Abstract
Although consequences of malaria in pregnancy are well known, the period of pregnancy in which infection has the highest impact is still unclear. In Benin, we followed up a cohort of 1,037 women through pregnancy until delivery. The objective was to evaluate the relationship between the timing of infection and birth weight, and maternal anemia at delivery. At the beginning of pregnancy, peripheral infections were associated with a decrease in mean birth weight (−98.5 g; P = 0.03) and an increase in the risk of anemia at delivery (adjusted odds ratio [aOR] = 1.6; P = 0.03). Infections in late pregnancy were related to a higher risk of maternal anemia at delivery (aOR = 1.7; P = 0.001). To fully protect the women during the whole pregnancy, already implemented measures (insecticide-treated nets and intermittent preventive treatment) should be reinforced. In the future, a vaccine against pregnancy-associated malaria parasites could protect the women in early pregnancy, which seems to be a high-risk period.
Introduction
Every year, 30 million women are exposed to the risk of malaria in pregnancy (MiP) in malarious areas of Africa.1 Malaria infection during pregnancy is a significant cause of maternal, fetal, and infant mortality and morbidity. Malaria in pregnancy is associated with maternal anemia, premature delivery, and intrauterine growth retardation.2 The last two factors are both responsible for low birth weight (LBW) of the newborn, the single most important risk factor for infant mortality (causing 75,000–200,000 infant deaths every year).3–7
Although the consequences of MiP are well known, the period of malaria infection during pregnancy, which has the highest impact for the mother and the baby, is still an open question.
Most studies use placental infection as an indicator of malaria status during pregnancy.8–11 Indeed, placental thick blood smear is easy to perform at delivery, and it is probably a good reflection of the maternal malaria infections in the last month of pregnancy. On the other hand, the follow-up of women during their whole pregnancy through a cohort is time-consuming and expensive. However, a single measure at delivery cannot describe the frequency and the timing of malaria infection during the course of pregnancy.
To our knowledge, only three studies investigated the relationship between the timing of MiP and its consequences on the outcome of pregnancy.12–14 In these studies though, gestational age (GA) was not precisely assessed and women were usually seen at their first antenatal visit (ANV) late in pregnancy.
To study immunological responses against malaria in pregnancy, a prospective cohort in pregnant women: “Strategy To Prevent Pregnancy-Associated Malaria “(STOPPAM) was conducted in Benin and Tanzania. One thousand pregnant women were followed up in each site. Women were encouraged to consult early and ultrasound scans were systematically performed during pregnancy. One of the main goals of the project was to evaluate the relationship between the timing of infection and consequences of MiP. The characterization of the most harmful period of malaria infection during pregnancy should help to improve preventive policies during pregnancy. The current work reports the study conducted in Benin.
Materials and methods
Study area, population.
The study took place in the district of Comé in the Mono province, located 70 km West from the economical capital of Benin, Cotonou (Figure 1). The climate is subtropical with two rainy seasons: from April to July, and from September to November. The annual rainfall is over 1,300 mm. The principal malaria vectors are Anopheles gambiae s.s., and Anopheles funestus. The setting is a high malaria transmission area with two peaks during the rainy seasons. The entomological inoculation rate ranges from 35 to 60 infective bites per person and per year.15 The predominant parasite species (97%) causing malaria in this region is Plasmodium falciparum.
Figure 1.
Study area (Charlotte Pierrat UMR216, IRD).
In the study area, health care is provided through three health dispensaries, 11 private clinics, and a district hospital. Women were enrolled in the three dispensaries: Comé, Akodeha, and Ouedeme Pedah. Comé is a semi-rural site and the two other health centers are located in a rural setting. The principal occupations of the inhabitants are farming, fishing, and trading.
Enrollment.
The enrollment of pregnant women started in June 2008 and the last delivery occurred in September 2010. Local midwives provided clinical and gynecological examination. Five nurses were recruited and trained as “project assistants” to fill out questionnaires and to collect blood samples from the study participants. Midwives and project assistants worked in close collaboration. The inclusion criteria were gestational age under 24 weeks, living within 15 km from the dispensary for > 6 months, and having planned to deliver at the hospital. The study objectives were explained twice to the women: first by the midwife and then by the project assistant. Pregnant women were enrolled in the study after giving informed and signed consent.
On the initial visit, assistants and midwives collected information regarding the reproductive history and the current pregnancy, medical history, socio-economic indicators, and the use of bed nets. Axillary temperature, blood pressure, weight (SECA scale, Hamburg, Germany), height, and the mid-upper arm circumference (MUAC) of mothers were measured. After clinical examination, rapid diagnostic tests (RDT) and thick and thin blood smears were systematically made and venous blood samples (3 tubes: ethylenediaminetetraacetic acid [EDTA] [2 mL], dry [2 mL], and citrate-phosphate-dextrose with adenine (CPDA) [4 mL]) were taken. Albumin and sugar in urine were measured with a dipstick (Uriscan 7strip, YD Diagnostics Corporation, Kyunggi-do, South Korea).
