Abstract
Background
We previously demonstrated that macrophage LRP1 deficiency increases atherosclerosis despite anti-atherogenic changes including decreased uptake of remnants and increased secretion of apoE. Thus, our objective was to determine whether the atheroprotective effects of LRP1 require interaction with apoE, one of its ligands with multiple beneficial effects.
Methods and Results
We examined atherosclerosis development in mice with specific deletion of macrophage LRP1 (apoE−/−MΦLRP1−/−) and in LDLR−/− mice reconstituted with apoE−/−MΦLRP1−/− bone marrow. The combined absence of apoE and LRP1 promoted atherogenesis more than did macrophage apoE deletion alone in both apoE-producing LDLR−/− (+88%) and apoE−/− mice (+163%). The lesions of both mouse models with apoE−/−LRP1−/− macrophages had increased macrophage content. In vitro, apoE and LRP1 additively inhibit macrophage apoptosis. Furthermore, there was excessive accumulation of apoptotic cells in lesions of both LDLR−/− (+110%) and apoE−/−MΦLRP1−/− mice (+252%). The apoptotic cell accumulation was partially due to decreased efferocytosis as the ratio of free to cell-associated apoptotic nuclei was 3.5-fold higher in lesions of apoE−/−MΦLRP1−/− versus apoE−/− mice. Lesion necrosis was also increased (6-fold) in apoE−/−MΦLRP1−/− versus apoE−/− mice. Compared to apoE−/− mice, the spleens of apoE−/−MΦLRP1−/− mice contained 1.6 – and 2.4 –fold more total and Ly6-Chigh monocytes. Finally, there were 3.6- and 2.4-fold increases in Ly6-Chigh and CCR2+ cells in lesions of apoE−/−MΦLRP1−/− versus apoE−/− mice, suggesting that accumulation of apoptotic cells enhances lesion development and macrophage content by promoting the recruitment of inflammatory monocytes.
Conclusion
LRP1 exerts anti-atherogenic effects via pathways independent of apoE involving macrophage apoptosis and monocyte recruitment.
Keywords: atherosclerosis, apoE, LRP1, Ly6-Chigh monocytes, apoptosis
Macrophages play a critical role in atherogenesis by taking up lipoprotein particles trapped in the arterial intima and activating the inflammatory response, or turning into apoptosis-prone foam cells. Apolipoprotein E (apoE) and the LDL receptor-related protein 1 (LRP1) are critical for these processes1–3. Plasma apoE associates with lipoproteins and causes bulk clearance of remnants4. Macrophages contribute to less than 10% of plasma apoE5, but produce most of the apoE in the atheroma. Evidence supports an anti-atherogenic role for both systemic and macrophage apoE1, 2, 6. LRP1 is both a cargo-transporter and a signaling receptor. It binds over 30 distinct extracellular ligands and various cytoplasmic adaptor proteins7. LRP1 protects against atherosclerosis when expressed by vascular smooth muscle cells (SMCs)8, hepatocytes9, or macrophages3, 10.
LRP1 and apoE may work in common pathways in preventing atherosclerosis. ApoE on remnant lipoproteins serves as a ligand for their LRP1-mediated internalization9, and is one of several triggers of LRP1-activated downstream signaling7. ApoE signaling via LRP1 has been shown to promote cell survival11. Interaction of apoE with macrophage LRP1 initiates calcium mobilization and both apoE and LRP1 limit the macrophage inflammatory response by preventing NF-κβ activation12, 13. In addition, our previous studies demonstrated that macrophages lacking LRP1 cause increased atherosclerosis development in LDLR−/− mice despite up-regulation of apoE secretion,3, 14 suggesting that LRP1 mediates the beneficial effects of apoE. In these studies, we also showed that the LRP1/apoE axis limits macrophage inflammation and regulates the rate of efferocytosis of apoptotic macrophages14.
The objective of the current studies was to investigate whether LRP1 modulates atherosclerosis independently of apoE. We examined atherosclerosis development in mice with specific deletion of macrophage LRP1 (apoE−/−MΦLRP1−/−) and in LDLR−/− mice reconstituted with apoE−/−MΦLRP1−/− bone marrow. Our studies demonstrate that the pro-atherogenic effects of macrophage LRP1 deletion are enhanced in the absence of macrophage apoE and further enhanced in the absence of systemic apoE. The atherogenic effects of macrophage LRP1 deletion in the setting of complete apoE deficiency include enhanced intimal macrophage accumulation, excessive macrophage apoptosis, decreased efferocytosis, increased necrosis, and enhanced content of pro-infammatory Ly6-Chigh monocytes and CCR2+ macrophages15, 16.
Methods
A detailed description of all methods is available in the supplemental materials (available online at http://circ.ahajournals.org).
Mice
Mice lacking macrophage LRP1 (MΦLRP1−/−) were developed as described,3 and mated with apoE−/− mice to obtain apoE−/−MΦLRP1−/− mice. All mice were on C57BL/6 background. Animal procedures were performed in accordance with the Institutional Animal Care and Usage Committee of Vanderbilt University.
Atherosclerosis Analyses
LDLR−/− mice (12 week old) were lethally irradiated (9.5 Gy) using a cesium gamma source and transplanted with 5×106 bone marrow (BM) cells from female apoE−/−(n=10) or apoE−/−MΦLRP1−/− (DKO) mice (n=10) as described1, 2. After four weeks, the mice were placed on western-type diet for 8 weeks, and then the extent of atherosclerosis was examined. For experiments comparing atherosclerosis in apoE−/− (n=12) or apoE−/−MΦLRP1−/− mice (n=12), eight to ten week old female mice were fed a western-type diet for 8 weeks. The extent of atherosclerosis was examined both in oil red O–stained cross-sections of the proximal aorta (15 alternate 10-μm cryosections) and by en face analysis using the KS300 imaging system (Kontron Elektronik GmbH) as described 1.
Analyses of Macrophage Apoptosis
Peritoneal macrophages were seeded in chamber slides (Nalge Nunc International) and incubated for 16h in serum-free DMEM alone or containing lipopolysaccharide (LPS, 50ng/ml). Cell death was determined by TUNEL (Tdt-mediated dUTP nick end labeling) staining using the in situ detection kit (Roche).
Analyses of Lesion Apoptosis, Efferocytosis, and Necrosis
TUNEL staining was performed on 5-μm proximal aortic cryosections using the in situ cell death detection kit (Roche). The TUNEL positive cells were counted in four sections per mouse and normalized to Oil-red-O lesion area. For analysis of efferocytosis, 5-μm proximal aortic cryosections were stained with TUNEL using the TMR red (Roche) detection kit. Nuclei were counterstained with Hoechst and macrophage cytoplasm was detected using rabbit anti-macrophage antibody (Accurate Chemical and Scientific Corp.), goat anti-rabbit Alexa Fluor 488 conjugated secondary antibody (Molecular Probes, Inc.). The free versus macrophage associated apoptotic bodies were then counted in 4 sections per mouse. Necrosis was detected using Harris’s hematoxylin and eosin (H&E), and quantitated by measuring the H&E negative acellular area in the intima versus total intimal area.
