Abstract
A multiplex bead assay (MBA) was used to analyze serum samples collected longitudinally from children enrolled in a drug trial for treatment of filariasis in Leogane, Haiti. Recombinant antigens Bm14 and Bm33 from Brugia malayi, third polar tube protein (PTP3) from Encephalitozoon cuniculi, and merozoite surface protein-119 (MSP-119) from Plasmodium falciparum were coupled to carboxylated polystyrene microspheres. IgG responses to PTP3 and MSP-119 were not affected by albendazole (ALB), diethylcarbamazine (DEC), or combination of diethylcarbamazine and albendazole (DEC/ALB). However, IgG and IgG4 responses to Bm14 and Bm33 were significantly decreased (P < 0.001) by DEC and DEC/ALB treatment. Antibody responses to Bm14 and Bm33 decreased after DEC treatment (but not placebo) among children who were negative for microfilaremia and antigenemia at baseline, suggesting that these children harbored early stages of infection. The MBA is an excellent serologic technique for multiple antigens that offers substantial advantages over single-antigen based enzyme-linked immunosorbent assay in mass drug administration studies for monitoring changes in antibody levels.
Introduction
Numerous multiplex bead assays (MBAs) have been used to detect or quantify analytes in serum, culture supernatants, oral fluids, or other biological fluids from humans or animals.1–10 The popularity of the MBA is the result of 1) the relative ease of covalently coupling of an analyte-capture ligand to spectrally classified carboxylated microspheres; 2) the simultaneous collection of multiple data points from a single specimen, eliminating the one-data point per well enzyme-linked immunosorbent assay (ELISA); 3) the direct proportionality of the fluorescence intensity of the reporter fluorochrome to the amount of captured analyte; and 4) the use of a 96-well format with up to 100 differently classified beads per well, which yield a potential of 9,600 data points per plate. These attractive features of the MBA conserve specimens and save on labor, time, and cost when compared with the ELISA format. Furthermore, the MBA has been shown to be at least as sensitive as the ELISA.8,11
Thus far, the MBA has not been used to determine specific immunoglobulin antibody levels in humans infected with Wuchereria bancrofti, Brugia malayi, or Brugia timori, mosquito-transmitted nematodes that can cause lymphatic filariasis, which infects more than 120 million persons in more than 83 tropical and sub-tropical countries.12,13 Currently, the effectiveness of lymphatic filariasis mass drug administration (MDA) programs is determined by decreased levels of microfilaremia (MF), antigenemia, and antibody responses to various B. malayi antigens.14–16 For monitoring MDA programs, microfilaremia, antigenemia, and antibody levels provide measures of program impact. Antibody levels to relevant antigens can be assessed quantitatively by ELISA. Fortunately, for the evaluation of MDA success, antifilarial antibodies decrease after filarial antigens decrease, generally approximately 6–8 weeks post-drug administration, which is unlike some parasitic infections, such as schistosomiasis, in which antibody responses are not useful in differentiating past and present infections.17 Although the ELISA has provided useful information, it is not only expensive and laborious but requires relatively large volumes of serum or plasma to detect antibodies against multiple antigens. The need for improved serologic test for filariasis has been noted.18,19
We used MBA to measure antibody levels in serum specimens collected longitudinally from a subset of children in Haiti who were enrolled in a large, single-dose, placebo-controlled drug study that was initiated in October 1998 and monitored through May 1999.20 The MBA was originally a 23-plex bead assay consisting of recombinant antigens from blood-borne and enteric-borne protozoans and helminths, which is too large to describe in this report. In this study, Bm14 and Bm33 from B. malayi, merozoite surface protein-119 (MSP-119) from Plasmodium falciparum, third polar tube protein (PTP3) from Encephalitozoon cuniculi, and glutathione-S-transfersase (GST) from Schistosoma japonicum were coupled to beads and used for the detection of IgG. In addition, Bm14- and Bm33-coupled beads were used for the detection of IgG4.
Materials and Methods
Study population and design.
The study population and design have been described.20 The original study was reviewed and approved by the Centers for Disease Control and Prevention Institutional Review Board and by the Ethics Committee of Hôpital Sainte Croix, Leogane, Haiti. Briefly, our subset of 148 children, each with complete set of serum samples obtained at time points A, B, and C (described below) were 5–11 years of age at enrollment and were from the original study of 1,292 children who lived in Leogane, Haiti, a coastal town with a population of 10,000–15,000. After enrollment in October 1998, a finger prick blood specimen was collected between 7:30 pm and 9:30 pm for W. bancrofti MF density using a 20-μL thick– blood smear, and this was repeated in February and May 1999. These collections were designated time points A, B, and C. Upon enrollment and collection of pretreatment specimens, each child randomly received a single dose of a placebo, albendazole (ALB), diethylcarbamazine (DEC), or combined DEC/ALB as described.20 At the end of the study in May 1999, those children who had received placebo or ALB alone were treated with DEC and ALB, and those children who received the DEC/ALB combination or DEC alone received ALB.
For the 148 children, levels of IgG responses to the five antigens were determined. Of the 148 children, sufficient beads were available for a subset of 95 children who were used to determined IgG4 levels to Bm14 and Bm33 antigens. For antigenemia, the Og4C3 assay (James Cook University Tropical Biotechnology Pty. Ltd., Townsville, Queensland, Australia) was used at time points A and C as described.20
Recombinant antigen preparation and purification.
A recombinant B. malayi Bm14 antigen fused with six histidines (His6) was provided by the National Institutes of Health/National Institute of Allergy and Infectious Diseases Filariasis Research Reagent Repository Center (FR3), Molecular Resources Division (Smith College, Northampton, MA). Bm33 from B. malayi fused with His6 and GST was cloned, expressed, and purified as described below. The cloned DNA sequence included amino acids 18–224 of the B. malayi pepsin inhibitor analog protein (Bm33; GenBank accession no. L11001; the amino terminal signal peptide and nine hydrophobic residues at the carboxy terminus were excluded).21 The DNA sequence from an adult female worm cDNA library in Lambda Uni-Zap XR obtained from Smith College (Northampton, MA) was amplified by using a polymerase chain reaction.
The following deoxyoligonucleotide pair enabled directional cloning into the BamHI and EcoRI restriction sites of a modified pGEX 4T-2 plasmid vector (GE Healthcare, Piscataway, NJ): 5′-CGC GGA TCC GGT ATA GTG AAA AGG TAT AAC-3′ and 5′-GCG GAA TTC CTT CCG GTG CTT CAA CTG GCA C-3′. The plasmid had been modified by the insertion of the sequence 5′-GGA TCG AAG GTC GTC ACC ATC ACC ATC ACC ATT AA-3′ between the EcoRI and XhoI plasmid restriction sites so that a His6 tag and Factor Xa cleavage site would be added to the carboxy terminus of the GST fusion protein. AmpliTaq gold DNA polymerase was used as directed by the manufacturer (Perkin-Elmer Cetus, Foster City, CA). The resulting GST- and His6-tagged recombinant Bm33 protein was expressed in BL-21 Gold Escherichia coli cells (Stratagene, La Jolla, CA) and initially purified on a GST affinity column as directed by the manufacturer (GE Healthcare).
Glutathione-eluted proteins were precipitated by addition of ammonium sulfate to 55% saturation, and collected by centrifugation at 17,500 × g for 15 minutes. The protein pellet was dissolved in buffer containing 25 mM Na2PO4, pH 8.0, 1 M NaCl, and 2 M urea and clarified by centrifugation at 17,500 × g for 15 minutes. Final purification was accomplished by nickel affinity chromatography as directed by the manufacturer (HiTrap Chelating HP 1 mL column; GE Healthcare). Protein fractions containing the recombinant Bm33 fusion protein were combined and passed over a 5-mL desalting column (HiTrap; GE Healthcare) in buffer containing 25 mM 2-(N-morpholino) ethanesulfonic acid (MES), pH 6.0, 0.2 M NaCl, and 2 M urea.