According to the Beninese national recommendations, a kit including tablets of iron (200 mg to be taken daily during 1 month), folic acid (5 mg daily, 1-month treatment), mebendazole (500 mg during 3 days) for deworming, and an insecticide-treated net was given to the mother at enrollment.
Follow-up.
At each monthly ANV, symptoms experienced and illnesses having occurred since the last visit were noted. The same clinical and biological information were collected for inclusion. Following the national guidelines, two doses of sulfadoxine pyrimethamine (SP) (1,500 mg of sulfadoxine and 75 mg of pyrimethamine) given on the occasion of intermittent preventive treatment during pregnancy (IPTp) were administered at least 1 month apart in the second trimester of pregnancy under the supervision of midwives. Iron and folic acid tablets ensuring a daily intake for 1 month were given to the women at each ANV until delivery.
Any participant with documented fever (axillary temperature ≥ 37.5°) and malaria infection assessed by RDT received a treatment dose of quinine, or SP if it was the scheduled visit for IPTp intake. In this case, a control based on a blood smear was performed 7 days later.
In case of clinical symptoms between ANVs, women were asked to attend health facilities to get treatment. The same clinical and biological information were collected as during ANVs. These visits were identified as “unscheduled visits.”
In case of an illness unmanageable by the medical team of the dispensary, the woman was sent to the district hospital to get appropriate care. Diagnostic and treatment received at the reference hospital were noted.
Four ultrasound scans were planned and performed with a portable ultrasound system (Titan, Sonosite, Bothell, WA) by a midwife trained for ultrasound from August 2008 to March 2009 and by an obstetrician from April 2009 until the end of the study. The first scan aimed to determine the exact term of the pregnancy and the following to evaluate the intrauterine growth and fetal morphology.
Delivery.
At delivery, temperature, weight, and blood pressure of the mother were measured. Venous blood samples, thick and thin blood smears, and an RDT were obtained from the mother before delivery. The placenta was measured and weighed. The macroscopic aspect of the placenta was recorded. Blood placental samples and three placental biopsies were collected and two placental smears (thick and thin) were made. In case of positive RDT, a placenta perfusion with phosphate buffered saline plus CPDA solution was performed to collect placental Plasmodium parasites. Blood samples from the umbilical cord were drawn.
The newborn was examined clinically (APGAR score, icterus, and malformation) and anthropological measures were performed by the midwife: weight (electronic scale Seca), height, MUAC, head circumference, abdominal circumference, and foot length. The gestational age was assessed clinically by using the new Ballard score.16
For women delivering outside the study frame, birth outcomes were collected from the antenatal care book. Ballard score was assessed if the women presented at the study center within 2 days after delivery.
Laboratory procedures.
For the biological detection of plasmodial infections, we used Parascreen RDT (Zephyr Biomedical Systems, Goa, India) which detects P. falciparum histidine protein-2 (Pf HRP-2) and Plasmodium-species lactate dehydrogenase (Psp LDH).
Daily, all RDTs, thick and thin smears, and blood samples from the three health centers were collected, kept at 20°C, and sent to the laboratory in Cotonou. Thick blood smears were stained with Giemsa and read by two experienced parasitology technicians. Smears were considered negative if no asexual-stage Plasmodium parasite was detected after 500 leukocytes had been counted. Malaria parasites were counted against 200 leukocytes and parasite densities were estimated using leukocyte count of the hemogram. If results were discrepant, the slides were read by a third microscopist.
Blood samples were centrifuged and frozen for further immunological analyses and blood drops were deposited on filter paper for parasite genotyping. Analyses to determine hemogram and to measure alanine aminotransferase and creatinine concentrations were performed.
Statistical analysis.
Data were double-entered, validated, and cleaned using Access (Microsoft, version 2003, Redmond, WA). Stata version 11 for Windows (Stata Corp., College Station, TX) was used for all statistical analyses.
Plasmodium infection status was based on the results of thick blood smear. Low birth weight was defined as a birth weight < 2,500 g. We considered three periods during pregnancy: early pregnancy (0–4 months of gestation); mid-pregnancy (5–6 months), and late pregnancy (> 6 months). Because of the low number of women enrolled in the first trimester, we chose a 4-month limit for the first period of pregnancy instead of the more usual categorization by trimesters. The women's body mass index (BMI) was defined as the weight after delivery (kg)/height squared (m2) and then transformed into a binary variable: under and over the median (< 21; ≥ 21). Maternal anemia was defined as hemoglobin concentration under 11 g/dL. We excluded twin newborns from the analysis.
Our primary outcomes were the newborn's weight (LBW and decrease in mean birth weight) and maternal anemia at delivery. We first studied the association between each of these outcomes and the occurrence of a Plasmodium infection during the three gestational periods. We then considered other variables susceptible to have an influence on birth weight or maternal anemia, such as the infection of the placenta or the number of Plasmodium infections during the course of pregnancy (no infection, 1–2 infections, and more than 2 infections).
