Abstract
Development of new genetic approaches to either interfere with the ability of mosquitoes to transmit dengue virus or to reduce vector population density requires progressive evaluation from the laboratory to contained field trials, before open field release. Trials in contained outdoor facilities are an important part of this process because they can be used to evaluate the effectiveness and reliability of modified strains in settings that include natural environmental variations without releasing mosquitoes into the open field. We describe a simple and cost-effective semi-field system designed to study Aedes aegypti carrying a dominant lethal gene (fsRIDL) in semi-field conditions. We provide a protocol for establishing, maintaining, and monitoring stable Ae. aegypti population densities inside field cages.
Introduction
During the past decade, containment of genetically modified (GM) arthropods has become an important issue among people working in the field of vector-borne diseases. Development of genetically manipulated strains of arthropods unable to transmit pathogens1 or carrying sterility genes2 includes assessing the effects of genetic modifications on vector biology in semi-field conditions.3,4 Proper trials in semi-field systems, as defined by Ferguson and others,5 are essential to investigate possible changes in fitness and/or behavior of genetically modified vectors that may affect their compatibility with the natural environment and their competitiveness with wild-type conspecifics.6 If not detected, fitness costs and behavioral changes could cause genetic control strategies to fail.7
To test transgenic insect technologies and effector genes in semi-field conditions, it is important to meet certain requirements,5,8 such as, 1) developing semi-field structures of suitable size for target organisms that assure containment and prevent accidental release; 2) locating structures in an ecologically isolated area where the target vector and pathogen are already present; 3) reproducing as closely as possible all essential ecological conditions for the vector at the field site (i.e., temperature, humidity, solar radiation, wind, availability of larval development sites, mating sites, adult refugees, and food for immatures and blood hosts for adult females); 4) having the capacity to establish and maintain a stable, local vector population through several overlapping generations (i.e., caged populations should simulate free-ranging target populations as much as possible during GM arthropod evaluations); and 5) fine tuning methodology for measuring changes in population density (i.e., detect increases or decreases in the number, genotype and/or phenotype through time). Having facilities that are well characterized and validated procedures for establishing and maintaining a local population in a semi-field system for overlapping generations will allow researchers to test and modify, as necessary, the design and use of those facilities for evaluation of GM mosquitoes.
Herein, we report the design of a semi-field system and a protocol for its use. Our aim was to establish and maintain a stable wild-type Aedes aegypti population in an enclosed outdoor environment near Tapachula, Mexico, as a first step in conducting contained field trials with Ae. aegypti. In subsequent experiments we will evaluate Ae. aegypti carrying a dominant lethal gene (fsRIDL)2 (OX3604C) for suppression of local, wild-type mosquito populations in field cages similar to those we used in this study.
Materials and Methods
Study area.
Our study was carried out on a plot of land (14°51′41²N, −92°21′15²W) referred to hereafter as the “field site.” The land was located 11.2 km south-east from the center of Tapachula in the village of El Zapote and consisted of a 4.5 ha flat, rural area with 1.5 ha cultivated in cashew trees. The rest of the plot is grassland used for grazing cattle. Surrounding land is mainly cultivated with mango trees, soya beans, corn, and banana trees. Climate in the area is characterized by a rainy season during the summer months (May to October) with an average of 2,100-mm rainfall and a dry season during the winter months (November to April) with an average of 50-mm rain.
Cage design and maintenance.
Our semi-field system consisted of a 2.5 × 5.5 × 2 m (w × l × h) tent cage (Figure 1A and D) made of white tricot mesh, reinforced at angles and seams with white fabric (Figure 1B). It had four single zipper doors, one per side, which allows access to work in the internal space and entry into the cage through the side (where we observed the smallest number of mosquitoes resting on the tent walls). Entry through the side door minimized the possibility for mosquitoes to escape. The tent cage was supported by a steel frame designed for an outdoor canopy (model 1020-8, Galaxy Orion, China, Figure 1C), measured 3.0 × 6.0 × 3.0m (w × l × h) that had a white plastic roof, which partially protected the tent cage from rain and direct sunlight. Twelve windows in the canopy, six on each side of the roof, facilitated air circulation between the tent and the roof (Figure 1D) and decreased wind pressure on the roof itself. The canopy and tent cage were fastened on a wooden platform (8.0 × 5.0 × 0.8 m, Figure 1C). The platform was fastened to a metal frame that elevated the entire structure 80 cm above the ground and avoided flooding during the rainy season. A white plastic sheet covered the floor of the cage to ensure a tidy, easy to clean environment. The whole structure was isolated from the ground by 15 ant traps (i.e., metal buckets filled with water and soap; Figure 1E) under the legs of the platform, which was intended to minimize ground access to the cage by arthropods and small vertebrates. A plastic sheet laid on the ground below the platform (Figure 1E) prevented grass and other plants from growing under the cage and eventually touching the platform, which could be a means for arthropods and small animals to climb onto the platform and eventually into the cage. The following items were placed inside the cage to provide refugee for mosquitoes (Figure 1D and F): a potted plant (Dieffenbachia spp.), an open black closet made of wooden boxes (100 × 30 × 140 cm), two clay pots 10 L each containing 3 cm of soil, two stacks of clay bricks (50 × 50 × 50 cm) with an open inner space where mosquitoes could rest (Figure 1D and F), one dispenser holding water-soaked cotton, and two dispensers holding 10% sugar solution soaked cotton that were changed every second day, and one data-logger (Hobo Pro v2 temp/RH, Onset Computer Corporation, Bourne, MA) hanging in the middle of the cage so that it was protected from direct sunlight and could record temperature and humidity every 30 min. Clay resting sites were moistened every second day and the plant was watered as needed.
Figure 1.

