Abstract
Previous studies of Plasmodium vivax transmission to Anopheles spp. mosquitoes have not been able to predict mosquito infectivity on the basis of microscopic or molecular quantification of parasites (total parasites in the sample or total number of gametocytes) in infected blood. Two methods for production of P. vivax ookinete cultures in vitro, with yields of 106 macrogametocytes, 104 zygotes, and 103 ookinetes, respectively, per 10 mL of P. vivax-infected patient blood with approximately 0.01% parasitemia, were used to study P. vivax sexual stage development. The quantity of gametocytes, determined by counting Giemsa-stained blood smears, and quantity and type of gametocyte as determined by quantitative reverse transcriptase–polymerase chain reaction for Pvalpha tubulin II and macrogametocyte-specific pvg377 did not predict ookinete yield. Factors that affect the efficiency of in vitro P. vivax ookinete transformation remain poorly understood.
Introduction
Plasmodium vivax causes 70–80 million cases of malaria annually and predominates as the cause of malaria in South America and Asia.1–4 In the Amazon basin region of Peru, P. vivax incidence has increased since 1991 and remains the predominant cause of malaria in the Iquitos region where most cases occur.5,6
Plasmodium has a complex life cycle that consists of asexual development in a vertebrate host and sexual development in a mosquito vector. A small proportion of asexual parasites differentiate into sexually dimorphic microgametocytes and macrogametocytes within the vertebrate host. When mature gametocytes are taken up by the mosquito during a blood meal, specific molecular triggers initiate gametogenesis.7 Male microgametes fertilize female macrogametes to form zygotes that transform into ookinetes, the invasive form of the parasite that establishes infection in the mosquito. Successful transmission of the Plasmodium parasite depends on completion of the sexual cycle in the mosquito midgut. Interventions to block sexual development in the mosquito and thus prevent persons from infecting mosquitoes are actively being investigated for the development of transmission-blocking vacccines.8–11 The discovery of new transmission-blocking vaccine targets for P. vivax will be accelerated by the ability to generate sexual stage parasites in vitro for genetic, proteomic, cell biology, and biochemical analyses.
Two studies describing intentional P. vivax ookinete production in vitro have been published.12,13 Previous studies of P. vivax transmission to its Anopheles vector have not been able to define specific factors that predict parasite infectivity for mosquitoes on the basis of either microscopic examination of peripheral blood for the presence of P. vivax microgametocytes and macrogametocytes17,12,14 or molecular methods.15 Non-parasite factors, such as humoral immune mediators from the patient and mosquito innate immunity, potentially confounded those studies. To study P. vivax sexual stage development while minimizing the effects of potential confounding factors such as host cytokines, natural transmission-blocking antibodies in patient blood and/or mosquito-derived inhibitory factors, we tested the hypothesis that intrinsic parasite factors such as sex ratio and absolute gametocytemia might affect the production of in vitro P. vivax sexual stage parasites. Both classical parasitologic techniques and newly developed quantitative reverse transcription–polymerase chain reaction (qRT-PCR) assays based on sex-specific P. vivax markers were used to determine whether ookinete development could be correlated with gametocyte maturity or sex ratio in the P. vivax-infected blood.
Materials and Methods
Enrollment of P. vivax-infected patients.
Human participants were enrolled at local health posts in the region of Iquitos, Peru (Bellavista Nanay, Progreso, Masusa, Moronacocha, and San Juan) and hospitals (Hospital Apoyo Iquitos and Hospital Regional Loreto) in July 2009, after providing written informed consent in protocols approved by the Human Subjects Protection Program at the University of California San Diego and ethical committees at Universidad Peruana Cayetano Heredia, Lima, Peru and Asociación Benéfica PRISMA, Lima, Peru. This study was also registered with the Peruvian Ministry of Health, Directorate of Health (DISA, Loreto), Iquitos, Peru.
Patients were enrolled after presenting to local health posts and hospitals with a self-reported history of fever and a Giemsa-stained blood smear confirmed positive only for P. vivax malaria by at least two independent microscopists (a Ministry of Health health post technician and an independent malaria technician working at our Iquitos laboratory). Giemsa-stained thick blood smears were used to determine the number and type (sex) of gametocytes detected in a minimum of 10 oil-immersion high-powered (100× objective) fields until at least 200 leukocytes were counted.
Sampling and sample handling.
Approximately 10–20 mL of venous blood was collected into citrate-phosphate-dextrose anticoagulant–containing tubes and transported from the health post to the laboratory in a 37°C water bath within 1 hour of blood draw. After blood was obtained, patients were referred for treatment with primaquine and chloroquine according to National Peruvian Ministry of Health guidelines. Asexual and sexual stage parasite densities and microgametocytes and macrogametocytes were independently determined by two microscopists. No microscopic evidence of mixed P. falciparum infection was found.
Production of P. vivax ookinetes.
Plasmodium vivax-infected patient blood was first depleted of leukocytes by using a column of methylcellulose (CF-11; Whatman, Maidstone, United Kingdom). Autoclaved glass wool and CF-11 powder was packed into a sterile 20-mL syringe until the loosely packed volume of CF-11 was 10 mL. A 21-gauge needle was placed on the end of the syringe. The column was pre-warmed in a 37°C incubator and equilibrated with 10 mL of 37°C suspended animation (SA) solution, (10 mM Tris, 170 mM NaCl, 10 mM glucose, pH 7.4).13,16 Parasitized patient blood was pelleted by centrifugation, resuspended in three volumes of pre-warmed SA, and gravity filtered over the CF-11 column in a 37°C incubator with ambient gas conditions. Flow-through was collected and centrifuged to collect leukocyte-depleted parasitized erythrocytes.
