The cytoplasmic domain of FlhB from S. typhimurium has been purified and crystallized.
Keywords: flagellar type III secretion system, FlhB
Abstract
FlhB is a key protein in the regulation of protein export by the bacterial flagellar secretion system. It is composed of two domains: an N-terminal transmembrane domain and a C-terminal cytoplasmic domain (FlhBc). FlhBc from Salmonella typhimurium has been successfully crystallized using the vapour-diffusion method. The crystals diffracted to 2.45 Å resolution and belonged to space group P42212, with unit-cell parameters a = b = 49.06, c = 142.94 Å. A selenomethionine-containing variant of FlhBc has also been crystallized in the same space group and was used for initial phase calculation by the multiwavelength anomalous dispersion (MAD) method.
1. Introduction
Many bacteria swim in a liquid environment by means of flagella. The bacterial flagellum is a long filament that protrudes out of the cell body. It is a huge complex structure made up of more than 30 different proteins (Macnab, 2003 ▶). The flagellum is divided into three major substructures: the basal body, the hook and the filament. Most of the flagellar proteins are localized outside the cell and are exported across the cytoplasmic membrane by the flagellum-specific secretion apparatus, which shares similarity to the bacterial type III secretion system that is used to secrete effectors into the host-cell cytoplasm (Aizawa, 2001 ▶; Blocker et al., 2003 ▶). The flagellar type III secretion system is postulated to be located within the MS ring on the cytoplasmic side of the flagellar basal body. In the case of Salmonella it consists of six integral membrane proteins (FlhA, FlhB, FliO, FliP, FliQ and FliR) and three cytoplasmic proteins (FliH, FliI and FliJ) (Minamino & Macnab, 1999 ▶). The export apparatus is not only responsible for the translocation of flagellar proteins across the cell membrane but also regulates the order in which the proteins are exported. Upon completion of the hook, the secretion system switches substrate specificity from hook-type export to filament-type export. Three proteins, the membrane protein FlhB, the hook-length control protein FliK and Flk (also known as RflH or Fluke), play active roles in the switching (Hirano et al., 1994 ▶; Kutsukake et al., 1994 ▶; Williams et al., 1996 ▶; Kutsukake, 1997 ▶; Aldridge et al., 2006 ▶; Minamino et al., 2009 ▶; Erhardt et al., 2010 ▶).
Homologues of FlhB have been found in all bacterial type III secretion systems. The molecular weight of Salmonella FlhB is about 42 kDa and the protein consists of two domains: a hydrophobic N-terminal part consisting of 211 residues that is predicted to contain four transmembrane helices, and a C-terminal cytoplasmic domain, FlhBc, consisting of 172 residues (Minamino et al., 1994 ▶). FlhBc has been shown to interact with the soluble components of the type III secretion system: FliH, FliI, FliJ and the cytoplasmic part of the membrane protein FlhA (Minamino & Macnab, 2000a ▶; Zhu et al., 2002 ▶).
The wild-type cytoplasmic domain of Salmonella FlhB undergoes autocatalytic cleavage between amino-acid residues Asn269 and Pro270 within a highly conserved NPTH sequence (Minamino & Macnab, 2000b ▶; Zarivach et al., 2008 ▶). This autocleavage is essential for the switching process (Fraser et al., 2003 ▶; Ferris et al., 2005 ▶). Mutation of Asn269 to Ala prevents cleavage and locks the export apparatus in the hook-type specificity state.
To switch substrate specificity, FlhB receives a signal from FliK (Williams et al., 1996 ▶; Minamino et al., 2006 ▶). Cells with defective FliK have a very long hook (a so-called polyhook) without an attached filament (Hirano et al., 1994 ▶). Suppression mutations of FlhB that can lead to switching even in the absence of FliK have been described (Kutsukake et al., 1994 ▶; Williams et al., 1996 ▶). All of these mutations are found exclusively in FlhBc, leading to the assumption of an important role of this part of the protein in export regulation. The interaction between FliK and FlhBc has been investigated by optical biosensing methods (Morris et al., 2010 ▶). Recently, a model of the complex between FlhBc and the C-terminal domain of FliK has been proposed (Mizuno et al., 2011 ▶).
