Abstract
Type 2 diabetes mellitus (T2DM) is a progressive multisystemic disease that increases significantly cardiovascular morbidity and mortality. It is associated with obesity, insulin resistance, beta-cell dysfunction, and hyperglucagonemia, the combination of which typically leads to hyperglycemia. Incretin-based treatment modalities, and in particular glucagon-like peptide 1 (GLP-1) receptor agonists, are able to successfully counteract several of the underlying pathophysiological abnormalities of T2DM. The pancreatic effects of GLP-1 receptor agonists include glucose-lowering effects by stimulating insulin secretion and inhibiting glucagon release in a strictly glucose-dependent manner, increased beta-cell proliferation, and decreased beta-cell apoptosis. GLP-1 receptors are widely expressed throughout human body; thus, GLP-1-based therapies exert pleiotropic and multisystemic effects that extend far beyond pancreatic islets. A large body of experimental and clinical data have suggested a considerable protective role of GLP-1 analogs in the cardiovascular system (decreased blood pressure, improved endothelial and myocardial function, functional recovery of failing and ischemic heart, arterial vasodilatation), kidneys (increased diuresis and natriuresis), gastrointestinal tract (delayed gastric emptying, reduced gastric acid secretion), and central nervous system (appetite suppression, neuroprotective properties). The pharmacologic use of GLP-1 receptor agonists has been shown to reduce bodyweight and systolic blood pressure, and significantly improve glycemic control and lipid profile. Interestingly, weight reduction induced by GLP-1 analogs reflects mainly loss of abdominal visceral fat. The critical issue of whether the emerging positive cardiometabolic effects of GLP-1 analogs can be translated into better clinical outcomes for diabetic patients in terms of long-term hard endpoints, such as cardiovascular morbidity and mortality, remains to be elucidated with prospective, large-scale clinical trials.
Keywords: glucagon-like peptide 1, glucagonlike peptide 1 receptor, GLP-1 analogs, GLP-1 receptor agonists, incretins, type 2 diabetes mellitus, pleiotropic effects
Introduction
Type 2 diabetes mellitus (T2DM) is globally one of the most challenging public health problems of the twenty-first century. It is a complex, progressive multisystemic disease with serious, potentially life-threatening microvascular and macrovascular complications. According to current estimations, the overall prevalence of T2DM is expected to rise to 380 million by year 2025.1 If these ominous predictions for future diabetes prevalence finally come true, it is estimated that direct diabetes-associated healthcare costs will represent 7% to 13% of the global healthcare budget.2
Defective insulin secretion and impaired hepatic and peripheral insulin action constitute the major pathophysiological mechanisms underlying T2DM pathogenesis. However, it is the progressively deteriorating beta-cell function (pancreatic beta-cell failure), which ultimately leads to chronic hyperglycemia, and is critical to disease development and prognosis. Although beta-cell dysfunction is considered to be an acquired mechanism of diabetes progression, recent evidence suggests that the primary genetic defect contributing to T2DM is impaired beta-cell function, with genetically predisposed individuals possessing a reduced capacity to overcome peripheral insulin resistance.3 The beta-cell plays, therefore, a central pathogenetic role for T2DM, and treatment modalities exerting beneficial effects on beta-cell functional parameters are increasingly recognized as promising tools for T2DM management.
Successful treatment of T2DM is often complicated by the inevitably progressive nature of the disease and the need to balance target blood glucose levels against an increased risk of treatment-related adverse effects such as hypoglycemia and weight gain. Adherence of patients to treatment is often compromised by the fear of hypoglycemia and weight gain, as well as by complex therapeutic regimens. The convenience and flexibility of antidiabetic regimens is known to positively impact treatment compliance.4 For example, elderly patients, particularly those with some degree of cognitive dysfunction, are likely to experience difficulties in adhering to high-complexity regimens, involving frequent dosing, regular blood glucose monitoring, or meal planning.4 It becomes increasingly clear, that simplified antidiabetic regimens that reduce dosing frequency, minimize requirements for blood glucose testing and are not meal-dependent, can prove to be particularly beneficial for specific subgroups of difficult-to-treat patients.4 Of course, it is important to highlight that the safest and most effective way to manage T2DM without fear of drug-related side-effects is through comprehensive lifestyle modification and nonpharmacological weight loss, which is the recommended initial treatment approach for all T2DM patients. Although it is acknowledged that lifestyle interventions are difficult to ensure long-term compliance, it is important to emphasize that no pharmacological therapy is likely to be ultimately successful, without concomitant synergistic lifestyle changes. The role of lifestyle modification in optimizing outcomes of antidiabetic drug therapy is, therefore, of paramount importance, and should never be neglected or underestimated.
An optimal antidiabetic drug therapy should provide adequate glycemic control with minimal or no risk of hypoglycemia, augment the residual beta-cell functional capacity and in addition, target other T2DM-associated defects beyond hyperglycemia, such as obesity, hypertension, and dyslipidemia, so that a wide range of clinically significant cardiometabolic benefits may result. Despite the existing large armamentarium of oral antidiabetic agents, current therapy provides only a suboptimal solution, as the prevalence of adequate glycemic control remains unacceptably low (<40%).5 Many of the existing antidiabetic treatment options do not provide sustained glycemic control, can induce weight gain, and have negligible effect on the underlying beta-cell dysfunction.5–7 A relatively novel class of antidiabetic agents, incretin hormone glucagon-like peptide 1 (GLP-1) analogs or GLP-1 receptor agonists, represent a whole new therapeutic direction for T2DM management, and offer an attractive alternative approach to many aspects of diabetes pathophysiology.
The present review provides a concise overview of multiple GLP-1pancreatic and extrapancreatic physiological effects, and summarizes most recent preclinical and clinical data substantiating the role of GLP-1 analogs in current T2DM therapeutic algorithms.
The Incretin Effect and Glucagon-like Peptide 1 Therapy
GLP-1 is a 30-amino-acid polypeptide derived from the proglucagon gene that is rapidly secreted into the bloodstream from enteroendocrine L-cells in the distal small intestine and the colon in response to food ingestion.8 Within the L-cells of the small intestine, proglucagon peptide is processed by enzyme prohormone convertase 1/3 to GLP-1, GLP-2 (a key regulator of small intestinal growth), and glicentin.
GLP-1 is characterized as a pluripotent natural incretin hormone, which exerts multiple physiological actions throughout the human body. The name “incretin” is derived from the Latin word increscere (meaning “to increase”) and refers to the strong insulinotropic effect on pancreatic beta-cells of the incretin hormones GLP-1 and glucose-dependent insulinotropic polypeptide (GIP).
The incretin effect has been typically described by the observation that when glucose is administered orally, it results in a much more profound increase in plasma insulin concentrations compared with the insulin increase after intravenous glucose administration, despite equivalent blood glucose profiles.9 Incretin hormones (GLP-1 and GIP) account for 50% to 70% of postprandial insulin secretion from pancreatic beta-cells.9 The direct intraluminal contact between ingested nutrients and GLP-1 secreting L-cells is the major stimulus for GLP-1 secretion, while additional neuroendocrine signals (acetylcholine, gastrin-releasing peptide, and GIP) are also important contributing factors.9 GLP-1 postprandial secretion is actually biphasic: an early phase of 15–30 minutes, mediated by neural and endocrine factors, is subsequently followed by a more sustained phase of 30–60 minutes, stimulated by the contact of nutrients with the intestinal mucosa.10 Shortly after its secretion, GLP-1 is rapidly degraded by the ubiquitous enzyme dipeptidyl peptidase 4 (DPP-4), which cleaves off the two N-terminal residues of GLP-1, inactivating the hormone.11 DPP-4 is found in numerous human tissues. The soluble form of DPP-4, which is found in plasma, is responsible for the short half-life of the plasma circulating GLP-1 (approximately 1–1.5 minutes). Native GLP-1 has, therefore, limited therapeutic utility, as a continuous infusion would be required to achieve sustained in-vivo efficacy. To overcome the major therapeutic limitation of poor native GLP-1 viability due to its short half-life, longer-acting GLP-1 analogs have been clinically developed, providing all the positive effects of GLP-1 with a protracted mechanism of action.
