers also have an increased risk of prostate cancer, although these risks are not well delineated [7–9]. Prostate cancer in male carriers may also occur at an earlier age compared to sporadic cases of prostate cancer [9, 10]. Risks of other cancers in men with BRCA1/2 mutations are also elevated, including those of the breast and pancreas, although the lifetime risks of these cancers are under 10% [8]. Guidelines recommend that male mutation carriers undergo clinical breast exams twice a year, annual mammography if indicated (e.g., in men with gynecomastia or breast density), and prostate cancer screening [5, 7]. In addition to their own cancer risks, the children of male carriers are at 50% risk for inheriting their father’s mutation. Thus, the identification of male mutation carriers may have implications for their own medical management as well as that of their family.
The process and outcomes of BRCA1/2 testing in women have been well documented [11–13]. Evidence from observational studies to examine long-term behavioral and psychosocial outcomes among female BRCA1/2 carriers is accumulating [14, 15]. In contrast, despite initial qualitative studies, relatively few quantitative studies have evaluated the uptake and outcomes of BRCA1/2 genetic counseling and testing in men. The dearth of research conducted with men who pursue BRCA1/2 genetic counseling compared to the amount of research focused on women’s uptake of and outcomes following BRCA1/2 testing may be due to the higher degree of cancer risk among female carriers compared to male carriers, the lack of proven surveillance or risk management strategies available to male carriers, lower rate of BRCA1/2 testing uptake among men [16–18], or views that hereditary breast and ovarian cancer syndromes are more relevant to women than to men [18].
Uptake and predictors of testing
Several prior studies have reported higher rates of BRCA1/2 testing in women than in men [16–18], likely attributable to higher level of risk, greater options for risk management, salience of breast and ovarian cancer to women, and greater disclosure of information about hereditary cancer risks to women compared to men [19]. Despite discrepant rates of uptake, initial evidence suggests that men and women who opt for testing do so for similar reasons. Common motives for testing include concern for offspring, desire for information about cancer prevention and risk management, sense of perceived risk, feelings of familial obligation, guidance for reproductive decisions, and peace of mind [16, 18, 19]. Similarly, for both men and women, barriers to BRCA1/2 testing include concerns about genetic discrimination, psychological distress, guilt, and cost of testing.
Outcomes after BRCA1/2 testing
Although investigators have noted the importance of understanding the outcomes of BRCA1/2 testing among men due to the relatively few support programs and management strategies available to men [20], there has been limited quantitative research describing men’s psychological responses to BRCA1/2 testing over time. Qualitative and mixed-methods studies have reported a range of psychological responses among men who have received BRCA1/2 test results, including concerns about personal cancer risk, sadness about passing the disease risk down to children and relief from anxiety amongst non-carriers [21, 22]. To date, only a few studies have quantitatively assessed men’s psychological outcomes following BRCA1/2 genetic testing [14, 22–27]. Prior research includes smaller samples of men (e.g., 30–60 men) who pursued BRCA1/2 testing [14, 24, 27] and evaluated psychosocial and/or behavioral outcomes among male carriers and non-carriers. In one study, the small number of carriers prohibited statistical comparisons between carriers and noncarriers, although one of four identified male carriers and three male non-carriers reported high levels of post-testing distress [24]. Two studies reported general mental health outcomes among male carriers and non-carriers [14, 27]. Neither study reported differences between male carriers and non-carriers in general mental health outcomes at 1-year [27] or 3-years post-testing [14], nor were any significant changes in general mental health outcomes detected over time. Another investigation assessed the needs and experiences of 59 BRCA1/2 male carriers at an average of 2 years post result disclosure [22]. Slightly more than half of the men in this sample reported experiencing cancer-specific distress related to their cancer risk. Finally, among 87 men assessed following the receipt of genetic test results, male carriers who were the first in their families to receive genetic test results experienced short-term increases in psychological distress [26].
Given that men and women have an equal probability of inheriting a BRCA1/2 mutation, the similar stated reasons for test uptake between men and women, and the dearth of information about long-term psychosocial outcomes in male BRCA1/2 testers, we sought to expand upon the limited quantitative research in this area by prospectively evaluating the impact of BRCA1/2 testing in a larger sample of men. To date, we are unaware of any prospective studies with sufficient sample size to quantitatively evaluate general and cancer-specific psychosocial outcomes in male carriers and compare testing outcomes between male and female participants. Thus, the purpose of this investigation was to conduct a prospective longitudinal study to describe and compare uptake of and psychosocial outcomes following BRCA1/2 testing in a sample of men and women at high-risk for carrying a BRCA1/2 mutation.
Materials and methods
Participants
We recruited participants from an initial group of 1,134 individuals who participated in the genetic counseling and testing clinical research programs at the Lombardi Comprehensive Cancer Center (Washington, DC), Ruttenberg Cancer Center (New York, NY), or Englewood Hospital (NJ) between 2001 and 2005. In order to be eligible for this program, individuals had to be affected with breast or ovarian cancer, have a minimum 10% prior probability of carrying a BRCA1/2 mutation, or be a relative of a known mutation carrier. For the present report we focused on unaffected men and women with a known mutation in their family. We excluded all individuals who had been affected with breast cancer (n = 4 men, n = 594 women) or ovarian cancer (n = 67 women) due to the small number of affected men in the sample. We also excluded 29 individuals who did not complete the baseline assessment, 46 individuals who received uninformative BRCA1/2 test results and 53 women who had participated in a decision-making intervention following receipt of a positive BRCA1/2 test result. Thus, our final sample included 341 individuals unaffected with breast or ovarian cancer (n = 98 men; 243 women). All genetic counseling was provided free of charge. Genetic testing was provided free of charge at some but not all study sites and thus we evaluated the impact of study site in all analyses.
Procedure
All participants were self- or physician-referred to the genetic counseling program at one of the participating sites. Prior to the initial genetic counseling appointment, participants completed a baseline telephone interview to collect information on demographics, personal and family cancer history and psychological distress. After providing written informed consent, participants completed an initial genetic counseling session. Individuals who opted to pursue BRCA1/2 mutation testing provided a blood sample. Details of the content of the genetic counseling session are provided in previous reports [28]. After test results were available, participants completed a genetic counseling disclosure session, during which the test result was shared and implications of the test result were discussed, including associated prostate, breast and ovarian cancer risks, implications for family members, options for cancer prevention and surveillance, psychological issues related to disclosure, and referrals to other medical professionals for recommended follow-up. Following disclosure of test results, participants were contacted to complete follow-up telephone interviews at 6 and 12 months.