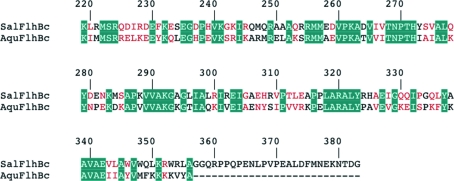
Several structures of the cytoplasmic domains of FlhB paralogues from bacterial needle type III secretion systems have been published (Zarivach et al., 2008 ▶; Deane et al., 2008 ▶; Lountos et al., 2009 ▶; Wiesand et al., 2009 ▶). Recently, we reported the successful crystallization of FlhBc from the hyperthermophilic bacterium Aquifex aeolicus (Meshcheryakov et al., 2011 ▶). Salmonella FlhBc and Aquifex FlhBc share 39% sequence identity (Fig. 1 ▶). However, the two proteins are evolutionarily very distant. Neither fliK nor flk were found in the Aquifex genome (Deckert et al., 1998 ▶), suggesting that the regulation of secretion differs in Salmonella and Aquifex. Comparison of the two proteins may provide us with additional information about the mechanism of FlhB function. Here, we describe the crystallization and preliminary X-ray diffraction study of the wild-type cleaved form of FlhBc from S. enterica serovar Typhimurium.
Figure 1.
Sequence alignment of FlhBc from S. typhimurium (SalFlhBc) and A. aeolicus (AquFlhBc). The numbering corresponds to Salmonella FlhB. Identical residues are shown with a teal background; similar residues are coloured red.
2. Materials and methods
2.1. Cloning, expression and purification
A DNA fragment encoding the cytoplasmic fragment of Salmonella FlhB (GeneID 1253435; amino-acid residues 219–383) was generated by PCR from genomic DNA of S. enterica as a template using the primers 5′-CAT ATG AAA TTA CGC ATG TCG CGG CAG G-3′ and 5′-GCT CTT CCG CAG GCA TCA GTA TTC TTC TCG TTC ATA AAA TC-3′, which contain NdeI and SapI restriction sites. The amplified DNA fragment was purified on agarose gel and ligated into pGEM-T vector (Promega) using A–T base pairing. After confirming the sequence, the DNA fragment was digested from the vector with NdeI and SapI, purified on agarose gel and cloned into the pTXB1 vector (New England BioLabs), generating an in-frame fusion with the intein and chitin-binding domain (CBD).
The recombinant vector containing the gene for the FlhBc-intein-CBD fusion protein was transformed into Escherichia coli strain BL21 (DE3) (Novagen). The transformed cells were cultured at 310 K to late exponential phase in 2 l Luria–Bertani medium containing 50 µg ml−1 ampicillin. Expression was induced with 1 mM isopropyl β-d-1-thiogalactopyranoside at an OD600 of 0.7–0.8. After 4 h incubation at 310 K, the cells were harvested by centrifugation at 8000g for 15 min. Further purification of Salmonella FlhBc was performed as described in Meshcheryakov et al. (2011 ▶). A typical preparation gives about 20–30 mg pure protein per litre of E. coli culture. The final protein contained no non-native amino-acid residues, with the exception that the C-terminal Gly residue was replaced by Ala in order to facilitate self-cleavage of the intein.
Selenomethionine-substituted FlhBc was expressed in methionine-auxotrophic E. coli strain B834 (DE3) (Novagen) using SeMet core medium (Wako Pure Chemical Industries). The purification of SeMet FlhBc was the same as that for the native protein. The incorporation of selenomethionine was confirmed by MALDI–TOF mass spectrometry.
2.2. Crystallization
The purified protein was transferred into 10 mM sodium acetate pH 4.5, 50 mM NaCl by gel filtration and concentrated using an Amicon Ultra system (Millipore) with a molecular-weight cutoff of 10 kDa. The protein concentration was measured by UV spectroscopy at 280 nm using a calculated molar extinction coefficient of 22 460 M −1 cm−1.
Preliminary screening of crystallization conditions was carried out by the sitting-drop vapour-diffusion method (150 nl protein solution was mixed with 150 nl reservoir solution and equilibrated against 120 µl reservoir solution) at 293 K in 96-well plates using an automated nanolitre liquid-handling system (Mosquito, TTP Labtech) and the following screening kits: Crystal Screen and Crystal Screen 2 (Hampton Research), Wizard I, II and III and Cryo I and II (Emerald BioSystems). Crystallization conditions were optimized by hanging-drop vapour diffusion using VDX plates (Hampton Research).
