Abstract
Upon mating, male mosquitoes transfer accessory gland proteins (Acps) that induce refractoriness to further mating in females. This can also occur because of cross-insemination by males of related species, a process known as mating interference (satyrization). This mechanism could explain the competitive displacement of resident Aedes aegypti by the invasive Aedes albopictus where they co-occur. We tested this hypothesis in mosquito populations in Florida. A new polymerase chain reaction species diagnostic applied to sperm dissected from 304 field-collected females revealed bidirectional cross-mating in five (1.6%) individuals. Cross-injections of females with Acps showed that Ae. albopictus males induced monogamy in heterospecific females but not Ae. aegypti males. Despite its low frequency in the areas under study, the first evidence of cross-mating in nature and the asymmetric effect of Acps on mating suggest that satyrization may have initially contributed to the observed competitive reduction of Ae. aegypti by invasive Ae. albopictus in many areas.
Introduction
Mating errors between biologically incompatible species may result in varying degrees of reproductive loss that decrease fitness.1 Ribeiro2 developed a model of asymmetric reproductive competition between two previously isolated species3 that considered the possible control of species, such as pests and vectors that have high reproductive rates. The model indicated that sterility induced by interspecific mating, also known as satyrization, was a more powerful force than Lotka-Volterra resource competition for producing competitive displacement or reduction.2,4 Although Ribeiro2 cited examples of the possible occurrence of satyrization in ticks, mosquitoes, and tsetse flies, knowledge about the impact of this phenomenon in natural populations has remained elusive.
Aedes albopictus (Skuse) and Aedes aegypti (L.) belong to different taxonomic divisions of the large subgenus Stegomyia and are native, respectively, to Asia and Africa.5,6 Both are notoriously invasive container species that play important roles as vectors of chikungunya, dengue, and other arboviruses that have extended their geographical distributions into temperate and tropical regions of all continents.7,8 In their native ranges, both species swarm and mate near hosts during the same peak morning and afternoon activity periods.9,10 Therefore, in the numerous areas of sympatry created by the spread of their invasive populations, virgin females of both species are likely to encounter heterospecific males attracted to the same hosts. This raises the question of how the two species, that share many morphological and behavioral traits but cannot produce viable hybrid offspring,11 avoid cross-mating in the field.
Working with multiple laboratory colonies, Leahy and Craig11 reported low frequencies of cross-mating in cages and identified structural incompatibilities of genitalia and differing responses to flight sounds as probable prezygotic isolating mechanisms that should discourage mating between these two species.12,13 Similar results and conclusions were reported on the basis of two Florida laboratory strains.14 However, another study found that cross-mating was common between Louisiana strains of Ae. aegypti and Ae. albopictus in the laboratory, and between wild Ae. albopictus and laboratory-reared Ae. aegypti females released in a Louisiana field site believed to contain only the former species.15 Importantly, this study also showed that, in cages, the rate of cross-insemination by Ae. albopictus males was significantly higher than that observed with Ae. aegypti males.15 These data indicate that asymmetric mating interference could constitute a potential explanation for the competitive reduction or displacement of Ae. aegypti in many habitats secondarily invaded by Ae. albopictus, such as the southeastern United States.4,16 However, some of the results of Nasci and others15 were not reproducible by other investigators.14 Whether interspecific mating occurs in natural populations of these species, at what frequency, and whether it is asymmetric has never been examined.
There is also evidence of differences in cross-reactivity of male accessory gland proteins (Acps), known to affect female physiology,17 between the two species. Cross-insemination experiments in cages showed that Ae. aegypti females oviposited sterile eggs after cross-mating with Ae. albopictus males, but that cross-inseminated Ae. albopictus females would not oviposit.18 Leahy and Craig19 also showed that heterologous male accessory gland implants resulted in oviposition of sterile eggs in Ae. aegypti females but no oviposition in Ae. albopictus females. These findings suggest that Ae. albopictus Acps trigger the switch to post-mating behavior—i.e., host finding, blood feeding, oogenesis, and oviposition—in Ae. aegypti females but that the converse does not apply for Ae. albopictus.
