Abstract
Breast cancer (BC) patients use alternative and natural remedies more than patients with other malignancies. Specifically, 63%–83% use at least one type of alternative medicine and 25%–63% use herbals and vitamins. Propolis is a naturopathic honeybee product, and CAPE (caffeic acid phenethyl ester), is a major medicinal component of propolis. CAPE, in a concentration dependent fashion, inhibits MCF-7 (hormone receptor positive, HR+) and MDA-231 (a model of triple-negative BC (TNBC) tumor growth, both in vitro and in vivo without much effect on normal mammary cells and strongly influences gene and protein expression. It induces cell cycle arrest, apoptosis and reduces expression of growth and transcription factors, including NF-κB. Notably, CAPE down-regulates mdr-1 gene, considered responsible for the resistance of cancer cells to chemotherapeutic agents. Further, CAPE dose-dependently suppresses VEGF formation by MDA-231 cells and formation of capillary-like tubes by endothelial cells, implicating inhibitory effects on angiogenesis. In conclusion, our results strongly suggest that CAPE inhibits MDA-231 and MCF-7 human breast cancer growth via its apoptotic effects, and modulation of NF-κB, the cell cycle, and angiogenesis.
Keywords: Breast Cancer, CAPE, Angiogenesis, Apoptosis, Cell Cycle
1. INTRODUCTION
The use of alternative remedies for the treatment of cancer is a $45 billion dollar industry, which includes a $2 billion practitioner channel supplement market [1]. Up to 80% of cancer patients use complementary or alternative medicine [2], while at least one third of them use alternative medicines in combination with allopathic treatment [3]. More often than not, cancer patients use these supplements despite recommendations by their physicians not to use them. Even more concerning is the fact that many cancer patients do not disclose the use of these alternative therapies while taking traditional hormonal or chemotherapeutic agents. These sentiments remain difficult to change despite the fact that most oncologists strongly discourage the use of such agents because of the lack of evidence showing benefit, the absence of supportive scientific data, and the concern about drug:drug interactions. Breast cancer (BC) patients, in particular, have become very enamored with the use of alternative and natural remedies for their cancer more than patients with other malignancies [4]. More specifically, up to 63% – 83% of BC patients use at least one type of alternative medicine and 25% – 63% take herbal and vitamin remedies [5–8].
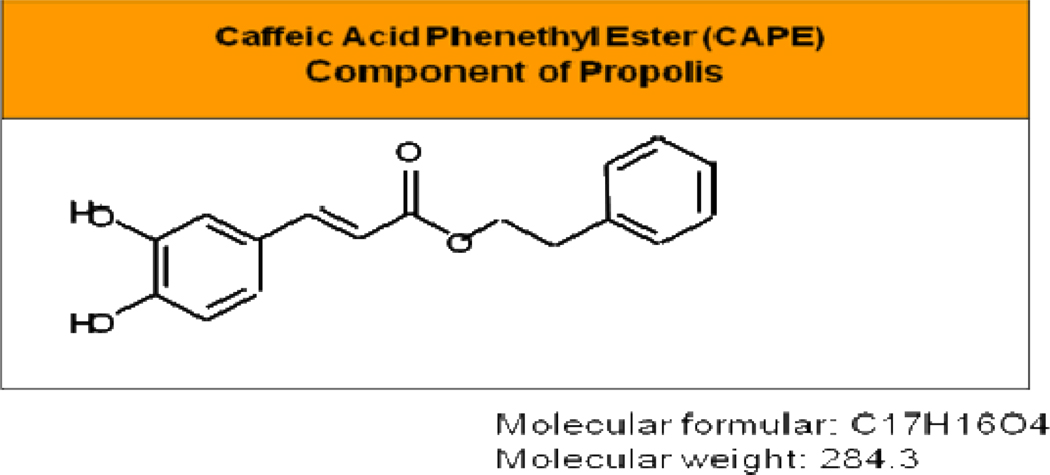
Caffeic acid phenethyl ester (CAPE; the structure is shown in Figure 1) is one of the main medicinal components of propolis. Propolis is a naturopathic formulation collected by honeybees from buds and exudates of conifer trees and plants. It is used by the bees as a protective barrier in the hive. Most importantly, it has been used safely since ancient times in folk medicine for its pharmaceutical properties [9–11]. There are different types of propolis based on geography, including Europe, New Zealand, Brazil, China and it contains a variety of chemical compounds such as flavonoids, phenolic acids, and their esters, terpenoids, steroids and amino-acids [12]. CAPE was synthesized in 1988 at Columbia University [13] and later found to be a potent anticancer ingredient in propolis [14–16]. CAPE possesses a plethora of important biological activities, including antibacterial, antiviral, antioxidant, anti-inflammatory, and anti-cancer [17–20]. CAPE is a well-known and well-documented inhibitor of the transcription factor nuclear factor kappa B (NF-κB) [21–23] as well as an inducer of apoptosis. CAPE-mediated apoptosis is accompanied by activation of caspase-3, down-regulation of Bcl-2, and up-regulation of Bax in human leukemic HL-60 cells, [24]. At low doses, CAPE also suppresses lipid peroxidation [25] and displays antioxidant activity [26, 15].
Fig. 1.
Molecular structure of CAPE
CAPE kills various types of cancer cells but is innocuous to normal cells. There are several studies reporting the in vitro and in vivo inhibitory effects of CAPE in multiple cancer models, such as colon cancer [27], lung cancer [28], melanoma [29], glioma [30], pancreatic cancer [31], gastric cancer [32], cholangiocarcinoma [33], hepatocellular carcinoma [34], and our data in breast cancer [35–39]. The role of CAPE as an inhibitor of angiogenesis, tumor metastasis and invasion has been demonstrated in several models of angiogenesis, both in vitro [40] and in vivo in colon cancer cells [41]. Another key growth factor in tumorigenesis is the epidermal growth factor receptor (EGFR) or ErbB family of receptor tyrosine kinases. In fact, ErbB2 is an important therapeutic target in breast tumors that overexpress the receptor HER2/neu [42], while EGFR (ErbB1/HER1) is operational in TNBC.
Breast cancer is a heterogeneous disease with a number of recognized biological subtypes, including triple negative breast cancer (TNBC). TNBC is significantly more aggressive than breast tumors of other molecular subtypes and although chemosensitiive, there are earlier and frequent recurrences. Thus, mortality of TNBC patients is significantly greater than that for patients with estrogen receptor-alpha (ER α) and progesterone receptor (PR) positive BC [43]. Response rates in patients with metastatic breast cancer progressively decline with advancing lines of treatment [44–45], possibly due to the tumor becoming resistant to a wide variety of anticancer therapies [46]. Although targeted anticancer therapies, such as trastuzumab, bevacizumab, and lapatinib have improved outcomes in patients with metastatic breast cancer, these agents are also subject to mechanisms involved in cancer resistance [47]. Unfortunately, drug resistance to all forms of systemic treatment (hormonal, chemotherapy, and targeted agents) is estimated to render solid tumors lethal in more than 90% of patients with metastatic disease [48]. Therefore, there is a great need for new agents that would increase survival of advanced BC patients and decrease treatment’s toxicity.
In this study, we investigated the effect of CAPE in a breast cancer model, including tumor growth both in vitro and in vivo and analyzed the effects on the cell cycle, apoptosis, and angiogenesis in the hormone receptor negative (HR-) MDA-231 and hormone receptor positive (HR+) MCF-7 breast cancer cell lines.
2. MATERIALS AND METHODS
2.1 Cells and Cell Culture
MDA-MB-231, MCF-7, MCF-10A and MCF-12A were purchased from the American Type Culture Collection (ATCC, Manassas, VA), and used for in vitro and in vivo experiments. MDA-MB-231 (MDA-231) cells were cultured in Leibowitz-15 (L-15) medium (Cellgro, Manassas, VA) supplemented with 5% heat-inactivated fetal bovine serum (FBS) (Cellgro, Manassas, VA) at 37 °C in a humidified incubator without CO2; MCF-7, MCF-10A and MCF-12A were cultured in DMEM (Cellgro, Manassas, VA) supplemented with 10% heat-inactivated FBS at 37°C in a humidified 5% CO2 incubator. All cells were passaged twice a week and maintained in exponential growth.
2.2 Cytotoxicity and Cell Cycle Experiments
Cells were cultured in 6-well tissue culture plates at 3×105/well in 3 ml media for 12 h, then incubated with different concentrations of CAPE (Sigma-Aldrich ) (0–40 µM) for another 72 h. The same volume of DMSO was used as the vehicle control for CAPE experiments at a final concentration of 0.1%. At the end of incubation, a tetrazolium dye colorimetric test (MTT test) was used to assess cell viability, as indicated by the conversion of tetrazolium salts to a colored product, formazan, the concentration of which was measured spectrophotometrically to calculate the IC50 value.
For apoptosis experiments, cells were seeded as above and then incubated with CAPE for 72 h. Cells were harvested, washed twice with PBS, and centrifuged. 1×105 cells were treated with Annexin V-FITC and propidium iodide (PI) using the apoptosis Detection Kit (BD Bioscience Pharmingen, San Jose, CA) and the manufacturer’s protocol. Annexin V-FITC and PI binding were analyzed by flow cytometry on FACScalibur (Becton Dickinson) without gating restrictions using 10,000 cells. Data were collected using logarithmic amplification of both the FL1 (FITC) and FL2 (PI) channels. Quadrant analysis of co-ordinate dot plots was performed with CellQuest software. Unstained cells were used to adjust the photomultiplier voltage and for compensation setting adjustment in order to eliminate spectral overlap between the FL1 and FL2 signals.