Cage design: (A) field cage; (B) tricot mesh reinforced at critical points with white fabric; (C) steel frame of an outdoor canopy located on a metal platform covered with wooden boards; (D) 12 windows, 6 on each side of the roof facilitate air flow are denoted by arrows; (E) metal containers filled with water and soap under legs of the platform to partially isolate the structure from the ground and blue plastic sheet under the platform to prevent plant growth; and (F) refugia to create places where adults can rest inside the tent cage.
We built two identical tent cages, labeled cage A and cage B. Cages were partially protected from sunlight by shade created by nearby cashew trees. Because the semi-field systems were used to contain locally derived Ae. aegypti, we did not include vestibules, a double layer of mesh for cage walls or equipment like air curtains as a means to increase containment.4 Cage tests were performed over an 11-month period from November 2008 to September 2009 (Table 1).
Table 1.
Schematic timeline field cage trials
| Nov 08 | Dec 08 | Jan 09 | Feb 09 | Mar 09 | Apr 09 | May 09 | Jun 09 | Jul 09 | Aug 09 | Sep 09 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Population dynamics | |||||||||||
| Female mortality dynamics | |||||||||||
| Adult production rate | |||||||||||
| Definitive trial |
Aedes aegypti strain.
In our trials we used mosquitoes from an Ae. aegypti colony established at the Centro Regional de Investigación en Salud Pública (CRISP) insectary in Tapachula during 2007. Aedes aegypti were colonized from eggs collected in El Zapote using 20 ovitraps. Subsequently, eggs were collected weekly in El Zapote and larvae were reared at the CRISP insectary. All adults that developed from those eggs (an average of 60 per week), were added to the colony to limit inbreeding effects. Larvae reared in the insectary and during experiments were fed a diet of laboratory rodent food (ground laboratory rodent food by LabDiet, Richmond, IN) and adult females imbibed blood from a rabbit (UC Davis Animal Care and Use protocol 15653). Adult males and females had access to water and a 10% sugar solution.
Temperature and relative humidity.
Temperature and relative humidity (RH) are important environmental parameters that affect adult mosquito biology.9 In semi-field systems, particular attention must be paid to fluctuations in temperature and RH values so that they are similar to those recorded in the open field surrounding the cages. Dataloggers were used to compare temperature and RH in cage A (the first cage built) to the open field outside the cage from June 23, 2008 to July 21, 2008 (rainy season). The datalogger inside the cage was hung from the roof in the middle of the tent. The other external datalogger was located less than 2 m from the cage in a shaded place, protected from rain, direct sunlight, and at the same height as the one located inside the cage. During a 28-day period dataloggers recorded temperatures and RH at the same time every 5 minutes for a total of 8,064 measurements. During the 76 days from 3 March to 7 May 2009 (dry to rainy season) temperature and RH were simultaneously recorded (every 30 min) in cages A and B to confirm that environmental conditions in the two cages were similar.
Field cage studies.
Field experiments (Table 1) are divided into 1) pre-trial tests of key aspects of Ae. aegypti biology and 2) a definitive trial, when we attempted to stabilize the density of an Ae. aegypti population in the cages.
Pre-trial tests.
Pre-trial tests were carried out to obtain data on Ae. aegypti population dynamics, adult production rate, and immature development time in the semi-field structure.
Population dynamics.
Two pre-trial tests were carried out between November 2008 and February 2009 to investigate whether the cages were suitable to host Ae. aegypti populations and to obtain data on population dynamics in the cages. During each pre-trial test, 300 2-day-old males and females (150 of each sex), were released into each cage. A restrained rabbit was provided daily for half an hour as a source of blood (UC Davis Animal Care and Use protocol 15653). Six water holding containers that were different in shape, size, color, and material were lined with paper strips, filled with purified water to have a total liquid high of about 10 cm, placed into each cage, and checked daily. Each day paper strips with eggs were removed and containers were refreshed with purified water and new paper strips.
The number of mosquitoes resting on the internal walls of each cage, females feeding on the rabbit and eggs laid on the paper strips were counted daily. The data were used as indirect measures of Ae. aegypti population dynamics. The two pre-trial tests ended after different time intervals. The first trial started during November 2008 and ended on Day 34. The second trial started during February 2009 and ended on Day 27.
Female mortality dynamics.
At the end of each trial, surviving mosquitoes were collected using backpack aspirators (model 1412, John W. Hock Company, Gainesville, FL), killed by freezing, counted, and sexed. To confirm our data on female mortality through time, three additional tests were performed as described previously during the rainy season (June–September 2009). Adults were introduced into cages as larvae and completed their larval development in the field cages. Six hundred first instar larvae were seeded in three mesh-covered buckets containing 2 L of water (200 larvae per container) in each field cage. Adults emerging from the containers were released into the cages until 300 males and females (150 of each sex) per cage were reached. Adults were maintained as described previously until they were collected. Adult mosquitoes were collected on Days 2, 10, and 15. To estimate the female mortality trajectory, nonlinear regression analysis was applied to the number of surviving females collected at each time interval.
Adult production rate.
To maintain a stable population inside the cages, it is necessary to introduce mosquitoes at certain time intervals to compensate for adult mortality. Among several different release approaches (i.e., release of adults, immature stages, or eggs) we based this study on eggs. By seeding mesh-covered containers inside cages with a certain number of recently oviposited eggs, we could obtain adults that experienced the same environmental conditions as larvae and control the exact number and sex of adults produced in the containers; freshly emerged adults were counted and sexed before introducing them into each cage.
