Abstract
Toxoplasma gondii is an important parasite in pregnant women. This case-controlled study assessed the seroprevalence of toxoplasmosis in 640 pregnant women in southern Thailand and identified their associated risk factors. The overall seroprevalence of toxoplasmosis was 181 (28.3%). Of this, 138 (21.6%) were positive for only anti-Toxoplasma immunoglobulin G (IgG) antibody, 43 (6.7%) were positive for both IgG and IgM antibodies, and none were positive for IgM antibody. Multivariate analysis revealed that increasing age (adjusted odds ratio [OR] = 1.64, 95% confidence interval [CI] = 1.01–2.67), living outside Songkhla province (adjusted OR = 1.56, 95% CI = 1.08–2.24), parity (adjusted OR = 1.65, 95% CI = 1.01–2.68), contact with cats (adjusted OR = 1.70, 95% CI = 1.20–2.43), and drinking of unclean water (adjusted OR = 1.70, 95% CI = 1.08–2.68) were factors associated with Toxoplasma seroprevalence. On the basis of the results obtained, a health surveillance program should be initiated as a primary preventive measure for congenital toxoplasmosis and focus on educating women of the child-bearing age group to avoid contact with cats and to strictly practice personal hygiene.
Introduction
Toxoplasma gondii, an obligate intracellular protozoan parasite, is an important public health concern because it causes congenital toxoplasmosis, which is acquired by a fetus from its infected pregnant mother. This congenital infection can lead to severe illnesses in the fetus and newborn infant, including brain and eye dysfunctions, or even a fatal condition.1 In the United States, among the ∼4.2 million live births per year, congenital toxoplasmosis occurs in ∼500 to 5,000 newborns.2 In Europe, the prevalence of congenital toxoplasmosis was 2.1/10,000 live-births.3 In South America, the estimated annual incidence of congenital toxoplasmosis varied from 9.5 to 10.6/1,000 live-births.4 In Thailand, antibodies to T. gondii varied from 2.3% to 29.6% among pregnant women and 7.18% to 13.14% in newborns.5–8 The risk of a congenital Toxoplasma infection in infants is directly related to their seropositive mothers.7 Toxoplasmosis is not only an increasingly important problem, but it also can lead to a significant social and economic burden. There has been little data available on Thai pregnant women in recent years and none has been reported from southern Thailand. Therefore, it is relevant to conduct an epidemiological study of this parasitic infection in our local community. This study was aimed to determine the seroprevalence of toxoplasmosis in pregnant women and to evaluate the association between risk factors and disease transmission.
Materials and Methods
Study site and population.
A prospective case control study was carried out at the antenatal clinic (ANC) at Songklanagarind Hospital, Hat Yai, Songkhla province, Thailand from October 2009 to June 2010. This hospital, with its capacity of 850 in-patient beds, is located in the south of Thailand and was built to facilitate the teaching, research, and training for medical personnel in various disciplines, and for the provision of healthcare to the general public, particularly among Southern Thais. The study included 640 eligible pregnant women who gave informed consent before this study. The inclusion criteria for the study subjects were 1) pregnant women with a gestational age ranging from 5 to 38 weeks who gave informed consent to participate in the study; 2) for women > 14 years of age, a random selection method was used to identify eligible pregnant women attending the antenatal care for the first time that were planning to have a routine blood test during the specified study period, and data were obtained using a standardized structured questionnaire; and 3) pregnant women with or without anti-human immunodeficiency virus (HIV) antibody status, indicated by the enzyme-linked immunosorbent assay (ELISA) technique.
The questionnaire was designed to detect socio-demographic and biologically plausible risk factors associated with toxoplasmosis, and clinical history and presenting signs and symptoms relating to toxoplasmosis (if any). An operational definition was used for the risk factors. A history of antibiotic use was defined as a person who had received antibiotic(s) related to anti-Toxoplasma therapy including co-trimoxazole, pyrimethamine, clindamycin, spiramycin, tetracyclines, macrolides, sulphonamides, antifolates, or trioxanes groups for treating an illness. Contact with cats was defined as a person who is the owner of at least one cat or has had close contact with cats by straying, playing, feeding, and sleeping in the house. Consumption of uncooked meat or raw meat was defined as a person who has a habit of eating uncooked meat, e.g., sausage, sashimi (a traditional Japanese dish consisting of very thin bite-size slices of fresh raw fish), satay (a Southeast Asian cuisine of grilled marinated meat, poultry, or seafood), barbecue, or any kinds of meats where the method of preparation could not be guaranteed for the absence of T. gondii. Blood transfusion was defined as a person who has received blood or blood products from her donors. Sources of drinking water were defined as a “clean source” if the person who consumes water as mineral, boiled, or filtered types, whereas an “unclean source” included water from a pipe, tap, or rain. Drinking milk was defined as a person who drinks either pasteurized/boiled milk or fresh milk directly from the cattle. Contact with soil was defined as a person who has a direct exposure to soil while gardening or any kind of outdoor activities. And, contact with other animals was defined as a person who has kept a dog or other warm-blooded animal as their pet, which could serve as an intermediate host for toxoplasmosis. An affected pregnant woman with a history of these risk assessments, at least a 3-month period before the study period, was recorded in this questionnaire.