There have been very few attempts to measure the frequency of interspecific mating of closely related, sympatric mosquito species, such as Ae. aegypti and Ae. albopictus, which encounter one another frequently throughout their broad invasive and native ranges.7,20 This is largely caused by the lack of adequate tools for effectively detecting cross-mating in nature. For example, most of the information on cross-mating avoidance by these species has been gathered through cage experiments followed by examination of spermathecal contents13,15,18 and, therefore, may not adequately reflect what happens in the natural setting.
We set out to detect cross-inseminations in wild sympatric populations of Ae. aegypti and albopictus in southern Florida using techniques of sperm analyses developed in studies of cross-mating and sperm usage in the malaria mosquito Anopheles gambiae.21–23 We also tested whether females of both species from the same area of sympatry in southern Florida were made refractory to mating by injections with heterospecific male accessory gland (MAG) extracts,24 which could induce asymmetric mating interference. Our results shed light on mechanisms of competition between these two medically important vector species and are important for understanding their population dynamics and the epidemiology of the diseases that they transmit.
Materials and Methods
Evaluation of cross-mating frequencies in sympatric field populations.
Field collections.
Adults of both species were collected with power aspirators (John Hoch Co., Gainesville, FL) between 2005 and 2007 from two auto salvage yards in southern Florida where an abundance of disused automotive parts provides larval habitats for both species, often leading to high mosquito densities: M&K Used Auto Parts, 45th Street (27.7° N, 80.4° W) in Vero Beach (seven collections in 2005–2007), and Belle Glade Auto Salvage, Canal Street (26.7° N, 80.6° W) in Belle Glade (two collections in 2007).
Dissection of spermathecae and extraction of sperm.
Wild-caught females morphologically identified as Ae. aegypti or Ae. albopictus were stored in ethanol until 24 h before dissection when they were transferred to a new tube with water for rehydration. One day later, they were dissected in a drop of water on a microscope slide coated in Sigmacote (Sigma-Aldrich, St. Louis, MO). Under a binocular microscope and using micro-pins, each female's terminal abdominal segment was removed and transferred to a new droplet of water for further dissection. The one medial and two lateral spermathecae of Ae. aegypti or Ae. albopictus differ in size and will be referred to as 1st (large medial), 2nd (medium-sized lateral), and 3rd (small lateral) spermathecae. They were readily identified as brown-pigmented-spherical organs and excised, removing as much surrounding tissue as possible. Each spermatheca was then transferred to an individual, clean drop of water, and ruptured by applying gentle pressure on its capsule. Storage in 70% ethanol caused the proteinaceous fluid in the spermatheca to coagulate, making it possible to collect a “sperm bundle,” with the tip of a micro-pin.
The sperm bundles from each spermatheca were transferred to separate 0.2 mL Eppendorf tubes (Eppendorf, Hamburg, Germany) containing 4.5 μL of sample buffer from an Illustra GenomiPhi V2 DNA amplification kit (GE Healthcare, Little Chalfont, UK) and kept on ice or frozen until the DNA amplification steps (see next section).
The presence or absence of sperm in each spermatheca was recorded for all individuals and variation in insemination frequency between populations and species was analyzed by logistic regression using JMP 7.02 software (SAS Institute, Inc., Cary, NC, 1989–2007).
Whole-genome amplification of sperm DNA.
In preparation for genetic analysis, sperm DNA extract was used as template for a multiple-displacement amplification using the protocol and reagents provided in the Illustra GenomePhi V2 DNA amplification kit (GE Healthcare). Following the amplification steps, 15 μL of water were added to each sample before proceeding with polymerase chain reaction (PCR) analyses.
Species-specific PCR diagnostics.