Differences in proportions and means were compared using the khi2 (or Fisher's exact test) and the Student t test, respectively. We used a logistic or a linear regression depending on the variable analyzed (LBW, birth weight, or anemia). Covariates were included in the initial models on the basis of the literature and on hypothesized underlying causal relationships directed acyclic graphs (DAG) method.17,18 We considered the following covariates: duration of pregnancy (transformed into a four class variable corresponding to the quartiles), sex of the newborn, parity (primigravidae or multigravidae), rainy season at delivery, human immunodeficiency virus (HIV) status, number of SP intakes, education, use of bed net, BMI, number of consultations (sum of the ANVs and unscheduled visits). We used a backward strategy to obtain the final multivariate model, a P value of < 0.05 was considered statistically significant.
During the first trimester, African women usually consult late for their first ANV; therefore, malaria status was missing for some women. At delivery, mostly because of a health workers strike in the area, some mothers' weights and malaria status (placental and peripheral smears) were missing. The HIV status was unknown for some women. To deal with missing data, we used the method of multiple imputations by chain equations (MICE)19 and we carried out 20 imputed datasets with 15 iterations for each.
Ethics clearance.
This study was approved by the ethics committees of the Research Institute for Development (IRD) in France and of the Science and Health Faculty (University of Abomey Calavi) in Benin. Written informed consent was given by all participants.
Results
As presented in the flowchart diagram (Figure 2), 1,037 pregnant women were enrolled in the STOPPAM study. Fifty-five women were excluded from the follow-up: 38 withdrawals (mainly because of fear of blood sampling), and 17 non-pregnant women confirmed after ultrasound scanning. Seventy-six women were lost to follow-up: 15 for outmigration and 61 with unstated reason.
Figure 2.
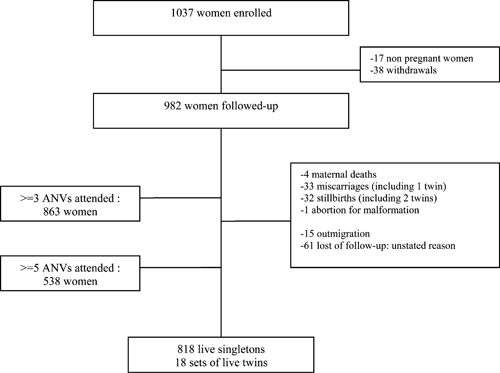
Study profile.
Age, gravidity, and education were similar between women who were lost to follow-up and those who remained in the cohort. Regarding ethnicity, there were more Mina and less Peda in the lost of follow-up group. At the end of the follow-up, 836 women gave birth to live newborns in the STOPPAM frame, including 18 sets of twins.
At delivery, placental smears and blood samples were available for 635 women. Age, gravidity, and education were similar between women with available data and those without.
Mina and Watchi were in the majority and Sahoue and Peda in the minority in the group of women with complete data (data not shown).
Table 1 presents the general characteristics of the 982 followed up mothers. On average mothers were 26.4 years of age (range 15–45), 18.2% were primigravidae, and 22.3% were secundigravidae. More than one-half (56%) did not attend school and the major ethnic group (29.6%) was the Peda group. At enrollment, the mean gestational age assessed by ultrasound was 17.2 weeks (SD = 4.7) with a minimum of 5 weeks. 26.7% of women reported the use of bed net at enrollment. The mean numbers of antenatal visits and unscheduled visits were 4.4 (SD = 1.6) and 0.8 (SD = 0.9), respectively, with a maxima of 8 and 5 visits, respectively. Among the 862 women tested, 98.1% were HIV negative and 1.9% was positive. Among infected women, the geometric mean parasite density was 933 parasites/μL (range: 16–53,708).
Table 1.
General characteristics of the pregnant women
| Characteristics | Mean (SD) or N (%) N = 982 |
|---|---|
| Age (years) | 26.4 (6.2) |
| Gestational age, assessed by ultrasound (weeks) | 17.2 (4.7) |
| Gravidity | |
| 1 | 179 (18.2%) |
| 2 | 219 (22.3%) |
| > 2 | 584 (59.5%) |
| Use of bed net at enrollment | 258 (26.2%) |
| Education | |
| None | 550 (56.0%) |
| Primary | 290 (29.5%) |
| Secondary | 142 (14.5%) |
| Ethnic group | |
| Peda | 291 (29.6%) |
| Watchi | 166 (16.9%) |
| Adja | 149 (15.2%) |
| Saha | 146 (14.9%) |
| Mina | 100 (10.2%) |
| Other | 130 (13.2%) |
| Number of ANVs | 4.4 (1.6) |
| Number of emergency visits | 0.8 (0.9) |
| HIV status† | |
| Negative | 846 (98.1%) |
| Positive | 16 (1.9%) |
ANV = antenatal visit; HIV = human immunodeficiency virus.
There were 862 women tested.
Birth weight.
Table 2 shows the effect of the timing of peripheral parasitemia in pregnant women on birth weight and LBW. The overall mean birth weight was 2998.2 g (SD = 474) and 10.9% were LBW. After adjustment, only early pregnancy infections were associated with a decrease in mean birth weight (−98.5 g; P = 0.03). The BMI, parity (decrease of 133.1 g in mean birth weight in primigravidae compared with multigravidae, P < 0.001), fetal sex (a 141.4 g increase in males compared with females, P < 0.001), the duration of pregnancy, the number of consultations, and the use of bed nets were also related to mean birth weight.