Ethical consideration.
This study was approved before its commencement by the ethical committee of the Faculty of Medicine, Prince of Songkla University, Thailand.
Serum samples.
Approximately 5 mL of venous blood samples were drawn from pregnant women who gave their consent to participate in this study. Their blood samples were kept in an ice box until being transferred to the Department of Microbiology, Faculty of Science for centrifuging at 4,000 RPM (rounds/minute) and subsequently their sera were kept at −20°C until further use.
Detection of IgG and IgM antibodies to Toxoplasma gondii.
Toxoplasmosis was screened by using a standard ELISA commercial kit (IgG-Trinity Biotech and IgM-Trinity Biotech, New York) in accordance with the manufacturer's instructions and performed at the Department of Microbiology, Faculty of Science, Prince of Songkla University, Hat Yai, Songkhla, Thailand. A result of > 1.10 ISR (immune status ratio) of anti-Toxoplasma IgG antibody was regarded as positive, and an indication of a latent or pre-existing Toxoplasma infection. A result > 1.10 ISR of anti-Toxoplasma IgM antibody was regarded as positive, and indicated a recently acquired Toxoplasma infection. A positive sample for the anti-Toxoplasma IgG antibody was also tested for its avidity using a standard ELISA commercial kit (IgG-NovaLisa and IgM- NovaLisa, Dietzenbach, Germany); high avidity (> 40%) indicated a past infection (of at least 4–5 months) and a low avidity (< 40%) indicated a recently acquired infection (within 4–5 months).
Statistical analysis.
Data obtained from both the questionnaire and laboratory tests were entered, edited, and analyzed using the statistical software SPSS version 10.0 (SPSS, Inc., Chicago, IL). The data with quantitative variables were expressed as the mean (±SD) and range, whereas qualitative variables were estimated and presented as frequencies and percentages. Univariate analyses and the χ2 test were used to investigate the association between Toxoplasma seropositivity as a dependent variable and possible demographic and risk factors as independent variables; P < 0.05 was regarded as being statistically significant. However, to retain all possible significant association, variables that showed an association with P ≤ 0.20 were used to apply to a multivariate logistic regression model (stepwise forward). Each dependent factor was modeled as dichotomous variables.
Results
During this study period, a total of 640 pregnant women were recruited. The age range was 15–45 with a mean of 28.6 ± 6.0 years. The main age group was between 26 and 35 years (346, 54.1%). The majority of these pregnant women had completed a tertiary level of education (376, 58.8%), laborers (260, 40.6%), those who stayed in Songkhla province (406, 63.4%), and those with no previous history of antibiotic use (618, 96.6%). A large number of pregnant women were in the first trimester of pregnancy (407, 63.6%), had ≤ 1 child (551, 86.1%), and had no history of abortion (493, 77%), as shown in Table 1. In addition, nonspecific clinical features such as fever (29), myalgia (80), joint pain (23), generalized weakness (102), blurred vision (30), headache (131), and rash (28) had been presented in pregnant women and particularly during their first trimester of pregnancies (data not shown).
Table 1.