Analyses of Lesion MOMA-2, Ly-6C, and CCR2
Macrophage content was detected using rabbit antibody against mouse MOMA-2 (Accurate Chemical & Scientific Corp.) as described3. Ly-6Cand CCR2 positive cells were detected in 4 cryosections per mouse using rat anti-mouse Ly6-C biotin (BD Pharmingen #557359), rabbit anti-mouse CCR2 monoclonal (AbCam #ab32144), either Streptavadin-AlexaFluor 488 (Invitrogen #S11223) or goat anti-rabbit AlexaFluor 647 (Invitrogen #A21244).
Flow Cytometry Analyses of Blood and Spleen Ly-6Chigh Monocytes
Wild type (WT, n=10), apoE−/− (n=10), MΦLRP1−/− (n=8), or apoE−/−MΦLRP1−/− (n=10) mice were fed a western diet for 8 weeks, and then blood was collected via cardiac puncture in sodium citrate (10mM sodium citrate, 13mM glucose, pH 6.5). Spleens were homogenized by disruption in sterile PBS pH 7.4 through a 70μM mesh screen. To distinguish monocytes from other blood and spleen cells, FITC fluorochrome tagged rat anti-mouse CD90.2, B220, GR1 (Pharmingen) and NK cells (Caltag) were used. Total monocytes were detected using rat anti-mouse CD11b-PE (Pharmingen) and Ly6-Chigh monocytes were quantitated using rat anti-mouse Ly6-C labeled with biotin (Pharmingen) and streptavadin-linked AlexaFluor 647 (Invitrogen).
Statistical Analysis
Differences between mean values were determined by one-way ANOVA (Bonferroni’s post test) and Mann-Whitney test using GraphPad PRISM. Prior to using one-way ANOVA (Bonferroni’s post test) to test for significance, the normality of the sample populations was tested by the Kolmogorov-Smirnov test. Significance was set for p<0.05.
Results
Impact of macrophage LRP1 deletion on atherosclerosis and intimal macrophage accumulation
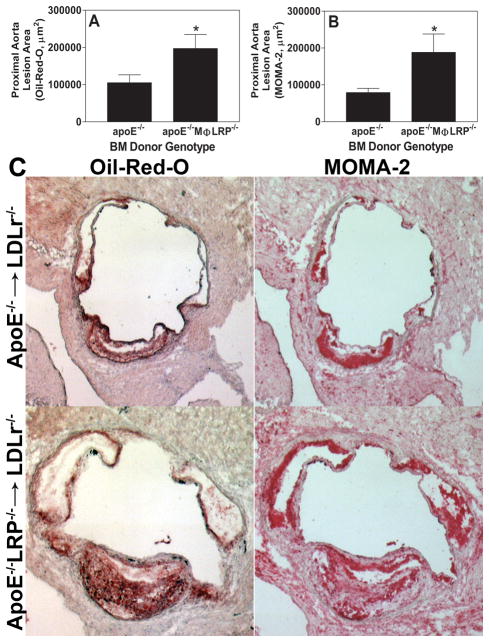
Our previous studies have shown that LDLR−/− mice recipient of apoE+/+MΦLRP1−/− or apoE−/−MΦLRP1+/+ BM develop 40% and 75% larger lesions in the aortic sinus, respectively, compared to LDLR−/− mice transplanted with WT BM2, 3. In addition, we have shown that LRP1−/−macrophages express and secrete more apoE compared to WT cells14. Since macrophage apoE is a potent anti-atherogenic agent2, the failure of increased expression of apoE in LRP1−/−macrophages to decrease atherosclerosis3 is consistent with the possibility that the atherogenic phenotype caused by LRP1−/− macrophages is due to the abrogation of apoE-mediated benefits. Therefore, we set out to study the role of an apoE/LRP1 interaction in atherogenesis by looking at the cellular and vascular consequences of removing either macrophage apoE alone or both systemic and macrophage apoE from mice with LRP1−/− macrophages. We first examined the role of macrophage apoE/LRP1 interaction by transplanting BM from either apoE−/− or apoE−/−MΦLRP1−/− mice into LDLR−/− recipients. We reasoned that, if the atheroprotective effects of macrophage LRP1 were mediated, exclusively or to a large extent, by its interaction with macrophage apoE, then atherosclerosis development would be similar in LDLR−/− mice transplanted with apoE−/− or apoE−/−/MΦLRP1−/− bone marrow. Four weeks after BM transplantation, recipient mice were placed on a Western diet for 8 weeks to raise plasma cholesterol levels and induce atherosclerosis. After 8 weeks on western diet, the plasma cholesterol and triglyceride levels as well as the lipoprotein cholesterol profiles were not different between the two groups (Supplemental Figure 1A). Compared to LDLR−/− mice receiving apoE−/− BM, those that received apoE−/−MΦLRP1−/− marrow had 88% more lipid-stainable lesion area in the proximal aorta (Figures 1A and 1C), and a 138% increase in MOMA-2 stainable intimal macrophages (Figures 1B and 1C). Aorta en face lesions were minimal and not significantly different between apoE−/−MΦLRP1−/− and apoE−/− BM recipient mice (0.91% vs 0.73%, p=0.36). These results are compatible with independent and additive effects of LRP1 and apoE on atherogenesis despite their cooperation in functional networks7, 9, 11.
Figure 1. Atherosclerosis in LDLR−/− mice transplanted with BM from apoE−/− or apoE−/−MΦLRP−/− mice.
Quantitation of the mean Oil-red-O (A) and MOMA-2 (B) stained cross-sectional area of proximal aortas from LDLR−/− mice transplanted with either apoE−/− (n=10) or apoE−/−MΦLRP−/− (n=10) BM. *p<0.05, Mann-Whitney test. C. Representative images show Oil-red-O and MOMA-2 stain in aortic root sections.
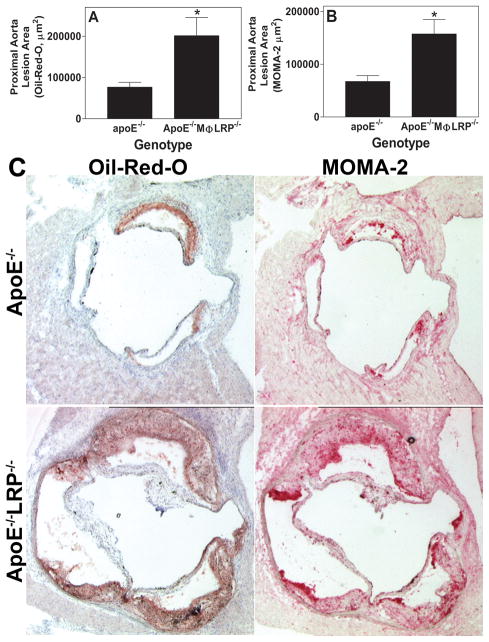
Studies have shown that even small amounts of circulating apoE can decrease atherosclerosis without changing plasma cholesterol17. Therefore, it is important to bear in mind that in the LDLR−/− mice receiving apoE−/− marrow, systemic apoE can navigate into the vessel wall and affect lesional macrophage function, therefore influencing atherogenesis. To eliminate the effects of plasma apoE, we compared plaque development in apoE−/− and apoE−/−/MΦLRP1−/−mice (n=12 each) fed a Western diet for 8 weeks. There were no differences in plasma cholesterol, triglyceride, and lipoprotein FPLC profiles between the two groups (Supplemental Figure 1B). ApoE−/−MΦLRP1−/− mice had 163% more Oil-red-O and 133% more MOMA-2 staining in the proximal aorta compared to apoE−/− mice (Figures 2A, 2B, and 2C). The fact that LRP1 modulates atherogenesis in the complete absence of apoE proves that LRP1 has apoE-independent effects in the vessel wall.