Gametocytes were then stimulated to undergo gametogenesis by using either exflagellation solution (XaES) containing xanthurenic acid (10 mM Tris, 170 mM NaCl, 10 mM glucose, 25 mM NaHCO3, 50–100 mM xanthenuric acid, 20% fetal calf serum, pH 8.417), or heat-inactivated AB+ human serum (HS) at pH 8.2–8.4 adjusted with NaOH (Figure 1) at approximately 25°C with ambient gas conditions. One hour after gametogenesis, parasites were centrifuged and resuspended at approximately 25°C in filter-sterilized ookinete media (RPMI 1640 medium, 25 mM HEPES, 2 mM l-glutamine, 2 g/liter of NaHCO3, 50 mg/liter of hypoxanthine, 15–20% heat-inactivated AB+ human serum, 100 units of penicillin/mL, 100 μg of streptomycin/mL, pH 8.2–8.4 adjusted with NaOH) to 20% hematocrit. Centrifugation was performed at 800 × g for 3 minutes. Cultures were incubated for 24–36 hours at approximately 25°C with ambient gas conditions hours to enable ookinete development.
Figure 1.
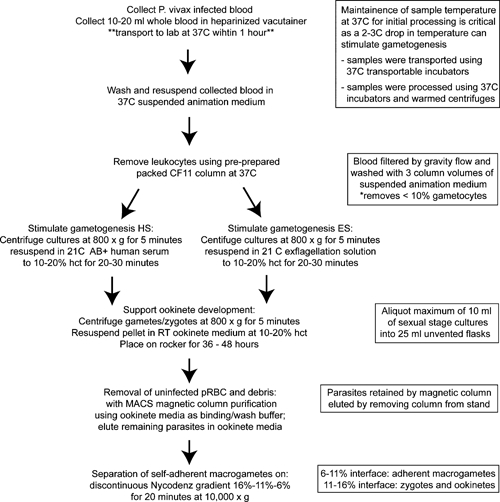
Flow chart of the Plasmodium vivax sexual state parasite culture protocol. pRBC = packed red blood cells; RT = room temperature (19–21°C); hct = hematocrit.
Purification of sexual stage parasites.
Most uninfected erythrocytes were removed from sexual stage parasite cultures by using density gradient centrifugation (Lympholyte-H; Cedarlane Laboratories, Burlington, NC) according to manufacturer's instructions. Parasites were collected from the gradient interface, washed twice in ookinete medium, and further purified by selection on a MidiMACS magnetic separator with an LD-50 column (Miltenyi Biotec, Bergisch Gladbach, Germany) according to manufacturer's instructions. For magnetic separation, ookinete media was used in place of MACS buffer. Positively selected parasites were centrifuged and resuspended in 100–500 μL of ookinete media; 10 μL of this suspension was used to make Giemsa-stained thin blood smears, and sexual stage parasites were quantified by using light microscopy. Ookinete yield was then calculated and reported as number of mature ookinetes/10 mL of infected patient blood.
Sexual stage and sex-specific gene expression analysis.
RNA and DNA were extracted from P. vivax-infected patient blood by using Trizol (Invitrogen, Carlsbad, CA) according to manufacturer instructions. DNA contamination was removed from isolated RNA samples by using RNase-free recombinant DNase I (DNA-free; Ambion, Austin, TX) according to manufacturer's instructions. RNA and DNA yields were quantified spectrophotometrically by using NanoDrop ND-1000 (Thermo Scientific, Wilmington, DE.
A PCR based on species-specific primers for P. falciparum Pfs25 (PF10_0303) was used to confirm the absence of P. falciparum mixed infections in all samples (Table 1). Expression of the gametocyte genes pvs230 (PVX_003905) and pvs28 (PVX_111180) and the microgametocyte-specific gene alpha tubulin II (PVX_090155) (predicted on the basis of P. falciparum alpha tubulin II)18 and the macrogametocyte-specific gene pvg377 (PVX_101400) were determined by using qRT-PCR (Table 1). Reverse transcription of RNA to cDNA was carried out by using a first strand synthesis kit (SuperScript III; Invitrogen) according to the manufacturer's instructions. Endpoint, non-quantitative PCR was performed with P. falciparum Pfs25-specific primers by using Platinum High Fidelity SuperMix (Invitrogen) and 250 nM primers for 40 cycles with an annealing temperature of 55°C and an extension temperature of 68°C. Quantitative PCR was performed by using gene-specific internal hybridization probes with an Opticon3 real-time thermal cycler (Bio-Rad, Hercules, CA), 40 cycles performed with High Fidelity SuperMix-UDG (Invitrogen), 250 nM primers and 150 nM of probe in 15-μL reaction volume, an annealing temperature of 52°C, and an extension temperature of 68°C. Standard curves for quantification were made using known quantities of plasmid standards containing the gene of interest. Three types of negative controls were included for each sample and gene-specific primer combination: 1) no DNA, 2) no probe and 3) RNA without reverse transcriptase. Samples were run in triplicate, and Student's t test was used to determine significance.
Table 1.
Plasmodium vivax gametocyte stage- and sex-specific gene primers*
| Primer or probe | Sequence |
|---|---|
| pvs230 F | 5′-TCAGTACAACCACCTGTTCAGCCA-3′ |
| pvs230 R | 5′-ACAAGTCCACGAGCTTCTTCACCT-3′ |
| pvs230 probe | 5′-6-FAM-AAATCGTCCCGCACAACTGCTTTGCCT-BHQ-3′ |
| pvs28 F | 5′-AACTGTGGAGACTACGCTGTGTGT-3′ |
| pvs28 R | 5′-ACATGGTGCTGTTCACATTAGCGG-3′ |
| pvs28 probe | 5′-6-FAM-AACGGCGTTCTGTGTGGAAAGGGAAA-BHQ-3′ |
| alpha tubulin II F | 5′-CTAGGGAAGGTACTCACAGATACG-3′ |
| alpha tubulin II R | 5′-CCCGACTGTCTATAAACTCTGC-3′ |
| alpha tubulin II probe | 5′-6-FAM-TGCTGGCCATCACCTCAAGTATCA-BHQ-3′ |
| Pfg377 F | 5′-CTGTACAGGTCTACAAGGCTTC-3′ |
| Pfg377 R | 5′-GAGTTGTATGGTTCCACCACAG-3′ |
| Pfg377 probe | 5′-6-FAM-ATAACTCCTACGATGCGGCGAAGAAG-BHQ-3′ |
| Pfs25 F | 5′-TCTTGTACATTGGGAACTTTGCCT-3′ |
| Pfs25 R | 5′-TGCGAAAGTTACCGTGGATACTG-3′ |
Primers and probes were used to determine expression of the following P. vivax sexual stage-specific genes: pvs230 (PVX_003905), pvs28 (PVX_111180), microgametocyte-specific gene alpha tubulin II (ATii, PVX_098630), and macrogametocyte-specific gene pvg377 (PVX_101400). BHQ = Black Hole Quencher. All primers and probes were obtained from Integrated DNA Technologies (San Diego, CA).