Recombinant MSP-119 from P. falciparum (isolate 3D7) fused with GST was obtained from the Zentrum für Molekulare Biologie der Universität Heidelberg, Universität Heidelberg (Heidelberg, Germany) and has been described.22 Preparation and purification of GST protein and the cleaved PTP3 from E. cuniculi (with no GST) have been described.10,23
Antigen coupling to beads.
Unless stated, all reagents were obtained from Sigma Chemical Co. (St. Louis, MO). Antigen coupling to 5.6-μm polystyrene microspheres (SeroMap Beads; Luminex Corporation, Austin, TX) has been described.11 Briefly, the carboxyl groups on each spectrally classified bead were chemically modified to an ester by using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (Pierce, Rockford, IL). The reaction of esters on the beads with primary amine groups on the antigens resulted in an amide covalent bond between bead and antigen. All antigens were soluble and coupled in phosphate-buffered saline (PBS), pH 7.2, except for Bm33, which required 25 mM MES, pH 6.0, 2 M urea, 0.2 M NaCl. Coupling efficiency was determined by using serum known to be highly reactive with the antigens, and 120 μg of antigen per 12.5 × 106 beads resulted in high fluorescence intensity by the reporter fluorochrome. The exception was Bm14/His6, which required 140 μg. After coupling, the beads were quantified by using a hemacytometer and stored at 4°C in PBS, 1.0% bovine serum albumin (BSA), 0.05% polyoxyethylenesorbitan monolaurate (Tween 20), 0.02% sodium azide. For each milliliter of bead suspension, the following protease inhibitors were included: 200 μg of pefabloc (4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride) (Roche Diagnostics, Indianapolis, IL), 200 μg EDTA, and 1 μg each of leupeptin and pepstatin A.
Multiplex bead assay.
A 1:200 dilution of serum in PBS containing 0.5% BSA, 0.05% Tween 20, 0.02% sodium azide, 0.5% polyvinyl alcohol (PVA), and 0.8% polyvinylpyrrolidone (PVP), designated PBN1, was incubated for 1 hour at 37°C and stored overnight at 4°C. The PVA and PVP reduced background without loss of sensitivity.24 We also included in the serum dilution 0.5% (v/v) E. coli crude extract suspension (concentration = 0.6 mg protein/mL) to absorb antibodies reactive to any residual E. coli proteins that may not have been eliminated in the antigen purification process. After centrifugation at 16,000 × g for 5 minutes, 25 μL of diluted and clarified serum was added to 25 μL of PBN1 containing 2,500 beads from each spectrally classified bead in each well in a 96-well filtered-bottom plate (Millipore, Bedford, MA) to yield a final 1:400 serum dilution. All serum samples were assayed in duplicate. The beads were suspended and gently shaken for 45 minutes at room temperature. Each well was washed three times with 100 μL of PBS, 0.05% Tween 20 (PBST) by using a vacuum device for filtered-bottom, 96-well plates (Millipore).
For total IgG detection, 50 μL of PBS containing 0.5% BSA, 0.05% Tween 20, 0.02% sodium azide, designated PBN2, and containing 50 ng of biotinylated mouse anti-human IgG (clone H2; Southern Biotech, Birmingham, AL) and 40 ng of biotinylated mouse anti-human IgG4 (clone HP6025; Invitrogen, South San Francisco, CA) was added to each well. Although clone H2 provided low background, it did not react well with human IgG4. For IgG4 detection alone, the solutions and concentrations were the same except that anti-human IgG was omitted. The beads were suspended and gently shaken for 45 minutes at room temperature and washed three times with PBST as described above. To each well, 50 μL of PBN2 containing 250 ng of streptavidin r-phycoerythrin (Invitrogen, Eugene, OR) was added, and the beads were suspended and gently shaken for 30 minutes at room temperature. After the beads were washed three times with PBST as described above, 50 μL of PBN2 was added to each well and the suspension was gently shaken for 30 minutes at room temperature. This step removed loosely bound antibodies, resulting in minimal variation in positive and negative controls between plates. The beads were then washed once with PBST and suspended in 125 μL of PBS per well. Data were acquired by using a reader (Luminex Corporation) equipped with Bio-Plex Manager 4.1 software (Bio-Rad, Hercules, CA).
Each parameter in the two-laser flow-base Luminex system has detectors with a dynamic range of more than four-log decades (channels ranging from 1 to 32,766, the possible fluorescence values without background subtracted) for placement of specific emitted light at variable intensities. The red laser excites the red and infra-red fluorochromes incorporated at different ratios within the beads, and the two emitted lights identify the bead classification. This laser also provides a light-scatter parameter that enables gated data acquisition on at least 100 monodispersed beads of each classification and excludes small and large debris and any clumped beads. The green laser excites r-phycoerythrin, the reporter fluorochrome, attached indirectly and externally to the beads, and the amount of light emitted is directly proportional to the amount of analyte bound to the beads. The mean from duplicate wells was reported as the median fluorescence intensity (MFI). For IgG, background fluorescence from a blank on each plate was subtracted from MFI (MFI-bg). For IgG4, MFI with no subtracted background was used to avoid negative numbers for some children. A positive control serum was diluted to yield mid-range fluorescence intensity and was used on each plate along with a negative control.
Establishment of cutoff.
Serum specimens from 67 adult citizens from the United States who had not traveled outside the United States were used to determine cutoffs to all antigen-coupled beads. For each antigen, the three highest responses from the 67 persons were eliminated, and the remaining responses were used to establish a mean plus 3 SD as the cutoff.
Semi-quantification of IgG4 responses to Bm14 and Bm33.
Of the 148 children, sufficient beads were available for 95 children for IgG4 detection to Bm14 and Bm33. Of the 95 children, 84 had MF and Og4C3 data at time point A; 54 of 84 children were MF and Og4C3 negative (MF–/Og4C3–), 21/84 were MF–/Og4C3+, and 9 of 84 were MF+/Og4C3+. Time point A serum samples from these 84 children were used to estimate the proportion of total IgG responses to Bm14 and Bm33 that was represented by IgG4. The MFI values of the IgG4 responses were divided by MFI-bg values of the IgG responses and multiplied by 100. Any percentage greater than 100 was assigned 100.
Elution of IgG4 from Bm14- and Bm33-coupled beads.
Approximately 80,000 beads coupled with Bm14 or Bm33 antigens were suspended in 500 μL of PBN1 containing 2.5 μL of serum specimen and incubated for 1 hour at room temperature on a rotator. For each serum, the bead-antibody suspension was transferred to two wells in a 96-well filtered-bottom plate and vacuum filtered. After washing each well six times with 100 μL of PBST, beads in each well were suspended in 600 μL of Gentle Ag/Ab elution buffer (Pierce) and pooled. The bead suspension was centrifuged at 16,000 × g for 5 minutes, and the supernatant was transferred to a concentration/dialysis device (Centricon 30; Millipore). After addition of 0.8 mL 1.0 M Tris-HCl, pH 8.0, 0.3 M NaCl (THS), the device was centrifuged at 6,000 rpm (SS-34 rotor, RC5 centrifuge; Sorvall, Miami, FL) until all solution passed through the device except for approximately 50 μL. An additional 2 mL of THS and 4 mL of PBS was then passed though the device. The concentrated dialysate, approximately 50 μL, was added to an equal volume of PBS, 1.0% BSA, 0.1% Tween 20, 0.02% sodium azide, 1.0% PVA, 1.6% PVP and stored at 4°C until exposure to coupled beads and data acquisition by MBA.
Statistical analysis.
The coefficient of variation was determined from positive controls that were diluted to produce moderate fluorescence activity on each plate. For comparison of prevalence of IgG4 and IgG responses to the antigens between time points, the z-test was used. The Tukey test was used to compare levels of IgG4 or IgG responses across the three time points within groups. The Mann-Whitney rank sum test and the Kruskal-Wallis test were used across two groups and across more than two groups, respectively. The Wilcoxon signed-rank test was used to determine differences in median Og4C3 values between time points A and C. The Spearman rank correlation was used (data on all children at time point A, plus children treated with placebo at B and C) to investigate the association between IgG4 and IgG responses to Bm14 and Bm33. P < 0.05 was considered to be statistically significant.