GLP-1 multiorgan and multisystemic activity is largely mediated by the GLP-1 receptor (GLP-1R), which is a member of family B1 of the seven-transmembrane G protein-coupled receptors.12 This receptor displays a wide tissue distribution and is localized in many organs, including the pancreatic islets, kidneys, heart, lungs, gastrointestinal tract (stomach and duodenum), pituitary, endothelium and parts of the peripheral and central nervous system.9 When GLP-1R binds and interacts with either endogenous GLP-1 ligand or exogenously administered GLP-1R agonists (GLP-1 analogs), pancreatic beta-cells are stimulated to secrete insulin in a glucose-dependent manner via activation of enzyme adenylcyclase, which results in increased intracellular levels of cyclic adenosine monophosphate, namely the crucial intracellular mediator of GLP-1 effects.9 In addition to potentiating insulin secretion, GLP-1 can also promote insulin biosynthesis from the pro-insulin gene, while it can significantly affect pancreatic alpha-cells, exerting a glucose-dependent suppressive effect on glucagon secretion.13,14 Given that GLP-1 stimulates insulin secretion and suppresses glucagon secretion only when blood glucose levels are truly elevated, it is important to emphasize that GLP-1-based therapy does not impair the counterregulatory response of glucagon to hypoglycemia, thus protecting against hypoglycemic episodes. Of note are intriguing experimental observations from recent animal and human in-vitro studies that GLP-1 is able to suppress cytokine-mediated beta-cell apoptosis, stimulate beta-cell neogenesis and proliferation, and substantially increase functional beta-cell mass.15
The above indicate that GLP-1 is able to successfully counteract multiple pathophysiological traits underlying T2DM, and not just defective insulin secretion. Table 1 describes in a schematic way the mechanisms, by which natural incretin GLP-1 and thus GLP-1-based therapy address the major abnormalities observed in T2DM patients.16
| T2DM abnormality | GLP-1 action |
|---|---|
| Defective insulin secretion | Glucose-dependent stimulation of insulin secretion |
| Blunted insulin response to meals | Improved insulin response tomeals (first-phase response) |
| Loss or reduction of incretin effect | Restoration of incretin activity, enhanced incretin effect |
| Hyperglucagonemia | Suppression of glucagon secretion at high glucose levels |
| Reduced beta-cell insulin content | Increased insulin biosynthesis |
| Reduced beta-cell mass | Increased beta-cell mass, differentiation of precursor cells into beta-cells |
| Abnormally high rate of beta-cell apoptosis | Inhibition of glucotoxicity-induced beta-cell apoptosis |
| Often overweight or obese | Suppression of appetite and decelerated gastric emptying, induction of satiety, weight loss |
GLP-1=glucagon-like peptide 1; T2DM=type 2 diabetes mellitus.
In healthy individuals, levels of GLP-1 are in the range of 5–10 pmol/L in the fasting state and 20–40 pmol/L postprandially.8 Conflicting findings exist concerning GLP-1 concentrations in patients with T2DM. One study demonstrated significantly reduced GLP-1 levels in a population of T2DM patients compared with appropriately matched healthy controls, although there was no difference between groups for the first 60 minutes following intake of a test meal.17 It is, however, well established that the beta-cell insulin secretory response to physiological levels of GLP-1 is severely impaired in T2DM.18 This defective insulin response (impaired incretin effect) can be restored to normal with sustained pharmacological levels of native GLP-1 (126 pmol/L).19
Incretin Biology Beyond the Pancreas
As a result of the broad tissue distribution of GLP-1R expression, GLP-1 exerts a pleiotropic range of favorable extrapancreatic effects, extending far beyond the Islets of Langerhans. These mainly affect gastrointestinal tract motility, centrally mediated appetite regulation, and cardiovascular function.
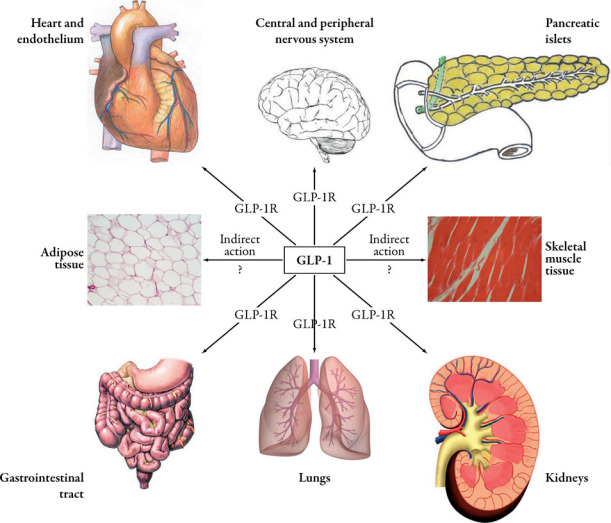
Figure 1 illustrates the manifold localization of GLP-1receptors throughout the human body, emphasizing the multifaceted physiology of this gut-derived hormone, which can also explain the pleiotropic effects of incretin-based therapy.
Gastrointestinal Effects of Glucagon-like Peptide 1
GLP-1 has been identified as a potent inhibitor of several upper gastrointestinal functions such as gastric acid secretion, pancreatic exocrine secretion, gastric emptying, and gastrointestinal motility, thereby decelerating the entry of nutrients into circulation and preventing exaggerated blood glucose excursions. These inhibitory actions of GLP-1 are thought to be part of the so-called “ileal brake effect”—namely the endocrine retrograde inhibition of upper gastrointestinal secretion and motility elicited by the presence of nutrients into the distal ileum.20 More specifically, GLP-1 has been found to significantly delay gastric emptying rate following meal consumption. In one study in 14 healthy volunteers, 6 hours after a solid 250 kcal mixed meal consumption, most of the food had gone from the stomach during placebo infusion, whereas approximately 50% still remained in the stomach, 360 minutes after the start of the GLP-1 infusion.21 The deceleration of gastric content evacuation, observed both in rodents and humans, is thought to involve the gut-brain axis and to be mediated by GLP-1Rs on afferent sensory vagal nerves. However, after vagal denervation, GLP-1 was still able to inhibit—to some extent—gastric motility in pigs, raising the possibility that GLP-1 regulates gastric emptying not only through afferent neural pathways, but also via direct gut-specific mechanisms.22 Apart from slowing gastric emptying, GLP-1 infusion has also been shown to significantly reduce gastric acid secretion via neurally mediated mechanisms.23
Central Nervous System Effects of Glucagon-like Peptide 1
GLP-1 is a proven key regulator of short-term and long-term energy homeostasis, considering that GLP-1Rs are abundantly expressed in brainstem and hypothalamic areas that regulate appetite and food intake. In an interesting study in T2DM patients, GLP-1 infusion increased significantly subjective feelings of fullness and postprandial satiety, while it decreased hunger sensation and prospective food intake.24 The observed anorexigenic effect induced by GLP-1 infusion in fasted individuals suggests that GLP-1 can affect satiety independently of delayed gastric emptying. In this context, it is important to note that GLP-1 is not only peripherally produced by L-cells of the small intestine, but is also centrally synthesized by a small distinct population of neurons within nucleus of solitary tract in the caudal brainstem.25 Current evidence suggests that both central and peripheral GLP-1 play a synergistic role in regulating food intake. According to the current model, food ingestion promotes GLP-1 release from the intestine. Along with gastric distension, this leads to activation of vagal afferent nerves, which in turn activate GLP-1-producing neurons projecting to the paraventricular nucleus of hypothalamus, where GLP-1R activation promotes anorexia and satiety.25 Further investigation is required to determine the relative contribution of peripheral and central GLP-1 to energy and glucose homeostasis, and delineate the specific sites of action for each function.
Another clinically relevant central effect of incretins consists in cognitive improvement and neuroprotection conferred by GLP-1 in rodents, rendering incretin-based therapy an attractive theoretical treatment modality for Alzheimer’s disease.26
Cardiovascular Effects of Glucagon-like Peptide 1
Cardiovascular risk factors can be definitely affected by the weight-reducing and glucose-lowering effects associated with GLP-1-based therapy. However, emerging studies suggest that GLP-1 and GLP-1 analogs exert additional beneficial effects on the cardiovascular system independently of their glucose-lowering potential.27
In experimental models, GLP-1 has been shown to exert a wide range of cardiovascular effects, such as modulation of heart rate, blood pressure, myocardial contractility, vascular tone, and protection against ischemia-reperfusion injury.27 Structural and functional cardiac abnormalities have been demonstrated in mice lacking the GLP-1R, suggesting that endogenous GLP-1 plays an important role in cardiovascular system integrity.28 Nikolaidis et al. showed that GLP-1 increased glucose uptake and left ventricular performance in dogs with pacing-induced dilated cardiomyopathy.29 Furthermore, a recent study showed that GLP-1 analog exenatide reduced infarct size and enhanced cardiac function in a porcine model of ischemia-reperfusion injury,30 while another recent study showed that 3 months of continuous infusion of GLP-1 resulted in increased survival and preserved cardiac function in a rat model of diabetes and hypertension.31 The GLP-1 analog liraglutide has also been shown to activate cytoprotective pathways and improve outcomes after experimental myocardial infarction in mice.32 In canine models of cardiac injury and heart failure, GLP-1 and its metabolically inactive metabolite GLP-1(9–36) improved myocardial function and cardiac output, while it decreased left ventricular end-diastolic pressure, heart rate, and systemic vascular resistance.29
In the human cardiovascular system, GLP-1R is expressed in cardiomyocytes, coronary endothelial cells, and vascular smooth muscle cells.27 Nikolaidis et al. reported that GLP-1 administration, when added to standard therapy, improved regional and global left ventricular function in patients with acute myocardial infarction after primary angioplasty.33 Furthermore, in a clinical setting, GLP-1augmented left ventricular ejection fraction and functional status in patients with congestive heart failure.34 The same research group reported that perioperative use of GLP-1 improved glycemic control without need for high-dose insulin or inotropic drugs in patients undergoing coronary artery bypass grafting.35 Interestingly, beneficial effects of GLP-1 on cardiovascular risk factors have been demonstrated in both healthy and diabetic subjects. Three years of exenatide treatment in T2DM patients resulted in significant reductions of systolic and diastolic blood pressure, without affecting the heart rate.36 Nikolaidis et al. reported that 72 hours of continuous GLP-1 infusion after coronary revascularization increased the ejection fraction and the regional and global wall motion indices in both diabetic and nondiabetic patients with acute myocardial infarction.33 Furthermore, intravenous GLP-1 infusion was shown to improve the blood pressure and cardiac function in the postoperative state after coronary artery bypass grafting in patients with T2DM.37 Clinical studies examining T2DM patients with coronary artery disease have also shown that GLP-1 infusion was able to improve the endothelial function, which is a surrogate marker of the overall atherosclerotic burden.38
It should be particularly emphasized that GLP-1-based therapy appears to improve a wide range of cardiovascular risk factors, including hypertension, dyslipidemia and subclinical low-grade inflammation.36 However, large-scale and well-designed studies are needed in order to investigate the impact of cardiovascular risk reduction induced by GLP-1-based treatment on hard cardiovascular endpoints, including morbidity and mortality.