Results
Description of human participants and sampling.
Twenty adults (> 18 years old) who came to the local health facilities in the region of Iquitos, Peru, with fever or complaints consistent with acute malaria were enrolled in this study. All were microscopically confirmed to have P. vivax malaria and had microscopically detectable gametocytes. Six patients had fewer than 1,000 gametocytes/μL of blood, 11 of 20 patients had 1,000–5,000 gametocytes/μL of blood, and three patients had more than 5,000 gametocytes/μL of blood. Five samples had readily detectable microgametocytes and macrogametocytes; in six samples, only microgametocytes were microscopically observed; and in nine samples, only macrogametocytes were microscopically observed (Table 2).
Table 2.
Summary of Plasmodium vivax-infected blood samples*
| Sample no. | Parasites | Gametocytes | Macro | Micro | Ookinete yield |
|---|---|---|---|---|---|
| 1 | 5.6 × 103 | 1.3 × 103 | + | + | – |
| 2 | 2.8 × 104 | 3.2 × 103 | + | – | |
| 3 | 3.7 × 103 | 7.2 × 103 | + | 6.2 × 103 | |
| 4 | 9.1 × 103 | 8.0 × 102 | + | + | 1.0 × 103 |
| 5 | 1.7 × 104 | 4.0 × 102 | + | – | |
| 6 | 1.4 × 104 | 4.2 × 103 | + | 1.1 × 102 | |
| 7 | 1.0 × 104 | 3.8 × 103 | + | 1.3 × 102 | |
| 8 | 1.4 × 103 | 4.3 × 103 | + | 1.4 × 103 | |
| 9 | 1.2 × 104 | 1.6 × 104 | + | 1.2 × 103 | |
| 10 | 2.4 × 103 | 2.1 × 103 | + | + | – |
| 11 | 1.5 × 103 | 6.0 × 102 | + | + | 3.0 × 103 |
| 12 | 1.1 × 104 | 2.9 × 103 | + | – | |
| 13 | 3.1 × 103 | 3.0 × 102 | + | – | |
| 14 | 1.6 × 103 | 7.0 × 102 | + | – | |
| 15 | 2.4 × 103 | 1.3 × 103 | + | 1.4 × 102 | |
| 16 | 3.6 × 103 | 2.4 × 103 | + | – | |
| 17 | 1.9 × 103 | 5.0 × 102 | + | – | |
| 18 | 5.9 × 103 | 1.6 × 103 | + | – | |
| 19 | 1.7 × 104 | 4.5 × 103 | + | + | – |
| 20 | 8.6 × 103 | 7.3 × 103 | + | – |
Total parasite density and total gametocyte density were calculated by determining the number of parasites per 200 leukocytes. Densities are shown as no. per microliter of blood. The presence of macrogametocytes (Macro) and/or microgametocytes (Micro) on blood smear is denoted as +. For cultures that generated ookinetes, yields are shown as no. ookinetes/10 mL of patient blood.
To determine whether sub-patent P. falciparum gametocytes might be present, isolated RNA was subjected to PCR by using primers for P. falciparum Pfs25.19 Only P. vivax-specific primers resulted in RT-PCR amplification of patient samples, confirming the absence of mixed P. falciparum infection in this patient population.
Effect of xanthurenic acid on P. vivax ookinete yield.
Experimental production of P. vivax ookinetes in vitro has been reported, which included the use of xanthurenic acid to stimulate gametogenesis.12,13,20 Although xanthurenic acid is known to enhance gametogenesis,12,21–23 empiric evidence has also demonstrated that serum alone can be sufficient for P. falciparum gametogenesis.24,25
To determine whether gametogenesis using xanthurenic acid or heat-inactivated serum improved ookinete yield, four patient blood samples were divided equally and each was treated with two different solutions to stimulate gametogenesis (Figure 2 and Table 3). Cultures treated with XaES contained more parasites with abnormal morphology compared with HS (Figure 2). For this reason, HS was used to stimulate gametogenesis in the remaining experiments.
Figure 2.
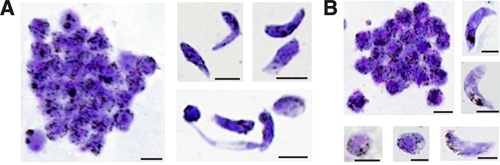
Comparison of xanthurenic acid and human serum on in vitro ookinete development of Plasmodium vivax. Giemsa-stained thin blood smears of P. vivax sexual stage parasite cultures derived from a single patient sample were equally divided and treated with two gametogenesis protocols using either exflagellation solution (XaES) or human serum (HS). A, Cultured sexual stage parasite forms generated by using XaES to stimulate gametogenesis. Elongated forms generated using this protocol showed normal immature and mature ookinete morphology and abnormal elongated forms. Approximately 30–40% of elongated parasites derived by using this method had either immature or abnormal morphology. B, Cultured sexual stage parasite forms generated by using HS to stimulate gametogenesis. More than 90% of elongated forms generated by using this protocol showed characteristic mature ookinete morphology. Scale bars = 5 μm.