Results
MBA controls and cutoffs.
Median and range of MFI and MFI-bg on each antigen and isotype detected are shown in Table 1 along with cutoff values. From all plates, the coefficient of variation for the positive controls, diluted to yield moderate reactivity, was less than 10%, and all negative controls were consistently below the cutoff values (Table 1). Beads coupled with GST showed no appreciable IgG responses (mean = 0.40 MFI-bg, range, –14 to 150 MFI-bg, n = 148) or IgG4 responses (mean = 42 MFI, range = 32–73 MFI, n = 95).
Table 1.
Antigen, isotype detected, cutoff, median, and range of MFI and MFI-bg for each antigen tested, Haiti*
| Antigen | Isotype | Cutoff | Median (range) of fluorescence intensities at time point A† |
|---|---|---|---|
| Bm14 | IgG | 134 | 18,854 (2–31,687) |
| Bm14 | IgG4 | 48 | 1,255 (40–32,289)‡ |
| Bm33 | IgG | 597 | 19,851 (13–30,600) |
| Bm33 | IgG4 | 105 | 127 (43–24,106) ‡ |
| PTP3 | IgG | 204 | 47 (5–25,988) |
| MSP-119 | IgG | 42 | 10 (–9 to 23,874) |
MFI = median fluorescence intensity; bg = background; PTP3 = third polar tube protein; MSP-119 = merozoite surface protein-119.
Possible MFI range is 1–32,766, and possible MFI-bg range can be a negative number to 32,766.
MFI data (IgG4) for 95 children. All other data are MFI-bg data (IgG) for 148 children.
Cutoff values were established for samples from 67 adult U.S. citizens. These donors had no history of foreign travel, and they were not likely to have been infected with or exposed to either filariasis or malaria because cutoffs for those antibody responses were less than 600 MFI-bg (Table 1). Similarly, E. cuniculi is not a common parasite in the general U. S. population; only 4.2% of 240 persons were considered antibody positive to PTP3-coated ELISA plates (Kucerova Z, unpublished data). In our study, the cutoff for PTP3 for U. S. citizen donors was only 204 MFI-bg (Table 1).
IgG responses to PTP3 and MSP-119.
As expected, prevalence of positive IgG responses to PTP3 and MSP-119 at time points A, B, and C were low and stable throughout the study. On the basis of the cutoff (Table 1) for IgG positivity to PTP3 (PTP3/IgG+), the prevalence was 12% at A, 11% at B, and 16% at C. Similar to PTP3, the cutoff (Table 1) for IgG positivity to MSP-119 (MSP-119/IgG+) yielded a prevalence of 19% at A, 18% at B, and 18% at C.
Prevalence of IgG4 and IgG responses to Bm14 and Bm33, MF, and Og4C3.
Prevalence of MF+, Og4C3+, and positive IgG4 and IgG antibody responses to Bm14 and Bm33 for time points A, B, and C in all treatment groups are shown in Table 2. For time points A, B, and C, prevalence of IgG4 and IgG responses to Bm14 and Bm33 decreased in children who received DEC or DEC/ALB but was only significant for Bm33/IgG4+ (P < 0.043) at time points B and C. Children who received placebo or ALB alone remained unchanged.
Table 2.
Prevalence of positive MF, Og4C3, IgG4, and IgG responses to Bm14 and Bm33 at time points A, B, and C for all treatment groups, Haiti*
| Observation | % Positive (no. positive/no. tested) in each treatment group | |||
|---|---|---|---|---|
| Placebo | ALB | DEC | DEC/ALB | |
| Pretreatment (time point A) | ||||
| Bm14 IgG | 73 (30/41) | 86 (31/36) | 72 (31/43) | 89 (25/28) |
| Bm33 IgG | 88 (36/41) | 94 (34/36) | 95 (41/43) | 93 (26/28) |
| Bm14 IgG4 | 82 (18/22) | 79 (19/24) | 67 (16/24) | 88 (22/25) |
| Bm33 IgG4 | 59 (13/22) | 58 (14/24) | 67 (16/24) | 60 (15/25) |
| Og4C3 | 44 (18/41) | 36 (13/36) | 28 (12/43) | 32 (9/28) |
| MF | 22 (10/37) | 12 (6/34) | 15 (6/39) | 4 (1/24) |
| Three months post-treatment (time point B) | ||||
| Bm14 IgG | 76 (31/41) | 83 (30/36) | 67 (29/43) | 86 (24/28) |
| Bm33 IgG | 88 (36/41) | 100 (36/36) | 88 (38/43) | 93 (26/28) |
| Bm14 IgG4 | 82 (18/22) | 79 (19/24) | 58 (14/24) | 84 (21/25) |
| Bm33 IgG4 | 59 (13/22) | 54 (13/24) | 46 (11/24)† | 36 (9/25) |
| Og4C3‡ | ||||
| MF | 24 (7/29) | 23 (5/22) | 14 (4/28) | 0 (0/19) |
| Six months post-treatment (time point C) | ||||
| Bm14/IgG | 78 (32/41) | 89 (32/36) | 65 (28/43) | 86 (24/28) |
| Bm33/IgG | 93 (38/41) | 100 (36/36) | 86 (37/41) | 89 (25/28) |
| Bm14/IgG4 | 82 (18/22) | 83 (20/24) | 54 (13/24) | 76 (19/25) |
| Bm33/IgG4 | 64 (14/22) | 54 (13/24) | 29 (7/24)† | 36 (9/25) |
| Og4C3 | 41 (17/41) | 33 (12/36) | 23 (10/43) | 32 (9/28) |
| MF | 24 (6/25) | 17 (4/24) | 14 (4/28) | 0 (0/17) |
MF = microfilaremia; ALB = albendazole; DEC = diethylcarbamazine.
Prevalence significantly lower (P < 0.043) than time point A (by z test).
Not tested.
For time points A, B, and C, prevalence of MF+ children did not increase in children who received DEC or DEC/ALB, and prevalence of MF+ children did show an increase, which was statistically insignificant, in children who received ALB alone or placebo (Table 2).
Prevalence of Og4C3+ children did not show a significant decrease in any of the treatment groups (Table 2). However, the median Og4C3 values were significantly lower (P < 0.001) at the end of the study (A: median = 17, mean = 2,263 and C: median = 15.0, mean = 639) for children who received DEC. The median Og4C3 values approached significance (P = 0.052) for children who received DEC/ALB (A: median = 19.0, mean = 1,060 and C: median = 18.5, mean = 433). In contrast, the median Og4C3 values for children who received placebo or ALB alone showed no significant decrease. Children who received placebo showed a significant increase (P = 0.039) (A: median = 22.0, mean = 3,086 and C: median = 33.0, mean = 3,713).
IgG responses to all antigens among 148 children.
At time point A, there were no significant differences in the median IgG levels to Bm14 and Bm33 among the four treatment groups, but as the study progressed, high levels of IgG responses to Bm14 and Bm33 decreased in the DEC and DEC/ALB groups (Table 3). Compared with time point A and within treatment groups, levels of IgG responses to Bm14 and Bm33 were significantly decreased (P < 0.001) at time points B and C in children who received DEC or DEC/ALB, but no significant differences were observed at time points A, B, and C in children who received placebo or ALB alone. Compared with children who received placebo or ALB alone, IgG responses to Bm14 and Bm33 were significantly lower at time point C (P < 0.005) in children who received DEC or DEC/ALB.
Table 3.