Whether GLP-1’s favorable effects on the cardiovascular system are mediated exclusively through GLP-1R or another as yet unidentified receptor has not been fully elucidated. However, some studies suggest that the presumably inactive metabolite GLP-1(9–36) may improve functional recovery in ischemic heart via mechanisms unrelated to GLP-1R.28
Other Extrapancreatic Effects of Glucagonlike Peptide 1
GLP-1 may exert an anabolic effect on the liver, resulting in increased glycogen synthesis from circulating glucose, while it also suppresses hepatic glucose output (gluconeogenesis) via inhibited pancreatic glucagon secretion.10 Moreover, recent data suggest that GLP-1-induced inhibition of the tubular Na+/H+ exchanger isoform 3 increases urinary sodium excretion, exerting thus natriuretic and diuretic effects, which could partly contribute to the observed blood pressure reduction achieved with human GLP-1 analogs.39 In addition, GLP-1 has shown to decrease glomerular hyperfiltration, and it has been speculated that this effect could possibly contribute to clinically significant renoprotection.40 Finally, GLP-1R mRNA has been found in rat type 2 pneumocytes, and both GLP-1 and exenatide have shown to stimulate secretion of phosphatidylcholine, which is a major component of lung surfactant.41 The clinical implications of the latter observation remain unresolved.
Glucagon-like Peptide 1 Analogs Versus Oral Dipeptidyl Peptidase 4 Inhibitors
DPP-4 inhibitors, such as sitagliptin, vildagliptin, and saxagliptin, are an alternative treatment strategy to using GLP-1 analogs. These oral agents prevent GLP-1 degradation, thus increasing the plasma levels of endogenous GLP-1 to physiological levels (in the range 10–25 pmol/L). However, data suggest that the relatively modest physiological levels of GLP-1 achieved with DPP-4 inhibitors may be the reason for the neutral bodyweight effect and less pronounced blood glucose reduction observed with this category, when compared with GLP-1R agonists.42 This is in contrast with the significant weight loss and HbA1c reduction observed with GLP-1 analogs, which provide high pharmacological steady-state levels of GLP-1R agonist sufficient to restore the impaired beta-cell insulin secretory response of T2DM patients to levels similar to those of healthy adults.
The differential characteristics of these two types of incretin-based treatments are schematically presented in Table 2.
| DPP-4 inhibitors | GLP-1R agonists |
|---|---|
| Both GLP-1 and GIP enhanced | Pure GLP-1 effect |
| Increased levels of GLP-1 in | Pharmacological levels of GLP-1 physiological range |
| Limited by endogenous secretion | Not limited by endogenous secretion |
| Moderate efficacy | Enhanced efficacy |
| Well tolerated | Nausea, gastrointestinal side-effects |
| No weight change | Weight loss |
| Oral route of administration | Subcutaneous injection |
DPP-4=dipeptidyl peptidase-4; GIP=glucose-dependent insulinotropic polypeptide; GLP-1=glucagon-like peptide 1; GLP-1R=glucagon-like peptide 1 receptor.
Clinical Efficacy of Daily (Short-acting) Glucagon-like Peptide 1 Analogs
Exenatide: a Twice-Daily Glucagon-like Peptide 1 Mimetic
Exenatide is a subcutaneously injected incretin mimetic, which needs to be preprandially administered twice per day. It is indicated as adjunctive therapy for patients with T2DM who are already receiving antidiabetic therapy with metformin, a sulfonylurea, or both, but continue to have suboptimal glycemic control.
Exenatide was originally isolated from the saliva of the venomous lizard Heloderma suspectum (Gila monster) in a search for biologically active peptides.43 It shares only 53% amino acid sequence homology with native GLP-1, but exenatide and naturally occurring GLP-1 are equipotent with regard to binding and activation of GLP-1Rs on pancreatic beta-cells.44 After subcutaneous administration, exenatide is rapidly absorbed, reaching peak concentrations in approximately 2 hours.45 As it is primarily cleared through the kidneys by glomerular filtration, the plasma half-life of subcutaneously administered exenatide is 2–3 hours, which is prohibitive for once-daily dosing.46
In three phase 3 placebo-controlled clinical trials that enrolled a total of 1446 patients who received 5 or 10 μg exenatide twice daily, or placebo for 30 weeks, the addition of exenatide to the pre-existing treatment with metformin, sulfonylurea, or both, was associated with significant reductions of HbA1c.47 Most exenatide-treated patients achieved a target HbA1c of ≤7%, a significant decrease in fasting plasma glucose concentrations, and a dose-dependent progressive weight loss.47 Nausea was the most commonly reported adverse event in the combined exenatide groups, reaching the level of 43.5%. Based on the limited homology of exenatide with native human GLP-1, the incidence of anti-exenatide antibodies was as high as 43% at 30 weeks in the exenatide phase 3 development program.48 According to some recent evidence, such high immunogenicity could attenuate, to some extent, the clinical efficacy of exenatide.49 Subjects completing 3 consecutive years of exenatide exposure in these phase 3 trials were all enrolled into an open-label clinical trial, aimed at evaluating the effects of prolonged exenatide therapy on glycemic control, bodyweight, cardiometabolic risk markers, and safety. Interestingly, HbA1c and bodyweight reductions were significantly sustained at 3 years, while it is noteworthy that exenatide-treated patients exhibited minor but significant reductions in total cholesterol, low-density lipoprotein cholesterol, triglycerides, and hepatic aminotransferases.36 The substantial improvement in the lipid profile and hepatic biochemistry of patients treated with exenatide was coupled with a progressive weight loss of approximately 5 kg from baseline to the end of the 3-year study period.36
The concomitant use of exenatide and insulin is currently not US Food and Drug Administration (FDA) approved, due to the lack of solid clinical evidence. However, a combined regimen of insulin and exenatide might be advantageous, especially for mitigating insulin-related weight gain in obese T2DM patients.50 Several small prospective and retrospective studies evaluating the potential advantages of combination therapy reported significant reductions in HbA1c, bodyweight, and total daily insulin dose requirements.50
In an additional open-label noninferiority trial, treatment with exenatide 10 μg twice daily and appropriately titrated insulin glargine for 16 weeks were interestingly associated with similar reductions in HbA1c, when combined with a single oral antidiabetic agent.51 Exenatide therapy proved to be more beneficial than insulin glargine in terms of reducing bodyweight and postprandial glucose excursions, whereas insulin glargine was more effective in improving fasting hyperglycemia. Based on the positive metabolic effects of exenatide use in conjunction with oral antidiabetic agents (metformin, sulfonylurea, or thiazolidinediones), Moretto et al. aimed to evaluate the efficacy and tolerability of exenatide as monotherapy in antidiabetic drug-naive T2DM patients, and suggested that exenatide monotherapy might be a viable treatment option for drug-naive T2DM patients who are inadequately controlled with diet or exercisealone.52
Liraglutide: a Once-Daily Human Glucagon-like Peptide 1 Analog
The structure of liraglutide is based on native human GLP-1. However, there are two important modifications to the native molecule that make liraglutide less susceptible to DPP-4 degradation, giving it a half-life of approximately 13 hours: the attachment of a C16 fatty acid side chain linked via a glutamoyl spacer to lysine at position 26, and replacement of lysine with arginine at position 34, providing only one binding site for the fatty acid side chain.53 As there is only one amino acid substitution, the resultant molecule of liraglutide shares 97% amino acid sequence identity (homology) with native human GLP-1. Following subcutaneous injection, the fatty acid side chain allows liraglutide to self-associate into heptamers at the injection site depot.53 The size of the heptamer and the strong self-association facilitate the prolonged and delayed absorption of liraglutide from subcutaneous tissue. After entering the bloodstream, the fatty acid chain allows reversible binding of liraglutide to serum albumin, providing partial stability and resistance to DPP-4 catabolism.16 The above pharmacokinetic mechanisms account for the protracted mechanism of action of liraglutide and are consistent with once-daily dosing.