Measures
Control and predictor variables
Sociodemographics
Participants provided demographic information at the baseline interview, including age, race, education, marital status, employment status, number and ages of daughters and sons, insurance status, ancestry and annual family income. We summarized the overall number of daughters and sons as well as the number of each who were age 18 and younger at the time of the baseline survey. We dichotomized the other demographic variables as follows: age (≤50 versus >50), race (Caucasian versus other), marital status (married versus other), education (college graduate versus < college graduate), employment (employed full time versus other), insurance status (yes versus no), ancestry (Jewish versus other), annual family income (<$75,000 versus ≥ $75,000), and study site (Georgetown versus other). We combined the New York and New Jersey sites as the number of participants from New Jersey (n = 5 men and n = 10 women) was small.
Medical
Participants provided information via self-report regarding family cancer history (first and second degree relatives with breast/ovarian cancer) and personal medical history, including history related to cancer diagnosis, screening, surgery, other treatment, and years since diagnosis.
Genetic test result
Genetic test results were classified as true negative (no mutation found in an individual with an identified mutation in the family) or positive test result (presence of a BRCA1 or BRCA2 mutation). As noted above, individuals who received uninformative BRCA1/2 test results were not included in the sample.
Outcome variables
Cancer-specific distress
We measured distress related to cancer or risk for cancer using the Impact of Event Scale (IES) [29], which consists of intrusion and avoidance subscales. Items are scored on a weighted 4-point scale (0 = not at all, 1 = rarely, 3 = sometimes, 5 = often; range 0–75). Participants were asked to rate how frequently each thought occurred during the past 7 days. The IES was completed at baseline and the 6- and 12-month post-test follow-up assessments, with good internal consistency for the overall IES scale at each time point with Cronbach’s alphas of 0.90, 0.93 and 0.94, respectively.
General distress
The anxiety and depression subscales of the Brief Symptom Inventory (BSI) [30] were used to measure general distress. Items on these subscales were summed to create a general distress scale. The measure consists of 12 items scored on a 4-point scale (1 = not at all, 4 = extremely; range 12–48). Participants were asked to rate how much discomfort each of the symptoms had caused them during the previous 2 weeks. The BSI has good reliability and validity and has been successfully used as a measure of distress in medical populations [31]. Internal consistency using Cronbach’s alpha was 0.89 at baseline, 0.88 at 6-months and 0.91 at 12-months.
Genetic testing distress
The Multidimensional Impact of Cancer Risk Assessment Questionnaire (MICRA) [32] contains 25 items that assess participants’ specific responses to learning their genetic test results (i.e., “feeling relieved about your test result”, “feeling guilty about your test result”, “being uncertain about what your test result means about your cancer risk”). The items are scored on a weighted 4-point scale (0 = not at all, 1 = rarely, 3 = sometimes, 5 = often; range 0–125). Participants were asked to provide ratings for the past week. The measure contains three factors, Distress, Uncertainty, and Positive Experiences, which were combined to obtain a total score in this study. This total score has been found to differentiate between distress levels of individuals who test positive for BRCA1/2 and those who do not [32]. Cronbach’s alpha in the present study was 0.79 at 6-months and 0.82 at the 12-month follow-up.
Statistical analyses
We generated descriptive statistics to characterize sociodemographic, clinical and distress variables. To evaluate bivariate predictors of psychosocial distress outcomes, we used χ2 tests, analysis of variance, Pearson correlations and t-tests, identifying variables related to our outcomes at an alpha level of level of P ≤ 0.10. To determine which variables were independently associated with our distress outcomes, we conducted multiple linear regression analyses using Generalized Estimating Equations to control for intra-familial correlations. As appropriate, we included baseline levels of each outcome, potential confounders and study site in the regression models for our outcome variables.
Results
Sample characteristics
As displayed in Table 1, men had a mean age of 50.6 years (SD = 14.3, Range = 22–84 years) and women had a mean age of 42.9 (SD = 11.9, Range = 19–86 years). Most participants were Caucasian (95%), married (73%), and college educated (78%), and slightly more than half were employed full-time (57%). On average, participants had 1.5 family members affected with breast and/or ovarian cancer (SD = 1.7; Range = 0–7).
Table 1.
Characteristics of sample by sex
| Characteristic | Men (n = 98) | Women (n = 243) | All (n = 341) |
|---|---|---|---|
| Mean age (SD) | 50.6 (14.3) | 42.9 (12.0) | 45.1 (13.1) |
| Marital status | |||
| Married (%) | 83 (84.7) | 166 (68.3) | 249 (73.0) |
| Unmarried (%) | 15 (15.3) | 77 (31.7) | 92 (27.0) |
| Education | |||
| No college (%) | 16 (16.3) | 60 (24.7) | 76 (22.3) |
| Some college/degree (%) | 82 (83.7) | 183 (75.3) | 265 (77.7) |
| Employed | |||
| Full time (%) | 82 (83.7) | 112 (46.1) | 194 (56.9) |
| < Full time (%) | 16 (16.3) | 131 (53.9) | 147 (43.1) |
| Annual income | |||
| < $75,000 (%) | 31 (31.6) | 119 (49.0) | 150 (44.0) |
| ≥$75,000 (%) | 67 (68.4) | 124 (51.0) | 191 (56.0) |
| Race | |||
| White (%) | 92 (93.9) | 232 (95.5) | 324 (95.0) |
| Other (%) | 6 (6.1) | 11 (4.5) | 17 (5.0) |
| Ethnicity | |||
| Jewish (%) | 49 (50.0) | 139 (57.2) | 188 (55.1) |
| Non-jewish (%) | 49 (50.0) | 104 (42.8) | 153 (44.9) |
| FDRs with breast or ovarian cancer | |||
| < 2 (%) | 64 (65.3) | 178 (73.3) | 242 (71.0) |
| ≥2 (%) | 34 (34.7) | 65 (26.8) | 99 (29.0) |
FDRs First degree relatives
Rates of BRCA1/2 genetic testing uptake
A total of 94 men (95.9%) opted to have genetic testing and 227 women (93.4%) opted for testing. Men and women did not differ on rate of genetic test uptake, (N = 341) = 0.79, P = 0.37. Of the men who opted for testing, 44 received positive BRCA1/2 genetic test results and 50 received true negative results. Of the 44 male carriers, 20 had BRCA1 mutations and 24 had BRCA2 mutations. Among women, 79 received positive results (n = 42 with BRCA1 mutations and n = 37 with BRCA2 mutations) and 148 received negative results. Due to the small number of men (n = 4) who did not opt to pursue testing, we were unable to evaluate predictors of test uptake. Study site was unrelated to test uptake ( , P = 0.15).