Table 2.
Effect of the timing of malaria infections on low birth weight and mean birth weight*
| Malaria infection | Mean birth weight after MICE (g) | Univariate analysis† | Multivariate‡ analysis† | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean difference (g) | 95% CI | P | Mean difference (g) | 95% CI | P | |||||||||
| 0–4 months of gestation | ||||||||||||||
| Positive (N = 133) | 2865.9 | −171.4 | [−188.5, −154.3] | < 0.001§ | −98.5 | [−188.5, −8.5] | 0.03§ | |||||||
| Negative (N = 425) | 3037.3 | |||||||||||||
| 5–6 months of gestation | ||||||||||||||
| Positive (N = 126) | 2978.2 | −23.9 | [−43.4, −4.3] | 0.02§ | 35.4 | [−41.7, 112.5] | 0.37 | |||||||
| Negative(N = 669) | 3002.1 | |||||||||||||
| > 6 months of gestation | ||||||||||||||
| Positive (N = 400) | 2932.2 | −136 | [−150.4, −121.6] | < 0.001§ | −21.9 | [−82.2, 38.5] | 0.48 | |||||||
| Negative (N = 380) | 3068.2 | |||||||||||||
| Malaria infection | % of LBW after MICE | OR | 95% CI | P | aOR | 95% CI | P | |||||||
| 0–4 months of gestation | ||||||||||||||
| Positive (N = 133) | 15.6 | 1.7 | [0.9, 3.3] | 0.08 | 1.2 | [0.6, 2.6] | 0.57 | |||||||
| Negative (N = 425) | 9.5 | |||||||||||||
| 5–6 months of gestation | ||||||||||||||
| Positive (N = 126) | 12.6 | 1.2 | [0.7, 2.2] | 0.5 | 1.02 | [0.5, 2.0] | 0.9 | |||||||
| Negative (N = 669) | 10.5 | |||||||||||||
| > 6 months of gestation | ||||||||||||||
| Positive (N = 400) | 14.8 | 2.4 | [1.5, 3.9] | < 0.001§ | 1.4 | [0.8, 2.5] | 0.2 | |||||||
| Negative (N = 380) | 6.7 | |||||||||||||
MICE = method of multiple imputation by chain equations; LBW = low birth weight.
Reference class is Negative malaria infection.
Adjusted for parity, newborn's sex, rainy season at delivery, maternal body mass index (BMI), education, duration of gestation, number of sulfadoxine pyrimethamine (SP) intakes, number of consultations, and use of bed net.
Statistically significant results (P < 0.05).
When we analyzed LBW, after adjustment, there was no relation with any of the three Plasmodium infection periods. The following cofactors were related with an increased risk of LBW: low BMI, short duration of pregnancy, and first pregnancy (data not shown).
We found that 11.5% (80 of 696) of the placentas were malaria infected. We did not find any association between placental infection and mean birth weight (−22.0 g; 95% confidence interval [CI] [−120; 76.1]; P = 0.66) after adjustment for cofactors. Similarly, placental infection was not related to LBW (adjusted odds ratio [aOR] = 1.1; 95% CI [0.5; 2.2]; P = 0.9).
We also considered the number of Plasmodium infections during pregnancy instead of the three Plasmodium infection periods. After adjustment, we found no significant association with a decrease in mean birth weight (1–2 Plasmodium infections versus no infection: −50.1 g; 95% CI 95 [−111.6; 11.4]; P = 0.11, and ≥ 3 Plasmodium infections versus no infection: −116.4 g; 95% CI [−245; 12.6]; P = 0.08). Similarly, there was no association between LBW and the number of Plasmodium infections after adjustment (1–2 Plasmodium infections versus no infection: aOR = 1.3; 95% CI [0.8; 2.3]; P = 0.3 and ≥ 3 Plasmodium infections versus no infection: aOR = 1.3; 95% CI [0.5; 3.5]; P = 0.6).
Maternal anemia.
The mean hemoglobin concentrations at enrollment and at delivery were 10.6 g/dL (1.24) and 11.0 g/dL (1.35), and 61.2% and 44.9% of the women were considered anemic, respectively. There were 0.5% (4 of 812) women at enrollment and 0.9% (6 of 635) women at delivery with severe anemia (< 7 g/dL).
Table 3 shows the analysis of maternal anemia at delivery in relation to the three possible peripheral Plasmodium infection periods. In multivariate analysis, women infected during early and late parts of pregnancy were more at risk of maternal anemia at delivery compared with uninfected women (early pregnancy: aOR = 1.6; P = 0.03 and late pregnancy: aOR = 1.7; P = 0.001). Women with a lower BMI had a 1.8-fold increased risk of having anemia at delivery.
Table 3.