Median IgG responses (MFI-bg) to Bm14, Bm33, PTP3, and MSP-119 from treatment groups in 148 children at time points A, B, and C, Haiti*
| Observation | Treatment group (no.) | |||
|---|---|---|---|---|
| Placebo (41) | ALB (36) | DEC (43) | DEC/ALB (28) | |
| Pretreatment (time point A) | ||||
| Bm14/IgG | 20,893 | 25,333 | 6,755 | 16,747 |
| Bm33/IgG | 15,905 | 20,596 | 15,555 | 20,991 |
| PTP3/IgG | 46 | 62 | 51 | 37 |
| MSP-119/IgG | 8 | 10 | 12 | 10 |
| Three months post-treatment (time point B) | ||||
| Bm14/IgG | 23,467 | 17,560 | 2,099† | 4,996† |
| Bm33/IgG | 16,077 | 18,325 | 8,894† | 8,587† |
| PTP3/IgG | 36 | 59 | 41 | 33 |
| MSP-119/IgG | 8 | 10 | 9‡ | 7 |
| Six months post-treatment (time point C) | ||||
| Bm14/IgG | 24,135 | 16,600 | 1,650†§ | 1,508† |
| Bm33/IgG | 13,000 | 16,882 | 5,166†¶# | 7,533†¶ |
| PTP3/IgG | 49 | 55 | 51 | 31 |
| MSP-119/IgG | 7 | 10 | 12 | 8 |
MFI = median fluorescence intensity; bg = background; ALB = albendazole; DEC = diethylcarbamazine.
Median values (MFI-bg, IgG responses) significantly lower (P < 0.005) compared with time point A in same treatment group (by Tukey test).
Median values (MFI-bg, IgG responses) significantly lower (P = 0.031) compared with time point A in same treatment group (by Tukey test).
Median values (MFI-bg, IgG responses) significantly lower (P < 0.005) compared with albendazole and placebo children at the same time point (by Kruskal-Wallis test).
Median values (MFI-bg, IgG responses) significantly lower (P < 0.001) compared with albendazole children at the same time point (by Kruskal-Wallis test).
Median values (MFI-bg, IgG responses) significantly lower (P < 0.001) compared with placebo children at the same time point (by Kruskal-Wallis test).
Median IgG levels to PTP3 and MSP-119, which were low, showed no significant differences among the four treatment groups except for MSP-119, which was significantly lower at time point B (P = 0.031) compared with time point A, but was not significantly different at time point C (Table 3).
IgG4 responses to Bm14 and Bm33 among 95 children.
Levels of IgG4 responses to Bm14 and Bm33 are shown in Table 4. Although the range of fluorescence intensities of IgG4 to Bm14 and Bm33 was comparable to those of IgG (Table 1), the levels of IgG4 responses to Bm14 and Bm33 were considerably lower (Table 4). Furthermore, the levels of IgG4 responses to Bm14 and Bm33 were significantly lower (P < 0.001) at time points B and C than at time point A in children who received DEC or DEC/ALB. There was a significant decrease (P < 0.022) in levels of IgG4 responses to Bm14 and Bm33 at time point C than at time point A in children who received ALB alone. Also, compared with time point A, there was a significant decrease (P = 0.013) in IgG4 responses to Bm14 at time point C in children who received placebo.
Table 4.
Median IgG4 responses (MFI) to Bm14 and Bm33 from treatment groups in 95 children at time points A, B, and C, Haiti*
| Observation (antigen and isotype responses) | Treatment group (no.) | |||
|---|---|---|---|---|
| Placebo (22) | ALB (24) | DEC (24) | DEC/ALB (25) | |
| Pretreatment (time point A) | ||||
| Bm14/IgG4 | 2,095 | 7,153 | 457 | 708 |
| Bm33/IgG4 | 163 | 117 | 122 | 133 |
| Three months post-treatment (time point B) | ||||
| Bm14/IgG4 | 1,449 | 764 | 79† | 163† |
| Bm33/IgG4 | 164 | 107 | 98† | 79† |
| Six months post-treatment (time point C) | ||||
| Bm14/IgG4 | 721‡ | 703‡ | 73† | 77† |
| Bm33/IgG4 | 121 | 115‡ | 76† | 73† |
MFI = median fluorescence intensity; bg = background; ALB = albendazole; DEC = diethylcarbamazine.
Median values (MFI, IgG4 responses) significantly lower (P < 0.001) compared with time point A in the same treatment group (by Tukey test).
Median values (MFI, IgG4 responses) significantly lower (P < 0.022) compared with time point A in the same treatment group (by Tukey test).
IgG4 and IgG responses to Bm14 and Bm33 among children in different infection groups.
Because of the small numbers of children who were MF+/Og4C3+ and that DEC was the most effective drug against filariasis, children who did not receive DEC (placebo and ALB treated) were compared with children who received DEC (DEC and DEC/ALB treated) as shown in Figures 1–3.
Figure 1.
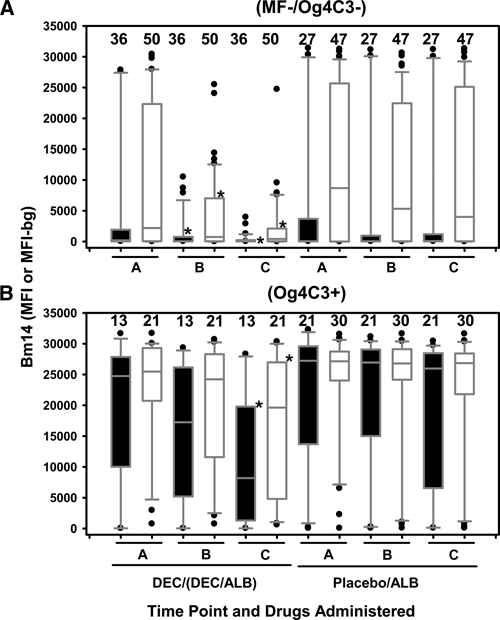
IgG4 and IgG responses to Bm14 for children of different infection groups, Haiti. Closed and opened box and whisker plots represent IgG4 responses from 95 children and IgG responses from 148 children, respectively. On the left are time points A, B, and C for children who received diethylcarbamazine (DEC) or DEC/albendazole (ALB) and on the right are time points A, B, and C for children who received placebo or ALB alone. A, Negative microfilaremia and antigenemia (MF–/Og4C3–) children. B, Positive antigenemia (Og4C3+) children, who include 37% (19 of 52) positive for MF. *Denotes significantly lower levels of IgG4 or IgG responses to Bm14 at time points B or C compared with time point A in the same treatment group and same infection status. Horizontal bars in box: low, 25%; middle, median; upper, 75%. Low whisker, 5%; upper whisker, 95%. Outliers are indicated by closed circles. The number of children is listed above the box.
Figure 2.
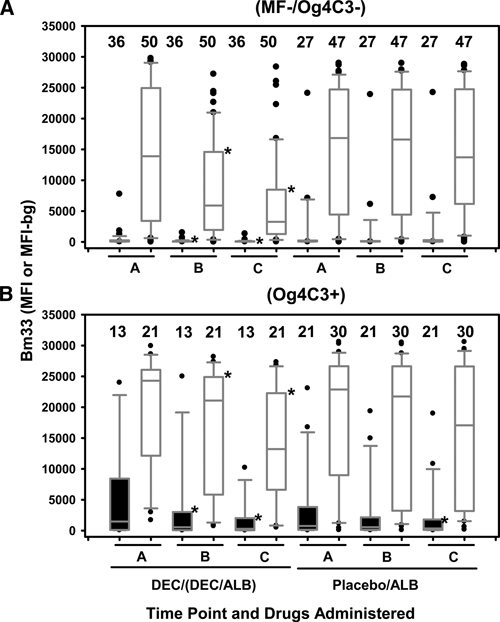
IgG4 and IgG responses to Bm33 for children of different infection groups, Haiti. Closed and opened box and whisker plots represent IgG4 responses from 95 children and IgG responses from 148 children, respectively. On the left are time points A, B, and C for children who received diethylcarbamazine (DEC) or DEC/albendazole (ALB) and on the right are time points A, B, and C on children who received placebo or ALB alone. A, Negative microfilaremia and antigenemia (MF–/Og4C3–) children; B, Positive antigenemia (Og4C3+) children, who include 37% (19 of 52) positive for MF. *Denotes significantly lower levels of IgG4 or IgG responses to Bm33 at time points B or C compared with time point A in the same treatment group and same infection status. Horizontal bars in box: low, 25%; middle, median; upper, 75%. Low whisker, 5%; upper whisker, 95%. Outliers are indicated by closed circles. The number of children is listed above the box.