Figure 2. Atherosclerosis in apoE−/− and apoE−/−MΦLRP−/− mice.
Quantitation of the mean Oil-red-O (A) and MOMA-2 (B) stained cross-sectional area of proximal aortas from apoE−/− and apoE−/−MΦLRP−/− mice (n=12 per group). *p<0.05, Mann-Whitney test. C. Images show Oil-redO and MOMA-2 stain in aortic root sections.
Effects of apoE and LRP1 deletion on lesion and macrophage apoptosis
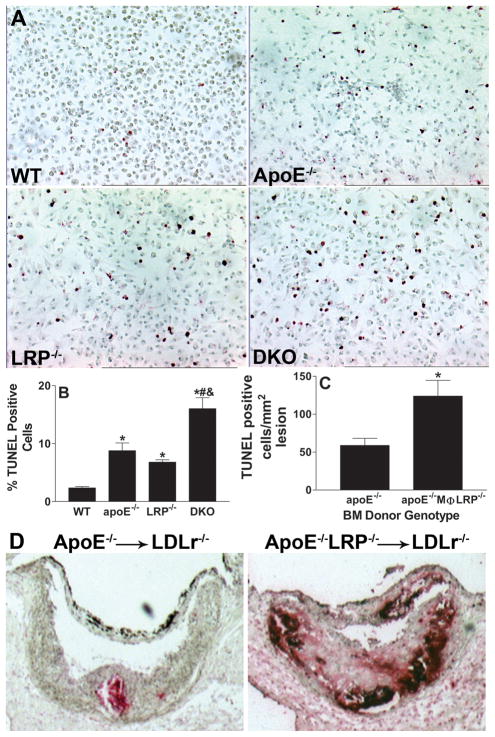
Macrophage apoptosis influences atherosclerosis development18, and both LRP1 and apoE play roles in cell survival and in efferocytosis of apoptotic macrophages11, 14, 19. Therefore, we next compared the level of apoptosis induction in peritoneal macrophages of the four genotypes under conditions of stress. To induce apoptosis, the cells were incubated for 16h in serum-free DMEM (nutritional stressor), and cell death was determined by TUNEL staining (Figures 3Aand 3B). Compared to wild-type macrophages, significantly more apoptotic cells were seen in cultures of apoE−/− (3.7-fold), LRP1−/− (2.5 fold), and apoE−/−LRP1−/− (6.7-fold) macrophages, suggesting additive effects of LRP1 and apoE on apoptotic susceptibility. Similar differences were observed when apoptosis was stimulated with LPS, where TUNEL positive cells were 10%, 21%, 24%, and 48% of lesions in wild-type, apoE−/−, LRP1−/−, and apoE−/−LRP1−/−macrophage cultures, respectively.
Figure 3. In vitro macrophage apoptosis and atherosclerotic lesion apoptosis.
A and B. Images show TUNEL positive (red) and negatively stained macrophages after 16h with DMEM (A). Quantitation of the percent TUNEL positive cells in WT, apoE−/−, LRP−/−, and apoE−/−LRP−/−cultures (n=6 per group). (B). *, #, and & denotes statistically significances compared to WT, apoE−/−, and LRP−/− cells, respectively, p<0.05, ANOVA with Bonferroni’s post test. C. Quantitation of apoptotic cells in proximal aorta sections from apoE−/− (n=6) or apoE−/−MΦLRP−/−(n=7) BM recipient LDLr−/− mice. *p<0.05, Mann-Whitney test. D. Images show TUNEL staining (dark red) of nuclei in aortic root sections.
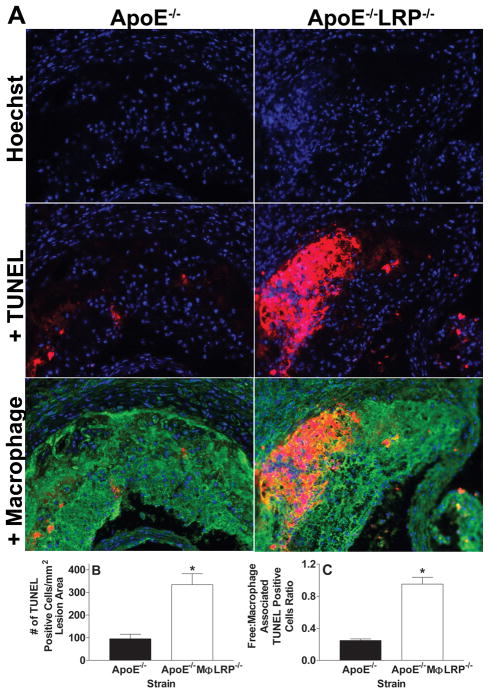
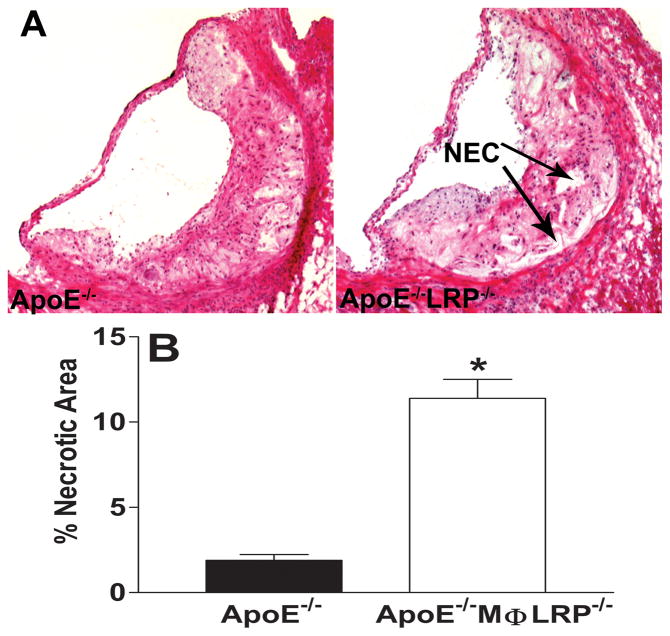
We next examined whether the enhanced atherosclerosis that occurs in the absence of macrophage LRP1 can be attributed to increased macrophage apoptosis. TUNEL staining analyses show that lesions of apoE−/−MΦLRP1−/−→LDLR−/− mice contained more than twice as many apoptotic cells per mm2 lesion area compared to the lesions of apoE−/−→LDLR−/− mice (Figures 3C and 3D). Compared to lesions of apoE−/− mice, there was a 3.5-fold increase in apoptotic cells in lesions of apoE−/−MΦLRP1−/− mice (Figures 4A and 4B), demonstrating that the effects of macrophage LRP1 deletion on lesional apoptotic cell accumulation are more exacerbated in the complete absence of apoE. The apoptotic cells co-localized with macrophage enriched areas of lesions in both apoE−/− and apoE−/− MΦLRP1−/− mice (Figure 4A). Staining of lesion apoptosis, nuclei, and macrophage cytoplasm enabled the quantitation of free versus macrophage associated apoptotic bodies as previously described in detail14 (also see Supplemental Methods). The ratio of free to macrophage associated apoptotic nuclei/bodies was 3.8-fold higher in lesions of apoE−/− MΦLRP1−/− mice compared to apoE−/− mice (Figure 4C), indicating that deletion of macrophage LRP1 impairs efferocytosis independently of apoE. Consistent with the excessive accumulation of apoptotic cells and defective lesional efferocytosis, the percent necrotic area was 6-fold greater in lesions of apoE−/− MΦLRP1−/− mice (Figures 5A and 5B).