To apply this introduction method during the definitive trial, it was necessary to determine the number of adults produced after seeding a known number of eggs into a container and the minimum and maximum larval development times. For this purpose, eight 7-L plastic buckets were filled with 2 L of purified water, seeded with a total of 300 embryonated eggs on paper strips, and containers were covered with a mesh to prevent deposition of additional eggs by wild mosquitoes. The buckets were placed on a table 2 m from the field cages in a shaded area. Larval food was added ad libitum. Paper strips were removed from the buckets on Day 5 to avoid mold development. Larvae were filtered and buckets were refreshed every fifth day with clean water and food to prevent excessive fungal and bacterial growth in larval rearing water. Development times from egg to adult emergence were recorded. Adults emerging from containers were aspirated out of containers, counted daily, and sexed. The trial ended when no larvae were detected in any bucket.
Definitive trial.
On the basis of results from pre-trial tests, the following protocol was developed and tested from 3 March to 17 May 2009 as a means of maintaining stable Ae. aegypti population densities inside field cages.
A cohort of 300 male and female (150 of each sex) 2-day-old Ae. aegypti was introduced into each tent cage. Mosquitoes had access to water and 10% sucrose solution (both renewed every 2 days). Once a week, a restrained rabbit was introduced into each cage to provide a blood meal for females.
Two days after blood feeding, three oviposition containers (2, 6, and 11 L, which corresponded to those containers that collected the highest number of eggs during pre-trial tests) were lined with filter paper and placed into each cage. Containers were removed after 48 hrs. Oviposited eggs were brought back to the CRISP laboratory, counted, dried for 24 hrs, and kept in a labeled zip-lock bag.
The number of mosquitoes resting on the internal walls of the cages was recorded daily between 8:00 AM and 10:00 AM. The number of females taking a full blood meal was visually estimated while the rabbit was in the cage. The number of eggs produced weekly was recorded.
Each week 300 eggs in a mesh-covered, 7-L plastic container filled with 2 L of water and food ad libitum were placed into the cage. All 300 eggs that had been produced the previous week by mosquitoes in one cage went back into the same cage the following week. To minimize using eggs laid by one or a few females, we cut small pieces of paper containing a maximum of 30 eggs from different paper strips until 300 eggs were obtained from Week 2 on. During Week 1, eggs from the insectary colony were used.
Adults emerging from the egg container were counted, sexed, and released into their respective cage. On the basis of pre-trial tests, we assumed that the development time from egg to adult took a maximum of 2 weeks and, therefore, on a weekly basis there were two containers in each cage that asynchronously produced adults. Containers were removed from cages after 14 days. In a few cases larvae (always < 5) were still developing and were discarded.
After 11 weeks, the trial ended and all mosquitoes in each cage were collected using back pack aspirators, brought back to the insectary, killed by freezing, counted, and sexed.
One of the key objectives of our study was to develop methodology for detecting population density changes in the field cages. Although measures of population density could be achieved using Back Pack Aspirators or BG-Sentinel Traps, which were specifically developed to collect Ae. aegypti adults,10 we tried to use approaches that would not damage mosquitoes or adversely affect their behavior or survival. Our aim was to develop a methodology for maintaining stable populations (Ro ∼ 1), without perturbing mosquito activity or survival, so that in subsequent trials we will be able to confidently detect fluctuations in population density through time that is attributable to the introduced genetically modified mosquitoes.
Statistical analysis.
SPSS 15.0 (SPSS Inc., Chicago, IL) was used for statistical analysis.
Temperature and Relative Humidity (RH).
Mann-Whitney U tests were performed to compare temperature and RH between indoor and outdoor environments and between cages A and B. Spearman rank correlation tests were used to compare the number of adults resting on the internal walls of cages to temperature and RH, which were recorded when mosquitoes were counted.
Pre-trial tests.
Population dynamics data from the first 27 days were not normally distributed and could not be normalized with log transformation. The nonparametric Mann-Whitney U test was used, therefore, to determine differences between replicates in distributions of adults, blood-fed females, and eggs. We tested for a difference in egg production between cage A and B during November's pre-trial test using the χ2 test. Kruskal-Wallis tests were performed to investigate differences in distributions of adults, fed females, and eggs between the two pre-trial tests. Linear regression analysis was applied to the mean number of adults counted daily in both trials and to the number of females collected in cages at different time intervals. The latter analysis was used to build best fit curves describing adult and female mortality dynamics. A Spearman rank correlation test was used to compare the distribution of the daily mean number of eggs collected with the expected number of females obtained from the curve describing female mortality dynamics in pre trials.
Definitive trial.
The Mann-Whitney U test was used to compare the mean number of adults produced in containers in cage A versus cage B. Linear regression analysis was applied to the number of eggs collected weekly from cages A and B.
Results and Discussion
Costs.
All components used to build the semi-field system were purchased in Tapachula and only the outdoor canopy was produced outside of Mexico. Wooden boards for the floor, fabric and tricot for the cage walls were easily obtained local materials that helped minimize costs. The most expensive component (75% of the total cost) was for the metal frame of the wooden platform, which was caused by the relatively high price of steel and labor cost. Each semi-field system cost the equivalent of US $2,500.
Temperature and RH.
Open field versus cage.
Temperature ranged outdoors between 21.2 and 38.1°C, median = 24.8°C, and inside cages between 21.2 and 39.4°C, median = 24.9°C. There were no significant differences in temperatures between environments inside and outside of the cage (U = 32,726,092, P = 0.48). During the same period RH ranged between 41.1% and 97.4% (outdoors) and 41.0% and 96.8% (indoors). The RH was slightly higher outside (median = 92.5%) than inside the cage (median = 91.8%) and the difference was statistically significant (U = 35,785,316.5, P = 0.002).
Cage A versus B.