Effect of the timing of malaria infections on maternal anemia at delivery*
| Malaria infection | % of maternal anemia after MICE | Univariate analysis† | Multivariate‡ analysis† | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | aOR | 95% CI | P | ||
| 0–4 months of gestation | |||||||
| Positive (N = 133) | 55.6 | 1.7 | [1.1, 2.6] | < 0.01§ | 1.6 | [1.05, 2.5] | 0.03§ |
| Negative (N = 425) | 41.8 | ||||||
| 5–6 months of gestation | |||||||
| Positive (N = 126) | 51.3 | 1.4 | [0.9, 2.1] | 0.15 | 1.1 | [0.7, 1.7] | 0.62 |
| Negative (N = 669) | 43.6 | ||||||
| > 6 months of gestation | |||||||
| Positive (N = 400) | 51.8 | 1.9 | [1.4, 2.7] | < 0.001§ | 1.7 | [1.3, 2.4] | 0.001§ |
| Negative (N = 380) | 35.9 | ||||||
MICE = method of multiple imputation by chain equations; OR = odds ratio; aOR = adjusted odds ratio; CI = confidence interval.
Reference class is Negative malaria infection.
Adjusted for parity, newborn's sex, rainy season at delivery, maternal BMI, education, duration of gestation, number of SP intakes, number of consultations, use of bed net, and HIV status.
Statistically significant results (P < 0.05).
When we replaced the three periods of Plasmodium infection by the placental infection, we also found an influence of the infection of the placenta and of the BMI on anemia (aOR = 2.2; 95% CI: [1.3, 3.7]; P = 0.002, and aOR = 1.9; 95% CI: [1.4, 2.6]; P < 0.001).
The risk of maternal anemia also increased with the number of Plasmodium infections detected during pregnancy (table 4) and the trend test was significant (P < 0.001). Having a BMI lower than 21 was also strongly associated with maternal anemia at delivery (aOR = 1.8; 95% CI: [1.3, 2.5]; P < 0.001).
Table 4.
Effect of the number of malaria infections on maternal anemia at delivery*
| Number of malaria infection | Number of maternal anemia (%) | Univariate analysis† | Multivariate‡ analysis† | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | aOR | 95% CI | P | ||
| 0 (N = 355) | 135 (38.0) | 1 | 1 | ||||
| 1–2 (N = 243) | 105 (43.2) | 1.7 | [1.5, 2.6] | 0.007§ | 1.7 | [1.2, 2.3] | 0.003§ |
| ≥ 3 (N = 37) | 25 (67.5) | 3.0 | [1.4, 6.4] | 0.002§ | 3.1 | [1.5, 6.3] | 0.003§ |
OR = odds ratio; CI = confidence interval; aOR = adjusted odds ratio.
Reference class is Negative malaria infection.
Adjusted for parity, newborn's sex, rainy season at delivery, maternal BMI, education, duration of gestation, number of SP intakes, number of consultations, use of bed net, and HIV status.
Statistically significant results (P < 0.05).
Discussion
Our results show that in Beninese pregnant women, Plasmodium infections seem to be particularly harmful at the beginning and at the end of pregnancy.
At the beginning of pregnancy (< 4 months of gestation), the main consequences were a decrease in mean birth weight (on average 100 g) and an increase in the risk of anemia at delivery. At the end of pregnancy, peripheral infections, placental infection, were also associated with a higher risk of maternal anemia.
Materno-fetal exchanges occur through the placenta, which plays a central role in the fetal growth. Cytoadherence of parasitized red blood cells (RBC) is not possible before the placenta is formed, approximately at the 14th week of gestation. Two explanations to our results are possible. It can be considered that in our population, only Plasmodium infections occurring after the placentation were able to induce a decrease in birth weight through cytoadherence. Alternatively, as suggested by Brabin and others20 and Rogerson and others,21 an early infection could alter the process of the placentation and then directly have an influence on fetal growth.
A limited number of studies have investigated the relation between the timing of Plasmodium infection in pregnancy and its consequences for the mother and the baby.
Taha and others14 conducted a case-control hospital study and a community cohort study between 1989 and 1990 in central Sudan and showed that the risk of LBW was higher when Plasmodium infection occurred during the first trimester of gestation. More recently, in a re-analysis of a chloroquine randomized trial of malaria prophylaxis conducted in Burkina Faso in the late 1980s, Cottrell and others12 evidenced the importance of the beginning and the end of pregnancy in terms of Plasmodium infection. They found that a peripheral infection after 6 months of gestation was related to a decrease in mean birth weight and a higher risk of LBW, although there was a trend between an early maternal infection (< 4 months) and a decrease in birth weight. In a third study, Kalilani and others13 followed a cohort of pregnant women in Malawi between 2002 and 2003. They found that the risk of LBW was highest during the second trimester of gestation (13–26 weeks), and that it increased with the number of malaria episodes during the gestation. They also found that maternal anemia at delivery was only influenced by the number, but not the timing of malaria episodes.