Figure 3.
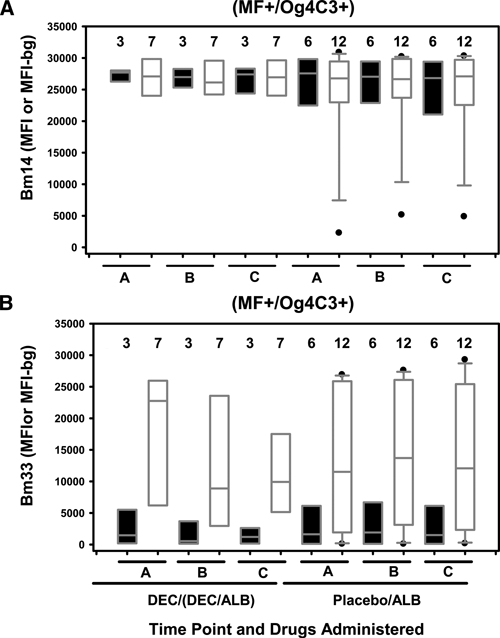
IgG4 and IgG responses to Bm14 and Bm33 for children positive for microfilaremia and antigenemia (MF+/Og4C3+), Haiti. A total of 9 MF+/Og4C3+ children (3 treated with diethylcarbamazine [DEC] or DEC/albendazole [ALB] and 6 treated with placebo or ALB) were tested for IgG4 responses, and a total of 19 MF+/Og4C3 + children (7 treated with DEC or DEC/ALB and 12 treated with placebo or ALB) were tested for IgG responses. Closed and opened box and whisker plots represent IgG4 and IgG responses, respectively. On the left are time points A, B, and C for children who received DEC or DEC/ALB and on the right are time points A, B, and C for children who received placebo or ALB alone. A, IgG4 and IgG responses to Bm14. B, IgG4 and IgG responses to Bm33. Horizontal bars in box: low, 25%; middle, median; upper, 75%. Low whisker, 5%; upper whisker, 95%. Outliers are indicated by closed circles. The number of children is listed above the box. Boxes with no whiskers are less than nine children.
We identified drug effects on levels of IgG4 and IgG responses to Bm14 at time points A, B, and C in children who were categorized as MF–/Og4C3– (Figure 1A) or Og4C3+ (Figure 1B) at time point A. Of all MF–/Og4C3– children at time point A, 67% (36 of 54) and 66% (56 of 85) were Bm14/IgG4+ and Bm14/IgG+, respectively. Compared with time point A, levels of IgG4 and IgG responses to Bm14 in MF–/Og4C3– children who received DEC or DEC/ALB (Figure 1A) were significantly lower (P < 0.001) at time points B and C. No significant changes in levels of IgG4 and IgG responses to Bm14 were observed in MF–/Og4C3– children who received placebo or ALB alone (Figure 1A).
Of all Og4C3+ children at time point A (Figure 1B), including 19 children (37%) who were MF+, 97% (32 of 33) and 98% (51 of 52) were Bm14/IgG4+ and Bm14/IgG+, respectively, which was significantly higher (P < 0.034) in prevalence than MF−/Og4C3- children (Figure 1A). Levels of IgG4 to Bm14 in Og4C3+ children (Figure 1B) were increased compared with those in MF–/Og4C3– children (Figure 1A). Compared with time point A, decreased levels of IgG4 and IgG responses to Bm14 were observed in the Og4C3+ children (Figure 1B) who received DEC or DEC/ALB, but this was significant (P < 0.001) only at time point C. However, these changes were not observed in Og4C3+ children who received placebo or ALB alone (Figure 1B).
Drug effects on levels of IgG4 and IgG responses to Bm33 at time points A, B, and C for children who were categorized as MF-/Og4C3- or Og4C3+ at time point A are shown in Figure 2. Of all MF–/Og4C3– children at time point A, 44% (24 of 54) and 89% (76 of 85) were Bm33/IgG4+ and Bm33/IgG+, respectively. Levels of IgG4 and IgG responses to Bm33 in MF–/Og4C3– children who received DEC or DEC/ALB (Figure 2A) were significantly lower (P < 0.001) at time points B and C, but not among antigen-negative children who received placebo or ALB alone (Figure 2A).
Of all Og4C3+ children at time point A, including 19 children (37%) who were MF+, 88% (29/33) and 98% (51/52) were Bm33/IgG4+ and Bm33/IgG+, respectively, which for Bm33/IgG4+ was significantly higher (P < 0.001) in prevalence than Bm33/IgG4+ in MF–/Og4C3– children. Decreased levels of IgG4 and IgG responses to Bm33 were observed in the Og4C3+ children (Figure 2B) who received DEC or DEC/ALB (P < 0.001) at time points B and C. No significant changes in the levels of IgG responses to Bm33 were observed at time points A, B, and C in Og4C3+ children who received placebo or ALB alone (Figure 2B). However, IgG4 levels to Bm33 at time point C were significantly lower (P = 0.002) than time point A in children who received placebo or ALB alone (Figure 2B).
Drug effects on levels of IgG4 and IgG responses to Bm14 and Bm33 at time points A, B, and C among children who were MF+/Og4C3+ are shown in Figure 3. Although the number of children was small, levels of IgG4 and IgG responses to Bm14 in MF+/Og4C3+ children (Figure 3A) remained relatively high and showed no significant change throughout the study independent of which drug was received. Levels of IgG4 and IgG responses to Bm33 in MF+/Og4C3+ children (Figure 3B) were relatively low and moderate, respectively, but remained increased and showed no significant change throughout the study.
Significant and positive correlations of IgG4 and IgG responses to Bm14 and Bm33 were observed. The strongest correlation was between IgG4 and IgG responses to Bm14 (r = 0.842, P < 0.001). A weaker correlation was observed between IgG4 and IgG responses to Bm33 (r = 0.553, P < 0.001).
Testing cross-reactivity of IgG4 to Bm14 and Bm33.
Of 95 children, 24 had IgG4 responses to Bm14 and Bm33, raising the question of cross-reactivity. For three children, each with serum samples at time points A, B, and C, IgG4 bound to Bm33 beads were eluted and exposed again to Bm14- and Bm33-coupled beads. Eluted IgG4 from Bm33-coupled beads reacted with fresh Bm33-coupled beads but did not react with fresh Bm14-coupled beads and did not show any cross-reactivity. The reverse effect was tested for one child, for whom eluted IgG4 from Bm14 reacted with fresh Bm14-coupled beads but did not react with fresh Bm33-coupled beads. Samples from this child also did not show any cross reactivity.
Semi-quantification of IgG4 responses to Bm14 and Bm33.
Serum samples from time point A from 84 of 95 children were used to roughly estimate the percentage of IgG4 levels in total IgG levels to Bm14 and Bm33. For 54 MF–/Og4C3–, 21 MF–/Og4C3+, and 9 MF+/Og4C3+ children, the mean percentage levels of IgG4 to Bm14 were 55%, 81%, and 93% respectively, and the mean percent levels of IgG4 to Bm33 were 11%, 18%, and 16%, respectively.