Table 3.
Comparison of Plasmodium vivax ookinete yields by using two gametogenesis protocols*
| Sample no. | Xanthurenic acid | Human serum | ||||
|---|---|---|---|---|---|---|
| Round | Ookinete | Ookinete yield | Round | Ookinete | Ookinete yield | |
| 1 | + | – | 0 | + | – | 0 |
| 2 | + | – | 0 | + | – | 0 |
| 3 | + | – | 0 | + | + | 3.8 × 103 |
| 4 | + | + | 6.0 × 102 | + | + | 5.0 × 102 |
Four patient samples were equally divided and stimulated to undergo gametogenesis using two induction solutions, either exflagellation solution with xanthurenic acid or heat-inactivated human serum. The presence of round forms, which include macrogametes and zygotes (Round), and of ookinete forms is noted by +. Ookinete yields for each sample are described as no. ookinetes/5 mL of patient blood; only mature ookinete forms were quantified.
Plasmodium vivax sexual stage parasite cultures in which HS was used contained round and elongated forms (Figure 3). Of the 20 samples collected for this study, HS-produced cultures generated round forms, either macrogametes and/or zygotes; seven (35%) produced ookinetes. The elongated morphology of ookinetes clearly distinguished these forms from round gametocytes, macrogametes, and zygotes. Round forms could represent macrogametocytes or microgametocytes, macrogametes, or zygotes. Round, intraerythrocytic gametocytes were rarely observed at the end of the sexual stage parasite culture period. Round, erythrocyte-free parasites had three distinct nuclear morphologies: a single small eosinophilic nucleus, a single large eosinophilic nucleus, and two eosinophilic nuclei. It is likely that the round forms with a single, large nucleus or multiple nuclei represented zygotes, which are diploid. Elongated forms represented ookinetes and had the typical banana-shaped morphology of Plasmodium ookinetes with one large nucleus and multiple cleared regions, which may constitute vacuoles (Figure 3).
Figure 3.
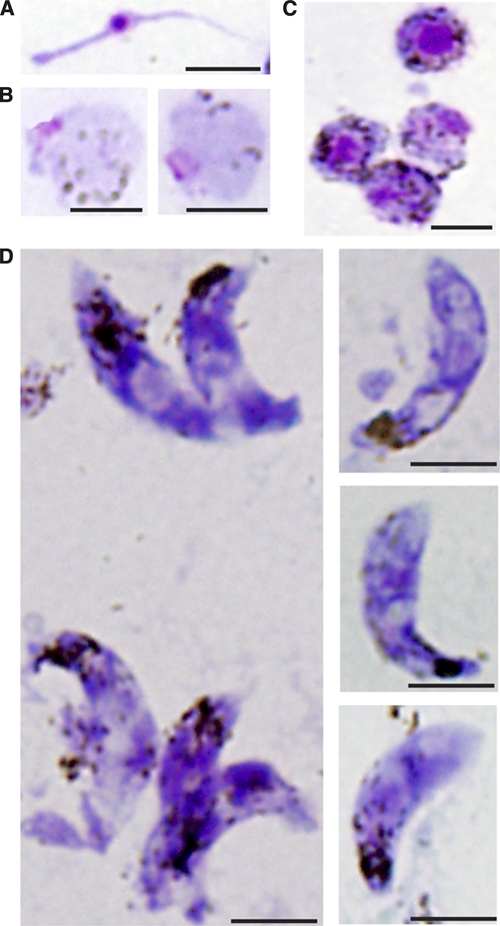
Giemsa-stained thin blood smears of in vitro generated Plasmodium vivax sexual stage parasites and Giemsa-stained thin blood smears of P. vivax sexual stage forms. A, Microgamete with eosinophilic nucleus. B, Round forms with basophilic cytoplasm, dispersed hemozoin, and a single small nucleus not contained within an erythrocyte. C, Round forms with basophilic cytoplasm, dispersed hemozoin, and a large eosinophilic nucleus not contained within an erythrocyte. D, Ookinetes with characteristic elongated form, localized hemozoin, and eosinophilic nuclei. Occasionally, two eosinophilic nuclei are visualized in a single ookinete. Additionally, parasites are seen with one to multiple, occasionally peri-nuclear, foci that do not stain well with Giemsa (→). Scale bars = 5 μm.
Senstivity of qRT-PCR for detection of P. vivax gametocytes compared with that of microscopy.
Under the conditions described in this report, morphologically mature ookinetes appeared within the 16–28 hours of culture initiation. Unlike P. falciparum, which has morphologically distinct gametocyte developmental stages, P. vivax gametocytes are thought to be fully functional when appearing simultaneously with asexual forms. Additionally, the low parasite and gametocyte densities common in P. vivax infection might have resulted in an underestimation of the microgametocytes or macrogametocytes present.
To determine whether a sensitive, molecular quantification method might provide a more accurate estimate of parasite density and sex ratio, qRT-PCR was performed on a subset of 12 samples using four sets of gene-specific primers and probes for gametocyte sex determination. The qRT-PCR and microscopic examination detected macrogametocytes in 83% (10 of 12) and 50% (6 of 12) of samples, respectively (Table 4). The qRT-PCR and microscopic analysis detected microgametocytes in 75% (9 of 12) and 67% (8 of 12) of samples, respectively. Both qRT-PCR and microscopic examination detected microgametocytes in 75% (6 of 8) samples that generated ookinetes and/or zygotes in sexual stage culture.
Table 4.