During the definitive trial we did not detect a significant difference in temperature between cages (range: cage A 15.7 to 41.3°C and cage B 15.6 to 41.7°C; median temperature cage A = B = 26.5°C, U = 6,542,179.5, P = 0.19). During the same period, RH ranged from 23.5% to 95.8% in cage A and 22.1% to 98.2% in cage B. Median RH was slightly higher in cage B (78.0%) than cage A (75.8%). The difference was statistically significant (Mann-Whitney U test: U = 6,894,466.5, P < 0.001).
The statistically significant differences in RH, but not in temperature, between inside and outside cage environments and between cage A and cage B are likely because of the equation for calculating RH-based ambient temperature (T) and dew point temperature (D): (RH = 100*[(D + d)/(T + d)]a*10b[1/(D+d)−1/(T+d)] where “a,” “b,” and “d” are constants).11 In this model the relationship between temperature and water vapor content in the air is exponential. This means that when barometric pressure and absolute humidity are constant, for each increase in temperature there is a relatively large decrease in RH. The small differences in temperature recorded during our study can, therefore, lead to statistically significant differences in RH that are not likely to be biologically meaningful. We concluded that temperature and RH for our cage design is a reasonable approximation to an open, natural habitat.
The first topic that arose before starting to populate the cages concerned the appropriate Ae. aegypti population density per cage. Ferguson and others5 state that “There are no general guidelines for the appropriate size of such a unit, but ideally it should be large enough to sustain a population of similar density to that encountered in the target environment for numerous generations.” Unfortunately, this is not feasible in the case of Ae. aegypti unless a semi-field system is the size of a small village, because Ae. aegypti adults are typically present in natural environments at low densities,12 which we confirmed for communities in the vicinity of our study area (Bond JG and others, unpublished data). To have populations of sufficient size to obtain statistically valid results (i.e., to avoid populations so small that outcomes of experiments occur by chance), Ae. aegypti densities in semi-field systems must be artificially increased. Data from previous studies on Ae. aegypti population management in large indoor insectaries at Colorado State University (Wise de Valdez MR, personal communication), indicate that experimental populations fluctuating between 300 and 500 individuals per cage of similar size to those used in this study is appropriate for meaningful experimental purposes.
Pre-trial tests.
Population dynamics.
During each of the two pre-trial tests, Mann-Whitney U tests did not detect differences between replicates (cage A versus cage B) in the distribution of adults (November U = 433.5, P = 0.53; February U = 281, P = 0.21), blood-fed females (November U = 581.5, P = 0.14; February U = 464.5, P = 0.24), or eggs (February U = 302.5, P = 0.85). The only significant difference was in egg production between cage A and cage B during November (U = 566.0, P = 0.02), when females in cage A produced fewer eggs compared with those in cage B (16,311 versus 25,422, χ2 = 1006.1, degrees of freedom [df] = 1, P < 0.01). This appears to be caused by unrecognized factor(s) that influenced oviposition in cage A. Data from cage A during November, therefore, were excluded from further statistical analysis because egg production was considered an outlier. Inclusion of adult and fed female distributions from cage A did not alter overall results.
During pre-trial tests between November and February no significant differences were observed in the distribution of adults (Kruskal-Wallis: H = 2.55, df = 2, P = 0.28) or eggs (Kruskal-Wallis: H = 0.85, df = 2, P = 0.65). The distribution of blood-fed females between the two trial periods were not comparable (Kruskal-Wallis: H = 10.28, df = 2, P < 0.01) and didn't provide useful information to understand Ae. aegypti population dynamics. Blood-fed females were, therefore, excluded from further statistical analysis. Figure 2 shows population dynamics based on the daily mean number of adults counted resting on cage walls during both pre-trial tests. The best fit curve for adult density based on this data was a logarithmic function y = −37.0ln(x) +123.8, R2 = 0.93. According to this model, at Day 1 (x = 1) only 123 mosquitoes would have survived inside the cage, which corresponds to a 41% survival rate after just 24 hours post release into the cage. After 1 week the number of adults would drop to 52 (17.3%) and then slowly decrease until Day 29 when the population in the cage would be extinct. This is inconsistent with our results for maximum female lifespan (33 days during November pre-trial) and highlights limitations in our ability to visually count resting mosquitoes. After release and blood feeding, many adults appeared to seek out humid and dark locations to rest. When they were in those locations it was impossible for us to accurately observe and count them. In addition, male lifespan is expected to be shorter than that of females.13 Consequently, it would be best to count the two genders separately, but it was not feasible for us to accurately distinguish between males and females while counting them through the mesh. For these reasons, we conclude that the number of adults resting on the internal walls of a cage is not a dependable means of measuring adult population dynamics.
Figure 2.
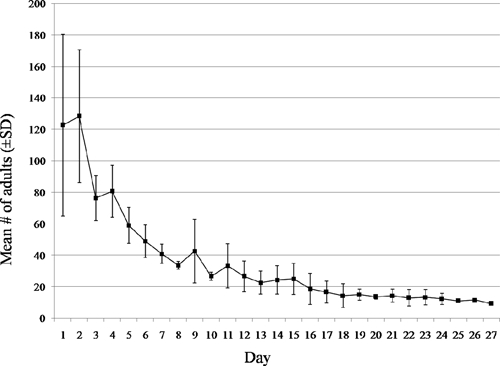
Observed daily mean number (bars represent standard deviation) of adults resting on the internal walls of cages during population dynamics pre-trial tests (300 adults released on Day 0).
Female mortality dynamics.