Figure 4. Macrophage apoptosis and efferocytosis in lesions of apoE−/− and apoE−/− MΦLRP−/− mice.
A Micrographs show nuclei (Hoechst, blue), nuclei + TUNEL positive staining (red), and merged images of macrophage cytoplasm (green), nuclei, and TUNEL in aortic root sections from apoE−/− and apoE−/−MΦLRP−/− mice. Quantitation of the number of apoptotic cells (B) and of the ratio of free versus macrophage associated TUNEL positive cells (C) in proximal aorta sections of lesions from apoE−/− and apoE−/−MΦLRP−/− mice (n=6 per group). *p<0.05, Mann-Whitney test.
Figure 5. Necrosis in lesions of apoE−/− and apoE−/−MΦLRP−/− mice.
A Images show hematoxylin and eosin staining of aortic root sections from apoE−/− and apoE−/−MΦLRP−/− mice. NEC denotes necrotic area. B. Quantitation of the percent necrotic area in the aortic lesions from apoE−/− and apoE−/−MΦLRP−/− mice (n=6 per group). *p<0.05, Mann-Whitney test.
Effects of apoE and LRP1 deletion on circulating, spleen, and lesion Ly-6Chigh monocytes
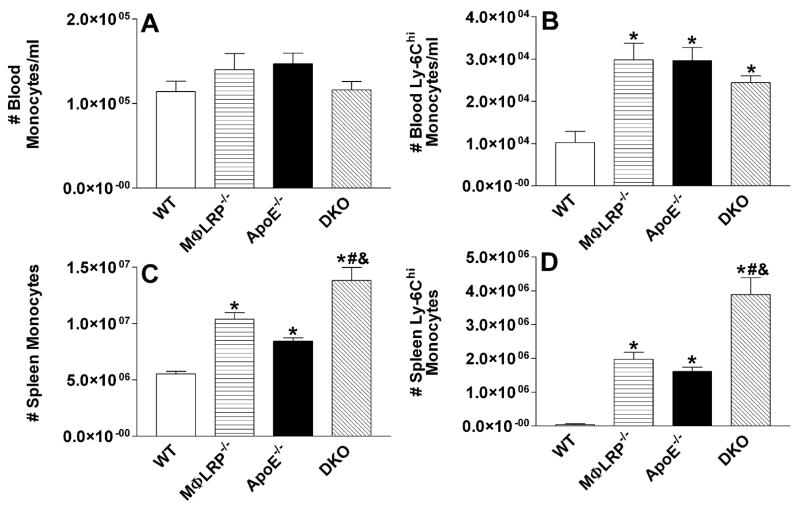
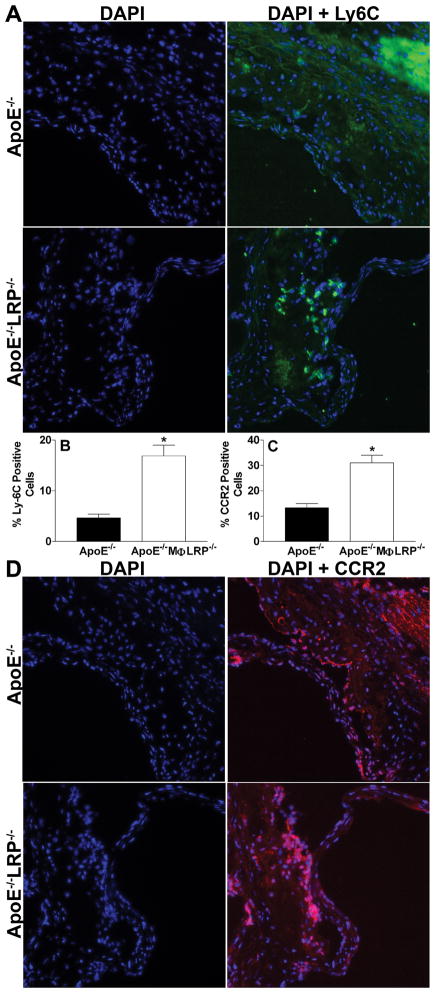
Studies have shown that apoE−/− mice on a western diet accumulate more pro-inflammatory Ly-6Chigh monocytes (Gr1+CCR2+CX3CR1low) in blood, spleen, and atherosclerotic lesions15. In addition, our previous studies have shown that deletion of macrophage LRP1 increases cell migration in response to the CCR2 ligand, MCP-1. Furthermore, studies suggest that apoptotic macrophages can enhance lesion development by promoting endothelial cell inflammation and the recruitment of monocytes18, 20, 21. Therefore, we investigated the impact of LRP1 expression on the accumulation of Ly-6Chigh monocytes. We first examined the effects of either single or combined deletion of apoE and LRP1 on circulating and splenic Ly-6Chigh monocytes in WT, MΦLRP1−/−, apoE−/−, and apoE−/−MΦLRP1−/− mice fed a western diet for 8 weeks, as measured by flow cytometry. The total number of blood monocytes was not significantly different among the four strains of mice (Figure 6A). Similar to what was previously reported,15 Ly-6Chigh monocyte levels in apoE−/− mice were 2.9-fold higher compared to WT mice (Figure 6B). Interestingly, Ly-6Chigh monocyte levels were equally increased in MΦLRP1−/− and apoE−/− MΦLRP1−/− mice (Figure 6B) suggesting that an apoE-LRP1 interaction regulates blood Ly-6Chigh monocytosis. Compared to WT spleens (Figure 6C), the number of total monocytes was 88%, 52%, and 150% higher in MΦLRP1−/−, apoE−/−, and apoE−/−MΦLRP1−/−spleens, respectively, indicating independent roles for apoE and LRP1 in spleen monocytosis. The number of spleen Ly-6Chigh monocytes were markedly increased in MΦLRP1−/−, apoE−/−, and apoE−/−MΦLRP1−/− mice compared to WT mice (Figure 6D) suggesting in large part that the increased monocytosis was due to accumulation of Ly-6Chigh monocytes. Consistent with independent roles of LRP1 and apoE in spleen Ly-6Chigh monocytosis, the number of Ly-6Chigh monocytes in apoE−/−MΦLRP1−/− spleens was 97% higher compared to MΦLRP1−/− spleens and 141% higher compared to apoE−/− spleens (Figure 6D). We next examined the distribution of Ly-6C positive cells in lesions from apoE−/− and apoE−/−MΦLRP1−/− mice (Figures 7A and 7B). Interestingly, the lesions of apoE−/−MΦLRP1−/− mice contained 3.6-fold more Ly-6C positive cells compared to lesions of apoE−/− mice, suggesting that LRP1 acts independently of apoE in controlling the accumulation of pro-inflammatory Ly-6Chigh monoctyes in the plaque. CCR2 is also a marker for pro-inflammatory monocytes and macrophages16 and is critical for the recruitment of Ly-6Chigh monocytes into the intima22. Consistent with the increased Ly-6Chigh monocyte content in apoE−/−MΦLRP1−/− lesions, these plaques also contained 2.4-fold more CCR2 positive cells compared to lesions of apoE−/− mice (Figures 7C and 7D).