Short- and long-term psychosocial outcomes
Impact of test result among men
In bivariate analyses, male carriers and non-carriers did not differ on general distress (t(n = 85) = −0.79, P = 0.43; t(n = 80) = 0.17, P = 0.87) or cancer-specific distress (t(n = 85) = 0.0, P = 0.99; t(n = 76) = −1.31, P = 0.19) at 6- or 12-months, respectively. However, male carriers did report significantly higher genetic testing distress compared to male non-carriers at both 6-months (t(n = 85) = 4.02, P < 0.0001) and 12-months (t(n = 79) = 2.82, P = 0.01) following genetic testing. Type of BRCA mutation (BRCA1 vs. BRCA2) did not have an impact on any of the psychosocial outcomes at 6- or 12-months following genetic testing. To determine whether test result (BRCA1/2 carrier vs. a true negative result) was an independent predictor of genetic testing distress, we used multiple linear regression models with generalized estimating equations (GEE) to account for potential confounders and correlated responses among family members. In these models, we controlled for baseline cancer-specific distress (since we did not have a measure of genetic testing distress prior to the receipt of test results), study site, and demographic/family history variables with significant bivariate associations with genetic testing distress. The results of these analyses are displayed in Table 2. In both multivariate models, carrier status remained a statistically significant predictor of genetic testing distress (6-months: Z = 4.48, P < 0.0001; 12-months: Z = 2.78, P < 0.01), with carriers reporting more genetic testing specific distress than non-carriers (see Table 2). Greater genetic testing distress at 6-and 12-months post-testing was also related to greater income.
Table 2.
Impact of BRCA1/2 test result on distress outcomes in men
| Variable | 6-Month outcomes Z scores
|
12-Month outcomes Z scores
|
||||
|---|---|---|---|---|---|---|
| Cancer-specific distress | General distress | Genetic-testing distress | Cancer-specific distress | General distress | Genetic-testing distress | |
| Associated baseline variablea | 2.76** | 4.97*** | 1.13 | 5.59*** | 3.92*** | 2.64** |
| Age | −2.96*** | −1.56 | −0.53 | −2.08* | −1.97* | −0.51 |
| # Daughters ≤ 18 years | −0.10 | −0.66 | −0.13 | 0.62 | −0.38 | 0.60 |
| Jewish ethnicity (vs. not) | 0.91 | 1.69b | 0.17 | 1.72b | 0.41 | −0.06 |
| Employed full-time (vs. not) | −2.31* | 1.15 | 1.05 | −1.37 | 0.18 | −0.86 |
| Annual income ≥ $75,000 (vs. not) | 1.02 | 0.55 | 2.19* | 0.21 | 1.04 | 2.25* |
| Study site (GU vs. NY/NJ) | 0.79 | 1.27 | −0.95 | 0.69 | 1.50 | 0.49 |
| BRCA1/2 carrier (vs. non-carrier) | −0.95 | 1.03 | 4.48*** | 0.62 | −0.15 | 2.78** |
We included the baseline variable associated with the specified outcome within the general estimating equation models. For genetic testing distress, we used cancer-specific distress at baseline, as we did not have a baseline measure of genetic testing distress
P < 0.10
P < 0.05;
P < 0.01;
P < 0.001
Comparisons between male and female BRCA1/2 carriers
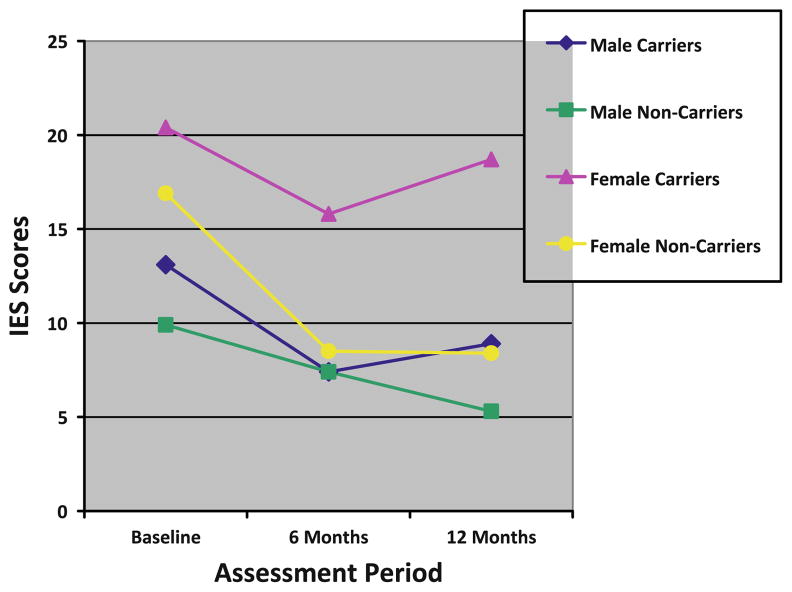
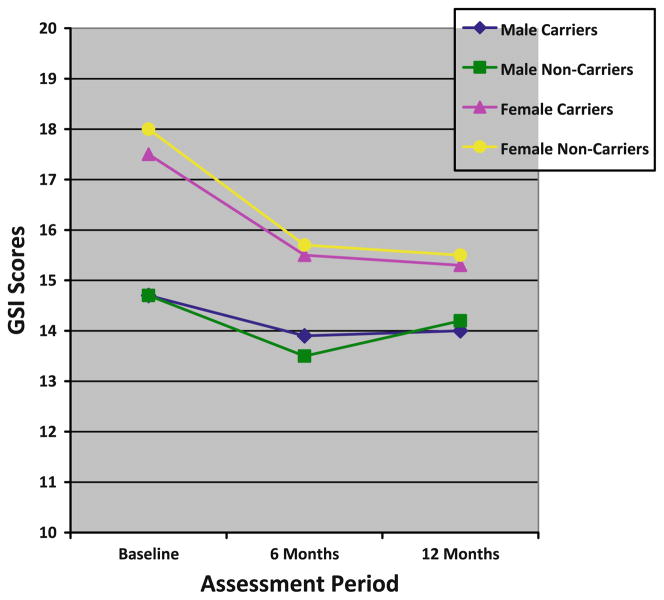
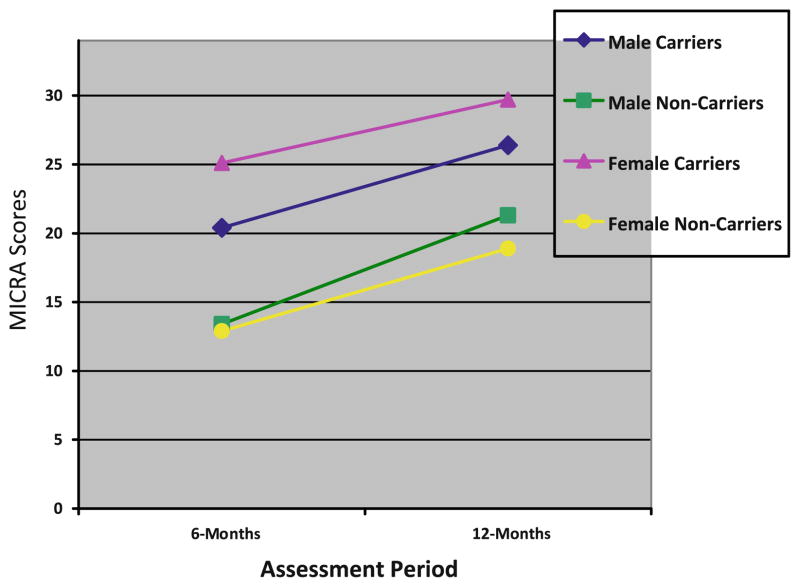