More reliable information was obtained from collecting adult mosquitoes. The number of females collected inside the cage when pre-trial tests were interrupted (130, 48, 30, 24, and 0 at Day 2, 10, 15, 27, and 34, respectively) was modeled using a logarithmic function (y = −43.7ln(x) + 156, R2 = 0.97) to describe female mortality through time (Figure 3). According to this equation, on Day 1 (x = 1) 125 females would be alive inside the cage (83.3% survival rate), 71 females would be alive on Day 7 (47.3%), and then their number would slowly decrease until Day 35 when all mosquitoes in the cage would be dead. These results are in agreement with what we observed in field cages. Consequently, this function was used to calculate the number of recently emerged females we needed to introduce into each cage (79 during the first week) to account for mortality.
Figure 3.
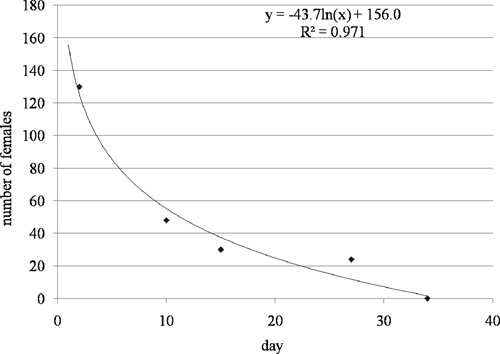
Female mortality dynamics described by the logarithmic function y = −43.7ln(x) + 156. Diamonds represent the number of females collected in the cages when pre-trial tests were interrupted.
Even though mortality was not constant during the first few weeks of the definitive trial, to develop a standardized protocol we decided to introduce a constant number of females weekly until the population stabilized. Because we assumed a 1:1 sex ratio, we decided to introduce a similar number of females and males into field cages each week.
After calculating the replacement rate, we sought indicators of adult abundance that could be used to detect fluctuations in population density. The indicator we selected was the trend in the daily mean number of eggs produced during pre-trial tests (Figure 4). The mean number of eggs laid per day in cages steadily decreased through time according to the expected number of females during the previous day, which was calculated from the mortality regression curve; the two datasets were significantly correlated (Spearman rank correlation test: ρ = 0.69, P < 0.01). It was not feasible to correlate eggs produced in a short temporal window (i.e., a few days) to the number of females because of variation in the length of gonotrophic cycles and to differences in oviposition behavior. Length of a gonotrophic cycle can differ among individuals14 and females can lay eggs over a series of days in different oviposition sites.15 These complications can create fluctuations in daily measures of egg production and make it difficult to retrospectively estimate over a short temporal window the number of ovipositing females and by extension the total number of females present in a cage. Our results do indicate, however, that eggs laid over a relatively long period of time, such as 4 weeks during pre-trial tests, is a reasonable indirect measure of fluctuations in female population density in our semi-field system.
Figure 4.
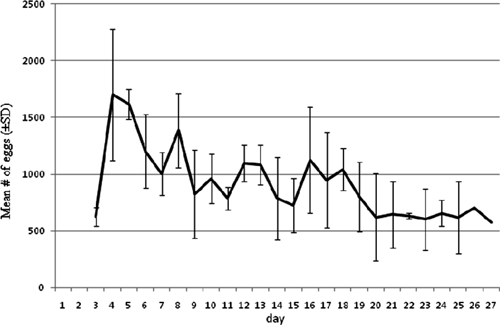
Mean number of eggs produced daily in population dynamics pre-trial tests.
Adult production from eggs and immature development time.
The average number of males and females produced from containers located close to the cages was 133 ± 15 (SD) and 124 ± 11 (SD), respectively. This means that in a time frame of 14 days, about 85% of seeded eggs developed into adults. Development time required from egg to adult ranged from 8 to 15 days; 90% of males emerged between Day 8 and 9, and 90% of females emerged between Day 9 and 11. More than 99% of adults emerged within 14 days. These results were useful for calculating the number of eggs (181) that should go back into a cage during the first week of the trial to compensate for death predicted by the female mortality curve (79 females). Because we expected that fluctuations of mosquito density caused by environmental factors at a field site could be high, we decided to increase the number of adults that we introduced into cages to avoid abrupt population reductions. For this reason, each week we added 300 eggs to containers in each cage (see Material and Methods, point 4).
Trends in female density were estimated on the basis of daily mortality of introduced females (150 at Day 0), followed by the daily mean number of females emerging from containers. Figure 5 shows results from a simulation in which female numbers decreas from Day 0–7 and then slowly increase until the population density stabilizes on Day 42, after which population density fluctuates between a minimum of 134 and a maximum of 190 females.
Figure 5.
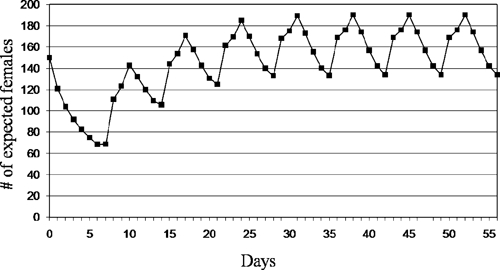
Simulation of expected female population dynamics in a field cage. A female mortality curve was applied to the number of females that were introduced into a cage on Day 0 and then to the daily mean number of females that emerged weekly from containers inside the cage.
Definitive trial.
Adults produced in cages.
During the definitive trial, eggs seeded in containers inside cages A and B produced females per week (mean of 87 [95% CI = 80.80–93.20], U = 25, P = 0.95), which were significantly lower than those obtained during the pre-trial test (mean of 124 [95% CI = 116.38–131.62]). The only difference between the pre-trial test and the definitive trial was that in the first case larvae were reared in the field, close to cages in a completely shaded area. During the definitive trial direct sunlight hit the containers located inside the cages for ∼2 hours daily. Because air temperature was not statistically different between the indoor and outdoor environment, sunlight may have adversely affected egg hatch rate and/or larval survival.