Discussion
The MBA provides a robust approach to the simultaneous analysis of antibody responses to multiple antigens. In the public health context, this assay platform has the potential to generate an epidemiologic snapshot of community exposures to infections of interest. Such a tool is valuable for baseline assessments and for monitoring changes in infection prevalence over time. In the context of rapidly expanding programs targeting neglected tropical diseases, including lymphatic filariasis, the MBA could be used to monitor program impact through school-based surveillance. We used existing samples from a school-based study of antifilarial treatment to demonstrate the utility of such an approach by assessing changes in antibody levels to filarial and non-filarial antigens after drug treatment. Among MF−/Og4C3+ children, antifilarial treatment significantly decreased antibody responses to recombinant filarial antigens, but not responses to unrelated antigens. Interestingly, antifilarial antibody responses also decreased significantly among filarial antigen-negative children who were treated with DEC, but not placebo. These observations may have implications for strategies to monitor filariasis elimination programs.
The subset of samples used in this study was representative of those collected from children enrolled in the larger drug treatment study.20 In our study, the prevalence of MF and intensity of antigenemia either remained static or significantly decreased (P < 0.001), respectively, among children who received DEC. For children who received placebo, the intensity of antigenemia significantly increased (P = 0.039) at the end of the study. As in other studies that showed decreased antibody responses to Bm14 by ELISA after DEC treatment,20,25–28 we showed decreases in antibody responses to Bm14 by MBA among children who received drugs containing DEC (Figure 1A and B). Interestingly, antibody responses to Bm14 and Bm33 decreased among children who received drugs containing DEC, even among children who were MF–/Og4C3– (Figures 1A and 2A). These decreases were not observed among MF–/Og4C3– children in the albendazole or placebo groups (Figures 1A and 2A), ruling out an effect of the intervention on transmission or a seasonal or secular trend in antibody levels. These results raise questions about the infection status of antigen-negative children. It is possible that many of these children harbor small numbers of adult or immature worms that do not produce detectable levels of antigen. In addition, the decrease in antibody levels in DEC-treated children argues that DEC is effective against early stages of the parasite, an observation consistent with the use of DEC for prophylaxis, both for loiasis in humans and Dirofilaria infections in pets.29–34 From the standpoint of monitoring antibody responses in children after MDA, these results emphasize the challenges inherent in distinguishing children who are infected from those who are exposed, but uninfected. From the programmatic perspective, it may be prudent to consider that antibody-positive children are actively infected.
Bm33 has not been used extensively to monitor antifilarial antibody levels in field settings. This protein, which is an aspartyl protease inhibitor, is strongly recognized by microfilaria-positive persons and a proportion of antigen-negative exposed persons.35 The seroprevalence of Bm33 was greater than that of Bm14. For example, among MF–/Og4C3– children, 76 (89%) of 85 were positive for IgG to Bm33 compared with 56 (66%) of 85 who were positive for IgG to Bm14. Thus, Bm33 could serve as an additional marker of filarial exposure. Interestingly, Di33, a homolog from Dirofilaria immitis, is strongly immunogenic and serves as the basis of a commercially available test for feline heartworm infection.36,37 We performed MBA on 15 adults suspected to have zoonotic filariasis. None were IgG positive to Bm14 and only 2 were IgG positive to Bm33, which suggests that cross-reactivity may be limited. Nonetheless, additional specificity testing of Bm33 should be carried out before it is used for large-scale field testing.
IgG and IgG4 responses to both antigens decreased after treatment, but the profile of isotype responses differed by antigen. We believe that the amount of IgG4 reactivity in the total IgG reactivity to Bm14 and Bm33 are good estimations because the seroprevalence of Bm14/IgG4+ and Bm14/IgG+ was 79% at time point A and there was a strong positive correlation (r = 0.842, P < 0.001) between IgG4 and IgG reactivity to Bm14. For Og4C3+ children at baseline, 81% and 93% of the total IgG responses to Bm14 were IgG4 in MF–/Og4C3+ and MF+/Og4C3+ children, respectively. Even among MF–/Og4C3– children, 55% of the total IgG responses to Bm14 were IgG4. In contrast, only 11%, 18%, and 16% of the total IgG responses to Bm33 were IgG4 in MF–/Og4C3–, MF–/Og4C3+, and MF+/Og4C3+ children, respectively. IgG1 is the major isotype elicited by Bm33 (Donaldson RA, unpublished data).38 These differences in isotype responses argue that Bm14 and Bm33 antibody responses are specific. We also showed that eluted IgG4 from either antigen does not cross-react with the other antigen.
The extent to which antibody levels decreased after treatment was inversely related to the infection load before treatment, as documented in other studies.13,39 In our study, for MF−/Og4C3− children who received drugs containing DEC, significant decreases (P < 0.001) in antibody responses to Bm14 and Bm33 occurred as early as time point B (Figures 1A and 2A) whereas Og4C3+ children who received drugs containing DEC did not show significant decreases (P < 0.001) in antibody responses to Bm14 until time point C (Figure 1B). MF+/Og4C3+ children, those with the highest antigen levels before treatment, showed no significant decrease in antibody responses to either Bm14 or Bm33 at any time point regardless of whether active drug was received (Figure 3A and B). Thus, for MF+/Og4C3+ persons, multiple doses of DEC or DEC/ALB are likely to be needed to reduce the parasite load.25
In contrast to the decreases in antibodies to filarial antigens, there were no significant changes in IgG responses to PTP3 from E. cuniculi. Little information was available on seroprevalence of PTP3 from E. cuniculi in Haiti. In the United States, 4.2% of 240 persons were considered to be antibody positive against PTP3 by ELISA (Kucerova Z, unpublished data). In our study, we found a prevalence of 12% for IgG against PTP3 at time point A, which remained stable throughout the study. The higher prevalence may be explained by poor environmental conditions in Haiti. Multiple doses of ALB are needed to eliminate symptoms of microsporidiosis and E. cuniculi load, and the single-dose ALB used in this study showed no effect on IgG responses to PTP3.40–42
Also, in contrast to the decreases in antibodies to filarial antigens, there were no significant changes in IgG responses to MSP-119 from P. falciparum. The MSP-119 includes the portion of the antigen found in the P. falciparum merozoite surface protein that is involved in the invasion of erythrocytes, and it predominantly elicits IgG1.43 Using a polymerase chain reaction, a molecular technique that determines infection at a single point in time, Eisele and others reported that 3.1% of persons in the Artibonite Valley in Haiti during a rainy season were positive for P. falciparum.44 In our study at baseline, the prevalence of MSP-119/IgG+ was 19% by MBA and remained stable at 18% through the remainder of the study. This finding may reflect cumulative exposure to P. falciparum among the enrolled children.
The MBA can monitor MDA program effectiveness by assessing immune responses to multiple filarial and non-filarial antigens simultaneously and economically. For example, in our next MDA study on filariasis, we intend to include the recombinant antigen from Wolbachia, an endosymbiotic bacterium, found in filarial nematodes.45 The MBA platform may find use in longitudinal studies in other neglected tropical disease programs, where the prevalence of antibodies to specific infections would be expected to decrease if the program decreases local transmission of the parasite. At the same time, antigens from waterborne pathogens can be included, which would enable simultaneous assessment of the impact of interventions aimed at improving water and sanitation. In addition, antigens from blood-borne pathogens such as Plasmodium can be included to assess anti-malaria drug treatment programs. These tasks can be accomplished concurrently with up to 100 antigens and without the extensive cost and labor and large quantity of serum or plasma specimens required by an ELISA. For the MBA, only 125 nL of serum specimen is required per well, and at current prices, one 10-plex assay plate cost $90.00–$95.00, not including labor and costs of antigens.
ACKNOWLEDGMENTS
We thank Drs. Udhayakumar Venkatachalam and Eric Tongren (Centers for Disease Control and Prevention) and Drs. Christian W. Kauth and Hermann Bujard (Zentrum fuer Molekulare Biologie Heidelberg, Universitaet Heidelberg, Heidelberg, Germany) for providing MSP-119/GST antigen, and Drs. M. Lizotte-Waniewski and S. A. Williams (Smith College, Northampton, MA) for providing the cDNA library for the λ Uni-ZapXR vector for Bm33.