Figure 6. Total and Ly-6Chigh monocytes in blood and spleens of WT, MΦLRP−/−, apoE−/−, and apoE−/−MΦLRP−/− mice.
A-D WT (n=10), MΦLRP−/− (n=8), apoE−/− (n=10), and apoE−/−MΦLRP−/− (n=10) mice were fed a western type diet for 8 weeks and then blood (A-B) and spleen (C-D) total (A,C) and Ly-6Chigh monocytes (B,D) were measured by flow cytometry analysis. A-D. *, #, and & denotes statistically significances compared to WT, apoE−/−, and MΦLRP−/− mice, respectively, p<0.05, ANOVA with Bonferroni’s post test.
Figure 7. Ly-6C and CCR2 positive cells in lesions of apoE−/− and apoE−/−MΦLRP−/− mice.
A and D Representative images show nuclei (blue, Dapi) and merged images of Ly-6C (A, green) and CCR positive staining (D, red) and nuclei. B and C. Quantitation of the percent Ly-6C (B) and CCR2 (C) positive cells in aortic root sections from apoE−/− and apoE−/−MΦLRP−/− mice (n=6 per group). *p<0.05, Mann-Whitney test.
Discussion
We previously demonstrated that macrophages lacking LRP1 enhance atherogenesis in the setting of increased apoE production3, 14. These results suggested that the established protective effects of apoE are mediated by its interaction with LRP1. The present studies were designed to determine the effects of macrophage LRP1 deletion in the absence of apoE, either from macrophages only or from all cells, with the aim of evaluating whether the atheroprotective effects of LRP1 are independent of its interaction with apoE. Our results show that LRP1 operates independently of apoE in regulating apoptosis, necrosis, and inflammatory Ly-6Chigh monocytosis, and that deletion of apoE, locally or systemically, amplifies the atherogenic effects of macrophage LRP1 deletion.
The atheroprotective effects of macrophage LRP1 are independent of apoE
We previously showed that MΦLRP1−/− BM recipient LDLR−/− mice develop 40% more atherosclerosis in the proximal aorta compared to LDLR−/− mice receiving control BM3. Prior to that, we had shown that deletion of macrophage apoE in LDLR−/− mice increases aortic sinus plaque burden by 75%2. These data demonstrate that LRP1 and apoE both exert atheroprotective functions. Given that apoE is a ligand for LRP1, we set out to study whether these proteins work exclusively in an interdependent pathway to maintain vascular integrity. Thus, we examined the effects of combined apoE and LRP1 deletion in macrophages. Our findings that apoE−/− MΦLRP1−/−BM→LDLR−/− mice develop 88% more atherosclerosis than recipients of apoE−/− BM proves that the combined deletion of these proteins has additive effects, thus favoring the hypothesis that the atheroprotective effects of macrophage LRP1 are largely independent of its interaction with apoE. In addition, the increased atherosclerotic effect of combined macrophage apoE/LRP deletion relative to that of single deletion of macrophage LRP13, 14 may be attributed to the fact that apoE−/−LRP−/− macrophages lack the compensatory benefit of increased macrophage apoE expression caused by isolated LRP1 deletion.
Because in LDLR−/− mice systemic apoE may interact with macrophage LRP1, we also compared atherosclerosis development in apoE−/− and apoE−/−MΦLRP1−/− mice, the latter lacking both systemic and macrophage apoE. Interestingly, apoE−/−MΦLRP1−/− mice developed 163% larger lesions than apoE−/− only mice. This result is consistent with recent studies of Hu and colleagues10 that showed a 114% increase in apoE−/−LDLR−/−MΦLRP1−/− mice compared to apoE−/−LDLR−/− mice. Based on all these studies, we conclude that macrophage LRP1 is atheroprotective regardless of the absence or presence of systemic or macrophage apoE and that other LRP1 ligands and/or signaling events must in part mediate the anti-atherogenic functions of macrophage LRP1. Conversely, apoE influences the process of plaque formation even in the absence of macrophage LRP1, as evidenced by the observations that the effects of LRP deletion on atherosclerosis and lesion apoptosis were much more pronounced in the setting of complete apoE deficiency. This is consistent with studies showing that even small amounts of circulating apoE can decrease atherosclerosis development17 and that exogenous apoE limits inflammation, apoptosis, and foam cell formation19, 23, 24.
LRP1 expression limits Ly-6Chigh monocytosis
Studies of Swirski and colleagues have shown that apoE−/− mice on a western diet develop enhanced Ly-6Chigh monocytosis that results from increased survival and proliferation as well as lower conversion of Ly-6Chigh monocytes to Ly-6Clow monocytes.25 A role for lipid loading in this process was suggested by the fact that statins prevented the Ly-6Chigh monocytosis whereas incubation with LDL promoted Ly-6Chigh monocyte survival25. Our studies demonstrate that deletion of macrophage LRP1 enhances circulating, splenic, and atheroma Ly-6Chigh monocytosis (Figures 6B, 6D, and 7B). The observation that Ly-6Chigh monocytosis in blood and spleen is similar in MΦLRP1−/− versus apoE−/− mice suggests that LRP1 plays a direct role in limiting Ly-6Chigh monocytosis as plasma cholesterol levels were much lower in MΦLRP1−/− mice compared to apoE−/− mice (101±12 vs. 817±40 mg/dl). Consistent with this concept is the observation that blood and spleen Ly-6Chigh monocytosis was markedly enhanced in MΦLRP1−/− mice compared to WT mice, which had similar plasma cholesterol levels (Figures 6B and 6D). The finding that blood Ly-6Chigh monocytosis was similar in MΦLRP1−/−, apoE−/−, and apoE−/−MΦLRP1−/− mice (Figure 6B) could indicate that an interdependent LRP1/apoE interaction regulates circulating Ly-6Chigh monocyte levels, where the absence of the ligand causes an equivalent effect to the absence of its receptor. However, it is worth noting that circulating Ly-6Chigh monocytes are maintained at low levels due to their rapid deployment to sites of inflammation26–28 and their shuttling back to the bone marrow compartment for conversion to Ly-6Clow monocytes26, raising the possibility that the lack of a difference in blood Ly-6Chigh monocyte levels is merely a reflection of increased recruitment of monocytes to inflammatory areas in apoE−/−MΦLRP1−/− mice. Spleen and lesion Ly-6Chigh monocyte levels were indeed markedly increased in apoE−/−MΦLRP1−/− mice compared to apoE−/− mice (Figure 6B), supporting an independent role for LRP1 in controlling Ly-6Chigh monocytosis. Splenic Ly-6Chigh monocytosis is increased during inflammation,29 and the spleen has been recently proven to serve as the source of Ly-6Chigh monocytes for recruitment to acute inflammatory sites, such as ischemic heart muscle28. Our previous studies showed that deletion of macrophage LRP1 enhances the expression of inflammatory cytokines including MCP-1, TNF-α, and IL-1β14, which could enhance recruitment of Ly-6Chigh monocytes to the spleen. In addition, the enhanced Ly-6Chigh monocytosis is likely due in part to activation of NF-κβ as increases in monocyte/macrophage survival and proliferation result from NF-κβ activation 30–32 and our studies demonstrated that expression of LRP1 reduces NF-κβ activation12. Another possibility is that LRP1 regulates Ly-6Chigh monocytosis by influencing cholesterol homeostasis, as cholesterol efflux reduces monocytosis33 and deletion of LRP1 decreases ABCA1 expression and cholesterol efflux in smooth muscle cells34.