A precise estimation of GA is important to investigate the timing of Plasmodium infection. The reference method is obstetric ultrasound, but in malaria-endemic areas, this technique is not widely available. Our study was able to afford an accurate ultrasound measure of the GA contrary to the three studies cited previously,12–14 and our results confirm, along with Cottrell and Taha's findings, that the beginning of pregnancy, when no SP can be administered to the woman, may be a high risk period for the newborn's weight. It is of importance to note that in Kalilani's study, pregnant women seen during the first trimester were discarded from the analysis because of their small number, which did not allow adequate statistical analysis. It is not uncommon in tropical areas that women are seen at their first ANV late in pregnancy. In our study, as the women's awareness had been raised by the team, more than two-thirds of them (558 of 818) consulted before 4 months of pregnancy, and we used an appropriate method (MICE) to deal with missing data related to Plasmodium infections in early pregnancy.
We did not find, as Cottrell did, that Plasmodium infection during the third trimester was associated with a decrease in mean birth weight or a higher risk of LBW. This discrepancy could be explained by the change in malaria prevention policies in pregnant women between the two studies. In 1987, the official recommendation in Burkina Faso was the prescription of a weekly chloroquine (CQ) prophylaxis, the drug being provided to the treatment group of the trial, whereas the control group was not given any preventive treatment. At that time, even if the women were made aware of the importance of the prophylaxis, there was no directly observed therapy (DOT) scheme. Therefore, it is likely that the overall protection of this population (both groups) at the end of pregnancy was not optimal, and it may explain why Plasmodium infection during the third trimester of pregnancy was a risk period for birth weight in Cottrell's analysis. In our study, IPTp with two doses of SP was currently implemented in Benin since 2004 following WHO guidelines as in the rest of sub-Saharan Africa, in the strict respect of the DOT scheme. Such policy probably ensured better protection during the end of pregnancy and prevented all possible consequences of Plasmodium infection in terms of birth weight. These findings are consistent with Kalilani's results, which also used SP IPTp with a second intake in the last part of pregnancy. In this study, the fact that Plasmodium infection during the second trimester was related to a higher risk of LBW is probably caused by a late enrollment of the women, and thus to a late intake of their first SP dose (very few women having consulted in the first trimester of gestation).
We also found that peripheral infections occurring early but also late in the course of pregnancy were related to a higher risk for maternal anemia at delivery.
MiP causes anemia through different mechanisms, the major being an important destruction of RBC by hemolysis.22 Iron and folic acid are necessary for the regeneration of RBC and the requirements of these two micronutrients increase physiologically at the end of the pregnancy.2 Folic acid and iron supplementations, as recommended by the WHO,23 suppose a daily intake until the delivery. However, similar to the unsupervised CQ administration previously recommended to protect from MiP, one can doubt the good adherence of the women to this treatment. The conjunction of the raising needs in micronutrients and the lack of compliance to the supplementation could lead to maternal anemia in case of late Plasmodium infections. The fetal growth is maximal between 20 and 30 weeks then slows down during the last month of pregnancy.1 The administration of a dose of SP IPTp during this period probably explains that the consequences of late Plasmodium infections are more marked on maternal hematological status than on birth weight.
Many studies have demonstrated that SP IPTp is more effective than CQ in reducing LBW and maternal anemia.24–29 Nevertheless in late pregnancy, as shown in our results, SP IPTp has incomplete capacity to limit consequences of MiP on maternal anemia at delivery.
Plasmodium infections at the beginning of pregnancy seem to have major effects, both in terms of birth weight and maternal anemia. Because SP IPTp can be given from the second trimester only (because of potential teratogenic effects during the first trimester) and women are seen late in pregnancy, they stay unprotected during the early period of gestation.
To fully protect the women through the whole duration of pregnancy, additional measures should be put forward, such as the use of impregnated bed nets and appropriate treatment of Plasmodium infections. An immediately available measure (which is presently proposed for low malaria transmission areas) would consist in a screening of pregnant women with RDTs, and a subsequent treatment in case of parasitemia (intermittent screening and treatment or IST). Such an early IST could improve the protection during the first trimester of gestation. However, an important limitation to this screening is the usual late attendance of women to ANVs in malarious areas. In the future, a vaccine against pregnancy-associated malaria parasites could be specifically efficacious on the early part of pregnancy, before first ANVs. It is one of the main goals of the STOPPAM project: to identify the most immunogenic epitopes of VAR2CSA (the major variable surface antigens of P. falciparum parasites infecting the pregnant women) to be included in a first generation candidate vaccine.30
ACKNOWLEDGMENTS
We are grateful to all the women who participated in the study. We thank all the medical, laboratory, and administrative staffs of Akodeha, Come central, Oudeme pedah Health centers, Hôpital de Zone, and Hôpital de la mere et de l'enfant de la lagune for their valuable contribution. We particularly thank Jacqueline Affedjou, Jean-Claude Sagbo, Marcel Ahlonsou, Bernadette Gandonou, Gbetognon Hounguevou, Clément Massenon, and Séverin Tossou-Vignibe, who were the field investigators, for their hard work and dedication to this study. We also thank Pr. René Xavier Perrin for his useful advices and help particularly for the ultrasound part of the study. We thank Franck Noumbissie for the management of the project, and John Lusingu, Adrian JF Luty, Thor Theander, and Marita Troye-Blomberg for their contribution to the design of the study.