Cells for cell cycle experiments were seeded and cultured as above and, after 24 h, cells were treated with varying doses of CAPE for 48 h. Samples were collected and fixed in cold 70% ethanol for 24 h. Fixed cells were washed with PBS and stained with propidium iodide containing RNase A at 10 µg/ml. Cells were collected by flow cytometry (FACScalibur, Becton Dickinson) and analyzed using ModFit Software.
2.3 Western Blotting
All antibodies used were obtained from Cell Signaling (Danvers, MA). Cells were seeded, cultured and treated with CAPE for 48 h. Whole cell protein lysates were prepared according to standard protocol using RIPA buffer (0.5 M Tris-HCl, pH 7.4, 1.5 M NaCl, 2.5% deoxycholic acid, 10% NP-40, 10 mM EDTA) (Upstate, CA). 30 µg protein was loaded per well of a 10% SDS PAGE gel using electrophoresis buffer (Biorad). After electrophoresis, the gel was transferred onto a PVD membrane (Biorad) using Running buffer (Biorad). Membranes were blocked in TBS-T with 5% skim milk and incubated overnight with the primary antibody (1:1000) then incubated with secondary peroxidase–linked antibody. Detection was done by enhanced chemoluminescence Western blotting detection system (Amersham Biosciences). Anti–β-actin or α-tubulin was used to ensure equal loading of protein on the gel.
2.4 Angiogenesis
CAPE effect on Tube Formation by Bovine Capillary Endothelial Cells (BCE) was examined as follows: BCE cells (5×104/well) were grown for 24 h in 24-well plates coated with Matrigel under normoxic or hypoxic conditions without treatment (controls), with 1.5-µM (1.5 nmol/well) CAPE or bFGF (basic fibroblast growth factor) and capillary formation assessed under the microscope.
To measure VEGF concentrations in culture medium, MDA-231 or MCF-7 cells were cultured at 3×105 cells/3 ml medium/well in 6-well tissue culture plates for 12 h, then treated with different CAPE concentrations (0–40 µM) for a further 48-h. Medium was harvested and VEGF in the medium quantified using an enzyme linked immunosorbent assay for human VEGF (ELISA, Invitrogen, Camarillo, CA).
2.5 Gene Expression Profile
Cells were seeded in 3 ml medium at 2.5×105/well of 6-well plates and allowed to attach overnight, washed once with PBS, fresh growth medium (3 ml) added, and cells were treated either with vehicle (DMSO) or CAPE in DMSO (10 µM stock solution) to reach a final concentration of 10 µM CAPE (or 30 nmol/well, which equals 0.12 pmol/cell). After 48 h, RNA was extracted using a Qiagen kit and analyzed by the gene array (Toxicology & Drug Resistance GeneArray, SABiosciences), as suggested by the manufacturer.
Gene array consisted of 288 genes, of which 6 were positive controls (GAPDH and ribosomal protein S27a) and two negative controls. Membranes were developed using chemiluminescence reagents (provided by the kit), visualized on an X-ray film, digitally transferred, and quantitated using Un-scan It software (Silk Sci. Corp.). The background was subtracted and signals (pixels) normalized using positive controls, with a normalizing factor being 1.08.
2.6 Breast Cancer Xenografts
All experiments using animals were performed according to the protocol approved by the Institutional Animal Care and Use Committee at New York University School of Medicine. MCF-7 cell injections: 1.25 × 106 cells in 0.2 ml/flank (1:1 Matrigel and growth medium) were subcutaneously injected into each of the two flanks of female (Ncr-nu-nu) mice (Taconic, Germantown, NY). Pellets containing 17-β estradiol (E2) (0.36 mg, 60-day time release; Innovative Research of America, Sarasota, FL) were implanted one week prior to cell injections. MDA-231 cell injections: 0.5 × 106 cells/in 0.2 ml/flank (1:1 Matrigel and medium) were subcutaneously injected into each of the two flanks of female nude mice. After one week acclimation on a control diet (7012 meal) with 1% sucrose, (Harlan Teklad, Madison, WI), diets containing CAPE at 0 or 10, 50, or 250 nmol/mouse/day were started 2 days prior to cell inoculation. The 7012 meal was irradiated prior to the addition of CAPE and sucrose. There were 8 mice per group. The age of mice at cell injection was 9 weeks, body weights were obtained weekly, mice checked for tumors twice/week, tumor measured using calipers, and volume quantitated according to L × W × H × 0.5332 formula. The experimental duration was about 60 days.
3. RESULTS
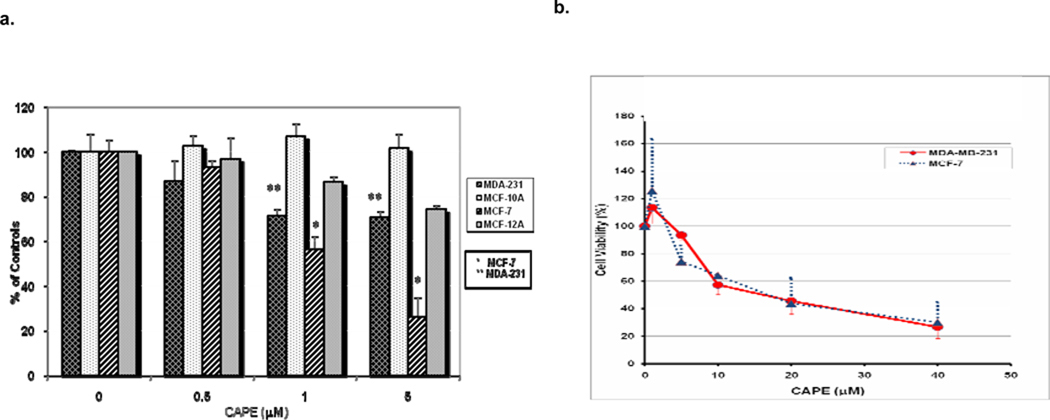
3.1 Cytotoxicity of CAPE (Figure 2)
Fig. 2. CAPE inhibits growth of breast cancer cells.
The cell lines were cultured and then incubated with different concentrations of CAPE and analyzed using the MTT assay as described in the Materials and Methods section. a) The viability of MDA 231 (ER−) and MCF-7 (ER+) breast cancer cells was decreased by CAPE in a dose-dependent manner. b) In contrast, exposure to CAPE had no significant cytotoxic effect on the control cells, the immortal but non-tumorigenic MCF-10A (ER−) cells and MCF-12A (ER+) cells. All the determinations were carried out on 3–7 replicates. Growth of MDA-231 cells (first left bar) was dose-dependently (1–5 µM CAPE) and significantly inhibited (30.6%±6.9; **p<0.0001 at 72 h) when compared to untreated controls, while growth of control MCF-10A cells (second bar) was not affected at all. MCF-7 cell growth (third bar) was also dose- and time-dependently inhibited by 1–5 µM CAPE when incubated for 72 h with 5 µM CAPE suppressing cell growth down to 25.8%±9.3; *p<0.0001 of the untreated cells, while the growth of control MCF-12A cells (right bar) was decreased to 74.3%±1.9, with the overall difference being about 50% between the two ER (+) cell lines (Figure 2a). As shown in Figure 2b, the viability of MDA-231 and MCF-7 breast cancer cells was further decreased by higher doses of CAPE (up to 40 µM), with an IC50 of approximately 15 µM. The experimental differences were determined by two-tailed Student’s t-test and p ≤0.05 was taken as a significant difference in all cases
The cytotoxic effects of various concentrations of CAPE on tumor and normal cells are shown in Figure 2. The viability of MDA-231 (ER−) and MCF-7 (ER+) breast cancer cells was decreased in a dose-dependent manner by low doses of CAPE, which suggests that CAPE-mediated inhibition of BC cell growth does not depend on the hormone receptors (ER and PR, (Fig. 2a, b). In contrast, CAPE exhibited no cytotoxic effects on immortal but non-tumorigenic MCF-10A (ER−) mammary cells, while growth of MCF-12A (ER+) cells was inhibited, but to a much lower extent than that of MCF-7 cells (Fig. 2a). As shown in Figure 2b, the viability of MDA-231 and MCF-7 breast cancer cells was further decreased by higher doses of CAPE (0 – 40 µM), with an IC50 of approximately 15 µM.