Comparison of quantitative real time PCR and light microscopy for gametocyte quantification and sexual differentiation of Plasmodium vivax*
| ID no. | Real-time RT-PCR | Light microscopy | ||||
|---|---|---|---|---|---|---|
| Pvg377 | ATii | Pvs28 | F Gc | M Gc | Gc | |
| 3† | + | – | 4.7 × 103 | – | + | 7.2 × 103 |
| 6† | – | – | 7.6 × 104 | + | – | 4.2 × 103 |
| 7† | + | + | 3.4 × 106 | + | – | 3.8 × 103 |
| 8† | + | + | 1.3 × 106 | – | + | 4.3 × 103 |
| 9† | + | + | 7.9 × 106 | – | + | 1.6 × 104 |
| 11† | + | + | 5.5 × 105 | + | + | 6.4 × 102 |
| 12 | + | + | 2.4 × 106 | – | + | 2.9 × 103 |
| 13 | + | – | 1.8 × 104 | – | + | 2.9 × 102 |
| 14 | – | + | 1.1 × 104 | – | + | 6.9 × 102 |
| 16 | + | + | 2.2 × 106 | + | – | 2.4 × 103 |
| 18 | + | + | 7.3 × 104 | + | – | 1.6 × 103 |
| 20 | + | + | 3.4 × 105 | + | + | 7.3 × 103 |
A subset of samples were analyzed by qRT-PCR to determine the sensitivity of gametocyte sex detection on the basis of expression of Pvg377 (PVX_101400) or ATii (PVX_098630) RNA from macro- or microgametocytes, respectively. Mature gametocyte density was quantified by expression of Pvs28 (PVX_111180) RNA from patient samples. Gametocyte density is expressed as no. parasites/μL of patient blood. PCR = polymerase chain reaction; ID = identification; RT = reverse transcription; FGc = macrogametes detected on slide; MGc = microgametes detected on slide; ATii = alpha tubilun ii; ii, Gc = gametocytes detected on slide; + = detected; – = not detected.
Samples that generated ookinetes.
Zygote and ookinete development did not correlate with levels of parasitemia or gametocytemia as determined either microscopically or by qRT-PCR. Samples with detectable microgametocytes and macrogametocytes appeared more likely to produce zygotes and ookinetes, but this was not statistically significant by Student's t test. Ookinete production did not correlate with total gametocyte density, macrogametocyte density, microgametocyte density, or the macrogametocytes:microgametocytes ratio.
Correlation of production of P. vivax sexual stage parasites in vitro with gametocyte density, maturity, or sex ratio.
Samples with microgametocytes and macrogametocytes (observed microscopically) in the ex vivo obtained P. vivax parasites appeared more likely to produce zygotes and ookinetes, but this was not statistically significant, likely because of small sample sizes. Although consistent with previous reports,7,12,26,27 data from in vitro cultivated P. falciparum ookinetes (where skewing of gametocytes can occur because of chromosomal deletions or other artifacts that affect gametocyte sex ratio28) suggest that ookinete development is strongly associated with gametocyte maturity and the macrogametocyte: microgametocyte sex ratio.
In P. falciparum, gametocyte development is morphologically distinct and can be readily differentiated into five forms by Giemsa staining. Additionally, gametocyte development is correlated with carefully regulated gene expression. For instance, Pfs230 is expressed early in gametocyte development but Pfs28 is not expressed until the gametocyte reaches stage V. Although we suspect that gene regulation is similarly regulated during P. vivax gametogenesis, gametocyte maturity cannot be assessed by morphology. Because P. vivax gametocytes cannot be routinely produced in a controlled, in vitro environment, this type of differential gametocyte gene regulation has not been definitively established. Therefore, we used Pvs230 and Pvs28 to quantify the number of P. vivax gametocytes by qRT-PCR.
The qRT-PCR more sensitively detected gametocytes than light microscopy. Therefore, we hypothesized that ookinete production would correlate with gametocyte density and sex ratio as determined by qRT-PCR. Surprisingly, this was not demonstrated (Figure 4B and Table 4). The production of zygotes and ookinetes did not significantly correlate with microscopically determined gametocyte density or detection of microgametocytes and macrogametocytes by either microscopy or qRT-PCR. Ookinete production did not correlate with gametocyte density, macrogametocyte density, microgametocyte density, or the macrogametocyte:microgametocyte ratio (Figure 4B).
Figure 4.
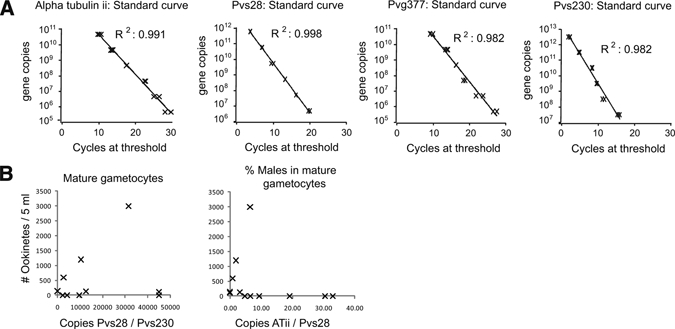
Quantitative reverse transcription–polymerase chain reaction (RT-PCR) used to analyze Plasmodium vivax gametocyte sex ratios. Parasite RNA was isolated by using Trizol reagent, treated with DNase, and used for RT-PCR. A, Standard curves were based on plasmid standards with the target PCR product for pvg377 (PVX_101400) associated with macrogametocytes; Pvalpha tubulin ii (ATii, PVX_098630) associated with microgametocyte; Pvs28 (PVX_111180) produced by both sexes of gametocytes; and the gametocyte gene Pvs230 (PVX_003905). B, No correlation was seen between ookinete production and the number of mature gametocytes in culture, as determined by the ratio of Pvs28 to Pvs230 by quantitative RT-PCR (qRT-PCR). No correlation could be demonstrated between ookinete production and the number of male gametocytes in culture, as determined by the ratio of ATii-to-Pvs28 by qRT-PCR. All qRT-PCR results were normalized to RNA concentration.