2.3. Data collection and processing
Diffraction data for both native FlhBc and SeMet-labelled FlhBc were collected on beamline BL44XU at SPring-8 using a MAR225 HE detector. Prior to data collection, the crystals were directly flash-cooled in their mother liquor using liquid nitrogen. X-ray diffraction data were recorded with an oscillation angle of 1°, an exposure time of 1 s per frame and a crystal-to-detector distance of 270 mm. Wavelengths for the MAD method were selected based on XAFS measurements. A complete SeMet-derivative data set was collected from a single crystal at wavelengths corresponding to the peak (0.97894 Å), the inflection point (0.97919 Å), a low remote (0.99490 Å) and a high remote (0.96396 Å). Diffraction data were indexed, integrated and scaled with MOSFLM (Leslie, 2006 ▶) and SCALA (Evans, 2006 ▶) from the CCP4 package (Winn et al., 2011 ▶).
3. Results
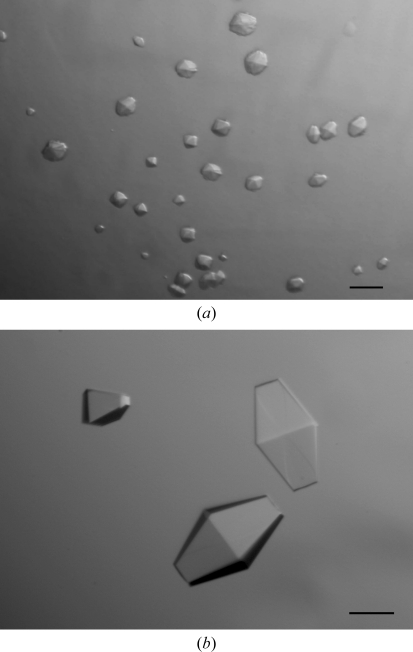
Microcrystals of Salmonella FlhBc were obtained using condition No. 26 of Cryo II (0.1 M MES pH 6.0, 0.2 M zinc acetate, 40% ethylene glycol; Fig. 2 ▶ a). However, the crystals showed no diffraction even using a synchrotron X-ray source. Diffraction-quality crystals were obtained after optimization of each component of the initial crystallization condition. The final crystallization conditions were 0.1 M sodium cacodylate pH 5.0, 0.2 M zinc acetate, 25% propylene glycol. Crystals appeared in 2–3 d after setup of the drop and grew to maximum dimensions of 0.3 × 0.1 × 0.1 mm within a week (Fig. 2 ▶ b).
Figure 2.
Crystals of Salmonella FlhBc grown in (a) 0.1 M MES pH 6.0, 0.2 M zinc acetate, 40% ethylene glycol and (b) 0.1 M sodium cacodylate pH 5.0, 0.2 M zinc acetate, 25% propylene glycol. Scale bar, 0.1 mm.
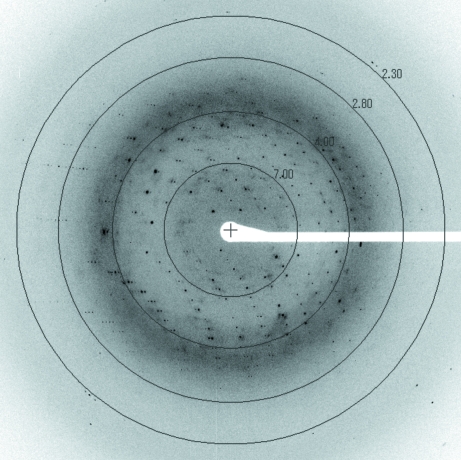
The crystals diffracted to 2.45 Å resolution (Fig. 3 ▶) and belonged to space group P42212, with unit-cell parameters a = b = 49.06, c = 142.94 Å, α = β = γ = 90.0°. Assuming one FlhBc molecule per asymmetric unit, the Matthews coefficient (Matthews, 1968 ▶) was calculated to be 2.31 Å3 Da−1, which corresponds to a solvent content of 46.8%.
Figure 3.
A typical X-ray diffraction pattern of a Salmonella FlhBc crystal. The oscillation range is 1°.