Univariate analysis for plausible demographic and obstetric profiles associated with Toxoplasma seropositive* pregnant women
| Variable | No. of pregnant women | P value | |
|---|---|---|---|
| Total (n, %) N = 640 | Toxoplasma seropositivity (n, %) N = 181 | ||
| Age | |||
| Range 15–45 (years) | |||
| Mean 28.6 (±6.01) years | |||
| Age group | 0.089 | ||
| 15–25 | 203 (31.7) | 49 (24.1) | |
| 26–35 | 346 (54.1) | 99 (28.6) | |
| 36–45 | 91 (14.2) | 33 (36.3) | |
| Education | 0.621 | ||
| Primary | 67 (10.5) | 21 (31.3) | |
| Secondary | 197 (30.8) | 59 (29.9) | |
| Tertiary | 376 (58.8) | 101 (26.9) | |
| Occupation | 0.627 | ||
| Laborer | 260 (40.6) | 72 (27.7) | |
| Non-laborer | 230 (35.9) | 62 (27) | |
| Other† | 150 (23.4) | 47 (31.3) | |
| Address | 0.031 | ||
| Songkhla province | 406 (63.4) | 103 (25.4) | |
| Living outside Songkhla province | 234 (36.6) | 78 (33.3) | |
| Past history of antibiotic use | 0.821 | ||
| No drug use | 618 (96.6) | 175 (28.3) | |
| Drug related to ATT‡ | 21 (3.3) | 6 (28.6) | |
| Drug unrelated to ATT | 1 (0.2) | – | |
| Trimester of pregnancy | 0.801 | ||
| First | 407 (63.6) | 112 (27.5) | |
| Second | 172 (26.9) | 52 (30.2) | |
| Third | 61 (9.5) | 17 (27.9) | |
| Parity (no. of children) | 0.013 | ||
| ≤ 1 | 551 (86.1) | 146 (26.5) | |
| ≥ 2 | 89 (13.9) | 35 (39.3) | |
| Abortion | 0.952 | ||
| No | 493 (77.0) | 138 (28) | |
| Yes with unknown cause | 59 (9.2) | 17 (28.8) | |
| Yes but unrelated to toxoplasmosis | 88 (13.8) | 26 (29.6) | |
Overall seroprevalence of anti-Toxoplasma antibodies in the study subjects.
Other includes retiree, unemployed, housewife, and students.
ATT = anti-Toxoplasma therapy.
The overall seroprevalence of toxoplasmosis was 181 (28.3%; 95% confidence interval [CI] = 24.9–31.8) in our pregnant women. Of these pregnant women, 138 (21.6%; 95% CI = 18.5–24.9) were positive for only anti-Toxoplasma IgG antibody and 43 (6.7%; 95% CI = 4.9–9.02) were positive for both anti-Toxoplasma IgG and IgM antibodies. All pregnant women with the seropattern of both antibodies were subsequently tested for IgG avidity and their results showed a high avidity, indicating a past infection with T. gondii. For another 39 (6.1%) pregnant women, who were positive for only IgM antibody, we repeated the test with their second samples 4 weeks apart, except for one pregnant woman who delivered before the second scheduled test, and the other one who was denied for this reason; however, there was no seroconversion found in these suspected mothers. In addition, we also performed detailed ultrasonography in all cases to identify any congenital anomalies related to Toxoplasma infection, i.e., intracranial calcification, periventricular calcification, and ventriculomegaly. The ultrasonography revealed that all of the fetuses appeared normal, and we found no congenital abnormalities at birth. It is noted that none of these seropositive pregnant mothers was coinfected with HIV during the time of this study.
In addition, we further tested for both anti-Toxoplasma IgG and IgM antibodies in all 80 newborns after their deliveries. Twenty-six newborns had only Toxoplasma IgG seropositivity and none was found to have anti-Toxoplasma IgM antibody (data are not shown).
Tables 1 and 2 show the seroprevalence of toxoplasmosis in pregnant women by their demographic and obstetric profiles and plausible risk factors. By univariate analysis, this study identified that the present address, parity, contact with cats, and sources of drinking water were statistically significant factors associated with seropositive pregnant women (P < 0.05). After the multivariate logistic regression analysis was applied, it was noted that the age group of ≥ 36 years, pregnant women who stayed outside Songkhla province, had contact with cats, and drank unclean water were identified as significant risk factors for infection with T. gondii (Table 3).
Table 2.
Univariate analysis for plausible risks factors associated with Toxoplasma seropositive* pregnant women
| Variable | No. of pregnant women | P value | |
|---|---|---|---|
| Total (n, %) N = 640 | Toxoplasma seropositivity (n, %) N = 181 | ||
| Awareness of toxoplasmosis | 0.540 | ||
| No | 553 (86.4) | 154 (27.8) | |
| Yes | 87 (13.6) | 27 (31) | |
| Contact with cat No Yes | 377 (58.9) 263 (41.1) | 90 (23.9) 91 (34.6) | 0.003 |
| Uncooked meat No Yes | 333 (52) 307 (48) | 90 (27) 91 (29.6) | 0.577 |
| Blood transfusion No Yes | 636 (99.4) 4 (0.6) | 181 (28.5) – | 0.208 |
| Source of drinking water Boiled Mineral/filtered water Piped/tap/rain water | 110 (17.2) 426 (66.6) 104 (16.3) | 36 (32.7) 107 (25.1) 38 (36.5) | 0.036 |
| Drinking milk No Boiled Pasteurized | 38 (5.9) 6 (0.9) 596 (93.1) | 12 (31.6) 3 (50) 166 (27.9) | 0.642 |
| Contact with soil No Yes | 199 (31.1) 441 (68.9) | 52 (26.1) 129 (29.3) | 0.233 |
| Contact with other animals No Yes | 289 (45.2) 351 (54.8) | 87 (30.1) 94 (26.8) | 0.353 |
Overall seroprevalence of anti-Toxoplasma antibodies in the study subjects.