Discussion
Using a refined approach to producing P. vivax ookinetes from ex vivo obtained gametocytes, we tested the hypothesis that sex ratios, as determined by using standard microscopy and with new qRT-PCR assays, would be associated with ookinete yield in culture. Although we observed a range of male:female gametocyte ratios in patient-derived samples, there was no quantitative association with ookinete yield. The use of xanthurenic acid, the so-called gametocyte exflagellation factor, was not necessary for optimal P. vivax ookinete production and was associated with abnormal ookinete morphology. Overall, given the typically low parasitemia found in P. vivax malaria, absolute yields of P. vivax ookinetes were relatively low: 103/10 mL of blood.
Molecular approaches to quantification of stage-specific gametocyte genes carried out by using qRT-PCR has promise for studying P. vivax gametocytes ex vivo. The qRT-PCR also has the advantage of potentially determining the proportion of gametocytes at a particular developmental stage. Using microgametocyte-specific gene primers for Pvalpha tubulin II and macrogametocyte-specific gene primers for pvg377, we found that qRT-PCR more sensitively and precisely detects and quantifies gametocytes, consistent with previous reports.25,29,30 For these reasons, a qRT-PCR approach was developed to more precisely assess gametocyte sex (Figure 4). Twenty patient samples were obtained and stimulated to generate sexual stage parasites in vitro. Although only 14 samples contained macrogametocytes detectable by light microscopy, all samples generated round forms (despite some of which lacked apparent macrogametocytes), which could represent macrogametes or zygotes, indicating that all samples contained macrogametocytes. Three samples that did not have microscopically detectable microgametocytes produced ookinetes. However, samples containing both macrogametocytes and microgametocytes may have failed to generate ookinetes. Thus, lack of ookinete development does not necessarily indicate lack of microgametocytes but the presence of ookinetes in culture necessitates that mature microgametocytes were present in the sample. Because ookinetes could not have been generated without the presence of both microgametocytes and macrogametocytes in culture, this clearly indicated that microscopy was inadequate for the accurate detection and quantification of P. vivax gametocytes in peripheral patient blood samples.
This study has at least four important limitations. First, parasitized blood was only obtained as convenience samples from patients with symptomatic acute P. vivax malaria and low parasitemia (approximately 0.1%). Obtaining blood from patients with higher parasitemias would likely improve ookinete yields. Whether gametocytes obtained from patients with asymptomatic parasitemias would more efficiently produce ookinetes also needs to be tested. An attractive hypothesis to test is whether human cytokine responses leading to reactive oxidative intermediates might damage gametocytes as has previously been suggested.31–33 Second, in vitro ookinete formation was not compared with natural ookinete formation within the mosquito gut of experimentally infected mosquitoes. In the previous study from Thailand, there was only a loose, not statistically significant association in ookinete formation when compared between mosquito midgut and in vitro culture conditions.12 Third, we did not assess the infectivity of mature P. vivax ookinetes for gliding motility or mosquito infectivity. Fourth, some recent work based on morphologic classification by light microscopy has demonstrated widely varying sex ratios of P. vivax in South Korea with only approximately half of observed patients having both sexes.34 A number of investigators have considered the importance of naturally occurring sex ratios in malaria transmission, studying rodent Plasmodium species,35,36 in vitro P. falciparum models and samples obtained ex vivo from infected humans.37 We speculate that sex ratios in outbred P. vivax parasite populations will have less relevance to transmission compared with that found in laboratory-based studies using inbred strains. Future work will address these limitations.
Of note, detection of microgametocytes and macrogametes in this study was done by light microscopy and quantification of these forms was done by using RT-PCR, but detection and quantification of sexual stage parasites was accomplished by light microscopy only. After the experimental work in this report was finished and the manuscript being revised, a report about promiscuous expression of the P. falciparum alpha tubulin II in males and female gametocytes was published38; antibodies specific to male-specific and female-specific P. vivax sexual stage forms are needed to resolve the discrepancy between gene expression and protein expression as previously described.18
The results in this report here build on previous work and contribute to the continued efforts at in vitro production of P. vivax sexual stage parasites. The availability of P. vivax sexual stage parasites will enable in-depth investigations into the mechanisms underlying successful transmission-blocking interventions. Obtaining P. vivax ookinetes will further promote our understanding of sexual stage parasite development in this parasite that is not amenable to continuous in vitro propagation, by facilitating genetic and biochemical analyses of sexual stage parasites.
ACKNOWLEDGMENTS
We thank the study participants for their cooperation; the Iquitos laboratory and field workers, especially Maribel Paredes and Haydee Guerra, for their assistance at the Iquitos Satellite Laboratory; and Paula Maguina for her expertise and assistance in coordinating ethics, logistics, and administrative research matters with health workers and health centers in Iquitos, Peru, and with oversight and compliance bodies in Iquitos and Lima, Peru and La Jolla, California.
Footnotes
Financial support: This study was supported by U.S. Public Health Service grants T32GM007198, K24AI068903, R01AI45999, U19AI089681, R01AI067727, and D43TW007120, and the Medicines for Malaria Venture, Geneva, Switzerland.
Disclosure: None of the authors have any conflicts of interest.
Authors' addresses: Viengngeun Bounkeua, Fengwu Li, Shira R. Abeles, and Colleen M. McClean, Division of Infectious Diseases, Department of Medicine, University of California San Diego, La Jolla, CA, E-mails: vbounkeua@ucsd.edu, f1li@ucsd.edu, sabeles@ucsd.edu, and mmcclean@gmail.com. Raul Chuquiyauri and Joseph M. Vinetz, Division of Infectious Diseases, Department of Medicine, University of California San Diego, La Jolla, CA and Instituto de Medicina Tropical Alexander von Humboldt, Universidad Peruana Cayetano Heredia, Lima, Peru, E-mails: jvinetz@ucsd.edu and rachuqui@ucsd.edu. Victor Neyra and Alejandro Llanos-Cuentas, Instituto de Medicina Tropical Alexander von Humboldt, Universidad Peruana Cayetano Heredia, Lima, Peru, E-mails: victorneyra2002@yahoo.es and elmer.llanos@upch.pe. Pablo P. Yori, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, E-mail: pyori@jhsph.edu.