Footnotes
Financial support: For their financial support, we thank Exxon Mobil and the GSK foundation (BTH funding).
Disclosure: This paper describes work undertaken in the context of the STOPPAM project, “Strategies To Prevent Pregnancy-Associated Malaria” (www.stoppam.org). STOPPAM is a Small and Medium Scale Collaborative Project supported by the European 7th Framework Programme, contract no.: 200889.
Authors' addresses: Bich-Tram Huynh, Nadine Fievet, Nicaise Tuikue Ndam, Philippe Deloron, and Michel Cot, Institut de Recherche pour le Développement (IRD), Mère et Enfant Face aux Infections Tropicales, Paris, France, and Faculté de Pharmacie, Université Paris Descartes, Paris, France, E-mails: bichtrambe@hotmail.com, fievet@ird.fr, nicaise.ndam@ird.fr, philippe.deloron@ird.fr, and michel.cot@ird.fr. Gildas Gbaguidi, Sébastien Dechavanne, and Sophie Borgella, Institut de Recherche pour le Développement (IRD), UMR216, Mère et Enfant Face aux Infections Tropicales, Paris, France, E-mails: gildas.gbaguidi@ird.fr, sebdechavanne@yahoo.fr, and sophie.borgella@ird.fr. Blaise Guézo Mévo, Hôpital de Comé, Comé, Bénin, E-mail: guemebla@yahoo.fr. Achille Massougbodji, Faculté des Sciences de la Santé (FSS), UER de Parasitologie, Université de Cotonou, Cotonou, Bénin, E-mail: massougbodjiachille@yahoo.fr.
References
- 1.World Health Organization . A Strategic Framework for Malaria Prevention and Control during Pregnancy in the African Region. Geneva, Switzerland: WHO; 2004. AFR/MAL/04/01. [Google Scholar]
- 2.Fleming AF. Tropical obstetrics and gynaecology. 1. Anemia in pregnancy in tropical Africa. Trans R Soc Trop Med Hyg. 1989;83:441–448. doi: 10.1016/0035-9203(89)90241-1. [DOI] [PubMed] [Google Scholar]
- 3.Bloland P, Slutsker L, Steketee RW, Wirima JJ, Heymann DL, Breman JG. Rates and risk factors for mortality during the first two years of life in rural Malawi. Am J Trop Med Hyg. 1996;55:82–86. doi: 10.4269/ajtmh.1996.55.82. [DOI] [PubMed] [Google Scholar]
- 4.Guyatt HL, Snow RW. Impact of malaria during pregnancy on low birth weight in sub-Saharan Africa. Clin Microbiol Rev. 2004;17:760–769. doi: 10.1128/CMR.17.4.760-769.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCormick MC. The contribution of low birth weight to infant mortality and childhood morbidity. N Engl J Med. 1985;312:82–90. doi: 10.1056/NEJM198501103120204. [DOI] [PubMed] [Google Scholar]
- 6.Murphy SC, Breman JG. Gaps in the childhood malaria burden in Africa: cerebral malaria, neurological sequelae, anemia, respiratory distress, hypoglycemia, and complications of pregnancy. Am J Trop Med Hyg. 2001;64:57–67. doi: 10.4269/ajtmh.2001.64.57. [DOI] [PubMed] [Google Scholar]
- 7.Steketee RW, Nahlen BL, Parise ME, Menendez C. The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg. 2001;64:28–35. doi: 10.4269/ajtmh.2001.64.28. [DOI] [PubMed] [Google Scholar]
- 8.Brabin BJ. An analysis of malaria in pregnancy in Africa. Bull World Health Organ. 1983;61:1005–1016. [PMC free article] [PubMed] [Google Scholar]
- 9.McGregor IA. Epidemiology, malaria and pregnancy. Am J Trop Med Hyg. 1984;33:517–525. doi: 10.4269/ajtmh.1984.33.517. [DOI] [PubMed] [Google Scholar]
- 10.Shulman CE, Marshall T, Dorman EK, Bulmer JN, Cutts F, Peshu N, Marsh K. Malaria in pregnancy: adverse effects on hemoglobin levels and birthweight in primigravidae and multigravidae. Trop Med Int Health. 2001;6:770–778. doi: 10.1046/j.1365-3156.2001.00786.x. [DOI] [PubMed] [Google Scholar]
- 11.Steketee RW, Wirima JJ, Hightower AW, Slutsker L, Heymann DL, Breman JG. The effect of malaria and malaria prevention in pregnancy on offspring birthweight, prematurity, and intrauterine growth retardation in rural Malawi. Am J Trop Med Hyg. 1996;55:33–41. doi: 10.4269/ajtmh.1996.55.33. [DOI] [PubMed] [Google Scholar]
- 12.Cottrell G, Mary JY, Barro D, Cot M. The importance of the period of malarial infection during pregnancy on birth weight in tropical Africa. Am J Trop Med Hyg. 2007;76:849–854. [PubMed] [Google Scholar]