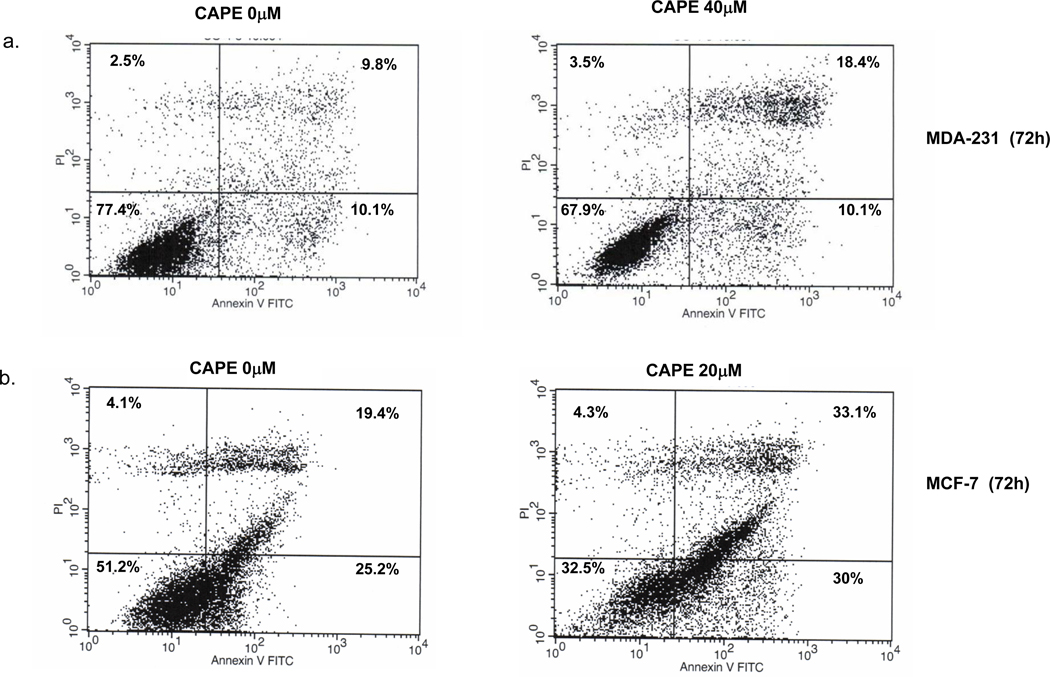
3.2 Effect of CAPE on apoptosis (Figure 3)
Fig. 3. CAPE induces apoptosis.
MDA-231 and MCF-7 Cells (3 × 105) were cultured and incubated with CAPE for 72 h then treated with Annexin V-FITC and propidium iodide (PI) according to the Materials and Methods. After 72h, 40 µM CAPE caused an increase in Annexin V-positive apoptotic cells (Upper Right and Lower Right quadrant (UR+LR)) as compared to 0 µM in MDA-231 cells. In MCF-7 cells, 20 µM CAPE treatment resulted in an increase in Annexin V-positive apoptotic cells (UR+LR) when compared to 0 µM
MDA-231 cells were incubated with or without CAPE, collected, and analyzed by flow cytometry without gating restrictions. Seventy two h treatment with 40 µM CAPE resulted in 28.5% Annexin V-positive apoptotic cells as compared to 19% at 0 µM CAPE (UR + LR). Fas (1 µg/ml) did not cause apoptosis (data not shown), which is consistent with literature documentation that MDA -231 cells are resistant to Fas-induced apoptosis. In MCF-7 cells, 20 µM CAPE treatment resulted in 66% Annexin V-positive apoptotic cells (UR+LR) compared to 44% at 0 µM CAPE (no further apoptosis was observed at 40µM CAPE).
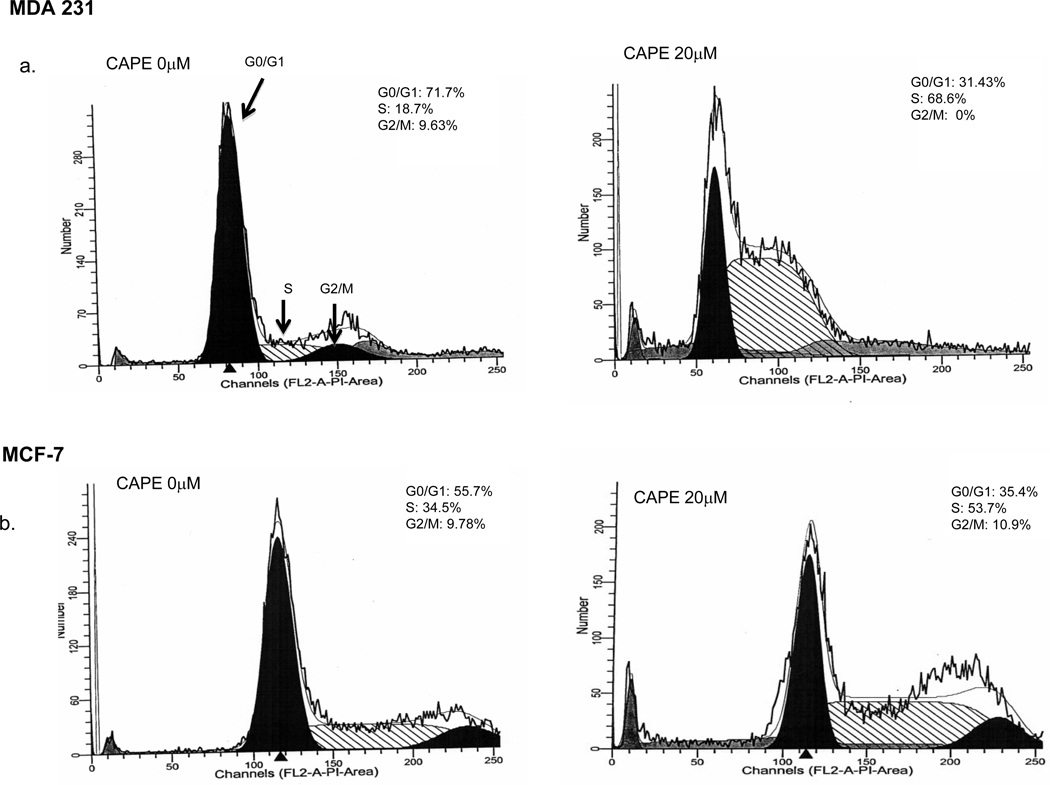
3.3 CAPE Effects on Cell-Cycle (Figure 4)
Fig. 4. CAPE induces cell cycle arrest.
MDA-231 and MCF-7 cells were treated with varying doses of CAPE for 48 h and cell cycle progression was determined using flow cytometry. CAPE exposure resulted in cell cycle arrest in the S-phase in a dose dependent manner for both MDA-231 (a) and MCF-7 (b) cells with an increase in the S-phase compartment and subsequent decrease in G0/G1 and G2/M phases. This figure is a representative of 3 independent experiments
MDA-231 and MCF-7 cells were treated with varying doses of CAPE for 48 h and cell cycle progression was determined using flow cytometry. CAPE induced cell cycle arrest in MDA-231 cells at the S phase, changing from 18.7% at 0 µM CAPE to 68.6 % at 20 µM CAPE with a concurrent decline in the G0/G1 phase from 71.7% at 0 µM CAPE to 31.4% at 20 µM CAPE and complete elimination of the G2/M phase from 9.6% at 0-µM CAPE to 0% at 20 µM CAPE (Fig 4a). Similarly, in MCF-7 cells, CAPE induced S phase cell cycle arrest changed from 34.5 % at 0 µM CAPE to 53.7% at 20 µM CAPE with a concurrent decline in the G0/G1 phase from 55.7% at 0 µM CAPE to 35.4% at 20 µM CAPE (Fig 4b).
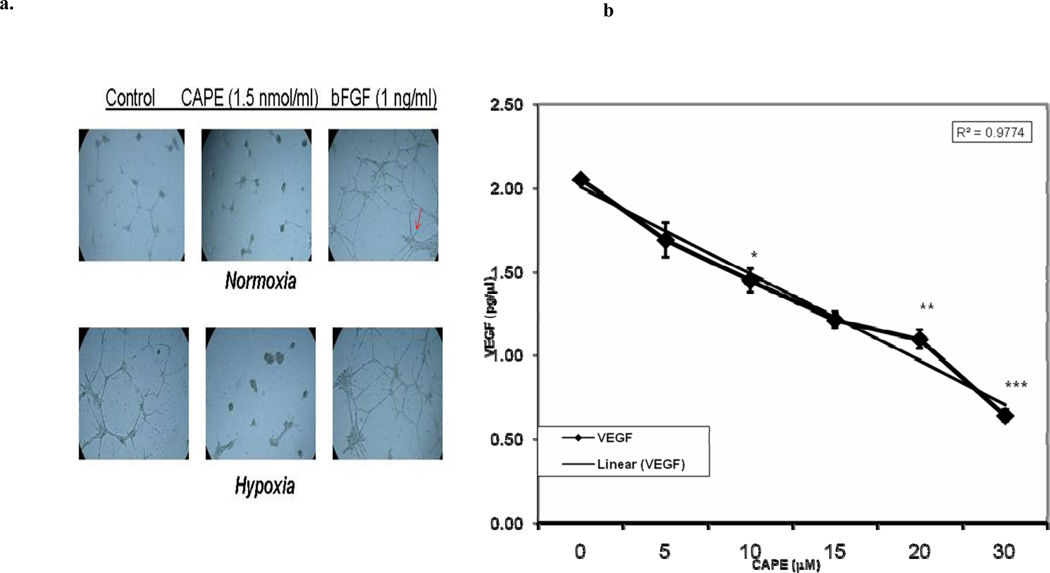
3.4 Inhibitory Effects of CAPE on Angiogenesis (Figure 5)
Fig. 5. CAPE inhibits angiogenesis.
a) Bovine capillary endothelial (BCE) cells were grown for 24 h in 24-well plates coated with Matrigel under normoxic or hypoxic conditions without treatment (controls), with 1.5-µM CAPE or bFGF (basic fibroblast growth factor, positive control). 1.5-µM CAPE inhibited tube formation under both normoxic and hypoxic conditions. b) MDA-231 cells were cultured at treated with different concentrations of CAPE (0–40 µM) and VEGF quantified in the media according to the Materials and Methods. CAPE inhibits VEGF secretion by MDA-231 cells in a dose dependent fashion from 0µM to 40 µM CAPE. This inhibition was statistically significant from 10µM CAPE (*p = 0.005), 20 µM CAPE (**p < 0.0001) up to 30µM CAPE (***p < 0.0004); n= 3. The experimental differences were determined by two-tailed Student’s t-test and p ≤0.05 was taken as a significant difference in all cases.