References
- 1.Mendis KN. The neglected burden of P. vivax malaria. Am J Trop Med Hyg. 2001;164:97–106. doi: 10.4269/ajtmh.2001.64.97. [DOI] [PubMed] [Google Scholar]
- 2.Guerra CA, Snow RW, Hay SI. Mapping the global extent of malaria in 2005. Trends Parasitol. 2006;22:353–358. doi: 10.1016/j.pt.2006.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hay SI, Guerra CA, Tatem AJ, Noor AM, Snow RW. The global distribution and population at risk of malaria: past, present, and future. Lancet Infect Dis. 2004;4:327–336. doi: 10.1016/S1473-3099(04)01043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Price RN, Tjitra E, Guerra CA, Yeung S, White NJ, Anstey NM. Vivax malaria: neglected and not benign. Am J Trop Med Hyg. 2007;77:79–87. [PMC free article] [PubMed] [Google Scholar]
- 5.Branch O, Casapia WM, Gamboa DV, Hernandez JN, Alava FF, Roncal N, Alvarez E, Perez EJ, Gotuzzo E. Clustered local transmission and asymptomatic Plasmodium falciparum and Plasmodium vivax malaria infections in a recently emerged, hypoendemic Peruvian Amazon community. Malar J. 2005;4:27. doi: 10.1186/1475-2875-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roshanravan B, Kari E, Gilman RH, Cabrera L, Lee E, Metcalfe J, Calderon M, Lescano AG, Montenegro-James S, Calampa C, Vinetz JM. Endemic malaria in the Peruvian Amazon region of Iquitos. Am J Trop Med Hyg. 2003;69:45–52. [PubMed] [Google Scholar]
- 7.Zollner GE, Ponsa N, Garman GW, Poudel S, Bell JA, Sattabongkot J, Coleman RE, Vaughan JA. Population dynamics of sporogony for Plasmodium vivax parasites from western Thailand developing within three species of colonized Anopheles mosquitoes. Malar J. 2006;5:68. doi: 10.1186/1475-2875-5-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saul A. Towards a malaria vaccine: riding the rollercoaster between unrealistic optimism and lethal pessimism. Southeast Asian J Trop Med Public Health. 1992;23:656–671. [PubMed] [Google Scholar]
- 9.Saul A. Mosquito stage, transmission blocking vaccines for malaria. Curr Opin Infect Dis. 2007;20:476–481. doi: 10.1097/QCO.0b013e3282a95e12. [DOI] [PubMed] [Google Scholar]
- 10.Gwadz RW. Successful immunization against the sexual stages of Plasmodium gallinaceum. Science. 1976;193:1150–1151. doi: 10.1126/science.959832. [DOI] [PubMed] [Google Scholar]
- 11.Lavazec C, Bourgouin C. Mosquito-based transmission blocking vaccines for interrupting Plasmodium development. Microbes Infect. 2008;10:845–849. doi: 10.1016/j.micinf.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Suwanabun N, Sattabongkot J, Tsuboi T, Torii M, Maneechai N, Rachapaew N, Yim-amnuaychok N, Punkitchar V, Coleman RE. Development of a method for the in vitro production of Plasmodium vivax ookinetes. J Parasitol. 2001;87:928–930. doi: 10.1645/0022-3395(2001)087[0928:DOAMFT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 13.McClean CM, Alvarado HG, Neyra V, Llanos-Cuentas A, Vinetz JM. Optimized in vitro production of Plasmodium vivax ookinetes. Am J Trop Med Hyg. 2010;83:1183–1186. doi: 10.4269/ajtmh.2010.10-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eyles DE, Young MD, Burgess RW. Studies on imported malarias; infectivity to Anopheles quadrimaculatus of asymptomatic Plasmodium vivax parasitemias. J Natl Malar Soc. 1948;7:125–133. [PubMed] [Google Scholar]
- 15.Bharti AR, Chuquiyauri R, Brouwer KC, Stancil J, Lin J, Llanos-Cuentas A, Vinetz JM. Experimental infection of the neotropical malaria vector Anopheles darlingi by human patient-derived Plasmodium vivax in the Peruvian Amazon. Am J Trop Med Hyg. 2006;75:610–616. [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Olayan EM BA, Butcher GA, Sinden RE, Hurd H. Complete development of mosquito phases of the malaria parasite in vitro. Science. 2002;295:677–679. doi: 10.1126/science.1067159. [DOI] [PubMed] [Google Scholar]
- 17.Westenberger SJ, McClean CM, Chattopadhya R, Dharia NV, Carlton JM, Barnwell JW, Collins WE, Hoffman SL, Zhou Y, Vinetz JM, Winzeler EA. A systems-based analysis of Plasmodium vivax lifecycle transcription from human to mosquito. PLoS Negl Trop Dis. 2010;4:e653. doi: 10.1371/journal.pntd.0000653. doi:10.1371/journal.pntd.0000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rawlings DJ, Fujioka H, Fried M, Keister DB, Aikawa M, Kaslow DC. Alpha-tubulin II is a male-specific protein in Plasmodium falciparum. Mol Biochem Parasitol. 1992;56:239–250. doi: 10.1016/0166-6851(92)90173-h. [DOI] [PubMed] [Google Scholar]
- 19.Babiker HA, Abdel-Wahab A, Ahmed S, Suleiman S, Ranford-Cartwright L, Carter R, Walliker D. Detection of low level Plasmodium falciparum gametocytes using reverse transcriptase polymerase chain reaction. Mol Biochem Parasitol. 1999;99:143–148. doi: 10.1016/s0166-6851(98)00175-3. [DOI] [PubMed] [Google Scholar]