Evidence from preclinical studies in animal models has shown that treatment with liraglutide improves aspects of beta-cell proliferation and function, ameliorates glycemic control, reduces bodyweight and positively affects cardiac function. After 6 weeks of treatment with liraglutide, Zucker diabetic fatty rats exhibited significantly increased beta-cell mass and a trend towards enhanced beta-cell proliferation compared with controls.54 In recent in-vitro studies using human pancreatic islet cells, liraglutide promoted beta-cell proliferation, inhibited interleukin-1 beta-induced apoptosis and increased pancreatic islet mass and size.55 Alongside its positive effects on beta-cells, liraglutide was also found to exert a significant effect on appetite suppression, food intake and weight in animal models of diabetes and obesity.56 Liraglutide treatment has also proven to have positive effects on the cardiovascular system, increasing survival, reducing cardiac rupture risk, and improving cardiac function in a mouse model of experimental myocardial infarction.32
In support of results from preclinical studies, early pharmacological studies in patients with T2DM have shown that liraglutide can increase beta-cell function in the fasting state by 30% compared with placebo, as assessed by homeostasis model assessment for beta-cell function.57 The same trial showed that maximum beta-cell secretory capacity was significantly higher after treatment with liraglutide, compared with placebo, and that proinsulin/insulin ratio (a measure of beta-cell stress and dysfunction) was reduced by 40% to 50%, a further indication of improved beta-cell function. Furthermore, liraglutide increases insulin secretion in a strictly glucose-dependent manner, and improves both first-phase and second-phase insulin responses in subjects with T2DM, as assessed by means of a hyperglycemic clamp.57 With liraglutide, glycemic control is improved by dose-dependent lowering of both fasting and postprandial plasma glucose concentrations in diabetic patients, and this effect lasts for the entire 24-hour dosing period. While establishing tight glycemic control remains the central objective of diabetes treatment, tackling comorbid obesity is also of major importance, as obesity per se constitutes a significant risk factor for a wide range of cardiometabolic complications in diabetic patients. Contrary to many existing antidiabetic agents, which are frequently associated with undesirable weight gain, liraglutide has shown positive effects on the mechanisms underlying weight loss. More specifically, it delays the rate of gastric emptying, reduces ad libitum energy intake and exerts a modest suppression of hunger sensation, as indicated by diverse appetite rating endpoints.58
Encouraging preclinical and phase 2 clinical trial results led to the comprehensive program LEAD (Liraglutide Effect and Action in Diabetes), involving over 4456 enrolled patients from more than 600 sites in 40 countries, of whom over 2739 individuals with T2DM were treated with liraglutide for up to 52 weeks. The program consisted of LEAD-1 to LEAD-6 (all including active comparator treatments), while the liraglutide-sitagliptin trial (Lira-DPP-4i) was a 26-week randomized head-to-head comparison of liraglutide against DPP-4 inhibitor sitagliptin, when added on to pre-existing metformin treatment. Table 3 provides a synopsis of LEAD studies. The LEAD program and Lira-DPP-4i trial have demonstrated that liraglutide, both as monotherapy and in combination with a variety of antidiabetic drugs, is associated with substantial improvements in HbA1c, fasting and postprandial glucose levels, and improved beta-cell function, as measured by HOMA-B and proinsulin/insulin ratio.59–65 Meta-analyses of the LEAD trials have consistently shown that liraglutide is associated with greater HbA1c reductions in patients with higher baseline HbA1c, and that the extent of HbA1c reduction with liraglutide is independent of concomitant weight loss experienced.66 It is noteworthy that liraglutide lowered HbA1c by up to 2.5% from baseline in patients with poor initial glycemic control (HbA1c <10%), which was significantly greater than active comparators. A meta-analysis of LEAD trials has also shown that significant reductions in systolic blood pressure (by up to 2.6 mmHg from baseline) could be achieved within 2 weeks of liraglutide treatment, before any major weight loss could be observed, and these reductions were independent of concomitant treatment with antihypertensive medication and sustained for up to 26 weeks.66
| Study | Number of patients | Study duration (weeks) | Compared randomized regimens |
|---|---|---|---|
| LEAD-1 | 1041 | 26 | Liraglutide+SU versus SU+TZD |
| LEAD-2 | 1091 | 26 | Liraglutide+MET versus MET+SU |
| LEAD-3 | 746 | 52 | Liraglutide monotherapy versus SU monotherapy |
| LEAD-4 | 533 | 26 | Liraglutide+MET+ TZD versus MET+TZD |
| LEAD-5 | 581 | 26 | Liraglutide+MET+SU versus glargine+MET+SU |
| LEAD-6 | 464 | 26 | Liraglutide+MET and/or SU versus exenatide+ MET and/or SU |
| Lira-DPP-4i | 665 | 26 | Liraglutide+MET versus sitagliptin+MET |
LEAD=Liraglutide Effect and Action in Diabetes ; Lira-DPP-4i=liraglutide-sitagliptin trial; MET=metformin; SU=sulfonylurea (glimepiride); TZD=thiazolidinedione (rosiglitazone).
In LEAD-1, liraglutide added to glimepiride was well tolerated and provided improved glycemic control and favorable weight profile, compared with adding rosiglitazone to glimepiride. In LEAD-2, liraglutide induced similar glycemic control, reduced bodyweight, and lowered the incidence of hypoglycemia, compared with glimepiride, when they were both added to pre-existing metformin treatment. In LEAD-3, liraglutide proved to be both safe and effective as initial pharmacological therapy for T2DM, and led to greater reductions in HbA1c, weight, hypoglycemia, and blood pressure than glimepiride monotherapy. In LEAD-4, liraglutide combined with metformin and rosiglitazone proved to be a well tolerated combination therapy for T2DM, and provided significant improvement in glycemic control. In LEAD-5, the strategy of adding liraglutide to combined treatment with glimepiride and metformin proved to be non-inferior to adding basal insulin glargine in terms of HbA1c reduction. In LEAD-6 head-to-head study of liraglutide once daily versus exenatide twice daily (as add-on to metformin and/or sulfonylurea therapy), mean HbA1c reduction was significantly greater with liraglutide treatment than with exenatide.59–65
Across all LEAD trials, liraglutide led to significant weight loss in T2DM patients, and primarily reduction of body fat. The majority of body fat lost proved to be visceral abdominal fat, while weight loss effect of liraglutide was becoming more prominent with increasing baseline body mass index.59–65 Regarding the total cardiovascular burden of diabetic patients treated with liraglutide, total cholesterol, low-density lipoprotein cholesterol, free fatty acids, triglycerides, brain-derived natriuretic peptide, and high-sensitivity C-reactive protein levels were all significantly decreased from baseline after 26-week treatment with liraglutide.66 These data suggest that beyond its established effects on glycemic control, weight, and systolic blood pressure, liraglutide might contribute to clinically relevant cardiovascular risk reduction via additional, as yet undetermined, physiological effects. Concerning the impact of antidiabetic treatment on patient-reported health-related quality of life, overall patient satisfaction was better with liraglutide versus comparators such as exenatide, sitagliptin, and glimepiride.59,64,67 Across the entire spectrum of LEAD phase 3 clinical trials, liraglutide was generally well tolerated, with mild to moderate transient nausea being the most commonly reported treatment-related adverse event. Furthermore, a pooled analysis of four LEAD trials demonstrated that only 8.6% of patients developed anti-liraglutide antibodies, and this immunologic response has never been associated with reduced efficacy of liraglutide.68
Clinical Efficacy of Once-Weekly (Long-acting) Glucagon-like Peptide 1 Analogs
Albiglutide
Albiglutide (formerly known as albugon) is a long-acting GLP-1R agonist, developed by GlaxoSmithKline through the genetic fusion of two repeats of human GLP-1(7–36) molecules to recombinant human albumin.69 The GLP-1 dimer was used in order to enhance the interaction of the GLP-1 monomer with its receptor in the presence of albumin. A single amino acid substitution at the DPP-4-sensitive hydrolysis site (conversion of alanine to glycine at position 8) renders the molecule resistant to DPP-4 inactivation.70 The structure of albiglutide provides an extended half-life of 5–8 days, which may allow weekly or less frequent dosing regimens. The randomized, double-blind, controlled phase 2 clinical study by Rosenstock et al. aimed to evaluate efficacy, safety, and tolerability of incremental doses of albiglutide, administered subcutaneously with three dosing schedules (4, 15, or 30 mg weekly; 15, 30, or 50 mg biweekly; 50 or 100 mg monthly) in 356 patients with T2DM, inadequately controlled with diet and exercise or metformin monotherapy.71 Significant dose-dependent reductions in HbA1c (from −0.79 to −0.87%) and weight loss (−1.1 to −1.7 kg) were observed within all albiglutide treatment groups.71 The incidence of gastrointestinal adverse events (nausea, vomiting, and flatulence) in subjects receiving albiglutide 30 mg weekly was less than that observed for the highest biweekly and monthly doses of albiglutide.71 Albiglutide is relatively impermeant to the central nervous system, which may have implications for a better gastrointestinal tolerability profile when compared with exenatide.72 Several clinical trials examining the use of albiglutide as combination therapy in T2DM are currently ongoing and their results are awaited with anticipation.