Disclaimer: Use of trade names is for identification only and does not imply endorsement by the Public Health Service or by the U.S. Department of Health and Human Services. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Authors' addresses: Delynn M. Moss, Waterborne Disease Prevention Branch, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: dmm3@cdc.gov. Jeffrey W. Priest, Waterborne Disease Prevention Branch, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: jip8@cdc.gov. Alexis Boyd, Office of Intramural Training and Education, National Institutes of Health, Bethesda, MD, E-mail: boyda2@od.nih.gov. Tiffany Weinkopff, Zuzana Kucerova, and Patrick J. Lammie, Division of Parasitic Diseases, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: tiffany.weinkopff@unil.ch 1600, zik0@cdc.gov, and pjl1@cdc.gov. Michael J. Beach, Division of Foodborne, Waterborne and Environmental Diseases, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: mjb3@cdc.gov.
References
- 1.Wagner B, Freer H. Development of a bead-based multiplex assay for simultaneous quantification of cytokines in horses. Vet Immunol Immunopathol. 2009;127:242–248. doi: 10.1016/j.vetimm.2008.10.313. [DOI] [PubMed] [Google Scholar]
- 2.Sharma RK, Rogojina AT, Chalam KV. Multiplex immunoassay analysis of biomarkers in clinically accessible quantities of human aqueous humor. Mol Vis. 2009;15:60–69. [PMC free article] [PubMed] [Google Scholar]
- 3.Teles RP, Likhari V, Socransky SS, Haffajee AD. Salivary cytokine levels in subjects with chronic periodontitis and in periodontally healthy individuals: a cross-sectional study. J Periodontal Res. 2009;44:411–417. doi: 10.1111/j.1600-0765.2008.01119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waterboer T, Sehr P, Michael KM, Franceschi S, Nieland JD, Joos TO, Templin MF, Pawlita M. Multiplex human papillomavirus serology based on in situ-purified glutathione S-transferase fusion proteins. Clin Chem. 2005;51:1845–1853. doi: 10.1373/clinchem.2005.052381. [DOI] [PubMed] [Google Scholar]
- 5.Wong SJ, Demarest VL, Boyle RH, Wang T, Ledizet M, Kar K, Kramer LD, Fikrig E, Koski RA. Detection of human anti-flavivirus antibodies with a West Nile virus recombinant antigen microsphere immunoassay. J Clin Microbiol. 2004;42:65–72. doi: 10.1128/JCM.42.1.65-72.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lobashevsky AL, Manwaring JE, Travis MM, Nord BL, Higgins NG, Serov YA, Arnoff TS, Hommel-Berrey GA, Goggins WC, Taber TE, Carter CB, Sr, Smith DS, Wozniak TC, O'Donnell JA, Turrentine MW. Effect of desensitization in solid organ transplant recipients depends on some cytokines genes polymorphism. Transpl Immunol. 2009;21:169–178. doi: 10.1016/j.trim.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Khan IH, Krishnan VV, Ziman M, Janatpour K, Wun T, Luciw PA, Tuscano J. A comparison of multiplex suspension array large-panel kits for profiling cytokines and chemokines in rheumatoid arthritis patients. Cytometry B Clin Cytom. 2009;76:159–168. doi: 10.1002/cyto.b.20452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kellar KL, Mahmutovic AJ, Bandyopadhyay K. Current Protocols in Cytometry Chapter 13: Unit13 1. New York: John Wiley; 2006. (Multiplexed microsphere-based flow cytometric immunoassays). [DOI] [PubMed] [Google Scholar]
- 9.Dondog B, Clifford GM, Vaccarella S, Waterboer T, Unurjargal D, Avirmed D, Enkhtuya S, Kommoss F, Wentzensen N, Snijders PJ, Meijer CJ, Franceschi S, Pawlita M. Human papillomavirus infection in Ulaanbaatar, Mongolia: a population-based study. Cancer Epidemiol Biomarkers Prev. 2008;17:1731–1738. doi: 10.1158/1055-9965.EPI-07-2796. [DOI] [PubMed] [Google Scholar]
- 10.Moss DM, Montgomery JM, Newland SV, Priest JW, Lammie PJ. Detection of Cryptosporidium antibodies in sera and oral fluids using multiplex bead assay. J Parasitol. 2004;90:397–404. doi: 10.1645/GE-3267. [DOI] [PubMed] [Google Scholar]
- 11.Kellar KL, Kalwar RR, Dubois KA, Crouse D, Chafin WD, Kane BE. Multiplexed fluorescent bead-based immunoassays for quantitation of human cytokines in serum and culture supernatants. Cytometry. 2001;45:27–36. doi: 10.1002/1097-0320(20010901)45:1<27::aid-cyto1141>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 12.Michael E, Bundy DA, Grenfell BT. Reassessing the global prevalence and distribution of lymphatic filariasis. Parasitology. 1996;112:409–428. doi: 10.1017/s0031182000066646. [DOI] [PubMed] [Google Scholar]
- 13.Ottesen EA, Duke BO, Karam M, Behbehani K. Strategies and tools for the control/elimination of lymphatic filariasis. Bull World Health Organ. 1997;75:491–503. [PMC free article] [PubMed] [Google Scholar]
- 14.Wattal S, Dhariwal AC, Ralhan PK, Tripathi VC, Regu K, Kamal S, Lal S. Evaluation of Og4C3 antigen ELISA as a tool for detection of bancroftian filariasis under lymphatic filariasis elimination programme. J Commun Dis. 2007;39:75–84. [PubMed] [Google Scholar]
- 15.Liang JL, King JD, Ichimori K, Handzel T, Pa'au M, Lammie PJ. Impact of five annual rounds of mass drug administration with diethylcarbamazine and albendazole on Wuchereria bancrofti infection in American Samoa. Am J Trop Med Hyg. 2008;78:924–928. [PubMed] [Google Scholar]
- 16.Atmadja AK, Atkinson R, Sartono E, Partono F, Yazdanbakhsh M, Maizels RM. Differential decline in filaria-specific IgG1, IgG4, and IgE antibodies in Brugia malayi-infected patients after diethylcarbamazine chemotherapy. J Infect Dis. 1995;172:1567–1572. doi: 10.1093/infdis/172.6.1567. [DOI] [PubMed] [Google Scholar]
- 17.Rabello AL, Garcia MM, Pinto da Silva RA, Rocha RS, Katz N. Humoral immune responses in patients with acute Schistosoma mansoni infection who were followed up for two years after treatment. Clin Infect Dis. 1997;24:304–308. doi: 10.1093/clinids/24.3.304. [DOI] [PubMed] [Google Scholar]
- 18.Muck AE, Pires ML, Lammie PJ. Influence of infection with non-filarial helminths on the specificity of serological assays for antifilarial immunoglobulin G4. Trans R Soc Trop Med Hyg. 2003;97:88–90. doi: 10.1016/s0035-9203(03)90033-2. [DOI] [PubMed] [Google Scholar]
- 19.Lammie PJ Weil G, Noordin R, Kaliraj P, Steel C, Goodman D, Lakshmikanthan VB, Ottesen E. Recombinant antigen-based antibody assays for the diagnosis and surveillance of lymphatic filariasis: a multicenter trial. Filaria J. 2004;3:9. doi: 10.1186/1475-2883-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox LM, Furness BW, Haser JK, Desire D, Brissau JM, Milord MD, Lafontant J, Lammie PJ, Beach MJ. Tolerance and efficacy of combined diethylcarbamazine and albendazole for treatment of Wuchereria bancrofti and intestinal helminth infections in Haitian children. Am J Trop Med Hyg. 2005;73:115–121. [PubMed] [Google Scholar]