- 13.Kalilani L, Mofolo I, Chaponda M, Rogerson SJ, Meshnick SR. The effect of timing and frequency of Plasmodium falciparum infection during pregnancy on the risk of low birth weight and maternal anemia. Trans R Soc Trop Med Hyg. 2010;104:416–422. doi: 10.1016/j.trstmh.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taha Tel T, Gray RH, Mohamedani AA. Malaria and low birth weight in central Sudan. Am J Epidemiol. 1993;138:318–325. doi: 10.1093/oxfordjournals.aje.a116861. [DOI] [PubMed] [Google Scholar]
- 15.Kelly-Hope LA, McKenzie FE. The multiplicity of malaria transmission: a review of entomological inoculation rate measurements and methods across sub-Saharan Africa. Malar J. 2009;8:19. doi: 10.1186/1475-2875-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ballard JL, Khoury JC, Wedig K, Wang L, Eilers-Walsman BL, Lipp R. New Ballard Score, expanded to include extremely premature infants. J Pediatr. 1991;119:417–423. doi: 10.1016/s0022-3476(05)82056-6. [DOI] [PubMed] [Google Scholar]
- 17.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37–48. [PubMed] [Google Scholar]
- 18.Hernan MA, Hernandez-Diaz S, Werler MM, Mitchell AA. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol. 2002;155:176–184. doi: 10.1093/aje/155.2.176. [DOI] [PubMed] [Google Scholar]
- 19.van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med. 1999;18:681–694. doi: 10.1002/(sici)1097-0258(19990330)18:6<681::aid-sim71>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 20.Brabin BJ, Johnson PM. Placental malaria and pre-eclampsia through the looking glass backwards? J Reprod Immunol. 2005;65:1–15. doi: 10.1016/j.jri.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Rogerson SJ, Boeuf P. New approaches to pathogenesis of malaria in pregnancy. Parasitology. 2007;134:1883–1893. doi: 10.1017/S003118200700011X. [DOI] [PubMed] [Google Scholar]
- 22.Menendez C, Fleming AF, Alonso PL. Malaria-related anemia. Parasitol Today. 2000;16:469–476. doi: 10.1016/s0169-4758(00)01774-9. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization . Pregnancy, Childbirth, Postpartum and Newborn Care: A guide for Essential Practice. Geneva, Switzerland: WHO; 2009. [PubMed] [Google Scholar]
- 24.Kayentao K, Kodio M, Newman RD, Maiga H, Doumtabe D, Ongoiba A, Coulibaly D, Keita AS, Maiga B, Mungai M, Parise ME, Doumbo O. Comparison of intermittent preventive treatment with chemoprophylaxis for the prevention of malaria during pregnancy in Mali. J Infect Dis. 2005;191:109–116. doi: 10.1086/426400. [DOI] [PubMed] [Google Scholar]
- 25.Njagi JK, Magnussen P, Estambale B, Ouma J, Mugo B. Prevention of anemia in pregnancy using insecticide-treated bed nets and sulfadoxine-pyrimethamine in a highly malarious area of Kenya: a randomized controlled trial. Trans R Soc Trop Med Hyg. 2003;97:277–282. doi: 10.1016/s0035-9203(03)90141-6. [DOI] [PubMed] [Google Scholar]
- 26.Schultz LJ, Steketee RW, Chitsulo L, Wirima JJ. Antimalarials during pregnancy: a cost-effectiveness analysis. Bull World Health Organ. 1995;73:207–214. [PMC free article] [PubMed] [Google Scholar]
- 27.Shulman CE, Dorman EK, Cutts F, Kawuondo K, Bulmer JN, Peshu N, Marsh K. Intermittent sulphadoxine-pyrimethamine to prevent severe anemia secondary to malaria in pregnancy: a randomised placebo-controlled trial. Lancet. 1999;353:632–636. doi: 10.1016/s0140-6736(98)07318-8. [DOI] [PubMed] [Google Scholar]
- 28.Sirima SB, Cotte AH, Konate A, Moran AC, Asamoa K, Bougouma EC, Diarra A, Ouedraogo A, Parise ME, Newman RD. Malaria prevention during pregnancy: assessing the disease burden one year after implementing a program of intermittent preventive treatment in Koupela District, Burkina Faso. Am J Trop Med Hyg. 2006;75:205–211. [PubMed] [Google Scholar]
- 29.van Eijk AM, Ayisi JG, ter Kuile FO, Otieno JA, Misore AO, Odondi JO, Rosen DH, Kager PA, Steketee RW, Nahlen BL. Effectiveness of intermittent preventive treatment with sulphadoxine-pyrimethamine for control of malaria in pregnancy in western Kenya: a hospital-based study. Trop Med Int Health. 2004;9:351–360. doi: 10.1111/j.1365-3156.2004.01196.x. [DOI] [PubMed] [Google Scholar]
- 30.Tuikue Ndam N, Deloron P. Towards a vaccine against pregnancy-associated malaria. Parasite. 2008;15:515–521. doi: 10.1051/parasite/2008153515. [DOI] [PubMed] [Google Scholar]