The National Center for Biotechnology Information (NCBI) GenBank website was searched for published rDNA sequences from the two species. On the basis of an alignment of rDNA sequences, we identified conserved and diverged areas between the two species in the IGS and ETS region, as described previously.25 A primer set was developed and tested using DNA samples from Florida colonies of each species maintained at the Florida Medical Entomology Laboratory. The primer set features two species-specific forward primers, aegFw CTCTGCGTTGGATGAATGAT and albFw GATGATGGGTTCGCAAATAG, and a shared reverse primer AedesRev ATAGCGTGGTAGCCGTATG amplifying a 184-bp fragment in Ae. albopictus and a 284-bp fragment in Ae. aegypti (Figure 1). All primers had high priming efficiency resulting in sharp specific bands easily scored on an agarose gel. Additional tests showed that the PCR is able to detect the presence of DNA from either species in ratios from 1/1, 1/10, or higher (upper limit not known exactly but less than 1/100) (Figure 1), and no cross-amplification was ever observed. The same PCR diagnostics were applied to heads and thoraces of females from which spermathecae were dissected, to corroborate morphological identifications of field-collected specimens.
Figure 1.
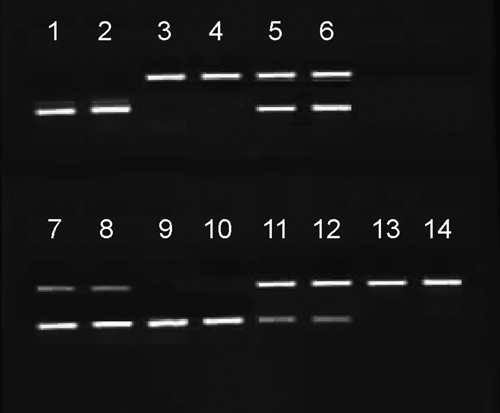
Test of the sensitivity of the species-specific polymerase chain reaction (PCR) assay (all PCR conducted with 1 μL of DNA). Lanes 1 and 2 contain DNA from Aedes aegypti alone and lanes 3 and 4 from albopictus alone, lanes 5 and 6 contain DNA from Aedes aegypti and albopictus in equal amounts, lanes 7–10 contain DNA from Ae. albopictus and aegypti in 1/10 and 1/100 (2 lanes each) and lanes 11–14 contain DNA from Ae. aegypti and albopictus in the same ratios.
Test of mating interference asymmetry by injection of MAG extracts.
Mosquito strains and rearing.
Aedes albopictus and Ae. aegypti used for these experiments were F3 progeny from simultaneous collections in 2009 in eastern Palm Beach County, Florida, where the two species occur in sympatry. Eggs were synchronously hatched in deoxygenated water, and larval cohorts were reared in enamel pans at 27°C and 14L:10D day length and fed daily a 1:1 mixture of yeast:lactalbumin. Trays were examined daily for pupae, which were removed and sexed under a dissecting microscope. Adults of the same species, sex, and age (±1 d) were allowed to emerge into the same 0.03 m3 cage.
Injections of male accessory gland extracts.
Injections of conspecific and heterospecific MAG extracts were performed following exactly the methodology first established for experimentally testing the effect of Acps by Craig24 and used in subsequent studies.26 We injected 4-day-old virgin Ae. aegypti and Ae. albopictus females intrathoracically with 1 μL of MAG extracts prepared from homogenates of accessory glands from 5-day-old virgin conspecific (control groups) or heterospecific males (25 pairs of glands in a 100 μL of 5% saline27). This resulted in four experimental groups.
Assessment of refractoriness to mating.
Females from each group were isolated in high humidity for 24 h before being transferred to cages (0.03 m3) with 100 conspecific virgin males for 24 h to assess the effect of MAG injection on mating. Because of variation among treatments in post-injection survivorship of females, male to female ratios in mating cages varied from 1.54 to 1.04. After exposures to males, females were knocked down in a freezer and transferred to 75% ethanol for a minimum of 24 h. Finally, their spermathecae were dissected out and scored for sperm presence. Differences in the proportion of females mated (mating frequency) among treatment groups were analyzed by logistic regression using JMP 7.02 software.28
Results
Spermathecal filling and DNA extraction of field-collected females.