In bivariate analyses, female carriers reported statistically significantly greater distress than male carriers prior to testing and at both follow-up assessments on all distress outcomes with one exception—women did not report greater genetic testing distress at 12-months than men (see Figs. 1, 2 and 3). As displayed in Table 3, we evaluated whether male and female carriers differentially responded to receipt of a positive BRCA1/2 test result using linear regression models with GEE to account for familial clustering. After adjusting for baseline distress differences and confounding demographic factors, we found no statistically significant differences between male and female carriers on any of the distress outcomes following receipt of test results (see Table 3).
Fig. 1.
Cancer-specific distress scores
Fig. 2.
General distress scores
Fig. 3.
Genetic testing distress scores
Table 3.
Impact of sex of BRCA1/2 carrier on distress outcomes controlling for baseline and demographic factors
| Variable | 6-Month outcomes Z scores
|
12-Month outcomes Z scores
|
||||
|---|---|---|---|---|---|---|
| Cancer-specific distress | General distress | Genetic-testing distress | Cancer-specific distress | General distress | Genetic-testing distress | |
| Associated baseline variablea | 8.82*** | 4.00*** | 5.35*** | 8.54*** | 3.08** | 4.86*** |
| Age | −1.17 | 0.12 | −1.98* | −2.08* | −1.48 | −2.03* |
| # Sons ≤ 18 years | 1.52 | 0.80 | 1.03 | 1.49 | 1.13 | 1.20 |
| # Daughters ≤ 18 years | 0.45 | 0.26 | −0.05 | −0.07 | 1.30 | 0.41 |
| Family history total | 1.90b | 1.92b | 0.52 | 0.29 | −0.08 | 0.45 |
| Married (vs. unmarried) | −1.23 | −1.14 | −0.65 | −2.03* | −2.16* | −2.15* |
| Jewish ethnicity (vs. not) | 3.17 | 2.01* | 3.18** | 1.66b | −0.27 | 1.16 |
| Employed full-time (vs. not) | −1.35 | −0.23 | −1.02 | −0.66 | −0.52 | −1.74b |
| Study site (GU vs. NY/NJ) | 3.00** | 1.65b | 1.59 | 2.19* | 1.17 | 1.52 |
| Sex (Male vs. Female) | −0.30 | 0.85 | −1.05 | 0.09 | 0.74 | −1.89b |
We included the baseline variable associated with the specified outcome within the general estimating equation models. For genetic testing distress, we used cancer-specific distress at baseline, as we did not have a baseline measure of genetic testing distress
P < 0.10
P < 0.05;
P < 0.01;
P < 0.001
Despite a lack of statistically significant differences in distress between male and female carriers in the multivariate models, we did find absolute differences between men and women on 6- and 12-months cancer-specific distress outcomes. Importantly, these differences reflected the significant pre-testing differences between these groups and not a differential response to receiving a positive test result. We did not find such absolute differences with general or genetic testing distress outcomes in the multivariate models.
Comparisons between male and female non-carriers
We also examined whether men and women who tested negative for the BRCA1/2 mutation reported differences in distress prior to testing or following receipt of their test results. We again used linear regression models with GEE to account for familial clustering. As among carriers, female non-carriers reported higher pre-test cancer-specific (Z = 2.04, P = 0.04) and general distress (Z = 2.05, P = 0.04) than male non-carriers. At the follow-up assessments, female non-carriers did not differ from male non-carriers on any of the distress outcomes after adjusting for baseline distress and confounding demographic factors. In terms of absolute differences, female non-carriers reported greater general distress than male non-carriers at 6-months, but these differences disappeared at 12-months. Likewise, we did not see any absolute differences between male and female non-carriers on cancer-specific or genetic testing distress at the follow-up assessments.