It has been shown that propolis inhibits angiogenesis in the chick embryo chorioallantoic membrane model and in calf pulmonary arterial endothelial proliferation assays [49]. CAPE, propolis’ component, also inhibited growth of blood vessels in these models [49]. We tested CAPE in a different model using BCE (bovine capillary endothelial) cells for growth in Matrigel using treatment with basic fibroblast growth factor (bFGF), a positive control that increases tube formation by endothelial cells, or with a low dose of CAPE (1.5-µM). As Figure 5a illustrates, treatment with bFGF increased tube formation, which is a measure of the ability to develop blood vessels. In contrast, 1.5 µM CAPE inhibited their formation under normoxia and, to an even greater extent, under hypoxic conditions. Such results indicate that CAPE can effectively prevent formation of blood vessels even under hypoxic conditions commonly occurring in solid tumors. MDA-231 cells are known to secrete vascular endothelial growth factor (VEGF) necessary for endothelial cell growth and important in tumor blood vessel formation (MCF-7 cells secrete very little VEGF; not shown). CAPE, from 0 to 40 µM, inhibits VEGF secretion by MDA-231 cells in a dose-dependent fashion from 0 to 40 µM CAPE, with >90% suppression at the highest dose (Figure 5b).
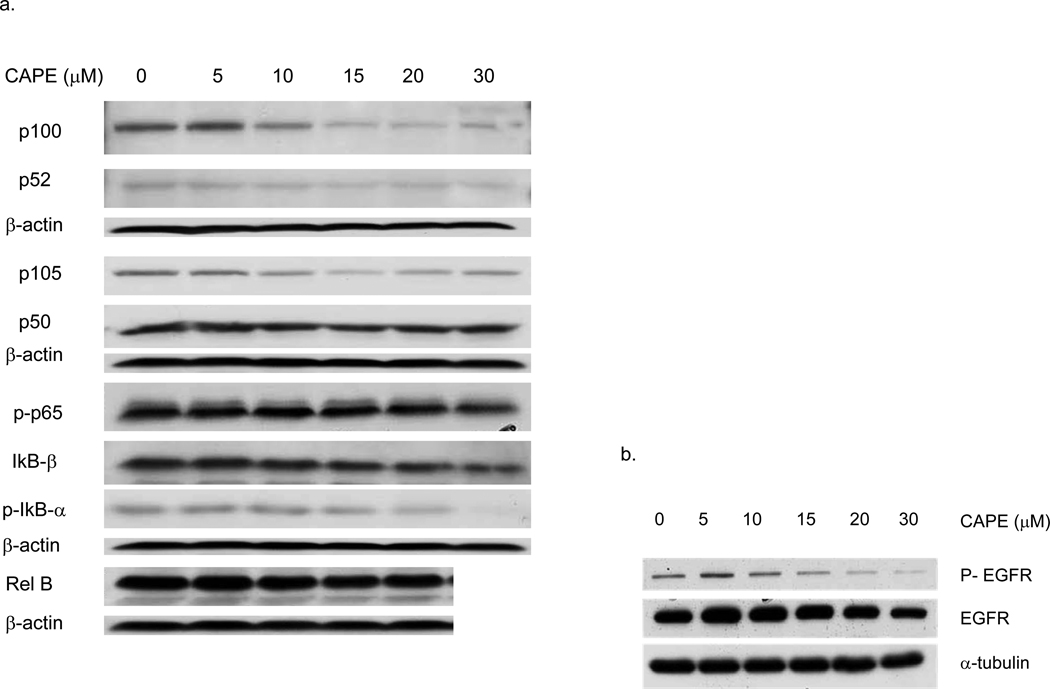
3.5 CAPE Effects on the NF-κB Transcription Factors (Figure 6, Table I)
Fig. 6. CAPE inhibits NFκB.
a) MDA-231 cells were cultured and treated with CAPE for 48 h and protein lysates made as described in Materials and Methods. In MDA-231 cells, immunoblotting show CAPE-induced dose-dependent decreases in protein expression:IκB-β, p52 (3-fold) and p-IκB-α (20-fold), and the NF κB subunits p50, Rel B (1.5-fold), p-p65, p105 (2-fold), and p100 (5-fold). There were no changes observed in IκB-α and C-rel protein levels (blots not shown). Autoradiographs were scanned and bands quantified by a densitometry scanner. Densitometry results were expressed relative to a normalized protein control (β actin). b) CAPE inhibits both the total and phosphorylated forms of EGFR protein expression in a dose dependent manner. Representative blots are shown
Table I.
Quantification of the NF-κB Pathway proteins
| CAPE (µM) |
Rel B | C-rel | p-p65 | IκB-β | IκB-α | p-IκBα | p100 | p52 | p105 | p50 |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| 5 | 79.7 | 123.8 | 85.8 | 78.6 | 95.14 | 84.9 | 96.5 | 82.4 | 88.1 | 109.4 |
| 10 | 81.3 | 113.6 | 80.8 | 72.0 | 93.16 | 92.4 | 44.7 | 75.1 | 60.6 | 105.0 |
| 15 | 78.7 | 108.8 | 82.7 | 65.6 | 85.07 | 66.0 | 17.1 | 52.5 | 24.2 | 79.1 |
| 20 | 73.0 | 112.6 | 75.8 | 53.5 | 110.18 | 53.4 | 19.3 | 76.0 | 44.8 | 94.4 |
| 30 | 58.6 | 30.4 | 105.55 | 6.5 | 19.5 | 35.7 | 49.3 | 85.9 | ||
CAPE modulates NF-κB, it inhibits NF-κB binding activity and decreases the expression of NF-κB1 (p50) and RelA (p65) [21, 22, 32, 33]. We show here that CAPE inhibits NF-κB activity in MDA-231 cells (Figure 6a). As shown by Western blotting, MDA-231 cells exhibited a marked dose-dependent decreases in the protein expression of both NF-κB1 & 2 (p105 & p100, respectively) as well as RelA (p65) when compared to cells not treated with CAPE. At 20 µM, CAPE inhibited p100 by ~90%, p52 by ~25%, p105 by ~55%, and phosphorylated (p)-p65 by ~40% (Table I). Further, p-IκB-α, an activated (phosphorylated) form of IκB-α, and IκB-β, inhibitors of NF-κB1&2, respectively, were decreased by CAPE to about the same degree (i.e., ~44% at 20 µM CAPE) (Table I), There were no changes observed in the levels of IκB-α and C-rel proteins. β-actin levels remained the same in vehicle control and CAPE treated cells. NF-κB is a known downstream signaling molecule for EGFR signaling, which is important in the proliferation, invasion and metastasis in breast cancer [50]. TNBC cells are known to overexpress EGFR and MDA-231 cells exemplify the TNBC phenotype model. Importantly, as shown in Figure 5b, CAPE decreased the total and phosphorylated (active) forms of EGFR protein expression, thus providing a mechanistic explanation for the findings reported above.
3.6 Effect of CAPE on Gene Expression and Protein Products in MCF-7 and MDA-231 cells (Tables II & III)
Table II.
Effects of CAPE on Toxicology & Drug Resistance Genes (Superarray)
| GENES (MDA 231 Cells) | Decreased (fold) | GENES (MCF-7 Cells) | Increased (fold) |
|---|---|---|---|
| Oncogenes & homologs (v-abl, ELK1, v-act | 11; 3-4 | Heme oxygenase 1 | 4.2 |
| Cytokines (TNF-a super-family, IL-6 GM-CSF, IL-1b) | 11 6; 4 | Decreased (fold) | |
| Cyclins (G1, D1, E1, C) | 7, 2 | Excision repair cross-complementing rodent repair deficiency, compl. Group 3 (XPB) & compl. Group1 | 5.6 & 3.5 |
| Egr-1; CDK-2 | 7; 4 | PCNA | 4.5 |
| Chaperonins containing TCP1 | 2.0 --3.2 | ||
| Retinoid X (α&β) & Retinoic acid receptor | 4; 2;2-4 | DNA damage-inducibe transcript 3 | 2.8 |
| Topoisomerase (DNA) I | 2.6 | ||
| Nuclear receptors subfamily 1 | 4 | Suppression of tumorigenicity 13 (Hsp70-interacting protein) | 2.5 |
| ABC subfamily B (MDR1/TAP) | 4 | Hsps (27, 70 & 90) & Hsp-interacting proteins | 1.4 -- 2.5 |
| IGF-like receptors 1&2; IGFBP6 | 3.5;2;2 | Retinoblastoma 1 (Rb-1 | 2.5 |
| Estrogen receptor 1 (ER-α) | 2.4 | ||
| NF-κB1 & NF-κB2; IkB α & β | 3.3;2 | Cytochrome P450s | 1.5 -- 2.3 |
| Glutathione reductase | 2.2 | ||
| ATM (complement. Groups A,C&D) | 3 | NF-kB1 (p105) | 2 |
| Retinoic acid receptor, β & α (RARs) | 2 & 1.5 | ||
| EGFR; Topoisomerase 1; PCNA | 3;2.5;2 | Cdk inhibitors (p27, Kip1 & p19), Cyclin E1 | 1.7 -- 1.9 |
| Chemokine (C-C motif) | 1.8 | ||
| BCL2-like 1&2; BCL2-assoc. athanog | 1.7 | P53 | 1.8 |
| Rb 1; TP53 | 1.7; 1.5 | E2F transcription factor 1 | 1.5 |
Table III.