According to the simulation described previously, lower production of adults will lead to different expected fluctuations in female density compared with those showed in Figure 5. In this case, once stabilized, expected mosquito density will fluctuate between 94 and 125 females.
Population dynamics.
Linear regression analysis indicated that the number of eggs collected weekly in cage A decreased over time (y = −28.17x + 2,264.65, r2 = 0.66, P < 0.01) until the end of the trial, indicating that the population in cage A did not stabilize (Figure 6). This conclusion was supported by the low number of adults collected in cage A at the end of the trial (48 females and 34 males). Conversely, in cage B (Figure 6) the number of eggs collected decreased only slightly over time as illustrated with the horizontal regression line (y = −7.09x + 1744.45, r2 = 0.049, P = 0.51). The number of females collected in cage B was close to the lower number expected based on the simulation, 85 and 94, respectively. We suspect that the difference in population dynamics between cage A and B was caused by damage to the plastic roof of cage A. On Day 41, during a storm, cashew fruits fell onto and tore the plastic roof, which could not be repaired. For the remainder of the trial, the damaged roof of cage A allowed direct sunlight and rainfall into the tent cage.
Figure 6.
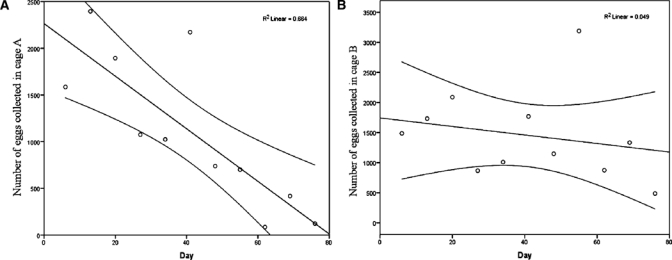
Regression line y = −28.17x + 2,264.65 representing the egg production trend in cage A (left), and regression line y = −7.09x + 1,744.45 representing the egg production trend in cage B (right) during definitive trial, with 95% confidence intervals.
Another potential complication affecting adult survival and egg production was predation. Even though we attempted to isolate cages from mosquito predators, when disassembled at the end of the trial, refugia in both cages contained Ae. aegypti legs and wings, something that is typical of predation by ants. This indicates that, although our study was carried out in confined and partially isolated cages, predation was probably an issue that to some extent influenced adult survival. We were not able to determine if the remains of the mosquitoes we found were caused by scavenged carcasses or insects that were killed by a predator. Adding vestibules with sleeved access to cages may help reduce predator entry into cages.
Visual counts of adults resting on the internal walls were not a useful indicator of population dynamics. This conclusion was confirmed by the observation that the number of adults counted resting on cage walls was inversely correlated to temperature recorded just before starting the count (Spearman rank correlation test: cage A ρ = −0.41, P < 0.01; cage B ρ = −0.24, P < 0.05). Unexpectedly, no correlation was found with RH (Spearman rank correlation test: cage A ρ = 0.18, P = 0.14; cage B ρ = 0.04, P = 0.70), although temperatures and RH recorded in cages were inversely correlated (Spearman rank correlation test: cage A ρ = −0.85, P < 0.01; cage B ρ = −0.87, P < 0.01). This suggests that the lower number of mosquitoes resting on cage walls was a function of temperature (i.e., lower densities at higher temperatures) and indicates that the tendency to seek dark and perhaps cooler places as the resting sites was influenced more by temperature than by RH. In future studies, earlier inspection during cooler times of day (e.g., 7:00–8:00 am) should be explored to increase the opportunity to observe and count adults.
According to the female mortality curve, the corresponding daily mean survival rate over a 33-day period was 0.88 ± 0.07 (SD). This is comparable with Ae. aegypti survival rates estimated by McDonald16 and Trpis and others17 during mark release recapture experiments; i.e., 0.89. Our results indicate, however, that survival for Ae. aegypti females in our semi-field system is age dependent. The highest daily survival rate recorded (> 0.92) was between Day 8 and 18. Other authors have similarly discussed the issue of age-dependent mosquito survival. In a laboratory study in which they monitored survival of > 100,000 Ae. aegypti, Styer and others18 reported age-dependent changes in mortality. It was low at young ages (< 10 days old), steadily increased at middle ages, and decelerated at older ages. Similarly, Ae. aegypti survivorship during mark-recapture studies was not constant over the recapture period and decreased with age.19–23
Conclusions
Results from our trials are consistent with use of our semi-field system design and procedures protocol for contained field trials with Ae. aegypti. Our cage design is intended for use in trials with a self-limiting system; i.e., fsRIDL. For trials that require assessment of gene flow (i.e., transgene drive systems) investigators will need to determine if modifications in containment features or cage structure are needed; i.e., capacity to expand or collapse connections between cages to simulate varying barriers to movement among populations.
Our methods for calculating female mortality and the number of new females that should be introduced into a field cage per unit time allowed us to maintain a reasonably stable mosquito density, which will be important for assessing population reduction and transgene spread strategies. Egg production through time was a good proxy to detect fluctuations in population densities. The ability to monitor changes in population densities could be improved if a method is developed to sample adults directly without adversely affecting their fitness. Temperature inside cages was equivalent to that recorded outside. Although RH was different between indoor and outdoor environments and between the two cages, those differences were < 7% and, thus, we did not consider them a biological impediment to field trials. Temperature and RH are the most important environmental parameters to monitor and to attempt to keep under control so that cages are suitable for establishing and maintaining Ae. aegypti populations through time. In future studies, these parameters should be compared with those of Ae. aegypti resting sites within the local habitat.
If future research with GM mosquitoes requires double layers of mesh for containment purposes, we expect that it will affect airflow through cages and thus may increase internal temperature and alter humidity, compared with the single layer of mesh we used. In those cases, investigators may need to modify cage design to account for environmental differences inside cages compared with natural Ae. aegypti resting sites.