Predictors of distress among male and female carriers
We conducted bivariate analyses to identify potential predictors of distress outcomes among male and female carriers. Among male carriers, Jewish participants reported more general distress at 6 months compared to non-Jewish participants (t(n = 85) = 2.03, P = 0.04) and men who were not employed full time reported greater general distress at 12 months (t(n = 80) = 2.22, P = 0.03) compared to men who were employed. Among female carriers, having daughters (t(n = 65) = −1.62, P = 0.10 with 12-months cancer-specific distress), having a greater family history of breast/ovarian cancer (r = 0.22, P = 0.07, with 6-months general distress), being unmarried, having lower income (t(n = 67) = 1.87, P = 0.07 and t(n = 67) = 1.88, P = 0.07 with 12-months general distress, respectively), and being Jewish (t(n = 66) = 1.63, P = 0.10 with 6-months genetic testing distress) were marginally associated with increased distress.
We used linear regression models with generalized estimating equations to account for familial clustering to look at independent predictors of distress in male BRCA1/2 carriers. In these models, we included the relevant baseline, demographic and clinical variables that had demonstrated significant relationships with distress outcomes among both male and female carriers at the P < 0.10 level. Although we used separate generalized estimating equation models for male carriers and female carriers, we controlled for the same set of variables across all of the models (see Table 4).
Table 4.
Predictors of distress in both male and female BRCA1/2 carriers
| Variable | 6-Months outcomes Z scores
|
12-Months outcomes Z scores
|
||||
|---|---|---|---|---|---|---|
| Cancer-specific distress | General distress | Genetic-testing distress | Cancer-specific distress | General distress | Genetic-testing distress | |
| Associated baseline variablea | ||||||
| Men | 3.66*** | 1.77c | 3.37*** | 4.44*** | 2.74** | 4.73*** |
| Women | 6.30*** | 3.58*** | 4.77*** | 6.22*** | 3.09** | 3.22** |
| Family history totalb | ||||||
| Men | 0.85 | 0.73 | 0.30 | −1.54 | 0.09 | 1.26 |
| Women | 1.62c | 1.88c | 0.13 | 0.71 | −0.26 | −0.37 |
| Having daughters (vs. not) | ||||||
| Men | 0.08 | 1.38 | 1.42 | 0.46 | 0.10 | 1.76c |
| Women | 1.04 | 0.25 | 0.06 | 1.43 | −0.07 | −0.20 |
| Married (vs. not) | ||||||
| Men | −1.11 | −1.55 | −1.32 | 1.90c | 0.05 | −2.18* |
| Women | −0.24 | −0.80 | −0.53 | −1.55 | −1.78c | −1.68 |
| Jewish ethnicity (vs. not) | ||||||
| Men | 1.12 | 0.93 | 1.78c | 1.52 | −0.23 | 0.68 |
| Women | 2.74** | 1.99* | 1.40 | 1.26 | −0.85 | −0.22 |
| Annual income ≥ $75,000 (vs. not) | ||||||
| Men | 1.19 | 1.60 | 1.55 | −1.15 | 1.81c | 1.14 |
| Women | 1.05 | 0.26 | −0.45 | 0.58 | −1.01 | −0.06 |
| Employed full-time (vs. not) | ||||||
| Men | −1.21 | 1.39 | 0.62 | −0.16 | 1.80c | −0.26 |
| Women | −0.67 | −0.98 | −1.50 | −0.79 | −1.52 | −1.47 |
| Study site (GU vs. NY/NJ) | ||||||
| Men | 0.96 | 0.46 | 0.31 | 0.37 | 1.47 | 1.67c |
| Women | 2.21* | 1.54 | 0.92 | 1.48 | −0.21 | 0.27 |
We included the baseline variable associated with the specified outcome within the general estimating equation models. For genetic testing distress, we used cancer-specific distress at baseline, as we did not have a baseline measure of genetic testing distress
Family history total represents the total number of first and second degree relatives diagnosed with breast or ovarian cancer
P < 0.10
P < 0.05;
P < 0.01;
P < 0.001
Cancer-specific distress
Among male carriers, baseline cancer-specific distress was independently associated with cancer-specific distress at 6- (Z = 3.66, P < 0.001) and 12-months (Z = 4.44, P < 0.0001). Not being married had a marginal effect on cancer-specific distress among male carriers at 12-months (Z = 1.90, P < 0.10). In female carriers, cancer-specific distress at baseline predicted cancer-specific distress at 6-months (Z = 6.30, P < 0.001), along with being Jewish (Z = 2.74, P < 0.01) and being from the Washington, DC testing site (Z = 2.21, P < 0.05). At 12-months, only greater baseline distress scores remained a significant predictor (Z = 6.22, P < 0.001) in female carriers.
General distress
Among male carriers, there were no independent predictors of general distress at 6-months. At 12-months, baseline general distress predicted greater general distress (Z = 2.74, P < 0.01). Lower income (Z = 1.81, P < 0.10) and not being employed (Z = 1.80, P < 0.10) were marginally associated with greater general distress at 12-months. Among female carriers, baseline levels also predicted general distress at 6-months (Z = 3.58, P < 0.001), as did Jewish ethnicity (Z = 1.99, P < 0.05), with trends for relationships between general distress and having a greater family history of breast/ovarian cancer (Z = 1.88, P < 0.10). At 12-months, general distress in female carriers was predicted by baseline levels (Z = 3.09, P < 0.01), with a trend for a relationship between general distress and being unmarried (1.80, P < 0.10).
Genetic testing distress
Predictors of higher genetic-testing specific distress among male carriers at 6-months were higher baseline cancer-specific distress (Z = 3.37, P < 0.001) with Jewish ethnicity (Z = 1.78, P < 0.10) showing a trend. At 12-months, higher genetic-testing specific distress was predicted by higher baseline cancer-specific distress (Z = 4.73, P < 0.0001), and being unmarried (Z = 2.18, P < 0.05), with trends for relationships with having daughters (Z = 1.76, P < 0.10) and study site (Z = 1.67, P < 0.10). Among female carriers, genetic testing distress at 6-months was predicted by baseline level of cancer-specific distress (Z = 4.77, P < 0.001). At 12-months, only baseline cancer-specific distress (Z = 3.22, P < 0.01) predicted genetic testing distress among female BRCA1/2 carriers.