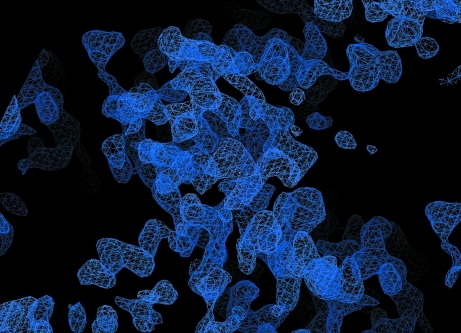
Selenomethionine-labelled FlhBc was prepared in order to perform a MAD experiment. The SeMet FlhBc crystals grew under slightly different conditions (0.1 M sodium cacodylate pH 5.0, 0.3 M zinc acetate, 20% propylene glycol) with a similar morphology to the native FlhBc crystals. These crystals belonged to space group P42212, with similar unit-cell parameters to the native protein (Table 1 ▶). A multiple-wavelength anomalous diffraction data set for the SeMet crystal was collected at four different wavelengths, but only two of them (peak and inflection point) were useful for phase calculation. Phase calculation and density modification were performed using SHELXD (Sheldrick, 2008 ▶), which resulted in an interpretable map (Fig. 4 ▶). Automatic model building with Buccaneer (Cowtan, 2008 ▶; Winn et al., 2011 ▶) provided an approximately 70% complete model. Manual model building and refinement are currently under way.
Table 1. Diffraction data statistics for FlhBc crystals.
Values in parentheses are for the highest resolution shell.
| SeMet derivative | |||
|---|---|---|---|
| Peak | Edge | Native | |
| Space group | P42212 | P42212 | P42212 |
| Unit-cell parameters (Å) | |||
| a = b | 48.75 | 48.82 | 49.06 |
| c | 143.08 | 143.2 | 142.94 |
| Wavelength (Å) | 0.978941 | 0.979187 | 0.9 |
| No. of images | 180 | 180 | 100 |
| Resolution (Å) | 48.75–2.80 (2.95–2.80) | 48.82–2.90 (3.06–2.90) | 40.45–2.45 (2.58–2.45) |
| Completeness (%) | 99.8 (100) | 99.8 (100) | 98.8 (100) |
| Total reflections | 63640 (9113) | 57393 (8460) | 52654 (7798) |
| Unique reflections | 4714 (647) | 4279 (601) | 6875 (984) |
| Multplicity | 13.5 (14.1) | 13.4 (14.1) | 7.7 (7.9) |
| Rmerge† (%) | 6.8 (34.0) | 6.4 (32.3) | 7.5 (38.0) |
| Mean I/σ(I) | 21.4 (7.5) | 23.3 (7.9) | 16.2 (5.7) |
R
merge = 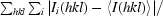
 , where Ii(hkl) is the intensity of the ith measurement of reflection hkl and 〈I(hkl)〉 is the mean value of Ii(hkl) for all i measurements.
, where Ii(hkl) is the intensity of the ith measurement of reflection hkl and 〈I(hkl)〉 is the mean value of Ii(hkl) for all i measurements.
Figure 4.
Experimental electron-density map at a contour level of 1.5σ calculated at a resolution of 2.8 Å.
Acknowledgments
This work was supported by direct funding provided by OIST. We thank Dr S.-Y. Park for his help in obtaining beam time.