Changes in Protein levels after CAPE treatment.
| Protein | MDA-231 | MCF-7 | |||
|---|---|---|---|---|---|
| p21 | 2-x ↑ | 3-5-x ↑ | |||
| p27 | 2-x ↑ | --- | |||
| Ki-67 | 5-x ↓ | 4-x ↓ | |||
| PCNA | 2-x ↓ | 2-x ↓ | |||
| p-EGFR | 3-x ↓ | ||||
| EGFR | ↓ | Not expressed | |||
| p-Erks&p-JNK | each 3-x ↑ | --- | |||
| p-p38 | 2-3-x ↑ | 2-3-x ↑ | |||
| NF-κB1/p105 | 3-x ↓ | --- | |||
| NF-κB2/p100 | 5-x ↓ | --- | |||
| p-IkB-α & β | 2-x ↓ each | --- | |||
| IkB-β | 2-x ↓ | --- | |||
| Ferritin-L | 3-x ↑ | 3-x ↑ | |||
| Ferritin-H | 2-3-x ↑ | 2-x ↓ | |||
| Stat-3; NQO1 | 2-x ↓ each | 2-x ↓ each | |||
| HO-1 | 2-x ↑ | 6-x ↑ | |||
Gene array studies using the Toxicology and Drug Resistance screen showed down-regulation of a number of genes in MDA-231 and MCF-7 cells treated with CAPE. Table 2 highlights several of these genes that are important in cell proliferation, apoptosis and inflammation such as oncogenes, inflammatory cytokines, cyclins, growth factors and receptors (i.e., EGFR, IGFR-like 1&2, RAR, Egr-1, and NF-κB transcription factors). Notably, CAPE down-regulates mdr-1 gene expression, considered responsible for the resistance of cancer cells to chemotherapeutic agents. Protein expression levels of important proliferation, inflammation, and transcription factors are also depicted in Table 3. CAPE exposure results in the decreased expression of proliferation proteins Ki-67 (4–5 fold), PCNA (proliferating cell nuclear antigen), (2-fold in MDA-231 and 4-fold in MCF-7 cells), and transcription factors NF-κB1&2 (Figure 6 and Table III), p-Erk, p-JNK, p-p38 (2–3 fold) and Stat 3 (2-fold) (Table 3). CAPE also led to the increased expression of ferritin (2–3 fold), an iron storage protein, and cell-cycle regulators p21 and p27 (2-fold in MDA-231 cells, 3–5 fold in MCF-7 cells) (Table III). Of the analyzed genes in CAPE-treated cells, only one (heme oxygenase, OH-1) was significantly elevated by 4.2-fold in MCF-7 cells, and a 2-fold increase in protein expression in MDA-231 and 6-fold in MCF-7 cells respectively (Table II, III), while 71 genes were down-regulated (Table II).
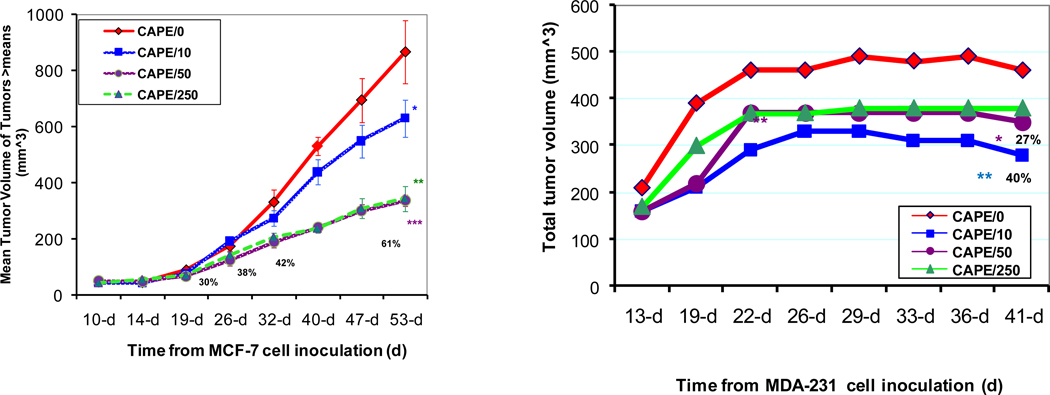
3.7 CAPE-induced Effects on Growth of Breast Cancer Tumor Xenografts (Figure 7)
Fig. 7. CAPE inhibits breast cancer xenograft tumor growth.
MDA-231 or MCF-7 cells/in 0.2 ml/flank (1:1 Matrigel and medium) were subcutaneously injected into each of the two flanks of female nude mice and fed with a diet containing cape or control diet. CAPE decreased the tumor volume of mouse xenografts by about 60% for MCF-7 (CAPE 50 nmol/mouse/day) xenografts with tumor volume greater than the means and 40% for MDA 231 xenografts (CAPE 10 nmol/mouse/day). The most consistent effects were evident using 50 nmol CAPE/ mouse/day diet, which caused statistically significant decline in growth of large tumors in MCF-7-inoculated mice 19, 26, 32, and 53 days after cell implantation, with 30% 38% 42% and 61% decreases in tumor volume, respectively, with p < 0.005 at CAPE 50 and 250 nmol/mouse/day at the end of the experiment. In MDA-231 cells, CAPE diet caused decreases in tumor volume at all the doses tested but, notably, there were more significant decreases in tumor volume (~40%; **p = 0.009 ) at the lowest dose of 10 nmol CAPE/mouse/day as compared to 50 and 250 nmol CAPE diets that yielded about 27% decrease in tumor volume (*p = 0.02). The experimental differences were determined by two-tailed Student’s t-test and p ≤0.05 was taken as a significant difference in all cases
To study the effects of CAPE in vivo, we utilized the same breast cancer cell lines MCF-7 ER(+) and MDA-231 ER(−) differing in the ER status that were used in our in vitro experiments. NCR (Balb/c background) female nude mice were tested for responses to the dietary non-toxic doses of 10, 50, and 250 nmol CAPE/mouse/day. Mice pre-implanted with E2 (17β-estradiol) were injected with MCF-7 cells in both flanks and fed either regular or one of the three CAPE-containing diets. Results showed that CAPE diets caused dose- and time-dependent decreases in rates of tumor growth, as assessed by tumor volume. The most consistent effects were evident using 50 nmol CAPE-containing diet, which caused statistically significant decline in growth of large tumors in MCF-7-inoculated mice 19, 26, 32, and 53 days after cell implantation, with 30%, 38%, 42% and 61% decreases in tumor volume, respectively, with p < 0.005 at CAPE 50 and 250 nmol/mouse/day at the end of the experiment. The same type of experiments were performed using MDA-231 cells, but without E2 implantation. After 22 days past cell inoculation, CAPE diet caused decreases in tumor volume at all the doses tested but, notably, there were more significant decreases in tumor volume (~40%; p = 0.009 ) at the lowest dose of 10 nmol CAPE/mouse/day as compared to 50 and 250 nmol CAPE diets that yielded about 27% decrease in tumor volume (p = 0.02). There was no toxicity evident at these (10, 50, and 250 nmol CAPE/mouse/day for 2 months as well as much higher CAPE doses (0.15%/mouse/day for 4 months), as evaluated by tumor weight and during necropsies (not shown).
4. DISCUSSION
CAPE is known to inhibit growth of different types of cancer cells and here we show that it also inhibits growth of breast cancer cells, both the estrogen receptor positive as well as the estrogen receptor negative (TNBC) subtypes. In contrast, exposure to CAPE had no significant cytotoxic effect on immortalized, but non-tumorigenic breast cancer cells serving as the controls (Figs 2a & b). This in vitro inhibitory effect was recapitulated in the in vivo pre-clinical model where we demonstrated that within 3–4 weeks, CAPE decreases the tumor volume of mouse xenografts by about 40% and over 60% for MDA-231 and MCF-7 xenografts, respectively (Fig 7). Interestingly, the more aggressive phenotype, MDA-231 (TNBC) xenografts were more sensitive to CAPE requiring only 10 nmol CAPE/mouse/day to achieve this degree of tumor volume reduction compared to 50 nmol CAPE/mouse/day required for MCF-7 xenografts. Further, both xenograft models support the observation that low doses of CAPE result in greater effects on tumor growth inhibition as shown by 10 nmol CAPE producing the most inhibitory effects on MDA-231 when compared to 50 nmol and 250 nmol, as well in MCF-7 xenografts, where 250 nmol CAPE did not cause any further decrease in tumor growth when compared to 50 nmol (Fig 7).