Table 3.
Multivariate logistic regression analysis for risk factors associated with Toxoplasma seropositive* pregnant women
| Variable | Adjusted odds ratio (OR) | 95% CI | P value | |
|---|---|---|---|---|
| Age ≥ 36 | 1.64 | 1.01 | 2.67 | 0.04 |
| Living outside Songkhla province | 1.56 | 1.08 | 2.24 | 0.01 |
| Parity ≥ 2 | 1.65 | 1.01 | 2.68 | 0.04 |
| Contact with cat | 1.70 | 1.20 | 2.43 | 0.002 |
| Drinking unclean water (pipe/tap/rain) | 1.70 | 1.08 | 2.68 | 0.02 |
Overall seroprevalence of anti-Toxoplasma antibodies in the study subjects. Adjusted variables include age group, address, parity, contact with cat, and source of drinking water in this statistical analysis.
Figure 1 shows the epidemiological surveillance data of toxoplasmosis reported in pregnant women from the central (Bangkok), northern (Chiang Mai), and northeastern (Khon Kaen and Ubon Ratchathani) parts of Thailand. The shaded area identifies the location of this study area on toxoplasmosis in pregnant women attending the ANC at Songklanagarind Hospital, Prince of Songkla University, Hat Yai, Songkhla, southern Thailand.
Figure 1.
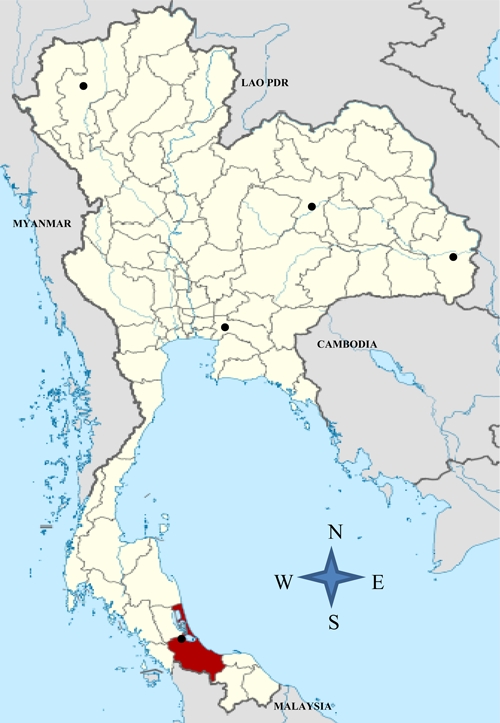
The epidemiological surveillance data of toxoplasmosis reported in pregnant women from central (Bangkok), northern (Chiang Mai), and northeastern (Khon Kaen and Ubon Ratchathani) parts of Thailand. The shaded area identifies the location of this study area on toxoplasmosis in pregnant women attending the antenatal clinic (ANC) at Songklanagarind Hospital, Prince of Songkla University, Hat Yai, Songkhla, southern Thailand.
Discussion
Studies on toxoplasmosis, an important parasitic disease in pregnant women, have been consistently reported from many parts of the world. In our study, the result revealed that 28.3% of 640 pregnant women were seropositive to Toxoplasma and indicated a lifelong latent or subclinical toxoplasmosis. To the best of our knowledge, this is the first study reported from southern Thailand. Surprisingly, this study produced a higher prevalence rate when compared with earlier studies in this region (Figure 1), such as 2.5% to 27.2%5,7,9–11 from the central/capital (Bangkok), 2.8%6 from the north (Chiang Mai), and 3–12%12,13 from the northeastern parts of Thailand. This is also a higher rate than reported from other Asian countries, including rates of 3.7% in Korea,14 7.7% in Vietnam,15 10.6% in China,16 and 17.2% in Singapore.17 In general, the Toxoplasma prevalence rate remains high in many parts of the world, e.g., 40.8% in Nigeria,18 48.6% in Albania,19 49% in Malaysia,20 and 68.6% in Brazil.21 This increasing number of Toxoplasma serostatus further highlights the need for routine screening in women during the childbearing age, particularly from endemic areas of toxoplasmosis, and to monitor the trend of Toxoplasma infections. In addition, all seronegative women should be tested for their Toxoplasma serostatus to prevent their seroconversion during pregnancy.