- 20.Hisaeda H, Stowers AW, Tsuboi T, Collins WE, Sattabongkot JS, Suwanabun N, Torii M, Kaslow DC. Antibodies to malaria vaccine candidates Pvs25 and Pvs28 completely block the ability of Plasmodium vivax to infect mosquitoes. Infect Immun. 2000;68:6618–6623. doi: 10.1128/iai.68.12.6618-6623.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Billker O, Lindo V, Panico M, Etienne AE, Paxton T, Dell A, Rogers M, Sinden RE, Morris HR. Identification of xanthurenic acid as the putative inducer of malaria development in the mosquito. Nature. 1998;392:289–292. doi: 10.1038/32667. [DOI] [PubMed] [Google Scholar]
- 22.Billker O, Miller AJ, Sinden RE. Determination of mosquito bloodmeal pH in situ by ion-selective microelectrode measurement: implications for the regulation of malarial gametogenesis. Parasitology. 2000;120((Pt 6)):547–551. doi: 10.1017/s0031182099005946. [DOI] [PubMed] [Google Scholar]
- 23.Garcia GE, Wirtz RA, Barr JR, et al. Xanthurenic acid induces gametogenesis in Plasmodium, the malaria parasite. The Journal of Biological Chemistry. 1998;273:20. doi: 10.1074/jbc.273.20.12003. [DOI] [PubMed] [Google Scholar]
- 24.Muhia DK, Swales CA, Deng W, Kelly JM, Baker DA. The gametocyte-activating factor xanthurenic acid stimulates an increase in membrane-associated guanylyl cyclase activity in the human malaria parasite Plasmodium falciparum. Mol Microbiol. 2001;42:553–560. doi: 10.1046/j.1365-2958.2001.02665.x. [DOI] [PubMed] [Google Scholar]
- 25.McRobert L, Taylor CJ, Deng W, Fivelman QL, Cummings RM, Polley SD, Billker O, Baker DA. Gametogenesis in malaria parasites is mediated by the cGMP-dependent protein kinase. PLoS Biol. 2008;6:e139. doi: 10.1371/journal.pbio.0060139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eyles DE. Anopheles mosquito production of the plant communities of Reelfoot Lake. J Tenn Acad Sci. 1948;23:139–147. [PubMed] [Google Scholar]
- 27.Nicastri E, Bevilacqua N, Sane Schepisi M, Paglia MG, Meschi S, Ame SM, Mohamed JA, Mangi S, Fumakule R, Di Caro A, Capobianchi MR, Kitua A, Molteni F, Racalbuto V, Ippolito G. Accuracy of malaria diagnosis by microscopy, rapid diagnostic test, and PCR methods and evidence of antimalarial overprescription in non-severe febrile patients in two Tanzanian hospitals. Am J Trop Med Hyg. 2009;80:712–717. [PubMed] [Google Scholar]
- 28.Guinet F, Dvorak J, Fujioka H, Keister D, Muratova O, Kaslow D, Aikawa M, Vaidya A, Wellems T. A development defect in Plasmodium falciparum male gametogenesis. Journal of Cell Biology. 1996;135:269–278. doi: 10.1083/jcb.135.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carter R, Ranford-Cartwright L, Alano P. The culture and preparation of gametocytes of Plasmodium falciparum for immunochemical, molecular, and mosquito infectivity studies. Methods Mol Biol. 1993;21:67–88. doi: 10.1385/0-89603-239-6:67. [DOI] [PubMed] [Google Scholar]
- 30.Arai M, Billker O, Morris HR, Panico M, Delcroix M, Dixon D, Ley SV, Sinden RE. Both mosquito-derived xanthurenic acid and a host blood-derived factor regulate gametogenesis of Plasmodium in the midgut of the mosquito. Mol Biochem Parasitol. 2001;116:17–24. doi: 10.1016/s0166-6851(01)00299-7. [DOI] [PubMed] [Google Scholar]
- 31.Naotunne TDS, Karunaweera ND, Giudice GD, Kularatne MU, Grau GE, Carter R, Mendis KN. Cytokines kill malaria parasites during infection crisis: extracellular complementary factors are essential. J Exp Med. 1991;173:523–529. doi: 10.1084/jem.173.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karunaweera ND, Carter R, Grau GE, Kwiatkowski D, Del Giudice G, Mendis KN. Tumour necrosis factor-dependent parasite-killing effects during paroxysms in non-immune Plasmodium vivax malaria patients. Clin Exp Immunol. 1992;88:499–505. doi: 10.1111/j.1365-2249.1992.tb06478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karunaweera ND, Grau GE, Gamage P, Carter R, Mendis KN. Dynamics of fever and serum levels of tumor necrosis factor are closely associated during clinical paraxysms in Plasmodium vivax malaria. Proc Natl Acad Sci USA. 1992;89:3200–3203. doi: 10.1073/pnas.89.8.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huh AJ, Kwak YG, Kim ES, Lee KS, Yeom JS, Cho YK, Kim CS, Park JW. Parasitemia characteristics of Plasmodium vivax malaria patients in the Republic of Korea. J Korean Med Sci. 2011;26:42–46. doi: 10.3346/jkms.2011.26.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reece SE, Duncan AB, West SA, Read AF. Sex ratios in the rodent malaria parasite, Plasmodium chabaudi. Parasitology. 2003;127:419–425. doi: 10.1017/s0031182003004013. [DOI] [PubMed] [Google Scholar]
- 36.Drew DR, Reece SE. Development of reverse-transcription PCR techniques to analyse the density and sex ratio of gametocytes in genetically diverse Plasmodium chabaudi infections. Mol Biochem Parasitol. 2007;156:199–209. doi: 10.1016/j.molbiopara.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Babiker HA, Schneider P, Reece SE. Gametocytes: insights gained during a decade of molecular monitoring. Trends Parasitol. 2008;24:525–530. doi: 10.1016/j.pt.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwank S, Sutherland CJ, Drakeley CJ. Promiscuous expression of alpha-tubulin II in maturing male and female Plasmodium falciparum gametocytes. PLoS One. 2010;5:e14470. doi: 10.1371/journal.pone.0014470. [DOI] [PMC free article] [PubMed] [Google Scholar]