Flow cytometric analysis shows that CAPE induces an S-phase cell-cycle arrest as evidenced by the accumulation of MCF-7 and MDA-231 cells in the S phase, from 34.5% (no CAPE) to 53.7% (at 20 µM CAPE) in MCF-7 cells and from 18.7% (no CAPE) to 68.6% (at 20 µM CAPE) in MDA-231 cells, respectively, with a concomitant decrease in cell accumulation in the G0/G1 phase of the cell cycle and complete elimination of the G2/M phases (Fig 4). The validity of these findings is supported by the increased protein expression of p21 and p27 and gene down-regulation of Rb, TP53, cyclins G1, D1, E1 & C, and CDK2, (Tables 2, 3) where the combined effect of these regulatory factors produce a net result of cell cycle arrest.
We examined the role of apoptosis in CAPE-induced tumor inhibition. In MDA-231 cells, we noticed a small (from 19 to 28%) but significant increase in Annexin V staining in 0 and 40-µM CAPE-treated cells, respectively (Fig. 2). While small, this effect is significant given that MDA-231 cells are known to be resistant to apoptosis, in particular, fas-induced apoptosis (control fas antibody did not induce apoptosis in MDA-231 cells, data not shown). Similarly, a modest increase in Annexin V-positive cells was observed in MCF-7 cells from 44% (no CAPE) to 66% (20 µM CAPE); higher concentrations of CAPE did not result in further apoptosis increases. Together, these results suggest that the tumor growth inhibition induced by CAPE is due in part to both cell-cycle arrest and apoptosis as has been shown by others but, in our breast cancer model system, cell-cycle arrest appears to be playing a more significant role.
Angiogenesis, or new blood vessel growth, is defined as a process in which a network of new blood vessels emerges from preexisting vessels [51]. It has been shown that exponential tumor growth over a few mm3 in size is dependent on the recruitment of its own nutrition and oxygen supply by angiogenesis [52]. The components of propolis have been studied in this regard and CAPE was demonstrated to have significantly strong anti-angiogenic effects on tube formation and growth of human umbilical vein endothelial cells (HUVECs) [40]. We sought to determine whether the tumor inhibitory effects of CAPE could also be a consequence of angiogenesis inhibition in breast cancer. We were able to recapitulate in our laboratory the anti-angiogenic effect of CAPE in our breast cancer model system first on tube formation by bovine capillary endothelial cells (Fig 5a) then on human mammary endothelial cells (HMECs) where CAPE at low doses from 0 to 5-µM effectively inhibited HMECs in a dose-dependent manner (data not shown). When we tested for CAPE’s effect on VEGF in MDA-231 cells, we observed a dose-dependent inhibition of VEGF secretion (Fig 5b). (MCF-7 cells do not secrete any significant levels of VEGF, which we verified (data not shown).
The NF-κB signal transduction pathway is deregulated in a variety of human cancers [53–54]. NF-κB plays a critical regulatory role in the expression of numerous target genes that control cell death, proliferation, differentiation, and immune and inflammatory responses [55]. In most types of cancer cells, NF-κB is constitutively active. [54, 56–58]. However, in breast cancer cells, high-level NF-κB activation is found in 86% of HER2-positive but ER-negative cancers, 33% of basal-like cancers (HER2-negative and ER-negative, and only weakly in ER-positive cancers [50]. The absence of constitutive NF-κB activation in ER-positive cancers is remarkable, and probably explained by specific inhibitory crosstalk between NF-κB and ER signaling [50].
Blocking NF-κB has been shown to stop tumor cells from proliferating, to die, or to become more sensitive to the action of antitumor agents, especially antioxidants [59]. Inhibition of NF-κB activation produces a corresponding increase in apoptosis, indicating that the balance of cell viability versus cell death is preserved by the degree of NF-κB activation [58]. Therefore, agents capable of down-modulating the activation of NF-κB have a potential for use in therapeutic interventions. CAPE inhibits NF-κB 1&2 in MDA-231 breast cancer cells. Further, p-IκB-α, an activated (phosphorylated) form of IκB-α, and IκB-β, inhibitors of NF-κB1&2, respectively, were decreased by CAPE to about the same degree (i.e., ~44% at 20 µM CAPE) (Table I). Thus, low doses of CAPE down-regulate the activation of NF-κB and could become an agent that is useful for therapeutic intervention. This is particularly true in TNBC cells that overexpress EGFR, a molecular feature important in their tumorigenesis and which acts on downstream NF-κB activation. CAPE’s ability to inhibit EGFR lends further support for its therapeutic potential given the number of targeted therapeutic agents against EGFR available in clinical oncology today for malignancies including head and neck cancer, lung cancer and colon cancer, to name a few [60].
Gene arrays showed that CAPE causes extensive changes in gene expression in both ER+ and ER− types of breast cancer cells. Heme oxygenase 1, an inducible stress protein that protects against oxidative damage, was increased in both MDA-231 and MCF-7 cells (Table III). As listed in Table II, the down-regulated genes in both cell lines include DNA damage-inducible genes, heat shock proteins, chaperonins, PCNA, topoisomerase I, and NF-κB1 (p105). Additional CAPE down-regulated genes in MCF-7 cells include ERα, RAR, cytochrome P-450s, and glutathione reductase. In MDA-231 cells, down-regulated genes are inflammatory cytokines, cyclins, RXR, ATM, BCL2-like, BCL-2 associated athanogene (an anti-apoptotic protein), various oncogenes, thus showing decreases in oxidative stress and cell proliferation, and an increase in a pro-apoptotic potential. Importantly, CAPE inhibited expression of IGFR-like 1&2. The majority of preclinical data show that insulin growth factor receptor (IGFR1) activation reinforces and potentially amplifies the biological effects of ER activation in breast cancer [61]. Studies showing anti-estrogens, like tamoxifen, can inhibit IGF mediated growth [62–63] and the finding that anti-IGF strategies can inhibit estrogen-mediated growth have led many to suggest that IGFs are partially responsible for estrogen-mediated signaling. However, there is also evidence for a role for IGFR1 in TNBC. IGFR1 is amplified in TNBC and studies have shown that treatment with anti IGF1 therapy in combination with chemotherapy in a TNBC primary human tumorgraft with high levels of both IGF-IR expression and activity, showed significant growth inhibition [64].
Importantly, we found that CAPE also inhibits expression of the mdr-1 gene (Table 2) known to confer resistance to chemotherapeutic drugs in cancer cells. Hence, it is likely that this decrease in multi-drug resistance gene levels (~4-fold) is the reason for a strong inhibition of growth and cell-cycle arrest by a concurrent treatment of CAPE and Taxol in vitro and in vivo when each is used at suboptimal doses (37, Omene C et al, manuscript in preparation). A decline in this gene should decrease not only the risk of drug resistance, but also allow the use of lower doses of therapeutics, e.g, paclitaxel (Taxol), with greater effectiveness and fewer side effects. These results suggest that CAPE could be used in adjuvant capacity to standard chemotherapeutic drugs as well.
Overall, our results strongly suggest that CAPE inhibits MDA-231 and MCF-7 human breast cancer cell growth due to its cytotoxicity in tumor but not normal cells and its effects on apoptosis, cell cycle, NF-κB and angiogenesis. We propose that CAPE is a viable candidate as a new therapeutic agent by itself as well as an adjuvant to standard chemotherapeutic drugs; the ongoing research should further delineate the mechanism(s) of CAPE effects on breast cancer.
AKNOWLEDGEMENTS
We thank Dr. William Carroll and Dr. Owen OA O’Connor for their critical review of this manuscript.