Taspoglutide
Taspoglutide (R1583/BIM51077) is a novel human GLP-1 analog developed by Ipsen SA, which is now licensed and has been moved into phase 3 clinical trials by Hoffmann-La Roche.73 Taspoglutide is similar in structure to native human GLP-1(7–36) amide, except for two amino acid substitutions (93% sequence homology with native GLP-1) in positions 8 and 35 with aminoisobutyric acid, which render the molecule resistant to degradation by DPP-4.73 Short-term efficacy of taspoglutide was clinically evaluated by Kapitza et al. in patients with T2DM.74 Three cohorts with 12 diabetic patients in each were given single subcutaneous injections of taspoglutide (1, 8, or 30 mg) and four diabetic patients were randomized to placebo. The highest doses of 8 and 30 mg resulted in significant improvements in glycemic parameters such as fasting blood glucose, 24-hour blood glucose profile, and 5-hour postprandial blood glucose levels.74 With the 30 mg dose of taspoglutide, the maximum effect on metabolic parameters was observed 14 days after injection, but was still evident for up to 28 days after injection. In the double-blind, placebo-controlled study by Nauck et al. in T2DM patients inadequately controlled with metformin alone, taspoglutide at doses 5/10/20 mg weekly or 10/20 mg biweekly improved significantly fasting and postprandial glucose control and elicited substantial weight loss, when combined with metformin.75 The magnitude of improvement in glycemic control observed with taspoglutide after 8 weeks (−1.1% decrease in HbA1c from a baseline of 7.9%) compared favorably with that seen with other GLP-1R agonists, such as exenatide and liraglutide. Phase 3 clinical trials are currently in progress.
Exenatide Long-Acting Release Formulation
Exenatide has been developed by Amylin Pharmaceuticals in a sustained/long-acting release formulation (LAR) planned for once-weekly subcutaneous administration. This LAR is based on a common biodegradable medical polymer, used in bioabsorbable sutures and other extended-release pharmaceuticals containing 3% exenatide peptide.76,77 In patients with T2DM who are inadequately controlled with metformin and/or diet, 0.8 and 2.0 mg of exenatide once weekly for 15 weeks resulted in HbA1c reductions of 1.4% and 1.7% compared with an increase of 0.4% in the placebo group.78 The 2 mg group experienced a weight loss of 3.8 kg versus no weight change in the other groups. Drucker et al. compared the effect of 10 μg exenatide twice daily and 2 mg exenatide LAR once weekly in 295 patients with T2DM, who were suboptimally controlled on lifestyle intervention and/or oral antidiabetic treatment.79 In this study, exenatide LAR once weekly was superior to exenatide twice daily in terms of glycemic parameters. Interestingly, glucagon levels decreased significantly more with exenatide once weekly versus exenatide twice daily.79 Additionally, significantly greater reductions in total and low-density lipoprotein cholesterol levels were observed with the once-weekly exenatide formulation, compared with twice-daily exenatide.79 Both treatments elicited equally significant improvements in fasting triglyceride levels, systolic and diastolic blood pressure. Anti-exenatide antibodies were formed in 67% of exenatide LAR-treated patients78 and were comparatively higher among exenatide LAR-treated patients as opposed to exenatide twice-daily-treated patients.79 It cannot be excluded that high titers of such antibodies may be associated with diminished therapeutic efficacy.
Safety Issues and Overall Concerns Associated With Incretin-based Therapy
In early preclinical studies in rodents, long-term exposure to the GLP-1R agonist liraglutide was associated with upregulation of calcitonin gene expression and thyroid C-cell hyperplasia, adenomas, or even carcinomas at the highest doses.80 Although in-vitro rodent experiments have suggested involvement of GLP-1R in C-cell neoplasia,81 it is important to acknowledge the species-specific differences between rodents and humans in GLP-1R expression and activity at the level of thyroid tissue, which need to be seriously considered, while trying to interpret the preclinical observations. In rodents, there is high expression and activity of GLP-1R on thyroid C-cells, while humans have low thyroid expression of GLP-1R, and GLP-1R agonists do not appear to stimulate calcitonin release to a clinically relevant degree.81 In the clinical setting, LEAD trials with up to 2 years duration and over 2700 enrolled patients suggested no direct carcinogenic or growth-promoting effects of liraglutide, as no patient developed medullary thyroid carcinoma.59–65
Another theoretical risk associated with incretin-based therapy is the risk of acute or chronic pancreatitis, which has been raised during postmarketing surveillance both for GLP-1 analogs and DPP-4 inhibitors. A small number of cases with pancreatitis have been reported in patients treated with exenatide, liraglutide, sitagliptin, and saxagliptin,82 without, however, being able to establish the causal or incidental nature of this relationship, as T2DM patients are predisposed to develop pancreatitis at a higher rate than the general population regardless of type of antidiabetic treatment due to concomitant obesity and hypertriglyceridemia.83 Up-to-date clinical and experimental datasets linking GLP-1R agonists and DPP-4 inhibitors to pancreatitis remain incomplete.
Further concerns related to treatment with GLP-1R agonists include the potential fear of self-injections and the commonly observed difficulty in tolerating the gastrointestinal side-effects, in particular nausea, during the first weeks of treatment. Despite the ongoing effort to minimize the number of injections to once-weekly or once-monthly regimens, the concept of injectable treatments causes considerable anxiety and distress to a subgroup of patients, who strongly insist on being treated with oral agents, even at the cost of suboptimal glycemic control. As far as nausea is concerned, although it is mild in severity and transient in duration in the majority of cases, it can be perceived as a greatly unpleasant and worrisome adverse event by some patients, who are prone to abandon treatment shortly after nausea of any severity is experienced. Beyond safety and tolerability issues, the pharmacoeconomic parameters of incretin-based treatment merit also serious consideration, as the high cost of GLP-1 analogs and DPP-4 inhibitors can become prohibitive for some healthcare systems. It is important that large-scale cost-effectiveness analyses are performed in the future in order to conclude whether the high cost of these agents can be counterbalanced by the cost savings associated with prevention of complications or reducing the frequency of blood glucose monitoring.
Unresolved Issues and Future Perspectives
The critical question that remains unanswered in the field of incretin research is whether incretin-based therapies are able to exhibit a long-term favorable impact on beta-cell mass and function, suggesting the potential to alter the natural course of T2DM. Given the current lack of noninvasive imaging methods that enable direct in-vivo quantitative assessment of beta-cell mass in humans, a reasonable option to test the validity of this intriguing theory would be to perform long-term clinical trials in order to evaluate the durability of glycemic control that can be achieved with GLP-1R agonists or DPP-4 inhibitors. If long-term glycemic control is established with these agents, it could be assumed that they exert chronic positive effects on beta-cell health, as the major factor underlying worsened glycemic control under treatment is the progressive loss of islet mass.
There are also a number of issues related to incretin-based therapy that warrant further investigation. For example, the preclinical findings suggesting cardioprotective actions of theoretically inactive metabolite GLP-1(9–36) through GLP-1R-independent mechanisms,28 needs further confirmation. The validation of these findings would indicate that DPP-4 inhibitors might be deprived of such cardioprotective potential by blocking the conversion of the active GLP-1 metabolite to its inactive form. Another interesting theory that merits further research is that once-daily GLP-1R agonists, such as liraglutide, are more effective in controlling fasting rather than postprandial hyperglycemia, while twice-daily GLP-1R agonists, such as exenatide, and DPP-4 inhibitors are more effective in controlling postprandial rather than fasting hyperglycemia.84 Another interesting area of research is delineating the exact mechanisms by which GLP-1 analogs suppress glucagon secretion from pancreatic alpha-cells, as it remains controversial whether GLP-1Rs are expressed on the surface of human alpha-cells. It has been shown that GLP-1Rs could not be detected in purified rat alphacells, while their direct exposure to GLP-1 did not alter glucagon secretion.85,86 As a result, an indirect action, mediated by the stimulatory effect of GLP-1 on neighboring beta-cells or delta-cells and resulting in intra-islet paracrine inhibition of glucagon release, might also be a biologically plausible explanation. Further research in this field may provide insight into how inappropriate glucagon secretion could be therapeutically controlled in T2DM.