Our study found that 6.1% of our pregnant women had false positive reactions for anti-Toxoplasma antibody, which was later confirmed by no seroconversion to indicate that there was no risk of congenital infection. The seroconversion is important, particularly in congenital infection. If women are infected before pregnancy, there is no immediate effect on the fetus. Furthermore, the gestational age at seroconversion is also important, as congenital infection rates and its severity are different. To our surprise, this is the first time IgG avidity has been introduced for the routine screening of Toxoplasma infection among pregnant women in Thailand and in our neighboring Southeast Asian countries.15,17,20 From this study, we can ensure that IgG avidity is a confirmatory test to differentiate between recently acquired and past infections, and it is useful to exclude acquired infection within the last 4 to 5 months.22,23 Moreover, IgG avidity can assist during the first trimester of pregnancy when recent maternal infection reveals low IgG avidity to indicate a high risk of congenital infection.24
The epidemiological study on toxoplasmosis in pregnant women would be incomplete without assessing their significant risk behaviors. The seroprevalence of Toxoplasma IgG antibody in our pregnant women increased with age,25–28 and can be explained because even though some of these countries are located in different geographical zones, we definitely share certain similarities in terms of our environment, culture, and life styles. When considering residential areas, the prevalence rate of Toxoplasma infection seems to be higher in rural or suburban areas16,27,29,30 than for people living in urban or cosmopolitan areas, which was also evidenced in our study. In Thailand, local people in general and rural communities in particular are still living in a low socio-economic condition and some could have been infected with T. gondii outside of Songkhla province. However, a further study needs to be carried out on a larger scale to clarify this association before any conclusion can be made. It was of interest that Toxoplasma infection is more likely to occur in women who had multiparity.20,27,31,32 This is the first of its kind, to our knowledge, reported in Thailand and the number of children that have contributed to the increasing Toxoplasma prevalence in women is unknown. Possible explanations for this problem are that women with children are more likely to have contact with plausible risk factors to Toxoplasma acquisition or they are careless when preparing food that can lead to contamination32 or because their children have more contact with stray cats. Nevertheless, an earlier study stated that children have not been identified as an independent risk for prevalence of Toxoplasma.33 This study confirms that contact with cats among our pregnant women has also posed a significant risk factor for Toxoplasma infection.16,26,34,35 In Thailand, we can find a large number of stray cats roaming the streets, fresh/wet markets, public places, and Buddhist temples in both urban and rural areas. There are not only stray cats that always defecate anywhere but also many owners of an indoor cat that allow their cats to defecate outside their homes; consequently, there is a high chance of T. gondii oocysts contaminating the environment and being transmitted to man.36 In contrast, a previous study pointed out that having an indoor cat and direct contact with cats was not enough to increase the transmission rate of infection with T. gondii.34 Unclean (pipe, rain, or tap) water was identified as a potential source of transmission for T. gondii found among our pregnant women. This is considered to be a new finding of an association with human waterborne toxoplasmosis ever documented in Thailand and also in Southeast Asia. Water has been implicated as a source of Toxoplasma infection and has been reported globally for pregnant women.28,30,37–40 On the basis of these findings, serial screening for Toxoplasma infection in the first, second, and third trimesters might make it feasible to detect when seroconversion occurs, particularly among high-risk pregnant women.
In conclusion, the results obtained from this study certainly provide some baseline data on the prevalence and risk factors of toxoplasmosis in pregnant women from southern Thailand. Therefore, we propose that a health surveillance program be used as a primary preventive measure for congenital toxoplasmosis and focus on educating women in the reproductive age group to avoid contact with feral cats and to strictly practice personal hygiene. Moreover, future studies are recommended to be carried out not only in seronegative pregnant women but also in seropositive HIV pregnant mothers to investigate seroprevalence-related risk factors of T. gondii and its vertical transmission.
ACKNOWLEDGMENT
We thank Brian Hodgson and Sucheep Phiriyasamith for their assistance and comments of this manuscript.
Footnotes
Financial support: This study was supported by the University of Malaya Research Grant (UMRG 094/09HTM), Kuala Lumpur, Malaysia and Faculty of Medicine Research Foundation (no.: 01/2553), Prince of Songkla University, Songkhla province, Thailand.