LRP1 prevents macrophage apoptosis independently of apoE
Macrophage apoptosis plays a role in atherosclerosis development18. Studies have shown that LRP1 mediates the phagocytosis of apoptotic macrophages and promotes cell survival11, 14. ApoE has also been implicated in mediating phagocytic14, 19 and anti-apoptotic pathways in cells35. In some cell types, the anti-apoptotic effects of apoE are mediated by LRP1/apoE interaction11. Our in vitro studies show that both LRP1 and apoE deletion increase macrophage apoptosis, and that the combined deletion of LRP1 and apoE produces an additive effect in increasing the number of apoptotic macrophages (Figures 3A and 3B). This observation suggests that apoE and LRP1 have independent roles in macrophage apoptosis. Thus, apoE may protect macrophages from apoptosis through interacting with proteins other than LRP1. As an example, SR-BI has been shown to mediate the phagocytic clearance of apoptotic cells and bind with high affinity to apoE/phospholipid complexes36. However, our recent studies demonstrate that macrophage LRP1 deletion in LDLR−/− mice promotes the accumulation of lesion apoptotic macrophages by decreasing efferocytosis despite increased apoE secretion compared to WT cells14. This suggests that in vivo the independent antiapoptotic mechanisms of apoE cannot compensate for the absence of LRP1. Consistent with the in vitro studies, we demonstrate that combined deletion of macrophage LRP1 and apoE in vivo results in markedly increased numbers of lesion apoptotic cells both in the presence of systemic apoE (Figure 3C) and in the complete absence of apoE (Figure 4B). Furthermore, the accumulation of apoptotic macrophages resulting from combined deletion of LRP1 and apoE was associated with impaired efferocytosis (Figure 4C). LRP1 ligands that can regulate apoptotic cell homeostasis in vivo include prosaposin37, α2-macroglobulin38, β2 glycoprotein-139, and calreticulin40.
In recent years, it has become clear that macrophage apoptosis has divergent effects on atherosclerosis18, 41 depending on the balance between generation of apoptotic cells and efficient phagocytic clearance. Studies by our group and others have shown that in the early fatty streak phase18, 42, 43, modest increases in apoptotic macrophages coincide with decreased atherosclerosis due to efficient efferocytosis. In later lesion stages, the accumulation of apoptotic cells has been proposed to promote plaque instability41. The non-internalized apoptotic cells secrete inflammatory cytokines14, 44, driving more post-apoptotic necrosis resulting in plaque instability14, 41. Consistent with the accumulation of inflammatory apoptotic macrophages promoting plaque instability, the necrotic area was markedly increased in lesions of apoE−/−MΦLRP1−/− mice compared to lesions in apoE−/− mice (Figure 5) after only 8 weeks of consuming a western diet.
Besides impacting plaque stability, the excessive accumulation of apoptotic macrophages in lesions of mice with combined deficiency of apoE and LRP likely contributes to the accelerated atherosclerosis development by facilitating the recruitment of monocytes. Studies have demonstrated that apoptotic macrophages contain oxidized phospholipid, which promote secretion of inflammatory cytokines, endothelial cell activation, and recruitment of monocytes18,21. The current studies show that the combined deficiency of macrophage apoE and LRP1 enhance the macrophage content in lesions of both LDLR−/− and apoE−/− mice. Consistent with this possibility also is the finding that the lesions of apoE−/−MΦLRP1−/− contained more Ly-6Chigh monocytes (Figure 7B). Compared to Ly-6Clow monocytes, Ly-6Chigh monocytes preferentially bind to activated endothelium and migrate into atherosclertic lesions15. CCR2 is a marker for pro-inflammatory monocytes and macrophages16 and is critical for the recruitment of Ly-6Chigh monocytes into the intima15. Thus, the increased content of CCR2 positive cells (Figure 7C) in apoE−/−MΦLRP1−/− lesions is also consistent with enhanced recruitment of pro-inflammatory Ly-6Chigh monocytes. Other studies have shown that accumulation of lesion apoptotic macrophages is associated with enhanced atherosclerosis progression and monocyte recruitment18, 45, 46, particularly in the presence of inflammation resulting from defective efferocytosis20, 47, 48.
In conclusion, our study demonstrates that macrophage LRP1 slows atherosclerosis development by limiting lesion macrophage death and Ly-6Chigh monocytosis. These atheroprotective functions occur via mechanisms that are independent of its interaction with apoE. Thus, apoE and LRP1, two abundant atheroma proteins that physically interact with each other to regulate cell survivial, remnant uptake, and activate downstream signaling,7, 9, 11 actually influence atherogenesis through non-redundant pathways.
Supplementary Material
Clinical Perspective.
ApoE is a plasma protein that regulates both clearance of VLDL and maturation of HDL. It is also expressed at high levels by macrophages, and found to have strong anti-atherogenic effects in mouse models. In humans, HDL-associated apoE correlates with presence of CAD and may become a biomarker for this common disease. ApoE binds to multiple receptors, including LRP1, a member of the LDL receptor family. LRP1 binds multiple ligands and can both internalize cargo and trigger signaling-mediated downstream effects. Both proteins control cellular cholesterol trafficking and plaque volume via regulation of cell death. These functions are key targets for the development of therapeutic strategies aiming at inducing plaque regression, an elusive and highly prized objective. We previously determined that macrophages lacking LRP1 cause accelerated atherosclerosis, a paradoxical finding given that in these cells: a) atherogenic lipoproteins are internalized with reduced efficiency; b) secretion of apoE is significantly up-regulated. Since this observation was made in mice expressing normal amounts of systemic and macrophage apoE, it was not possible to determine whether the negative effect of LRP1 removal was either caused by the interruption of an “apoE-LRP1 axis” or attenuated by the over-expression of apoE. The current studies clearly show that most functions of apoE and LRP1 in the artery wall occur through mutually independent pathways, and that the absence of apoE greatly magnifies the effects of LRP1 deficiency on cell death. Our results help understand the forces controlling plaque volume expansion or contraction, and may inform development of regression-inducing agents.