Future directions for research related to incretin-based therapy include clinical trials with combined regimens of basal or prandial insulin with GLP-1R agonists or DPP-4 inhibitors, novel indications based on the emerging neuroprotective and cardioprotective properties of GLP-1R agonists, and a comprehensive pharmacoeconomic evaluation of the cost-effectiveness profile of incretin-based therapies in order to answer the question of whether their high cost can be offset by their overall clinical benefit. In terms of safety issues, monitoring of pancreatic function and serum calcitonin levels in patients chronically treated with incretin-based therapies in the context of ongoing, long-term, prospective, controlled clinical trials is considered to be a prudent approach.
Conclusion
The development of incretin-based therapies promises to dramatically alter the current therapeutic landscape of T2DM. The glucose-lowering properties of GLP-1 analogs are now well established. In addition, continuing research suggests that GLP-1-based therapy has potential benefits far more wide-ranging than its isolated effects on glucose metabolism and bodyweight. In order for a glucose-lowering agent to improve cardiovascular outcomes, which is the ultimate goal for T2DM management, it may need to impact multiple key drivers of atherosclerotic complications, such as dyslipidemia, hypertension, subclinical inflammation, endothelial dysfunction, and increased thrombogenicity. It becomes increasingly evident that GPL-1 mimetics are able to counteract some of the above critical abnormalities.
The optimal therapy for T2DM patients should be able to secure all of the following clinical benefits: adequate glycemic control with minimal risk of hypoglycemia, substantial improvement of the beta-cell function, clinically meaningful weight loss, effective control of diabetes-related cardiovascular risk factors such as hypertension and dyslipidemia, and a simple and flexible treatment regimen. It is true that the majority of currently available oral antidiabetic agents cannot sufficiently address the critical therapeutic issues mentioned above. Based on the accumulating and encouraging clinical evidence reviewed herein, GLP-1R agonists may be able to provide some reliable solutions to the intractable problem of suboptimal T2DM management and have the potential to fulfill the currently unmet therapy needs.
However, whether the positive cardiometabolic effects of GLP-1R agonists can also be translated into better clinical outcomes for diabetic patients in terms of long-term cardiovascular morbidity and mortality is currently unknown, and remains to be further elucidated within the context of prospective, well-designed, large-scale clinical trials.
Acknowledgments
The authors declare no personal, commercial, academic, or financial conflicts of interest related to this manuscript. No funding or sponsorship has been received by the authors in relation to this article.
Open Access. This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source arecredited.
Footnotes
This article is published with open access at Springerlink.com
References
- 1.International Diabetes Federation. United Nations Resolution 61/225: World Diabetes Day. 2008. Available at: www.idf.org/webdata/docs/World_Diabetes_Day_Media_Kit.pdf. Accessed: January 28, 2011.
- 2.International Diabetes Federation. Diabetes Atlas. Executive Summary. second edition. Brussels: International Diabetes Federation; 2003. [Google Scholar]
- 3.Wajchenberg B.L. Beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev. 2007;28:187–218. doi: 10.1210/10.1210/er.2006-0038. [DOI] [PubMed] [Google Scholar]
- 4.Munshi M. Managing the “geriatric syndrome” in patients with type 2 diabetes. Consult Pharm. 2008;23(suppl.B):12–16. [PubMed] [Google Scholar]
- 5.Fox K. M., Gerber R.A., Bolinder B., Chen J., Kumar S. Prevalence of inadequate glycemic control among patients with type 2 diabetes in the United Kingdom general practice research database: A series of retrospective analyses of data from 1998 through 2002. Clin Ther. 2006;28:388–395. doi: 10.1016/j.clinthera.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Nathan D.M., Buse J.B., Davidson M.B., et al. Management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2006;29:1963–1972. doi: 10.2337/dc06-9912. [DOI] [PubMed] [Google Scholar]
- 7.Inzucchi S.E. Oral antihyperglycemic therapy for type 2 diabetes: scientific review. JAMA. 2002;287:360–372. doi: 10.1001/jama.287.3.360. [DOI] [PubMed] [Google Scholar]
- 8.Orskov C., Wettergren A., Holst J.J. Secretion of the incretin hormones glucagon-like peptide-1 and gastric inhibitory polypeptide correlates with insulin secretion in normal man throughout the day. Scand J Gastroenterol. 1996;31:665–670. doi: 10.3109/00365529609009147. [DOI] [PubMed] [Google Scholar]
- 9.Baggio L.L., Drucker D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 10.Kieffer T.J., Habener J.F. The glucagon-like peptides. Endocr Rev. 1999;20:876–913. doi: 10.1210/er.20.6.876. [DOI] [PubMed] [Google Scholar]
- 11.Vilsbøll T., Knop F.K., Krarup T., et al. The pathophysiology of diabetes involves a defective amplification of the late-phase insulin response to glucose by glucose-dependent insulinotropic polypeptide-regardless of etiology and phenotype. J Clin Endocrinol Metab. 2003;88:4897–4903. doi: 10.1210/jc.2003-030738. [DOI] [PubMed] [Google Scholar]
- 12.Runge S., Thøgersen H., Madsen K., Lau J., Rudolph R. Crystal structure of the ligand-bound glucagonlike peptide-1 receptor extracellular domain. J Biol Chem. 2008;283:11340–11347. doi: 10.1074/jbc.M708740200. [DOI] [PubMed] [Google Scholar]
- 13.Nauck M.A., Kleine N., Orskov C., et al. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7–36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1993;36:741–744. doi: 10.1007/BF00401145. [DOI] [PubMed] [Google Scholar]
- 14.Drucker D.J., Philippe J., Mojsov S., Chick W.L., Habener J.F. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc Natl Acad Sci USA. 1987;84:3434–3438. doi: 10.1073/pnas.84.10.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buteau J., Foisy S., Joly E., Prentki M. Glucagon-like peptide 1 induces pancreatic beta-cell proliferation via transactivation of the epidermal growth factor receptor. Diabetes. 2003;52:124–132. doi: 10.2337/diabetes.52.1.124. [DOI] [PubMed] [Google Scholar]
- 16.Drucker D.J., Nauck M.A. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 17.Toft-Nielsen M.B., Damholt M.B., Madsbad S., et al. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol. Metab. 2001;86:3717–3723. doi: 10.1210/jc.86.8.3717. [DOI] [PubMed] [Google Scholar]
- 18.Nauck M., Stöckmann F., Ebert R., Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1986;29:46–52. doi: 10.1007/BF02427280. [DOI] [PubMed] [Google Scholar]
- 19.Vilsbøll T., Krarup T., Madsbad S., Holst J.J. Defective amplification of the late phase insulin response to glucose by GIP in obese Type II diabetic patients. Diabetologia. 2002;45:1111–1119. doi: 10.1007/s00125-002-0878-6. [DOI] [PubMed] [Google Scholar]
- 20.Spiller R.C., Trotman I.F., Higgins B.E., et al. The ileal brake—inhibition of jejunal motility after ileal fat perfusion in man. Gut. 1984;25:365–374. doi: 10.1136/gut.25.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meier J. J., Gethmann A., Götze O., et al. Glucagonlike peptide 1 abolishes the postprandial rise in triglyceride concentrations and lowers levels of non-esterified fatty acids in humans. Diabetologia. 2006;49:452–458. doi: 10.1007/s00125-005-0126-y. [DOI] [PubMed] [Google Scholar]
- 22.Nagell C.F., Wettergren A., Ørskov C., Holst J.J. Inhibitory effect of GLP-1 on gastric motility persists after vagal deafferentation in pigs. Scand J Gastroenterol. 2006;41:667–672. doi: 10.1080/00365520500408253. [DOI] [PubMed] [Google Scholar]
- 23.Wettergren A., Wojdemann M., Meisner S., et al. The inhibitory effect of glucagon-like peptide-1 (GLP-1) 7–36 amide on gastric acid secretion in humans depends on an intact vagal innervation. Gut. 1997;40:597–601. doi: 10.1136/gut.40.5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zander M., Madsbad S., Madsen J.L., Holst J.J. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet. 2002;359:824–830. doi: 10.1016/S0140-6736(02)07952-7. [DOI] [PubMed] [Google Scholar]