Disclosure: This work was presented in part at the XIIth International Congress on Parasitology (ICOPA XII), Melbourne, Australia, 15–20 August 2010.
Authors' addresses: Veeranoot Nissapatorn and Lau Yee Ling, Department of Parasitology, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia, E-mails: veeranoot@um.edu.my and lauyeeling@um.edu.my. Chitkasaem Suwanrath and Verapol Chandeying, Department of Obstetrics and Gynecology, Faculty of Medicine, Prince of Songkla University, Hat Yai, Songkhla, Thailand, E-mails: schitkas@yahoo.co.uk and verapol.c@psu.ac.th. Nongyao Sawangjaroen, Department of Microbiology, Faculty of Science, Prince of Songkla University, Hat Yai, Songkhla, Thailand, E-mail: nongyao.s@psu.ac.th.
References
- 1.Remington JS, McLeod R, Thulliez P, Desmonts G. In: Infectious Diseases of the Fetus and Newborn Infant. Fifth edition. Remington JS, Klein JO, editors. Philadelphia, PA: Saunders; 2001. pp. 205–346. (Toxoplasmosis). [Google Scholar]
- 2.Jones JL, Dargelas V, Roberts J, Press C, Remington JS, Montoya JG. Risk factors for Toxoplasma gondii infection in the United States. Clin Infect Dis. 2009;49:878–884. doi: 10.1086/605433. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt DR, Hogh B, Andersen O, Fuchs J, Fledelius H, Petersen E. The national neonatal screening program for congenital toxoplasmosis in Denmark: results from the initial four years, 1999–2002. Arch Dis Child. 2006;91:661–665. doi: 10.1136/adc.2004.066514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandes RC, Vasconcellos VP, Araújo LC, Medina-Acosta E. Vertical transmission of HIV and Toxoplasma by reactivation in a chronically infected woman. Braz J Infect Dis. 2009;13:70–71. doi: 10.1590/s1413-86702009000100015. [DOI] [PubMed] [Google Scholar]
- 5.Nabnien K. Seroepidemiological Study of Toxoplasmosis in the Central Part of Thailand. Bangkok: Mahidol University; 1979. [master's thesis] [Google Scholar]
- 6.Morakote N, Thamasonthi W, Charuchinda K, Khamboonruang C. Prevalence of Toxoplasma antibodies in Chiang Mai population. Southeast Asian J Trop Med Public Health. 1984;15:80–85. [PubMed] [Google Scholar]
- 7.Chintana T, Sukthana Y, Bunyakai B, Lekkla A. Toxoplasma gondii antibody in pregnant women with and without HIV infection. Southeast Asian J Trop Med Public Health. 1998;29:383–386. [PubMed] [Google Scholar]
- 8.Wanachiwanawin D, Sutthent R, Chokephaibulkit K, Mahakittikun V, Ongrotchanakun J, Monkong N. Toxoplasma gondii antibodies in HIV and non-HIV infected Thai pregnant women. Asian Pac J Allergy Immunol. 2001;19:291–293. [PubMed] [Google Scholar]
- 9.Chintana T. Pattern of antibodies in toxoplasmosis of pregnant women and their children in Thailand. Southeast Asian J Trop Med Public Health. 1991;22:107–110. [PubMed] [Google Scholar]
- 10.Sukthana Y. Difference of Toxoplasma gondii antibodies between Thai and Austrian pregnant women. Southeast Asian J Trop Med Public Health. 1999;30:38–41. [PubMed] [Google Scholar]
- 11.Tantivanich S, Amarapal P, Suphadtanaphongs W, Siripanth C, Sawatmongkonkun W. Prevalence of congenital cytomegalovirus and Toxoplasma antibodies in Thailand. Southeast Asian J Trop Med Public Health. 2001;32:466–469. [PubMed] [Google Scholar]
- 12.Maleewong W, Lulitanond V, Pipitgool V, Auwijitaroon Y, Kuttsarejariya S, Morakote N. Prevalence of Toxoplasma antibodies in blood donors and pregnant women in Khon Kaen Province. J Med Assoc Thai. 1989;72:256–259. [PubMed] [Google Scholar]
- 13.Daenseekaew W, Maleewong W, Luevisadpaibul V, Molagool S, Rajanuwong A. Seroprevalence of Toxoplasma gondii in pregnant women in Ubon Ratchathani province. J Med Assoc Thai. 1992;75:609–610. [PubMed] [Google Scholar]