[This work was supported in part by grants BCTR0600476 & ES00260]
Footnotes
CONFLICT OF INTEREST
The authors do not have any conflicts of interest to disclose
REFERENCES
- 1.Nutritional business journal (NBJ) Integrative Medicine Report. 2009 [Google Scholar]
- 2.Boon H, Brown JB, Gavin A, Kennard MA, Stewart M. Breast Cancer Survivors' Perceptions of Complementary/Alternative Medicine (CAM): Making the Decision to use or not to use. Qual Health Res. 1999;9:639. doi: 10.1177/104973299129122135. [DOI] [PubMed] [Google Scholar]
- 3.Institute for Health and Healing at California Pacific Medical Center. 2002. Mar, [PubMed] [Google Scholar]
- 4.DiGianni LM, Garber JE, Winer EP. Complementary and Alternative Medicine Use Among Women with Breast Cancer. J Clin Oncol. 2002;20(18):34S–38S. [PubMed] [Google Scholar]
- 5.Crocetti E, Crotti N, Feltrin A, Ponton P, Geddes M, Buiatti E. The use of complementary therapies by breast cancer patients attending conventional treatment. Eur J Cancer. 1998;34:324–328. doi: 10.1016/s0959-8049(97)10043-0. [DOI] [PubMed] [Google Scholar]
- 6.Sparber A, Bauer L, Curt G, Eisenberg D, Levin T, Parks S, Steinberg SM, Wootton J. Use of complementary medicine by adult patients participating in cancer clinical trials. Oncol Nurse Forum. 2000;27:623–630. [PubMed] [Google Scholar]
- 7.Richardson MA, Sanders T, Palmer JL, Greisinger A, Singletary SE. Complementary/ alternative medicine use in a comprehensive cancer center and the implications for oncology. J Clin Oncol. 2000;18:2505–2514. doi: 10.1200/JCO.2000.18.13.2505. [DOI] [PubMed] [Google Scholar]
- 8.Morris K, Johnson N, Homer L, Walts D. A comparison of complementary therapy use between breast cancer patients and patients with other primary tumor sites. Am J Surg. 2000;179:407–411. doi: 10.1016/s0002-9610(00)00358-5. [DOI] [PubMed] [Google Scholar]
- 9.Lotfy M, Badra G, Burham W, Alenzi FQ. Combined use of honey, bee propolis and myrrh in healing a deep, infected wound in a patient with diabetes mellitus. Br J Biomed Sci. 2006;63(4):171–173. doi: 10.1080/09674845.2006.11732742. [DOI] [PubMed] [Google Scholar]
- 10.Castaldo S, Capasso F. Propolis, an old remedy used in modern medicine. Fitoterapia. 2002;73 Suppl 1:S1–S6. doi: 10.1016/s0367-326x(02)00185-5. [DOI] [PubMed] [Google Scholar]
- 11.Ansorge S, Reinhold D, Lendeckel U. Propolis and some of its constituents down-regulate DNA synthesis and inflammatory cytokine production but induce TGF-beta 1 production of human immune cells. Z Naturforsch [C] 2003;58:580–589. doi: 10.1515/znc-2003-7-823. [DOI] [PubMed] [Google Scholar]
- 12.Burdock GA. Review of the biological properties and toxicity of bee propolis (propolis) Food chem toxicology. 1998;36(4):347–363. doi: 10.1016/s0278-6915(97)00145-2. [DOI] [PubMed] [Google Scholar]
- 13.Grunberger D, Banerjee R, Eisinger K, Oltz EM, Efros L, Caldwell M, Estevez V, Nakanishi K. Preferential cytotoxicity on tumor cells by caffeic acid phenethyl ester isolated from propolis. Experientia. 1988;44:230–232. doi: 10.1007/BF01941717. [DOI] [PubMed] [Google Scholar]
- 14.Grunberger D, Frenkel K. Inhibition of cataract formation, diseases resulting from oxidative stress, and HIV replication by caffeic acid esters. 5,591,773. United States patent. 1997
- 15.Frenkel K, Wei H, Bhimani R, Ye J, Zadunaisky JA, Huang MT, Ferraro T, Conney AH, Grunberger D. Inhibition of tumor promoter mediated processes in mouse skin and bovine lens by caffeic acid phenethyl ester. Cancer Res. 1993;53:1255–1261. [PubMed] [Google Scholar]
- 16.Huang MT, Ma W, Yen P, Xie JG, Han J, Frenkel K, Grunberger D, Conney AH. Inhibitory effects of caffeic acid phenethyl ester (CAPE) on 12-O-tetradecanoylphorbol-13-acetate-induced tumor promotion in mouse skin and the synthesis of DNA, RNA and protein in HeLa cells. Carcinogenesis. 1996;17:761–765. doi: 10.1093/carcin/17.4.761. [DOI] [PubMed] [Google Scholar]
- 17.Son S, Lewis B. A, Free radical scavenging and antioxidative activity of caffeic acid amide and ester analogues: Structure-activity relationship. J. Agric. Food Chem. 2002;50:468–472. doi: 10.1021/jf010830b. [DOI] [PubMed] [Google Scholar]
- 18.Koltuksuz U, Mutus HM, Kutlu R, Ozyurt H, Cetin S, Karaman A, Gürbüz N, Akyol O, Aydin NE. Effects of caffeic acid phenethyl ester and epidermal growth factor on the development of caustic esophageal stricture in rats. J. Pediatr. Surg. 2001;36:1504–1509. doi: 10.1053/jpsu.2001.27032. [DOI] [PubMed] [Google Scholar]
- 19.Michaluart P, Masferrer JL, Carothers AM, Subbaramaiah K, Zweifel BS, Koboldt C, Mestre JR, Grunberger D, Sacks PG, Tanabe T, Dannenberg AJ. Inhibitory effects of caffeic acid phenethyl ester on the activity and expression of cyclooxygenase-2 in human oral epithelial cells and in a rat model of inflammation. Cancer Res. 1999;59:2347–2352. [PubMed] [Google Scholar]
- 20.Borrelli F, Izzo AA, Di Carlo G, Maffia P, Russo A, Maiello FM, Capasso F, Mascolo N. Effect of a propolis extract and caffeic acid phenethyl ester on formation of aberrant crypt foci and tumors in the rat colon. Fitoterapia. 2002;73:S38–S43. doi: 10.1016/s0367-326x(02)00189-2. [DOI] [PubMed] [Google Scholar]
- 21.Natarajan K, Singh S, Burke TR, Jr, Grunberger D, Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-κB. Proc Natl Acad Sci USA. 1996;93:9090–9095. doi: 10.1073/pnas.93.17.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watabe M, Hishikawa K, Takayanagi A, Shimizu N, Nakaki T. Caffeic acid phenethyl ester induces apoptosis by inhibition of NF-κB and activation of Fas in human breast cancer MCF-7 cells. J Biol Chem. 2004;279:6017–6026. doi: 10.1074/jbc.M306040200. [DOI] [PubMed] [Google Scholar]
- 23.Fitzpatrick LR, Wang J, Le T. Caffeic acid phenethyl ester, an inhibitor of nuclear factor-κB, attenuates bacterial peptidoglycan polysaccharide-induced colitis in rats. J Pharmacol Exp Ther. 2001;299:915–920. [PubMed] [Google Scholar]
- 24.Chen YJ, Shiao MS, Hsu ML, Tsai TH, Wang SY. Effect of caffeic acid phenethyl ester, an antioxidant from propolis, on inducing apoptosis in human leukemic HL-60 cells. J Agric Food Chem. 2001;49(11):5615–5619. doi: 10.1021/jf0107252. [DOI] [PubMed] [Google Scholar]
- 25.Kimura Y, Okuda H, Okuda T, Hatano T, Agata I, Arichi S. Studies on the activities of tannins and related compounds from medicinal plants and drugs. VII. Effects of extracts of leaves of Artemisia species, and caffeic acid and chlorogenic acid on lipid metabolic injury in rats fed peroxidized oil. Chem Pharm Bull (Tokyo) 1985;33:2028–2034. doi: 10.1248/cpb.33.2028. [DOI] [PubMed] [Google Scholar]
- 26.Laranjinha J, Vieira O, Madeira V, Almeida L. Two related phenolic antioxidants with opposite effects on vitamin E content in low density lipoproteins oxidized by ferrylmyoglobin: consumption vs regeneration. Arch Biochem Biophys. 1995;323:373–381. doi: 10.1006/abbi.1995.0057. [DOI] [PubMed] [Google Scholar]
- 27.Xiang D, Wang D, He Y, Xie J, Zhong Z, Li Z, Xie J. Caffeic acid phenethyl ester induces growth arrest and apoptosis of colon cancer cells via the beta-catenin/T-cell factor signaling. Anti cancer drugs. 2006;17(7):753–762. doi: 10.1097/01.cad.0000224441.01082.bb. [DOI] [PubMed] [Google Scholar]
- 28.Chen MF, Wu CT, Chen YJ, Keng PC, Chen WC. Cell killing and radiosensitization by caffeic acid phenethyl ester (CAPE) in lung cancer cells. J Radiation Res. 2004;45(2):253–260. doi: 10.1269/jrr.45.253. [DOI] [PubMed] [Google Scholar]
- 29.Kudugunti SK, Vad NM, Ekogbo E, Moridani MY. Investigational New Drugs Efficacy of Caffeic Acid Phenethyl Ester (CAPE) in skin B16-F0 melanoma tumor bearing C57BL/6 mice. 2011;29:52–62. doi: 10.1007/s10637-009-9334-5. [DOI] [PubMed] [Google Scholar]
- 30.Kuo HS, Kuo WH, Lee YJ, Lin WL, Chou FP, Tseng TH. Inhibitory effect of caffeic acid phenethyl ester on the growth of C6 glioma cells in vitro and in vivo. Cancer letters 28. 2006;234(2):199–208. doi: 10.1016/j.canlet.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 31.Chen MJ, Chang WH, Lin CC, Liu CY, Wang TE, Chu CH, Shih SC, Chen YJ. Caffeic acid phenethyl ester induces apoptosis of human pancreatic cancer cells involving caspase and mitochondrial dysfunction. Pancreatology. 2008;8(6):566–576. doi: 10.1159/000159843. [DOI] [PubMed] [Google Scholar]