- 25.Williams D.L. Minireview: finding the sweet spot: peripheral versus central glucagon-like peptide 1 action in feeding and glucose homeostasis. Endocrinology. 2009;150:2997–3001. doi: 10.1210/en.2009-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.During M.J., Cao L., Zuzga D.S., et al. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med. 2003;9:1173–1179. doi: 10.1038/nm919. [DOI] [PubMed] [Google Scholar]
- 27.Grieve D.J., Cassidy R.S., Green B.D. Emerging cardiovascular actions of the incretin hormone glucagon-like peptide-1: potential therapeutic benefits beyond glycaemic control? Br J Pharmacol. 2009;157:1340–1351. doi: 10.1111/j.1476-5381.2009.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ban K., Noyan-Ashraf M.H., Hoefer J., et al. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and independent pathways. Circulation. 2008;117:2340–2350. doi: 10.1161/CIRCULATIONAHA.107.739938. [DOI] [PubMed] [Google Scholar]
- 29.Nikolaidis L.A., Elahi D., Hentosz T., et al. Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation. 2004;110:955–961. doi: 10.1161/01.CIR.0000139339.85840.DD. [DOI] [PubMed] [Google Scholar]
- 30.Timmers L., Henriques J.P., de Kleijn D.P., et al. Exenatide reduces infarct size and improves cardiac function in a porcine model of ischemia and reperfusion injury. J Am Coll Cardiol. 2009;53:501–510. doi: 10.1016/j.jacc.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 31.Poornima I., Brown S.B., Bhashyam S., et al. Chronic glucagon-like peptide-1 infusion sustains left ventricular systolic function and prolongs survival in the spontaneously hypertensive heart failureprone rat. Circ Heart Fail. 2008;1:153–160. doi: 10.1161/CIRCHEARTFAILURE.108.766402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noyan-Ashraf M.H., Momen M.A., Ban K., et al. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes. 2009;58:975–983. doi: 10.2337/db08-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nikolaidis L.A., Mankad S., Sokos G.G., et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109:962–965. doi: 10.1161/01.CIR.0000120505.91348.58. [DOI] [PubMed] [Google Scholar]
- 34.Sokos G.G., Nikolaidis L.A., Mankad S., et al. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail. 2006;12:694–699. doi: 10.1016/j.cardfail.2006.08.211. [DOI] [PubMed] [Google Scholar]
- 35.Sokos G.G., Bolukoglu H., German J., et al. Effect of glucagon-like peptide-1 (GLP-1) on glycemic control and left ventricular function in patients undergoing coronary artery bypass grafting. Am J Cardiol. 2007;100:824–829. doi: 10.1016/j.amjcard.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 36.Klonoff D.C., Buse J.B., Nielsen L.L., et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin. 2008;24:275–286. doi: 10.1185/030079908x253870. [DOI] [PubMed] [Google Scholar]
- 37.Mussig K., Oncu A., Lindauer P., et al. Effects of intravenous glucagon-like peptide-1 on glucose control and hemodynamics after coronary artery bypass surgery in patients with type 2 diabetes. Am J Cardiol. 2008;102:646–647. doi: 10.1016/j.amjcard.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 38.Basu A., Charkoudian N., Schrage W., et al. Beneficial effects of GLP-1 on endothelial function in humans: dampening by glyburide but not by glimepiride. Am J Physiol. 2007;293:E1289–1295. doi: 10.1152/ajpendo.00373.2007. [DOI] [PubMed] [Google Scholar]
- 39.Carraro-Lacroix L.R., Malnic G., Girardi A.C.C. Regulation of Na+/H+ exchanger NHE3 by glucagon-like peptide 1 receptor agonist exendin-4 in renal proximal tubule cells. Am J Physiol. 2009;29:F1647–1655. doi: 10.1152/ajprenal.00082.2009. [DOI] [PubMed] [Google Scholar]
- 40.Gutzwiller J.P., Tschopp S., Bock A., et al. Glucagon-like peptide 1 induces natriuresis in healthy subjects and in insulin-resistant obese men. J Clin Endocrinol Metab. 2004;89:3055–3061. doi: 10.1210/jc.2003-031403. [DOI] [PubMed] [Google Scholar]
- 41.Benito E., Blazquez E., Bosch M.A. Glucagon-like peptide-1-(7–36)amide increases pulmonary surfactant secretion through a cyclic adenosine 3, 5-monophosphate-dependent protein kinase mechanism in rat type II pneumocytes. Endocrinology. 1998;139:2363–2368. doi: 10.1210/en.139.5.2363. [DOI] [PubMed] [Google Scholar]
- 42.Vilsbøll T. Liraglutide: a once-daily GLP-1 analogue for the treatment of type 2 diabetes mellitus. Expert Opin Investig Drugs. 2007;16:231–237. doi: 10.1517/13543784.16.2.231. [DOI] [PubMed] [Google Scholar]
- 43.Eng J., Kleinman W.A., Singh L., et al. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J Biol Chem. 1992;267:7402–7405. [PubMed] [Google Scholar]
- 44.Thorens B., Porret A., Bühler L., et al. Cloning and functional expression of the human islet GLP-1 receptor. Demonstration that exendin-4 is an agonist and exendin-(9-39) an antagonist of the receptor. Diabetes. 1993;42:1678–1682. doi: 10.2337/diabetes.42.11.1678. [DOI] [PubMed] [Google Scholar]
- 45.European Medicines Agency: EPARs for authorised medicinal products for human use. Byetta: Scientific Discussion (6). Available at: www.ema.europa.eu/humandocs/Humans/EPAR/byetta/byetta.htm. Accessed: January 28, 2011.
- 46.Kolterman O.G., Kim D.D., Shen L., et al. Pharmacokinetics, pharmacodynamics and safety of exenatide in patients with type 2 diabetes mellitus. Am J Health Syst Pharm. 2005;62:173–181. doi: 10.1093/ajhp/62.2.173. [DOI] [PubMed] [Google Scholar]
- 47.Iltz J.L., Baker D.E., Setter S.M., Keith Campbell R. Exenatide: an incretin mimetic for the treatment of type 2 diabetes mellitus. Clin Ther. 2006;28:652–665. doi: 10.1016/j.clinthera.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 48.DeFronzo R.A., Ratner R.E., Han J., Kim D.D., Fineman M.S., Baron A.D. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28:1092–1100. doi: 10.2337/diacare.28.5.1092. [DOI] [PubMed] [Google Scholar]
- 49.Buse J.B., Sesti G., Schmidt W.E., et al. Switching to once-daily liraglutide from twice-daily exenatide further improves glycemic control in patients with type 2 diabetes using oral agents. Diabetes Care. 2010;33:1300–1303. doi: 10.2337/dc09-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tzefos M., Olin J.L. Glucagon-like peptide-1 analog and insulin combination therapy in the management of adults with type 2 diabetes mellitus. Ann Pharmacother. 2010;44:1294–1300. doi: 10.1345/aph.1P047. [DOI] [PubMed] [Google Scholar]
- 51.Barnett A.H., Burger J., Johns D., et al. Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, openlabel, two-period, crossover noninferiority trial. Clin Ther. 2007;29:2333–2348. doi: 10.1016/j.clinthera.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 52.Moretto T.J., Milton D.R., Ridge T.D., et al. Efficacy and tolerability of exenatide monotherapy over 24 weeks in antidiabetic drug-naive patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2008;30:1448–1460. doi: 10.1016/j.clinthera.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 53.Knudsen L.B., Nielsen P.F., Huusfeldt P.O., et al. Potent derivatives of glucagon-like peptide-1 with pharmacokinetic properties suitable for once daily administration. J Med Chem. 2000;43:1664–1669. doi: 10.1021/jm9909645. [DOI] [PubMed] [Google Scholar]
- 54.Sturis J., Gotfredsen C.F., Rømer J., et al. GLP-1 derivative liraglutide in rats with beta-cell deficiencies: influence of metabolic state on betacell mass dynamics. Br J Pharmacol. 2003;140:123–132. doi: 10.1038/sj.bjp.0705397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Toso C., McCall M., Emamaullee J., et al. Liraglutide, a long-acting human glucagon-like peptide 1 analogue, improves human islet survival in culture. Transpl Int. 2010;23:259–265. doi: 10.1111/j.1432-2277.2009.00984.x. [DOI] [PubMed] [Google Scholar]
- 56.Raun K., von Voss P., Gotfredsen C.F., Golozoubova V., Rolin B., Knudsen L.B. Liraglutide, a long-acting glucagon-like peptide-1 analog, reduces body weight and food intake in obese candy-fed rats, whereas a dipeptidyl peptidase-IV inhibitor, vildagliptin, does not. Diabetes. 2007;56:8–15. doi: 10.2337/db06-0565. [DOI] [PubMed] [Google Scholar]