Discussion
To our knowledge, this is the first prospective study conducted in the United States that quantitatively evaluates BRCA1/2 genetic testing and psychosocial outcomes among men, and the first US study to directly compare psychosocial outcomes between male and female carriers over time. Unlike prior studies [16, 17, 33], we found no difference in BRCA1/2 test uptake between men and women. Earlier studies were conducted within 5–6 years following the clinical availability of testing. In our work, individuals were offered testing 10 or more years following the advent of clinical testing. Thus, the lack of difference in uptake between men and women may be due to the increased awareness and clinical acceptance of BRCA1/2 testing. Also, our study focused on unaffected individuals with a known mutation in their family. If we had included affected individuals and those without known familial mutations, it is possible that test uptake differences between men and women may have emerged.
Accumulating evidence suggests that female BRCA1/2 carriers do not experience long-term increases in cancer-specific or general distress following the receipt of positive test results [11, 14, 34–36]. Consistent with this, we found that male BRCA1/2 carriers did not show significant increases in cancer-specific or general distress and did not differ from non-carriers at either the 6- or 12-month follow-up time points on these outcomes. In contrast, male carriers did report higher levels of genetic-testing specific distress than male non-carriers. This finding is consistent with data among female carriers indicating greater increases in genetic-testing specific distress among carriers compared to noncarriers [32].
Among male carriers, those who were unmarried and had higher cancer-specific distress prior to genetic testing were most at risk for experiencing genetic testing distress over time. In addition, Jewish ethnicity and having daughters were marginally related to genetic testing distress, although larger samples of male carriers would be needed to more thoroughly examine these trends. Perhaps the unmarried men in our sample were concerned about the potential impact their carrier status has on their own cancer risk or the cancer risk in future offspring, given prior findings that many men report pursuing genetic testing out of a sense of familial obligation [16, 19]. The lack of differences between male carriers and non-carriers on outcomes of general distress over time are consistent with earlier reports that examined general mental health outcomes in men who had BRCA1/2 testing [14, 27]. These findings suggest that differences on genetic testing distress due to carrier status may not extend to more global detriments in psychosocial functioning.
After controlling for pre-test levels of distress, male and female carriers largely did not differ on psychosocial outcomes; however, significant absolute differences remained over time such that women continued to report more distress than men. The one exception to these results is that male and female carriers did not differ on reports of genetic testing distress 1 year after receipt of their positive test results. Some evidence suggests that certain female BRCA1/2 carriers may experience short term increases in distress [37, 38], but that carrier status is not predictive of long-term psychosocial outcomes [15, 34]. Perhaps as women begin adjusting to and implementing risk management strategies, distress associated with genetic testing may begin to dissipate. For example, among women affected with breast cancer who then pursue BRCA1/2 testing and learn they are mutation carriers, those who are most distressed even before genetic counseling and testing are most likely to obtain a prophylactic contralateral mastectomy within the year following testing [39]. Similar work can explore whether and how risk management decisions impact psychosocial outcomes among BRCA1/2 carriers unaffected with cancer.
Of note, the overall level of cancer-specific distress for female non-carriers is very close to the level observed among the male carriers in the present sample. As the personal cancer risk in male carriers is much lower than the risk in female carriers, the observed differences between male and female carriers in overall level of cancer-specific distress is not surprising and is consistent with prior research [23]. The way men and women cope with and respond to information about genetic risk for cancer is likely different given evidence from prior studies that men may minimize the emotional impact of their carrier status, interpreting the information more in terms of familial versus personal impact [21, 24]. Future research can further explore use of additional support and coping resources among both male and female carriers.
The current study has a number of strengths, including the prospective longitudinal design, comparison of male and female carriers and non-carriers and use of GEE regression models to account for correlations in responses among family members. Limitations to the study include the homogenous sample in terms of education, race, and health insurance status; lack of assessment of reasons the study participants pursued BRCA1/2 genetic counseling and testing, and our inability to explore predictors of test uptake among men due to the small number of men who elected not to pursue testing. Prospective studies with larger sample sizes could address this limitation. We did not assess some of the nuanced reasons that may contribute to men’s distress, such as direct worry about daughters’ cancer risks or frustration over the lack of clear management strategies. Additional inquiry into specific targets for psychosocial support to male carriers is warranted. Finally, we are uncertain why study site differences emerged for a small number of our study outcomes. Future work could explore potential regional differences in distress related to genetic testing in terms of local norms, knowledge of genetic risk for cancer, or differences in the support services offered to carriers across clinical settings.
Given our results that, compared to non-carriers, male BRCA1/2 carriers report elevated levels of genetic testing distress up to 12-months following receipt of results and findings in prior work of a lack of support services specific to male carriers, identification of men at greater risk for distress over time may be helpful. Similar to several other reports, our work confirms that pre-testing levels of distress is highly predictive of distress over time in both male and female BRCA1/2 carriers [35, 40, 41]. These convergent findings across multiple studies and various high-risk samples suggest that having genetic counselors or health-care professionals conduct brief pre-test assessments to identify patients most at risk for poorer psychosocial outcomes may be beneficial [40]. Both men and women at greater potential risk for experiencing heightened distress could then be connected with appropriate support resources and/or have additional psychosocial support programs [42] during and following the BRCA1/2 testing process.
Importantly, the level of distress reported does not appear to be clinically significant for most of the men and women in the present sample, although subgroups may be more at risk over time. Our results suggest that creation of educational materials specifically tailored to the needs and concerns of male carriers may be appropriate. Moreover, having genetics and other health care professionals acknowledge the lack of clear medical management guidelines and explore men’s reactions to being at risk for breast and other cancers may help address some concerns among male carriers. Men from hereditary breast and ovarian cancer families may have encountered challenges in discussing their risk for breast cancer and/or requests for screening for the disease. Future work can further explore whether tools regarding communication with physicians about personal risk or their children’s risk would be useful for male carriers. As more evidence accumulates about the types of counseling, support and decision aid resources that are most efficacious for female BRCA1/2 carriers [28], similar questions can be explored with men from hereditary breast and ovarian cancer families to provide the most appropriate type of education and resources to this understudied but important population of at-risk individuals.
Acknowledgments
This study is Supported by National Cancer Institute Grants K07 CA131172 (KDG), R01 CA01846 (MDS), U56 CA101429 (Peter Shields) and The Jess and Mildred Fisher Center for Familial Cancer Research.