- 14.Han K, Shin DW, Lee TY, Lee YH. Seroprevalence of Toxoplasma gondii infection and risk factors associated with seropositivity of pregnant women in Korea. J Parasitol. 2008;94:963–965. doi: 10.1645/GE-1435.1. [DOI] [PubMed] [Google Scholar]
- 15.Buchy P, Follézou JY, Lien TX, An TT, Tram LT, Tri DV, Cuong NM, Glaziou P, Chien BT. Serological study of toxoplasmosis in Vietnam in a population of drug users (Ho Chi Minh city) and pregnant women (Nha Trang) Bull Soc Pathol Exot. 2003;96:46–47. [PubMed] [Google Scholar]
- 16.Liu Q, Wei F, Gao S, Jiang L, Lian H, Yuan B, Yuan Z, Xia Z, Liu B, Xu X, Zhu XQ. Toxoplasma gondii infection in pregnant women in China. Trans R Soc Trop Med Hyg. 2009;103:162–166. doi: 10.1016/j.trstmh.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Wong A, Tan KH, Tee CS, Yeo GS. Seroprevalence of cytomegalovirus, Toxoplasma and parvovirus in pregnancy. Singapore Med J. 2000;41:151–155. [PubMed] [Google Scholar]
- 18.Akinbami AA, Adewunmi AA, Rabiu KA, Wright KO, Dosunmu AO, Dada MO, Adeyemo TA. Seroprevalence of Toxoplasma gondii antibodies amongst pregnant women at the Lagos State University Teaching Hospital, Nigeria. Niger Postgrad Med J. 2010;17:164–167. [PubMed] [Google Scholar]
- 19.Maggi P, Volpe A, Carito V, Schinaia N, Bino S, Basho M, Dentico P. Surveillance of toxoplasmosis in pregnant women in Albania. New Microbiol. 2009;32:89–92. [PubMed] [Google Scholar]
- 20.Nissapatorn V, Noor Azmi MA, Cho SM, Fong MY, Init I, Rohela M, Khairul Anuar A, Quek KF, Latt HM. Toxoplasmosis: prevalence and risk factors. J Obstet Gynaecol. 2003;23:618–624. doi: 10.1080/01443610310001604376. [DOI] [PubMed] [Google Scholar]
- 21.Sroka S, Bartelheimer N, Winter A, Heukelbach J, Ariza L, Ribeiro H, Oliveira FA, Queiroz AJ, Alencar C, Jr, Liesenfeld O. Prevalence and risk factors of toxoplasmosis among pregnant women in Fortaleza, northeastern Brazil. Am J Trop Med Hyg. 2010;83:528–533. doi: 10.4269/ajtmh.2010.10-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montoya JG. Laboratory diagnosis of Toxoplasma gondii infection and toxoplasmosis. J Infect Dis. 2002;185:S73–S82. doi: 10.1086/338827. [DOI] [PubMed] [Google Scholar]
- 23.Remington JS, Thulliez P, Montoya JG. Recent developments for diagnosis of toxoplasmosis. J Clin Microbiol. 2004;42:941–945. doi: 10.1128/JCM.42.3.941-945.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cruz ML, Cardoso CA, Saavedra MC, Santos ED, Melino T. Congenital toxoplasmosis infection in an infant born to an HIV-1-infected mother. Braz J Infect Dis. 2007;11:610–611. doi: 10.1590/s1413-86702007000600017. [DOI] [PubMed] [Google Scholar]
- 25.Spalding SM, Amendoeira MR, Klein CH, Ribeiro LC. Serological screening and toxoplasmosis exposure factors among pregnant women in South of Brazil. Rev Soc Bras Med Trop. 2005;38:173–177. doi: 10.1590/s0037-86822005000200009. [DOI] [PubMed] [Google Scholar]
- 26.Lopes FM, Mitsuka-Breganó R, Gonçalves DD, Freire RL, Karigyo CJ, Wedy GF, Matsuo T, Reiche EM, Morimoto HK, Capobiango JD, Inoue IT, Garcia JL, Navarro IT. Factors associated with seropositivity for anti-Toxoplasma gondii antibodies in pregnant women of Londrina, Paraná, Brazil. Mem Inst Oswaldo Cruz. 2009;104:378–382. doi: 10.1590/s0074-02762009000200036. [DOI] [PubMed] [Google Scholar]
- 27.Mohammad HI, Amin TT, Balaha MH, Moghannum MS. Toxoplasmosis among the pregnant women attending a Saudi maternity hospital: seroprevalence and possible risk factors. Ann Trop Med Parasitol. 2010;104:493–504. doi: 10.1179/136485910X12786389891443. [DOI] [PubMed] [Google Scholar]