- 57.Degn K.B., Juhl C.B., Sturis J., et al. One week’s treatment with the long-acting glucagonlike peptide 1 derivative liraglutide (NN2211) markedly improves 24-h glycemia and alpha- and beta-cell function and reduces endogenous glucose release in patients with type 2 diabetes. Diabetes. 2004;53:1187–1194. doi: 10.2337/diabetes.53.5.1187. [DOI] [PubMed] [Google Scholar]
- 58.Horowitz M., Flint A., Doran S., et al. The once-daily human GLP-1 analogue liraglutide decreases postprandial hunger and energy intake [Abstract 555-P] Diabetes. 2008;57(suppl.1):A165. [Google Scholar]
- 59.Buse J.B., Rosenstock J., Sesti G., LEAD-6 Study Group et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, openlabel trial (LEAD-6) Lancet. 2009;374:39–47. doi: 10.1016/S0140-6736(09)60659-0. [DOI] [PubMed] [Google Scholar]
- 60.Garber A., Henry R., Ratner R., LEAD-3 et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473–481. doi: 10.1016/S0140-6736(08)61246-5. [DOI] [PubMed] [Google Scholar]
- 61.Marre M., Shaw J., Brändle M., LEAD-1 SU study group et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with Type 2 diabetes (LEAD-1 SU) Diabet Med. 2009;26:268–278. doi: 10.1111/j.1464-5491.2009.02666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Russell-Jones D., Vaag A., Schmitz O., Liraglutide EffectAction in Diabetes 5 et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia. 2009;52:2046–2055. doi: 10.1007/s00125-009-1472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zinman B., Gerich J., Buse J.B., LEAD-4 Study Investigators et al. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD) Diabetes Care. 2009;32:1224–1230. doi: 10.2337/dc08-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pratley R.E., Nauck M., Bailey T., 1860-LIRADPP-4 Study Group et al. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet. 2010;375:1447–1456. doi: 10.1016/S0140-6736(10)60307-8. [DOI] [PubMed] [Google Scholar]
- 65.Pratley R.E., Nauck M., Frid A., Hermansen K., LEAD-2 Study Group et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32:84–90. doi: 10.2337/dc08-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fonseca V.A., Zinman B., Nauck M.A., Goldfine A.B., Plutzky J. Confronting the type 2 diabetes epidemic: the emerging role of incretin-based therapies. Am J Med. 2010;123:S2–S10. doi: 10.1016/j.amjmed.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 67.Bode B.W., Testa M.A., Magwire M., LEAD-3 Study Group et al. Patient-reported outcomes following treatment with the human GLP-1 analogue liraglutide or glimepiride in monotherapy: results from a randomized controlled trial in patients with type 2 diabetes. Diabetes Obes Metab. 2010;12:604–612. doi: 10.1111/j.1463-1326.2010.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Montanya E., Sesti G. A review of efficacy and safety data regarding the use of liraglutide, a oncedaily human glucagon-like peptide 1 analogue, in the treatment of type 2 diabetes mellitus. Clin Ther. 2009;31:2472–2488. doi: 10.1016/j.clinthera.2009.11.034. [DOI] [PubMed] [Google Scholar]
- 69.Bush M.A., Matthews J.E., De Boever E.H., et al. Safety, tolerability, pharmacodynamics and pharmacokinetics of albiglutide, a long-acting glucagon-like peptide-1 mimetic, in healthy subjects. Diabetes Obes Metab. 2009;11:498–505. doi: 10.1111/j.1463-1326.2008.00992.x. [DOI] [PubMed] [Google Scholar]
- 70.Tomkin G.H. Albiglutide, an albumin-based fusion of glucagon-like peptide 1 for the potential treatment of type 2 diabetes. Curr Opin Mol Ther. 2009;11:579–588. [PubMed] [Google Scholar]
- 71.Rosenstock J., Reusch J., Bush M., Yang F., Stewart M., Albiglutide Study Group Potential of albiglutide, a long-acting GLP-1 receptor agonist, in type 2 diabetes: a randomized controlled trial exploring weekly, biweekly, and monthly dosing. Diabetes Care. 2009;32:1880–1886. doi: 10.2337/dc09-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baggio L.L., Huang Q., Brown T.J., Drucker D.J. A recombinant human glucagon-like peptide (GLP)-1-albumin protein (albugon) mimics peptidergic activation of GLP-1 receptor-dependent pathways coupled with satiety, gastrointestinal motility, and glucose homeostasis. Diabetes. 2004;53:2492–2500. doi: 10.2337/diabetes.53.9.2492. [DOI] [PubMed] [Google Scholar]
- 73.Retterstøl K. Taspoglutide: a long acting human glucagon-like polypeptide-1 analogue. Expert Opin Investig Drugs. 2009;18:1405–1411. doi: 10.1517/13543780903164205. [DOI] [PubMed] [Google Scholar]
- 74.Kapitza C., Heise T., Birman P., et al. Pharmacokinetic and pharmacodynamic properties of taspoglutide, a once-weekly, human GLP-1 analogue, after single-dose administration in patients with type 2 diabetes. Diabet Med. 2009;26:1156–1164. doi: 10.1111/j.1464-5491.2009.02854.x. [DOI] [PubMed] [Google Scholar]
- 75.Nauck M.A., Ratner R.E., Kapitza C., Berria R., Boldrin M., Balena R. Treatment with the human once-weekly glucagon-like peptide-1 analog taspoglutide in combination with metformin improves glycemic control and lowers body weight in patients with type 2 diabetes inadequately controlled with metformin alone: a double-blind placebo-controlled study. Diabetes Care. 2009;32:1237–1243. doi: 10.2337/dc08-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tracy M.A., Ward K.L., Firouzabadian L., et al. Factors affecting the degradation rate of poly(lactideco-glycolide) microspheres in vivo and in vitro. Biomaterials. 1999;20:1057–1062. doi: 10.1016/S0142-9612(99)00002-2. [DOI] [PubMed] [Google Scholar]
- 77.Verspohl E.J. Novel therapeutics for type 2 diabetes: incretin hormone mimetics (glucagon-like peptide-1 receptor agonists) and dipeptidyl peptidase-4 inhibitors. Pharmacol Ther. 2009;124:113–118. doi: 10.1016/j.pharmthera.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 78.Kim D., MacConell L., Zhuang D., et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care. 2007;30:1487–1493. doi: 10.2337/dc06-2375. [DOI] [PubMed] [Google Scholar]
- 79.Drucker D.J., Buse J.B., Taylor K., et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, noninferiority study. Lancet. 2008;372:1240–1250. doi: 10.1016/S0140-6736(08)61206-4. [DOI] [PubMed] [Google Scholar]
- 80.Food and Drug Administration Center for Drug Evaluation and Research (CDER) Endocrinologic and Metabolic Drugs Advisory Committee Meeting. Hilton Hotel, Washington DC/Silver Spring, Maryland. Summary Minutes. 2009. Available at: www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugs/AdvisoryCommittee/UCM192028.pdf. Accessed: January 28, 2011.
- 81.Lamari Y., Boissard C., Moukhtar M.S., Jullienne A., Rosselin G., Garel J.M. Expression of glucagonlike peptide 1 receptor in a murine C cell line: regulation of calcitonin gene by glucagon-like peptide 1. FEBS Lett. 1996;393:248–252. doi: 10.1016/0014-5793(96)00895-2. [DOI] [PubMed] [Google Scholar]
- 82.Dore D.D., Seeger J.D., Arnold Chan K. Use of a claims-based active drug safety surveillance system to assess the risk of acute pancreatitis with exenatide or sitagliptin compared to metformin or glyburide. Curr Med Res Opin. 2009;25:1019–1027. doi: 10.1185/03007990902820519. [DOI] [PubMed] [Google Scholar]
- 83.Noel R.A., Braun D.K., Patterson R.E., Bloomgren G.L. Increased risk of acute pancreatitis and biliary disease observed in patients with type 2 diabetes: a retrospective cohort study. Diabetes Care. 2009;32:834–838. doi: 10.2337/dc08-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Drucker J., Sherman S.I., Gorelick F.S., et al. Incretinbased therapies for the treatment of type 2 diabetes: evaluation of the risks and benefits. Diabetes Care. 2010;33:428–433. doi: 10.2337/dc09-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Franklin I., Gromada J., Gjinovci A., Theander S., Wollheim C.B. β-cell secretory products activate α-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes. 2005;54:1808–1815. doi: 10.2337/diabetes.54.6.1808. [DOI] [PubMed] [Google Scholar]
- 86.Moens K., Heimberg H., Flamez D., et al. Expression and functional activity of glucagon, glucagon-like peptide I, and glucose-dependent insulinotropic peptide receptors in rat pancreatic islet cells. Diabetes. 1996;45:257–261. doi: 10.2337/diabetes.45.2.257. [DOI] [PubMed] [Google Scholar]