- 32.Wu CS, Chen MF, Lee IL, Tung SY. Predictive role of nuclear factor-kappa B activity in gastric cancer: a promising adjuvant approach with caffeic acid phenethyl ester. J clin gastroenterol. 2007;41(10):871–873. doi: 10.1097/MCG.0b013e31804c707c. [DOI] [PubMed] [Google Scholar]
- 33.Onori P, DeMorrow S, Gaudio E, Franchitto A, Mancinelli R, Venter J, Kopriva S, Ueno Y, Alvaro D, Savage J, Alpini G, Francis H. Caffeic acid phenethyl ester decreases cholangiocarcinoma growth by inhibition of NF-kappa B and induction of apoptosis. Int J, cancer. 2009;125(3):565–576. doi: 10.1002/ijc.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee KW, Kang NJ, Kim JH, Lee KM, Lee DE, Hur HJ, Lee HJ. Caffeic acid phenethyl ester inhibits invasion and expression of matrix metalloproteinase in SK-Hep1 human hepatocellular carcinoma cells by targeting nuclear factor kappa B. Genes Nutr. 2008;2(4):319–322. doi: 10.1007/s12263-007-0067-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu J, Horton L, Bosland M, Karkoszka J, Frenkel K. Caffeic Acid Phenethyl Ester (CAPE) as a preventive agent in a preclinical model of breast cancer. Proceedings of the American Association for Cancer Research. 2007 Abstract # 4202. [Google Scholar]
- 36.Wu J, Bukkapatnam U, Eckard J, Frenkel K. Caffeic acid phenethyl ester (CAPE, a product of propolis) as an inhibitor of human breast cancer growth in a pre-clinical study and its effects on factors involved in cell cycle, angiogenesis, and drug resistance. Proceedings of the American Association for Cancer Research. 2008 Abstract #5710. [Google Scholar]
- 37.Wu J, Omene C, Karkoszka J, Klein CB, Smith J, Frenkel K. CAPE, a honeybee product, inhibits growth of human breast cancer xenografts by oral and topical routes and suppresses self-renewal of breast cancer stem cells. Proceedings of the American Association for Cancer Research. 2009 Abstract #2345. [Google Scholar]
- 38.Wu J, Omene C, Smith J, Frenkel K. Inhibition of breast cancer stem cells (CSC) self-renewal and growth by CAPE, a product of propolis. Proceedings of the American Association for Cancer Research. 2010 Abstract #3555. [Google Scholar]
- 39.Omene C, Wu J, Qu S, Smith J, Frenkel K. CAPE-induced Inhibition of Breast Cancer Stem Cells (CSC) Self-renewal and Growth by Differentiation to a less Malignant Phenotype. Proceedings of the American Association for Cancer Research. 2011 Abstract # 3344. [Google Scholar]
- 40.Ahn MR, Kunimasa K, Kumazawa S, Nakayama T, Kaji K, Uto Y, Hori H, Nagasawa H, Ohta T. Correlation between anti-angiogenic activity and antioxidant activity of various components from propolis. Molecular nutrition & food research. 2009;53(5):643–651. doi: 10.1002/mnfr.200800021. [DOI] [PubMed] [Google Scholar]
- 41.Liao HF, Chen YY, Liu JJ, Hsu ML, Shieh HJ, Liao HJ, Shieh CJ, Shiao MS, Chen YJ. Inhibitory effect of Caffeic Acid phenethyl Ester on Angiogenesis, Tumor Invasion and Metastasis. J Agric Food Chem. 2003;51:7907–7912. doi: 10.1021/jf034729d. [DOI] [PubMed] [Google Scholar]
- 42.Foley J, Nickerson NK, Nam S, Allen KT, Gilmore JL, Nephew KP, Riese DJ., 2nd EGFR signaling in breast cancer: Bad to the bone. Semin Cell Dev Biol. 2010 doi: 10.1016/j.semcdb.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carey LA. Directed therapy of subtypes of triple-negative breast cancer. Oncologist. 2010;15 Suppl 5:49–56. doi: 10.1634/theoncologist.2010-S5-49. [DOI] [PubMed] [Google Scholar]
- 44.Conlin AK, Seidman AD. Taxanes in breast cancer: an update. Curr Oncol Rep. 2007;9:22–30. doi: 10.1007/BF02951422. [DOI] [PubMed] [Google Scholar]
- 45.Jones SE. Metastatic breast cancer: the treatment challenge. Clin Breast Cancer. 2008;8:224–233. doi: 10.3816/CBC.2008.n.025. [DOI] [PubMed] [Google Scholar]
- 46.Schwartz J, Wong ST. Novel combinations for treating metastatic breast cancer: improving the odds, Introduction. Am J Health Syst Pharm.1. 2009;66(23 Suppl 6):S1–S2. doi: 10.2146/ajhp090435. [DOI] [PubMed] [Google Scholar]
- 47.Wong ST, Goodin S. Overcoming drug resistance in patients with metastatic breast cancer. Pharmacotherapy. 2009;29(8):954–965. doi: 10.1592/phco.29.8.954. [DOI] [PubMed] [Google Scholar]
- 48.Longley DB, Johnston PG. Molecular mechanisms of drug resistance. J Pathol. 2005;205:275–292. doi: 10.1002/path.1706. [DOI] [PubMed] [Google Scholar]
- 49.Song YS, Park EH, Jung KJ, Jin C. Inhibition of angiogenesis by propolis. Arch Pharm Res. 2002;25(4):500–504. doi: 10.1007/BF02976609. [DOI] [PubMed] [Google Scholar]
- 50.Biswas D, Iglehart JD. Linkage Between EGFR Family Receptors and Nuclear Factor Kappa B (NF-κB) Signaling in Breast Cancer. J Cell Physiol. 2006;209:645–652. doi: 10.1002/jcp.20785. [DOI] [PubMed] [Google Scholar]
- 51.Folkman J. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 52.Naumov GN, Akslen LA, Folkman J. Role of angiogenesis in human tumor dormancy. Cell Cycle. 2006;5:1779–1787. doi: 10.4161/cc.5.16.3018. [DOI] [PubMed] [Google Scholar]
- 53.Zhang X, Jin B, Huang C. The PI3K/Akt pathway and its downstream transcriptional factors as targets for chemoprevention. Curr Cancer Drug Targets. 2007;7:305–316. doi: 10.2174/156800907780809741. [DOI] [PubMed] [Google Scholar]
- 54.Lu T, Stark GR. Cytokine overexpression and constitutive NF-κB in cancer. Cell Cycle. 2004;3:1114–1117. [PubMed] [Google Scholar]
- 55.Kucharczak J, Simmons MJ, Fan Y, Gelinas C. To be, or not to be: NF-κB is the answer—role of Rel/NF-κB in the regulation of apoptosis. Oncogene. 2003;22:8961–8982. doi: 10.1038/sj.onc.1207230. [DOI] [PubMed] [Google Scholar]
- 56.Weisz L, Damalas A, Liontos M, Karakaidos P, Fontemaggi G, Maor-Aloni R, Kalis M, Levrero M, Strano S, Gorgoulis VG, Rotter V, Blandino G, Oren M. Mutant p53 enhances nuclear factor κB activation by tumor necrosis factor a in cancer cells. Cancer Res. 2007;67:2396–2401. doi: 10.1158/0008-5472.CAN-06-2425. [DOI] [PubMed] [Google Scholar]
- 57.Fabre C, Grosjean J, Tailler M, Boehrer S, Ades L, Perfettini JL, de Botton S, Fenaux P, Kroemer G. A novel effect of DNA methyltransferase and histone deacetylase inhibitors: NF-κB inhibition in malignant myeloblasts. Cell Cycle. 2008;7:2139–2145. doi: 10.4161/cc.7.14.6268. [DOI] [PubMed] [Google Scholar]
- 58.Beg AA, Baltimore D. An essential role for NF-κB in preventing TNF-α-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 59.Escarcega RO, Fuentes-Alexandro S, Garcia-Carrasco M, Gatica A, Zamora A. The transcription factor nuclear factor-κB and cancer. Clin Oncol (R Coll Radiol) 2007;19:154–161. doi: 10.1016/j.clon.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 60.Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ. UBM Medica. 13th Edition 2010. Cancer management: A Multidisciplinary Approach, Medical, Surgical & Radiation Oncology. [Google Scholar]
- 61.Fagan DH, Yee D. Crosstalk between IGF1R and estrogen receptor signaling in breast cancer. J Mammary Gland Biol Neoplasia. 2008;13(4):423–429. doi: 10.1007/s10911-008-9098-0. [DOI] [PubMed] [Google Scholar]
- 62.Freiss G, Rochefort H, Vignon F. Mechanisms of 4-hydroxytamoxifen anti-growth factor activity in breast cancer cells: alterations of growth factor receptor binding sites and tyrosine kinase activity. Biochem Biophys Res Commun. 1990;173:919–926. doi: 10.1016/s0006-291x(05)80873-3. [DOI] [PubMed] [Google Scholar]
- 63.Wakeling AE, Newboult E, Peters SW. Effects of antioestrogens on the proliferation of MCF-7 human breast cancer cells. J Mol Endocrinol. 1989;2:225–234. doi: 10.1677/jme.0.0020225. [DOI] [PubMed] [Google Scholar]
- 64.Litzenburger BC, Creighton CJ, Tsimelzon A, Chan BT, Hilsenbeck SG, Wang T, Carboni JM, Gottardis MM, Huang F, Chang JC, Lewis MT, Rimawi MF, Lee AV. High IGF-IR activity in triple-negative breast cancer cell lines and tumorgrafts correlates with sensitivity to anti-IGF-IR therapy. Clin Cancer Res. 2010 doi: 10.1158/1078-0432.CCR-10-1903. [DOI] [PMC free article] [PubMed] [Google Scholar]