- 28.Ertug S, Okyay P, Turkmen M, Yuksel H. Seroprevalence and risk factors for Toxoplasma infection among pregnant women in Aydin province, Turkey. BMC Public Health. 2005;5:66. doi: 10.1186/1471-2458-5-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alvarado-Esquivel C, Alanis-Quiñones OP, Arreola-Valenzuela MA, Rodríguez-Briones A, Piedra-Nevarez LJ, Duran-Morales E, Estrada-Martínez S, Martínez-García SA, Liesenfeld O. Seroepidemiology of Toxoplasma gondii infection in psychiatric inpatients in a northern Mexican city. BMC Infect Dis. 2006;6:178. doi: 10.1186/1471-2334-6-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nijem KI, Al-Amleh S. Seroprevalence and associated risk factors of toxoplasmosis in pregnant women in Hebron district, Palestine. East Mediterr Health J. 2009;15:1278–1284. [PubMed] [Google Scholar]
- 31.Kapperud G, Jenum PA, Stray-Pedersen B, Melby KK, Eskild A, Eng J. Risk factors for Toxoplasma gondii infection in pregnancy. Results of a prospective case-control study in Norway. Am J Epidemiol. 1996;144:405–412. doi: 10.1093/oxfordjournals.aje.a008942. [DOI] [PubMed] [Google Scholar]
- 32.Jenum PA, Kapperud G, Stray-Pedersen B, Melby KK, Eskild A, Eng J. Prevalence of Toxoplasma gondii specific immunoglobulin G antibodies among pregnant women in Norway. Epidemiol Infect. 1998;120:87–92. doi: 10.1017/s0950268897008480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qublan HS, Jumaian N, Abu-Salem A, Hamadelil FY, Mashagbeh M, Abdel-Ghani F. Toxoplasmosis and habitual abortion. J Obstet Gynaecol. 2002;22:296–298. doi: 10.1080/01443610220130616. [DOI] [PubMed] [Google Scholar]
- 34.Avelino MM, Campos D, Jr, Parada JB, Castro AM. Risk factors for Toxoplasma gondii infection in women of childbearing age. Braz J Infect Dis. 2004;8:164–174. doi: 10.1590/s1413-86702004000200007. [DOI] [PubMed] [Google Scholar]
- 35.Ayi I, Edu SA, Apea-Kubi KA, Boamah D, Bosompem KM, Edoh D. Sero-epidemiology of toxoplasmosis amongst pregnant women in the greater accra region of Ghana. Ghana Med J. 2009;43:107–114. doi: 10.4314/gmj.v43i3.55325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dabritz HA, Conrad PA. Cats and Toxoplasma: implications for public health. Zoonoses Public Health. 2010;57:34–52. doi: 10.1111/j.1863-2378.2009.01273.x. [DOI] [PubMed] [Google Scholar]
- 37.López-Castillo CA, Díaz-Ramirez J, Gómez-Marín JE. Risk factors for Toxoplasma gondii infection in pregnant women in Armenia, Colombia. Rev Salud Publica (Bogota) 2005;7:180–190. doi: 10.1590/s0124-00642005000200006. [DOI] [PubMed] [Google Scholar]
- 38.Asthana SP, Macpherson CN, Weiss SH, Stephens R, Denny TN, Sharma RN, Dubey JP. Seroprevalence of Toxoplasma gondii in pregnant women and cats in Grenada, West Indies. J Parasitol. 2006;92:644–645. doi: 10.1645/GE-762R.1. [DOI] [PubMed] [Google Scholar]
- 39.Hung CC, Fan CK, Su KE, Sung FC, Chiou HY, Gil V, da Conceicao dos Reis Ferreira M, de Carvalho JM, Cruz C, Lin YK, Tseng LF, Sao KY, Chang WC, Lan HS, Chou SH. Serological screening and toxoplasmosis exposure factors among pregnant women in the Democratic Republic of Sao Tome and Principe. Trans R Soc Trop Med Hyg. 2007;101:134–139. doi: 10.1016/j.trstmh.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 40.Lin YL, Liao YS, Liao LR, Chen FN, Kuo HM, He S. Seroprevalence and sources of Toxoplasma infection among indigenous and immigrant pregnant women in Taiwan. Parasitol Res. 2008;103:67–74. doi: 10.1007/s00436-008-0928-1. [DOI] [PubMed] [Google Scholar]