References
- Aizawa, S.-I. (2001). FEMS Microbiol. Lett. 202, 157–164. [DOI] [PubMed]
- Aldridge, P., Karlinsey, J. E., Becker, E., Chevance, F. F. & Hughes, K. T. (2006). Mol. Microbiol. 60, 630–643. [DOI] [PMC free article] [PubMed]
- Blocker, A., Komoriya, K. & Aizawa, S. (2003). Proc. Natl Acad. Sci. USA, 100, 3027–3030. [DOI] [PMC free article] [PubMed]
- Cowtan, K. (2008). Acta Cryst. D64, 83–89. [DOI] [PMC free article] [PubMed]
- Deane, J. E., Graham, S. C., Mitchell, E. P., Flot, D., Johnson, S. & Lea, S. M. (2008). Mol. Microbiol. 69, 267–276. [DOI] [PMC free article] [PubMed]
- Deckert, G., Warren, P. V., Gaasterland, T., Young, W. G., Lenox, A. L., Graham, D. E., Overbeek, R., Snead, M. A., Keller, M., Aujay, M., Huber, R., Feldman, R. A., Short, J. M., Olsen, G. J. & Swanson, R. V. (1998). Nature (London), 392, 353–358. [DOI] [PubMed]
- Erhardt, M., Hirano, T., Su, Y., Paul, K., Wee, D. H., Mizuno, S., Aizawa, S. & Hughes, K. T. (2010). Mol. Microbiol. 75, 1272–1284. [DOI] [PMC free article] [PubMed]
- Evans, P. (2006). Acta Cryst. D62, 72–82. [DOI] [PubMed]
- Ferris, H. U., Furukawa, Y., Minamino, T., Kroetz, M. B., Kihara, M., Namba, K. & Macnab, R. M. (2005). J. Biol. Chem. 280, 41236–41242. [DOI] [PubMed]
- Fraser, G. M., Hirano, T., Ferris, H. U., Devgan, L. L., Kihara, M. & Macnab, R. M. (2003). Mol. Microbiol. 48, 1043–1057. [DOI] [PubMed]
- Hirano, T., Yamaguchi, S., Oosawa, K. & Aizawa, S. (1994). J. Bacteriol. 176, 5439–5449. [DOI] [PMC free article] [PubMed]
- Kutsukake, K. (1997). J. Bacteriol. 179, 1268–1273. [DOI] [PMC free article] [PubMed]
- Kutsukake, K., Minamino, T. & Yokoseki, T. (1994). J. Bacteriol. 176, 7625–7629. [DOI] [PMC free article] [PubMed]
- Leslie, A. G. W. (2006). Acta Cryst. D62, 48–57. [DOI] [PubMed]
- Lountos, G. T., Austin, B. P., Nallamsetty, S. & Waugh, D. S. (2009). Protein Sci. 18, 467–474. [DOI] [PMC free article] [PubMed]
- Macnab, R. M. (2003). Annu. Rev. Microbiol. 57, 77–100. [DOI] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol. 33, 491–497. [DOI] [PubMed]
- Meshcheryakov, V. A., Yoon, Y.-H. & Samatey, F. A. (2011). Acta Cryst. F67, 280–282. [DOI] [PMC free article] [PubMed]
- Minamino, T., Ferris, H. U., Moriya, N., Kihara, M. & Namba, K. (2006). J. Mol. Biol. 362, 1148–1158. [DOI] [PubMed]
- Minamino, T., Iino, T. & Kutuskake, K. (1994). J. Bacteriol. 176, 7630–7637. [DOI] [PMC free article] [PubMed]
- Minamino, T. & Macnab, R. M. (1999). J. Bacteriol. 181, 1388–1394. [DOI] [PMC free article] [PubMed]
- Minamino, T. & Macnab, R. M. (2000a). Mol. Microbiol. 35, 1052–1064. [DOI] [PubMed]
- Minamino, T. & Macnab, R. M. (2000b). J. Bacteriol. 182, 4906–4914. [DOI] [PMC free article] [PubMed]
- Minamino, T., Moriya, N., Hirano, T., Hughes, K. T. & Namba, K. (2009). Mol. Microbiol. 74, 239–251. [DOI] [PMC free article] [PubMed]
- Mizuno, S., Amida, H., Kobayashi, N., Aizawa, S.-I. & Tate, S. (2011). J. Mol. Biol. 409, 558–573. [DOI] [PubMed]
- Morris, D. P., Roush, E. D., Thompson, J. W., Moseley, M. A., Murphy, J. W. & McMurry, J. L. (2010). Biochemistry, 49, 6386–6393. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Wiesand, U., Sorg, I., Amstutz, M., Wagner, S., van den Heuvel, J., Lührs, T., Cornelis, G. R. & Heinz, D. W. (2009). J. Mol. Biol. 385, 854–866. [DOI] [PubMed]
- Williams, A. W., Yamaguchi, S., Togashi, F., Aizawa, S.-I., Kawagishi, I. & Macnab, R. M. (1996). J. Bacteriol. 178, 2960–2970. [DOI] [PMC free article] [PubMed]
- Winn, M. D. et al. (2011). Acta Cryst. D67, 235–242.
- Zarivach, R., Deng, W., Vuckovic, M., Felise, H. B., Nguyen, H. V., Miller, S. I., Finlay, B. B. & Strynadka, N. C. (2008). Nature (London), 453, 124–127. [DOI] [PubMed]
- Zhu, K., González-Pedrajo, B. & Macnab, R. M. (2002). Biochemistry, 41, 9516–9524. [DOI] [PubMed]