Cages should be built in an open space that minimizes or eliminates the risk of falling objects damaging the structure. Shade, which is important to prevent cages from overheating, can be provided with shade netting or other structural features that preserve desired environmental attributes without risking damage to the integrity of the structure.
Blood meals should be provided to females at least twice a week. This will allow at least two ovipositions per week and, thus, a larger number of data points that can be used to calculate and assess fluctuations in population density.
Although experiments were conducted in structures designed to be contained environments, invasion by predators can be an issue affecting target species mortality. For example, small airborne spiders can land on the cage and ants can forage or start new colonies in cages, even if attempts are made to isolate the cages from ants. Because the use of any insecticide must be avoided, in a few months cages located in a tropical environment can become a favorable habitat for predators that exploit prey enclosed in a limited setting. Predation can be minimized by carefully cleaning cages before starting an experiment and regularly checking them, and using a platform to support cages for evidence of predator invasion. In our trials, although very few ants were detected in the cages during normal trial activities, at the end of the trial ant colonies were discovered concealed between wooden boards on the platform, inside one flowerpot, and under the white plastic sheet covering the floor of a tent cage.
Environmental heterogeneity in cages is an important aspect for semi-field trials, because it gives Ae. aegypti the possibility to exploit different microhabitats in an enclosed environment. However, we emphasize that the more heterogeneous the cage environment, the more difficult it becomes to visually inspect the cages and detect mosquito predators. We suggest using simple resting sites such as black plastic buckets and avoiding structures or material because that could possibly create an environment in which predators can hide and establish populations; i.e., potted plants.
The semi-field system we built and used during the trial is cost-effective and has low maintenance costs, ∼US $300 per year. The plastic roof should be changed after 18 months and the tricot mesh cage after 10 months of use because plastic and fabric deteriorate in tropical areas caused by exposure to sunlight and high humidity.
With the addition of a vestibule, openings with sleeves to work inside the cage without having to enter the cage, and the use of proper security operation procedures, our semi-field system is suitable for contained field trials with genetically engineered mosquitoes, like those bearing sterility genes (i.e., RIDL) that do not require high security facilities.
ACKNOWLEDGMENTS
We are grateful to Juan Carlos Joo Chang, Luís Antonio García Rodas, Crystian Hidalgo Citalán Uriel, Hugo Cigarroa de Los Reyes, and Nallely Sofía Maza Ramos for technical assistance.
Footnotes
Financial support: This research was supported by funds from the Regents of the University of California from the Foundation for the National Institutes of Health through the Grand Challenges in Global Health Initiative (GC7 #316) and by a Pasteur Institute-Cenci Bolognetti Foundation grant to Laura Valerio.
Disclosure: This research benefited from discussions with working groups in the Research and Policy for Infectious Disease Dynamics (RAPIDD) program of the Science and Technology Directorate, Department of Homeland Security, and the Fogarty International Center, National Institutes of Health.
Authors' addresses: Luca Facchinelli and Laura Valerio, Department of Entomology, University of California, Davis, CA, E-mails: lfacchinelli@ucdavis.edu and lvalerio@ucdavis.edu. J. Guillermo Bond, Janine M. Ramsey, and M. Casas-Martinez, Centro Regional de Investigación en Salud Pública (CRISP), Instituto Nacional de Salud Pública (INSP), Chiapas, Mexico, E-mails: gbond@insp.mx, jramsey@insp.mx, and mcasas@insp.mx. Megan R. Wise de Valdez, Department of Microbiology, Immunology, and Pathology, Colorado State University, Fort Collins, CO, E-mail: megwise@lamar.colostate.edu. Laura C. Harrington, Department of Entomology, Cornell University, Ithaca, New York, E-mail: lch27@cornell.edu. Thomas W. Scott, Department of Entomology, University of California, Davis CA, and Fogarty International Center, National Institutes of Health, Bethesda, MD, E-mail: twscott@ucdavis.edu.