Contributor Information
Kristi D. Graves, Email: kdg9@georgetown.edu, Cancer Control Program, Department of Oncology, Lombardi Comprehensive Cancer Center, Georgetown University, 3300 Whitehaven Street, Suite 4100, Washington, DC 20007, USA. Fisher Center for Familial Cancer Research, Lombardi Comprehensive Cancer Center, Georgetown University, Washington, DC, USA
Rhoda Gatammah, University of the District of Columbia, Washington, DC, USA.
Beth N. Peshkin, Cancer Control Program, Department of Oncology, Lombardi Comprehensive Cancer Center, Georgetown University, 3300 Whitehaven Street, Suite 4100, Washington, DC 20007, USA. Fisher Center for Familial Cancer Research, Lombardi Comprehensive Cancer Center, Georgetown University, Washington, DC, USA
Ayelet Krieger, Cancer Control Program, Department of Oncology, Lombardi Comprehensive Cancer Center, Georgetown University, 3300 Whitehaven Street, Suite 4100, Washington, DC 20007, USA. Fisher Center for Familial Cancer Research, Lombardi Comprehensive Cancer Center, Georgetown University, Washington, DC, USA.
Christy Gell, Cancer Control Program, Department of Oncology, Lombardi Comprehensive Cancer Center, Georgetown University, 3300 Whitehaven Street, Suite 4100, Washington, DC 20007, USA.
Heiddis B. Valdimarsdottir, Mount Sinai School of Medicine, New York, NY, USA. Department of Health and Education, Reykjavik University, Reykjavik, Iceland
Marc D. Schwartz, Cancer Control Program, Department of Oncology, Lombardi Comprehensive Cancer Center, Georgetown University, 3300 Whitehaven Street, Suite 4100, Washington, DC 20007, USA. Fisher Center for Familial Cancer Research, Lombardi Comprehensive Cancer Center, Georgetown University, Washington, DC, USA
References
- 1.Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1998;62:676–689. doi: 10.1086/301749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Juan AS, Wakefield CE, Kasparian NA, Kirk J, Tyler J, Tucker K. Development and pilot testing of a decision aid for men considering genetic testing for breast and/or ovarian cancer-related mutations (BRCA1/2) Genet Test. 2008;12:523–532. doi: 10.1089/gte.2008.0035. [DOI] [PubMed] [Google Scholar]
- 3.Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25:1329–1333. doi: 10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. [Accessed 12/3/10];Genetic/Familial High-Risk Assessment: Breast and Ovarian–v.1.2010. 2010 from http://www.nccn.org/professionals/physician_gls/PDF/genetics_screening.pdf.
- 6.The Breast Cancer Linkage Consortium. Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst. 1999;91:1310–1316. doi: 10.1093/jnci/91.15.1310. [DOI] [PubMed] [Google Scholar]
- 7.Mitra AV, Bancroft EK, Barbachano Y, et al. Targeted prostate cancer screening in men with mutations in BRCA1 and BRCA2 detects aggressive prostate cancer: preliminary analysis of the results of the IMPACT study. BJU Int. 2010 doi: 10.1111/j.1464-410X.2010.09648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liede A, Karlan BY, Narod SA. Cancer risks for male carriers of germline mutations in BRCA1 or BRCA2: a review of the literature. J Clin Oncol. 2004;22:735–742. doi: 10.1200/JCO.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 9.Thompson D, Easton DF Breast Cancer Linkage Consortium . Cancer incidence in BRCA1 mutation carriers. J Natl Cancer Inst. 2002;94:1358–1365. doi: 10.1093/jnci/94.18.1358. [DOI] [PubMed] [Google Scholar]
- 10.Thompson D, Easton D Breast Cancer Linkage Consortium . Variation in cancer risks, by mutation position, in BRCA2 mutation carriers. Am J Hum Genet. 2001;68:410–419. doi: 10.1086/318181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graves KD, Schwartz MD. Psychological and behavioral impact of genetic testing. In: Isaacs C, Rebbeck TR, editors. Hereditary breast cancer: risk, prevention, and management. Informa Healthcare; New York: 2008. pp. 255–275. [Google Scholar]
- 12.Hamilton JG, Lobel M, Moyer A. Emotional distress following genetic testing for hereditary breast and ovarian cancer: a meta-analytic review. Health Psychol. 2009;28:510–518. doi: 10.1037/a0014778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meiser B, Halliday JL. What is the impact of genetic counselling in women at increased risk of developing hereditary breast cancer? A meta-analytic review. Soc Sci Med. 2002;54:1463–1470. doi: 10.1016/s0277-9536(01)00133-2. [DOI] [PubMed] [Google Scholar]
- 14.Foster C, Watson M, Eeles R, et al. Predictive genetic testing for BRCA1/2 in a UK clinical cohort: three-years follow-up. Br J Cancer. 2007;96:718–724. doi: 10.1038/sj.bjc.6603610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Oostrom I, Meijers-Heijboer H, Lodder LN, et al. Long-term psychological impact of carrying a BRCA1/2 mutation and prophylactic surgery: a 5-years follow-up study. J Clin Oncol. 2003;21:3867–3874. doi: 10.1200/JCO.2003.10.100. [DOI] [PubMed] [Google Scholar]
- 16.d’Agincourt-Canning L. Experiences of genetic risk: disclosure and the gendering of responsibility. Bioethics. 2001;15:231–247. doi: 10.1111/1467-8519.00234. [DOI] [PubMed] [Google Scholar]
- 17.Goelen G, Rigo A, Bonduelle M, De Greve J. Moral concerns of different types of patients in clinical BRCA1/2 gene mutation testing. J Clin Oncol. 1999;17:1595–1600. doi: 10.1200/JCO.1999.17.5.1595. [DOI] [PubMed] [Google Scholar]
- 18.Hallowell N, Ardern-Jones A, Eeles R, et al. Men’s decision-making about predictive BRCA1/2 testing: the role of family. J Genet Couns. 2005;14:207–217. doi: 10.1007/s10897-005-0384-3. [DOI] [PubMed] [Google Scholar]