Acknowledgments
Sources of Funding
This study was supported by NIH (HL57986 to SF and HL086988 to MFL). We acknowledge support from the Atherosclerosis Core of Vanderbilt Mouse Metabolic Phenotyping Centers (NIH DK59637).
Footnotes
Disclosures:
None
References
- 1.Linton MF, Atkinson JB, Fazio S. Prevention of atherosclerosis in apolipoprotein E-deficient mice by bone marrow transplantation. Science. 1995;267:1034–1037. doi: 10.1126/science.7863332. [DOI] [PubMed] [Google Scholar]
- 2.Fazio S, Babaev V, Burleigh M, Major A, Hasty A, Linton M. Physiologic expression of macrophage apoE in the artery wall reduces atherosclerosis in severely hyperlipidemic mice. J Lipid Res. 2002;43:1602–1609. doi: 10.1194/jlr.m200108-jlr200. [DOI] [PubMed] [Google Scholar]
- 3.Overton CD, Yancey PG, Major AS, Linton MF, Fazio S. Deletion of Macrophage LDL Receptor-Related Protein Increases Atherogenesis in the Mouse. Circ Res. 2007;100:670–677. doi: 10.1161/01.RES.0000260204.40510.aa. [DOI] [PubMed] [Google Scholar]
- 4.Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 5.Williams DL, Dawson PA, Newman TC, Rudel LL. Apolipoprotein E synthesis in peripheral tissues of nonhuman primates. J Biol Chem. 1985;260:2444–2451. [PubMed] [Google Scholar]
- 6.Plump A, Smith J, Hayek T, Aalto-Setala K, Walsh A, Verstuyft J, Rubin E, Breslow J. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- 7.Boucher P, Gotthardt M. LRP and PDGF signaling: a pathway to atherosclerosis. Trends in Cardiovasc Medicine. 2004;14:55–60. doi: 10.1016/j.tcm.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Boucher P, Gotthardt M, Li WP, Anderson RG, Herz J. LRP: role in vascular wall integrity and protection from atherosclerosis. Science. 2003;300:329–332. doi: 10.1126/science.1082095. [DOI] [PubMed] [Google Scholar]
- 9.Rohlmann A, Gotthardt M, Hammer RE, Herz J. Inducible inactivation of hepatic LRP gene by cre-mediated recombination confirms role of LRP in clearance of chylomicron remnants. Journal of Clinical Investigation. 1998;101:689–695. doi: 10.1172/JCI1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu L, Boesten LS, May P, Herz J, Bovenschen N, Huisman MV, Berbee JF, Havekes LM, van Vlijmen BJ, Tamsma JT. Macrophage low-density lipoprotein receptor-related protein deficiency enhances atherosclerosis in ApoE/LDLR double knockout mice. Arterioscler Thromb Vasc Biol. 2006;26:2710–2715. doi: 10.1161/01.ATV.0000249641.96896.e6. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi H, Campenot RB, Vance DE, Vance JE. Apolipoprotein E-containing lipoproteins protect neurons from apoptosis via a signaling pathway involving low-density lipoprotein receptor-related protein-1. J Neurosci. 2007;27:1933–1941. doi: 10.1523/JNEUROSCI.5471-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaultier A, Arandjelovic S, Niessen S, Overton CD, Linton MF, Fazio S, Campana WM, Cravatt BF, 3rd, Gonias SL. Regulation of tumor necrosis factor receptor-1 and the IKK-NF-kappaB pathway by LDL receptor-related protein explains the antiinflammatory activity of this receptor. Blood. 2008;111:5316–5325. doi: 10.1182/blood-2007-12-127613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jofre-Monseny L, Loboda A, Wagner AE, Huebbe P, Boesch-Saadatmandi C, Jozkowicz A, Minihane AM, Dulak J, Rimbach G. Effects of apoE genotype on macrophage inflammation and heme oxygenase-1 expression. Biochem Biophys Res Commun. 2007;357:319–324. doi: 10.1016/j.bbrc.2007.03.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yancey PG, Blakemore J, Ding L, Fan D, Overton CD, Zhang Y, Linton MF, Fazio S. Macrophage LRP-1 controls plaque cellularity by regulating efferocytosis and Akt activation. Arterioscler Thromb Vasc Biol. 2010;30:787–795. doi: 10.1161/ATVBAHA.109.202051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57:3239–3246. doi: 10.2337/db08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thorngate FE, Rudel LL, Walzem RL, Williams DL. Low levels of extrahepatic nonmacrophage ApoE inhibit atherosclerosis without correcting hypercholesterolemia in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2000;20:1939–1945. doi: 10.1161/01.atv.20.8.1939. [DOI] [PubMed] [Google Scholar]
- 18.Gautier EL, Huby T, Witztum JL, Ouzilleau B, Miller ER, Saint-Charles F, Aucouturier P, Chapman MJ, Lesnik P. Macrophage apoptosis exerts divergent effects on atherogenesis as a function of lesion stage. Circulation. 2009;119:1795–1804. doi: 10.1161/CIRCULATIONAHA.108.806158. [DOI] [PubMed] [Google Scholar]
- 19.Grainger DJ, Reckless J, McKilligin E. Apolipoprotein E modulates clearance of apoptotic bodies in vitro and in vivo, resulting in a systemic proinflammatory state in apolipoprotein E-deficient mice. J Immunol. 2004;173:6366–6375. doi: 10.4049/jimmunol.173.10.6366. [DOI] [PubMed] [Google Scholar]
- 20.Aprahamian T, Rifkin I, Bonegio R, Hugel B, Freyssinet JM, Sato K, Castellot JJ, Jr, Walsh K. Impaired clearance of apoptotic cells promotes synergy between atherogenesis and autoimmune disease. J Exp Med. 2004;199:1121–1131. doi: 10.1084/jem.20031557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang MK, Binder CJ, Miller YI, Subbanagounder G, Silverman GJ, Berliner JA, Witztum JL. Apoptotic cells with oxidation-specific epitopes are immunogenic and proinflammatory. J Exp Med. 2004;200:1359–1370. doi: 10.1084/jem.20031763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu D, Sharan C, Yang H, Goodwin JS, Zhou L, Grabowski GA, Du H, Guo Z. Apolipoprotein E-deficient lipoproteins induce foam cell formation by downregulation of lysosomal hydrolases in macrophages. J Lipid Res. 2007;48:2571–2578. doi: 10.1194/jlr.M700217-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Li FQ, Sempowski GD, McKenna SE, Laskowitz DT, Colton CA, Vitek MP. Apolipoprotein E-derived peptides ameliorate clinical disability and inflammatory infiltrates into the spinal cord in a murine model of multiple sclerosis. J Pharmacol Exp Ther. 2006;318:956–965. doi: 10.1124/jpet.106.103671. [DOI] [PubMed] [Google Scholar]