References
- 1.Franz AW, Sanchez-Vargas I, Adelman ZN, Blair CD, Beaty BJ, James AA, Olson KE. Engineering RNA interference-based resistance to dengue virus type 2 in genetically modified Aedes aegypti. Proc Natl Acad Sci USA. 2006;103:4198–4203. doi: 10.1073/pnas.0600479103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alphey L, Nimmo D, O'Connell S, Alphey N. Insect population suppression using engineered insects. Adv Exp Med Biol. 2008;627:93–103. doi: 10.1007/978-0-387-78225-6_8. [DOI] [PubMed] [Google Scholar]
- 3.Scott TW, Takken W, Knols BG, Boëte C. The ecology of genetic modified mosquitoes. Science. 2002;298:117–119. doi: 10.1126/science.298.5591.117. [DOI] [PubMed] [Google Scholar]
- 4.Benedict M, D'Abbs P, Dobson S, Gottlieb M, Harrington LC, Higgs S, James A, James S, Knols B, Lavery J, O'Neill S, Scott TW, Takken W, Toure Y. Guidance for contained field trials of vector mosquitoes engineered to contain a gene drive system: recommendations of a Scientific Working Group. Vector Borne Zoonotic Dis. 2008;8:127–166. doi: 10.1089/vbz.2007.0273. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson HM, Ng'habi KR, Walder T, Kadungula D, Moore SJ, Lyimo I, Russell TL, Urassa H, Mshinda H, Killeen GF, Knols BG. Establishment of a large semi-field system for experimental study of African malaria vector ecology and control in Tanzania. Malar J. 2008;20:158. doi: 10.1186/1475-2875-7-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helinski ME, Knols BG. Mating competitiveness of male Anopheles arabiensis mosquitoes irradiated with a partially or fully sterilizing dose in small and large laboratory cages. J Med Entomol. 2008;45:698–705. doi: 10.1603/0022-2585(2008)45[698:mcomaa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 7.Scott TW, Rasgon JL, Black WC, Gould F. In: Bridging Laboratory and Field Research for Genetic Control of Disease Vectors. Knols BG, Frontis LC, editors. Wageningen; The Netherlands: 2006. pp. 171–181.http://library.wur.nl/frontis/disease_vectors/16_scott.pdf (Fitness studies: developing a consensus methodology). Available at. Accessed February 2011. [Google Scholar]
- 8.Scott TW. Containment of arthropod disease vectors. ILAR J. 2005;46:53–61. doi: 10.1093/ilar.46.1.53. [DOI] [PubMed] [Google Scholar]
- 9.Kessler S, Guerin PM. Responses of Anopheles gambiae, Anopheles stephensi, Aedes aegypti, and Culex pipiens mosquitoes (Diptera: Culicidae) to cool and humid refugium conditions. J Vector Ecol. 2008;33:145–149. doi: 10.3376/1081-1710(2008)33[145:roagas]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 10.Williams CR, Long SA, Russell RC, Ritchie SA. Field efficacy of the BG-Sentinel compared with CDC Backpack Aspirators and CO2-baited EVS traps for collection of adult Aedes aegypti in Cairns, Queensland, Australia. J Am Mosq Control Assoc. 2006;22:296–300. doi: 10.2987/8756-971X(2006)22[296:FEOTBC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 11.Parish OO, Putnam TW. Equation for the Determination of Humidity from Dewpoint and Psychrometric Data. Washington DC: National Aeronautic and Space; 1977. Administration (NASA), Technical Note TN D-8401. [Google Scholar]
- 12.Scott TW, Morrison AC, Lorenz LH, Clark GG, Strickman D, Kittayapong P, Zhou H, Edman JD. Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: population dynamics. J Med Entomol. 2000;37:77–88. doi: 10.1603/0022-2585-37.1.77. [DOI] [PubMed] [Google Scholar]
- 13.Muir LE, Kay BH. Aedes aegypti survival and dispersal estimated by mark-release-recapture in northern Australia. Am J Trop Med Hyg. 1998;58:277–282. doi: 10.4269/ajtmh.1998.58.277. [DOI] [PubMed] [Google Scholar]
- 14.Reiter P. Oviposition, dispersal, and survival in Aedes aegypti: implications for the efficacy of control strategies. Vector Borne Zoonotic Dis. 2007;7:261–273. doi: 10.1089/vbz.2006.0630. [DOI] [PubMed] [Google Scholar]
- 15.Colton YM, Chadee DD, Severson DW. Natural skip oviposition of the mosquito Aedes aegypti indicated by codominant genetic markers. Med Vet Entomol. 2003;17:195–204. doi: 10.1046/j.1365-2915.2003.00424.x. [DOI] [PubMed] [Google Scholar]
- 16.McDonald PT. Population characteristics of domestic Aedes aegypti (Diptera: culicidae) in villages on the Kenya Coast I. Adult survivorship and population size. J Med Entomol. 1977;14:42–48. doi: 10.1093/jmedent/14.1.42. [DOI] [PubMed] [Google Scholar]
- 17.Trpis M, Hausermann W. Dispersal and other population parameters of Aedes aegypti in an African village and their possible significance in epidemiology of vector-borne diseases. Am J Trop Med Hyg. 1986;55:1263–1279. doi: 10.4269/ajtmh.1986.35.1263. [DOI] [PubMed] [Google Scholar]
- 18.Styer LM, Carey JR, Wang JL, Scott TW. Mosquitoes do senesce: departure from the paradigm of constant mortality. Am J Trop Med Hyg. 2007;76:111–117. [PMC free article] [PubMed] [Google Scholar]
- 19.Reisen WK, Mahmood F, Parveen T. Anopheles culicifacies Giles: a release-recapture experiment with cohorts of known age with implications for malaria epidemiology and genetical control in Pakistan. Trans R Soc Trop Med Hyg. 1980;743:307–317. doi: 10.1016/0035-9203(80)90089-9. [DOI] [PubMed] [Google Scholar]
- 20.Constantini C, Li S, della Torre A, Sagnon N, Coluzzi M, Taylor CE. Density, survival and dispersal of Anopheles gambiae complex mosquitoes in a West African Sudan savanna village. Med Vet Entomol. 1996;10:203–219. doi: 10.1111/j.1365-2915.1996.tb00733.x. [DOI] [PubMed] [Google Scholar]
- 21.Haramis LD, Foster WA. Survival and population density of Aedes triseriatus (Diptera: Culicidae) in a wood lot in central Ohio, USA. J Med Entomol. 1983;20:391–398. doi: 10.1093/jmedent/20.4.391. [DOI] [PubMed] [Google Scholar]
- 22.Harrington LC, Vermeylen F, Jones JJ, Kitthawee S, Sithiprasasna R, Edman JD, Scott TW. Age-dependent survival of the dengue vector, Ae. aegypti, demonstrated by simultaneous release and recapture of different age cohorts. J M Entomol. 2008;45:307–313. doi: 10.1603/0022-2585(2008)45[307:asotdv]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 23.Harrington LC, Edman JD, Costero AC, Clark GG, Kittayapong P, Scott TW. Analysis of survival of young and old Aedes aegypti (Diptera: Culicidae) from Puerto Rico and Thailand. 2001. J Med Entomol. 2001;38:537–547. doi: 10.1603/0022-2585-38.4.537. [DOI] [PubMed] [Google Scholar]