- 19.Daly MB. The impact of social roles on the experience of men in BRCA1/2 families: implications for counseling. J Genet Couns. 2009;18:42–48. doi: 10.1007/s10897-008-9183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daly PA, Nolan C, Green A, et al. Predictive testing for BRCA1 and 2 mutations: a male contribution. Ann Oncol. 2003;14:549–553. doi: 10.1093/annonc/mdg164. [DOI] [PubMed] [Google Scholar]
- 21.Dudok De Wit AC, Tibben A, Frets PG, Meijers-Heijboer EJ, Devilee P, Niermeijer MF. Males at-risk for the BRCA1 gene, the psychological impact. Psychooncology. 1996;5:251–257. [Google Scholar]
- 22.Liede A, Metcalfe K, Hanna D, et al. Evaluation of the needs of male carriers of mutations in BRCA1 or BRCA2 who have undergone genetic counseling. Am J Hum Genet. 2000;67:1494–1504. doi: 10.1086/316907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denayer L, Boogaerts A, Philippe K, Legius E, Evers-Kiebooms G. BRCA1/2 predictive testing and gender: uptake, motivation and psychological characteristics. Genet Couns. 2009;20:293–305. [PubMed] [Google Scholar]
- 24.Lodder L, Frets PG, Trijsburg RW, et al. Men at risk of being a mutation carrier for hereditary breast/ovarian cancer: an exploration of attitudes and psychological functioning during genetic testing. Eur J Hum Genet. 2001;9:492–500. doi: 10.1038/sj.ejhg.5200668. [DOI] [PubMed] [Google Scholar]
- 25.Lynch HT, Lemon SJ, Durham C, et al. A descriptive study of BRCA1 testing and reactions to disclosure of test results. Cancer. 1997;79:2219–2228. doi: 10.1002/(sici)1097-0142(19970601)79:11<2219::aid-cncr21>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 26.Smith KR, West JA, Croyle RT, Botkin JR. Familial context of genetic testing for cancer susceptibility: moderating effect of siblings’ test results on psychological distress one to two weeks after BRCA1 mutation testing. Cancer Epidemiol Bio-markers Prev. 1999;8:385–392. [PubMed] [Google Scholar]
- 27.Watson M, Foster C, Eeles R, et al. Psychosocial impact of breast/ovarian (BRCA1/2) cancer-predictive genetic testing in a UK multi-centre clinical cohort. Br J Cancer. 2004;91:1787–1794. doi: 10.1038/sj.bjc.6602207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwartz MD, Valdimarsdottir HB, DeMarco TA, et al. Randomized trial of a decision aid for BRCA1/BRCA2 mutation carriers: impact on measures of decision making and satisfaction. Health Psychol. 2009;28:11–19. doi: 10.1037/a0013147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: a measure of subjective stress. Psychosom Med. 1979;41:209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Derogatis LR, Melisaratos N. The brief symptom inventory: an introductory report. Psychol Med. 1983;13:595–605. [PubMed] [Google Scholar]
- 31.Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10:19–28. doi: 10.1002/1099-1611(200101/02)10:1<19::aid-pon501>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 32.Cella D, Hughes C, Peterman A, et al. A brief assessment of concerns associated with genetic testing for cancer: the multidimensional impact of cancer risk assessment (MICRA) questionnaire. Health Psychol. 2002;21:564–572. [PubMed] [Google Scholar]
- 33.Finlay E, Stopfer JE, Burlingame E, et al. Factors determining dissemination of results and uptake of genetic testing in families with known BRCA1/2 mutations. Genet Test. 2008;12:81–91. doi: 10.1089/gte.2007.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwartz MD, Peshkin BN, Hughes C, Main D, Isaacs C, Lerman C. Impact of BRCA1/BRCA2 mutation testing on psychologic distress in a clinic-based sample. J Clin Oncol. 2002;20:514–520. doi: 10.1200/JCO.2002.20.2.514. [DOI] [PubMed] [Google Scholar]
- 35.Smith AW, Dougall AL, Posluszny DM, Somers TJ, Rubinstein WS, Baum A. Psychological distress and quality of life associated with genetic testing for breast cancer risk. Psychooncology. 2008;17:767–773. doi: 10.1002/pon.1291. [DOI] [PubMed] [Google Scholar]
- 36.Wagner TM, Moslinger R, Langbauer G, et al. Attitude towards prophylactic surgery and effects of genetic counselling in families with BRCA mutations. Austrian Hereditary Breast and Ovarian Cancer Group. Br J Cancer. 2000;82:1249–1253. doi: 10.1054/bjoc.1999.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meiser B, Butow P, Friedlander M, et al. Psychological impact of genetic testing in women from high-risk breast cancer families. Eur J Cancer. 2002;38:2025–2031. doi: 10.1016/s0959-8049(02)00264-2. [DOI] [PubMed] [Google Scholar]
- 38.Tercyak KP, Lerman C, Peshkin BN, et al. Effects of coping style and BRCA1 and BRCA2 test results on anxiety among women participating in genetic counseling and testing for breast and ovarian cancer risk. Health Psychol. 2001;20:217–222. [PubMed] [Google Scholar]
- 39.Graves KD, Peshkin BN, Halbert CH, DeMarco TA, Isaacs C, Schwartz MD. Predictors and outcomes of contralateral prophylactic mastectomy among breast cancer survivors. Breast Cancer Res Treat. 2007;104:321–329. doi: 10.1007/s10549-006-9423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ertmanski S, Metcalfe K, Trempala J, et al. Identification of patients at high risk of psychological distress after BRCA1 genetic testing. Genet Test Mol Biomarkers. 2009;13:325–330. doi: 10.1089/gtmb.2008.0126. [DOI] [PubMed] [Google Scholar]
- 41.Schlich-Bakker KJ, ten Kroode HF, Ausems MG. A literature review of the psychological impact of genetic testing on breast cancer patients. Patient Educ Couns. 2006;62:13–20. doi: 10.1016/j.pec.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 42.Graves KD, Wenzel L, Schwartz MD, et al. Randomized controlled trial of a psychosocial telephone counseling intervention in BRCA1 and BRCA2 mutation carriers. Cancer Epidemiol Biomarkers Prev. 2010;19:648–654. doi: 10.1158/1055-9965.EPI-09-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]