- 25.Swirski FK, Pittet MJ, Kircher MF, Aikawa E, Jaffer FA, Libby P, Weissleder R. Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proc Natl Acad Sci U S A. 2006;103:10340–10345. doi: 10.1073/pnas.0604260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varol C, Landsman L, Fogg DK, Greenshtein L, Gildor B, Margalit R, Kalchenko V, Geissmann F, Jung S. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med. 2007;204:171–180. doi: 10.1084/jem.20061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, Leenen PJ. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 28.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jia T, Serbina NV, Brandl K, Zhong MX, Leiner IM, Charo IF, Pamer EG. Additive roles for MCP-1 and MCP-3 in CCR2-mediated recruitment of inflammatory monocytes during Listeria monocytogenes infection. J Immunol. 2008;180:6846–6853. doi: 10.4049/jimmunol.180.10.6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun X, Zhang W, Ramdas L, Stivers DN, Jones DM, Kantarjian HM, Estey EH, Vadhan-Raj S, Medeiros LJ, Bueso-Ramos CE. Comparative analysis of genes regulated in acute myelomonocytic leukemia with and without inv(16)(p13q22) using microarray techniques, real-time PCR, immunohistochemistry, and flow cytometry immunophenotyping. Mod Pathol. 2007;20:811–820. doi: 10.1038/modpathol.3800829. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Wang H, Piper MG, McMaken S, Mo X, Opalek J, Schmidt AM, Marsh CB. sRAGE induces human monocyte survival and differentiation. J Immunol. 2010;185:1822–1835. doi: 10.4049/jimmunol.0903398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hundal RS, Gomez-Munoz A, Kong JY, Salh BS, Marotta A, Duronio V, Steinbrecher UP. Oxidized low density lipoprotein inhibits macrophage apoptosis by blocking ceramide generation, thereby maintaining protein kinase B activation and Bcl-XL levels. J Biol Chem. 2003;278:24399–24408. doi: 10.1074/jbc.M209179200. [DOI] [PubMed] [Google Scholar]
- 33.Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, Tall AR. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 2010;328:1689–1693. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou L, Choi HY, Li WP, Xu F, Herz J. LRP1 controls cPLA2 phosphorylation, ABCA1 expression and cellular cholesterol export. PLoS One. 2009;4:e6853. doi: 10.1371/journal.pone.0006853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen YC, Pohl G, Wang TL, Morin PJ, Risberg B, Kristensen GB, Yu A, Davidson B, Shih Ie M. Apolipoprotein E is required for cell proliferation and survival in ovarian cancer. Cancer Res. 2005;65:331–337. [PubMed] [Google Scholar]
- 36.Svensson PA, Johnson MS, Ling C, Carlsson LM, Billig H, Carlsson B. Scavenger receptor class B type I in the rat ovary: possible role in high density lipoprotein cholesterol uptake and in the recognition of apoptotic granulosa cells. Endocrinology. 1999;140:2494–2500. doi: 10.1210/endo.140.6.6693. [DOI] [PubMed] [Google Scholar]
- 37.Misasi R, Garofalo T, Di Marzio L, Mattei V, Gizzi C, Hiraiwa M, Pavan A, Grazia Cifone M, Sorice M. Prosaposin: a new player in cell death prevention of U937 monocytic cells. Exp Cell Res. 2004;298:38–47. doi: 10.1016/j.yexcr.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 38.De Souza EM, Meuser-Batista M, Batista DG, Duarte BB, Araujo-Jorge TC, Soeiro MN. Trypanosoma cruzi: alpha-2-macroglobulin regulates host cell apoptosis induced by the parasite infection in vitro. Exp Parasitol. 2008;118:331–337. doi: 10.1016/j.exppara.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Maiti SN, Balasubramanian K, Ramoth JA, Schroit AJ. Beta-2-glycoprotein 1-dependent macrophage uptake of apoptotic cells. Binding to lipoprotein receptor-related protein receptor family members. J Biol Chem. 2008;283:3761–3766. doi: 10.1074/jbc.M704990200. [DOI] [PubMed] [Google Scholar]
- 40.Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, Bratton DL, Oldenborg PA, Michalak M, Henson PM. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 41.Thorp E, Cui D, Schrijvers DM, Kuriakose G, Tabas I. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of apoe−/− mice. Arterioscler Thromb Vasc Biol. 2008;28:1421–1428. doi: 10.1161/ATVBAHA.108.167197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Babaev VR, Chew JD, Ding L, Davis S, Breyer MD, Breyer RM, Oates JA, Fazio S, Linton MF. Macrophage EP4 deficiency increases apoptosis and suppresses early atherosclerosis. Cell Metab. 2008;8:492–501. doi: 10.1016/j.cmet.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu J, Thewke D, Su Y, MFL, Fazio S, Sinensky M. Reduced macrophage apoptosis is associated with accelerated atherosclerosis in low-density lipoprotein receptor-null mice. Arterioscl Thromb Vasc Biol. 2005;25:174–179. doi: 10.1161/01.ATV.0000148548.47755.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Y, Schwabe R, Devries-Seimon T, Yao P, Gerbod-Giannone M, Tall A, Davies R, Flavell R, Brenner D, Tabas I. Free cholesterol-loaded macrophages are an abundant source of TNF-alpha and IL-6. Model of NF-kappa B- and MAP kinase-dependent inflammation in advanced atherosclerosis. J Biol Chem. 2005;280:21763–21772. doi: 10.1074/jbc.M501759200. [DOI] [PubMed] [Google Scholar]
- 45.Thorp E, Li G, Seimon TA, Kuriakose G, Ron D, Tabas I. Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe−/− and Ldlr−/− mice lacking CHOP. Cell Metab. 2009;9:474–481. doi: 10.1016/j.cmet.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eto H, Miyata M, Shirasawa T, Akasaki Y, Hamada N, Nagaki A, Orihara K, Biro S, Tei C. The long-term effect of angiotensin II type 1a receptor deficiency on hypercholesterolemia-induced atherosclerosis. Hypertens Res. 2008;31:1631–1642. doi: 10.1291/hypres.31.1631. [DOI] [PubMed] [Google Scholar]
- 47.Ait-Oufella H, Kinugawa K, Zoll J, Simon T, Boddaert J, Heeneman S, Blanc-Brude O, Barateau V, Potteaux S, Merval R, Esposito B, Teissier E, Daemen MJ, Leseche G, Boulanger C, Tedgui A, Mallat Z. Lactadherin deficiency leads to apoptotic cell accumulation and accelerated atherosclerosis in mice. Circulation. 2007;115:2168–2177. doi: 10.1161/CIRCULATIONAHA.106.662080. [DOI] [PubMed] [Google Scholar]
- 48.Ait-Oufella H, Pouresmail V, Simon T, Blanc-Brude O, Kinugawa K, Merval R, Offenstadt G, Leseche G, Cohen PL, Tedgui A, Mallat Z. Defective mer receptor tyrosine kinase signaling in bone marrow cells promotes apoptotic cell accumulation and accelerates atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:1429–1431. doi: 10.1161/ATVBAHA.108.169078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.