- 21.Dissanayake S, Xu M, Nkenfou C, Piessens WF. Molecular cloning and serological characterization of a Brugia malayi pepsin inhibitor homolog. Mol Biochem Parasitol. 1993;62:143–146. doi: 10.1016/0166-6851(93)90191-y. [DOI] [PubMed] [Google Scholar]
- 22.Lucchi NW, Tongren JE, Jain V, Nagpal AC, Kauth CW, Woehlbier U, Bujard H, Dash AP, Singh N, Stiles JK, Udhayakumar V. Antibody responses to the merozoite surface protein-1 complex in cerebral malaria patients in India. Malar J. 2008;7:121. doi: 10.1186/1475-2875-7-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kucerova Z, Priest JW, Delbac F, Visvesvara GS, Secor WE. Expression and serologic assessment of a recombinant polar tube protein from Encephalitozoon cuniculi (PTP3) J Eukaryot Microbiol. 2006;53((Suppl 1)):S70–S71. doi: 10.1111/j.1550-7408.2006.00177.x. [DOI] [PubMed] [Google Scholar]
- 24.Waterboer T, Sehr P, Pawlita M. Suppression of non-specific binding in serological Luminex assays. J Immunol Methods. 2006;309:200–204. doi: 10.1016/j.jim.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Helmy H, Weil GJ, Ellethy AS, Ahmed ES, Setouhy ME, Ramzy RM. Bancroftian filariasis: effect of repeated treatment with diethylcarbamazine and albendazole on microfilaraemia, antigenaemia and antifilarial antibodies. Trans R Soc Trop Med Hyg. 2006;100:656–662. doi: 10.1016/j.trstmh.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 26.Tisch DJ, Bockarie MJ, Dimber Z, Kiniboro B, Tarongka N, Hazlett FE, Kastens W, Alpers MP, Kazura JW. Mass drug administration trial to eliminate lymphatic filariasis in Papua New Guinea: changes in microfilaremia, filarial antigen, and Bm14 antibody after cessation. Am J Trop Med Hyg. 2008;78:289–293. [PMC free article] [PubMed] [Google Scholar]
- 27.Ramzy RM, El Setouhy M, Helmy H, Ahmed ES, Abd Elaziz KM, Farid HA, Shannon WD, Weil GJ. Effect of yearly mass drug administration with diethylcarbamazine and albendazole on bancroftian filariasis in Egypt: a comprehensive assessment. Lancet. 2006;367:992–999. doi: 10.1016/S0140-6736(06)68426-2. [DOI] [PubMed] [Google Scholar]
- 28.Weil GJ, Ramzy RM, El Setouhy M, Kandil AM, Ahmed ES, Faris R. A longitudinal study of Bancroftian filariasis in the Nile Delta of Egypt: baseline data and one-year follow-up. Am J Trop Med Hyg. 1999;61:53–58. doi: 10.4269/ajtmh.1999.61.53. [DOI] [PubMed] [Google Scholar]
- 29.Yamagata GR, Gershwin LJ, Wong MM. Diethylcarbamazine-induced Dirofilaria immitis larval death, as indicated by immunoglobulin E concentration, in dogs with concurrent Ancylostoma caninum infection. Am J Vet Res. 1995;56:174–178. [PubMed] [Google Scholar]
- 30.Fujimaki Y, Sakamoto M, Shimada M, Kimura E, Aoki Y. Diethylcarbamazine: inhibitory effect on acetylcholinesterase of Dirofilaria immitis and Brugia pahangi. Southeast Asian J Trop Med Public Health. 1989;20:179–182. [PubMed] [Google Scholar]
- 31.Grieve RB, Mika-Johnson M, Jacobson RH, Cypess RH. Enzyme-linked immunosorbent assay for measurement of antibody responses to Dirofilaria immitis in experimentally infected dogs. Am J Vet Res. 1981;42:66–69. [PubMed] [Google Scholar]
- 32.Grover JK, Vats V, Uppal G, Yadav S. Anthelmintics: a review. Trop Gastroenterol. 2001;22:180–189. [PubMed] [Google Scholar]
- 33.Klion AD, Ottesen EA, Nutman TB. Effectiveness of diethylcarbamazine in treating loiasis acquired by expatriate visitors to endemic regions: long-term follow-up. J Infect Dis. 1994;169:604–610. doi: 10.1093/infdis/169.3.604. [DOI] [PubMed] [Google Scholar]
- 34.Winkler S, Paiha S, Winkler H, Graninger W, Marberger M, Steiner GE. Microfilarial clearance in loiasis involves elevation of Th1 and Th2 products and emergence of a specific pattern of T-cell populations. Parasite Immunol. 1996;18:479–482. doi: 10.1111/j.1365-3024.1996.tb01032.x. [DOI] [PubMed] [Google Scholar]
- 35.Scott AL, Ghedin E. The genome of Brugia malayi—all worms are not created equal. Parasitol Int. 2009;58:6–11. doi: 10.1016/j.parint.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frank GR, Mondesire RR, Brandt KS, Wisnewski N. Antibody to the Dirofilaria immitis aspartyl protease inhibitor homologue is a diagnostic marker for feline heartworm infections. J Parasitol. 1998;84:1231–1236. [PubMed] [Google Scholar]
- 37.Mejia JS, Nkenfou C, Southworth MW, Perler FB, Carlow CK. Expression of an Onchocerca volvulus Ov 33 homologue in Dirofilaria immitis: potential in immunodiagnosis of heartworm infection. Parasite Immunol. 1994;16:297–303. doi: 10.1111/j.1365-3024.1994.tb00352.x. [DOI] [PubMed] [Google Scholar]
- 38.Krushna NS, Shiny C, Dharanya S, Sindhu A, Aishwarya S, Narayanan RB. Immunolocalization and serum antibody responses to Brugia malayi pepsin inhibitor homolog (Bm-33) Microbiol Immunol. 2009;53:173–183. doi: 10.1111/j.1348-0421.2009.00114.x. [DOI] [PubMed] [Google Scholar]
- 39.Hussein O, El Setouhy M, Ahmed ES, Kandil AM, Ramzy RM, Helmy H, Weil GJ. Duplex Doppler sonographic assessment of the effects of diethylcarbamazine and albendazole therapy on adult filarial worms and adjacent host tissues in Bancroftian filariasis. Am J Trop Med Hyg. 2004;71:471–477. [PubMed] [Google Scholar]
- 40.Tremoulet AH, Avila-Aguero ML, Paris MM, Canas-Coto A, Ulloa-Gutierrez R, Faingezicht I. Albendazole therapy for Microsporidium diarrhea in immunocompetent Costa Rican children. Pediatr Infect Dis J. 2004;23:915–918. doi: 10.1097/01.inf.0000141724.06556.f9. [DOI] [PubMed] [Google Scholar]
- 41.Fournier S, Liguory O, Sarfati C, David-Ouaknine F, Derouin F, Decazes JM, Molina JM. Disseminated infection due to Encephalitozoon cuniculi in a patient with AIDS: case report and review. HIV Med. 2000;1:155–161. doi: 10.1046/j.1468-1293.2000.00022.x. [DOI] [PubMed] [Google Scholar]
- 42.Didier ES, Maddry JA, Brindley PJ, Stovall ME, Didier PJ. Therapeutic strategies for human microsporidia infections. Expert Rev Anti Infect Ther. 2005;3:419–434. doi: 10.1586/14787210.3.3.419. [DOI] [PubMed] [Google Scholar]
- 43.Stanisic DI, Richards JS, McCallum FJ, Michon P, King CL, Schoepflin S, Gilson PR, Murphy VJ, Anders RF, Mueller I, Beeson JG. Immunoglobulin G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infect Immun. 2009;77:1165–1174. doi: 10.1128/IAI.01129-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eisele TP, Keating J, Bennett A, Londono B, Johnson D, Lafontant C, Krogstad DJ. Prevalence of Plasmodium falciparum infection in rainy season, Artibonite Valley, Haiti, 2006. Emerg Infect Dis. 2007;13:1494–1496. doi: 10.3201/eid1310.070567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Punkosdy GA, Addiss DG, Lammie PJ. Characterization of antibody responses to Wolbachia surface protein in humans with lymphatic filariasis. Infect Immun. 2003;71:5104–5114. doi: 10.1128/IAI.71.9.5104-5114.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