The three spermathecae were dissected from a total of 176 Ae. aegypti and 141 Ae. albopictus females from M&K Auto and Belle Glade salvage yards (Table 1). All dissected females in both species and locations had sperm in the largest (1st) spermatheca. The proportion of large spermathecae that contained sperm whose DNA we could successfully extract, amplify, and identify to species ranged from 78.8% (Ae. albopictus in M&K Auto) to 89.5% (Ae. aegypti in Belle Glade) (Table 1). For the Ae. aegypti and albopictus populations from M&K Auto, nearly all medium (2nd) spermathecae (100% and 98.3%, respectively) were filled with sperm except for a single Ae. albopictus female. This was significantly higher than in Belle Glade where 95.3% and 89.4% of the 2nd spermathecae of Ae. aegypti and Ae. albopictus contained sperm (Logistic regression – Likelihood ratio tests: collection site χ2 = 10.8, degrees of freedom [df] = 1, P < 0.001; species χ2 = 2.7, df = 1, P = 0.099). Sperm was found in the smallest (3rd) spermatheca only in one Ae. albopictus from M&K Auto and in one Ae. aegypti from Belle Glade (Table 1).
Table 1.
Numbers and densities of field-collected Aedes aegypti and albopictus females, whose spermathecae were dissected and their sperm content analyzed genetically, from M&K Auto and Belle Glade in Florida*
| Location | Species | Mean no. per collection†(SE) | Adults dissected | Adults analyzed | Spermathecae | ||
|---|---|---|---|---|---|---|---|
| 1st (large) | 2nd (medium) | 3rd (small) | |||||
| M&K Auto | Ae. aegypti | 73.4 (18.7) | 90 | 85 | 79/11/0 | 79/11/0 | 0/–/90 |
| Ae. albopictus | 161.0 (69.0) | 75 | 72 | 59/16/0 | 58/16/1 | 1/–/74 | |
| Belle Glade | Ae. aegypti | 67.5 (2.5) | 86 | 85 | 78/8/0 | 73/9/4 | 1/1/84 |
| Ae. albopictus | 411.5 (170.5) | 66 | 62 | 54/12/0 | 42/17/7 | 0/–/66 | |
First number under each spermatheca shows those that contained sperm and were successfully genetically analyzed; second shows number filled with sperm but whose DNA analysis failed; third is number with no sperm present.
Means from N = 7 collections M&K (2005–07) and N = 2 collections Belle Glade (2007).
Frequencies of cross-insemination.
The overall frequency of heterospecific mating at the two collection sites was equal to 1.6%, with 5 of 304 females harboring sperm of the “wrong” species, as determined by PCR (Table 2). At M&K Auto, all Ae. aegypti females had mated with their own species, but a single Ae. albopictus (1.4%) had Ae. aegypti sperm in one of her spermathecae. At Belle Glade, three (3.6%) Ae. aegypti females were inseminated by male Ae. albopictus. A single Ae. albopictus female (1.6%) was also found to have cross-mated at that field site (Table 2).
Table 2.
Numbers of Aedes aegypti and Aedes albopictus females found with conspecific or interspecific sperm in one or more of their spermathecae in the M&K Auto and Belle Glade collections
| Location | Females | Sperm | Totals | |
|---|---|---|---|---|
| Ae. aegypti | Ae. albopictus | |||
| M&K Auto | Ae. aegypti | 85 | – | 85 |
| Ae. albopictus | 1 | 71 | 72 | |
| Belle Glade | Ae. aegypti | 82 | 3 | 85 |
| Ae. albopictus | 1 | 61 | 62 | |
| Totals | 169 | 135 | 304 | |
Patterns of spermathecal use in cross-insemination.
In two of the three cross-inseminated Ae. aegypti females, Ae. albopictus sperm was detected in their medium spermatheca and Ae. aegypti DNA was detected in the largest spermatheca. In the third female, despite two attempts, there was no successful amplification of DNA from the largest spermatheca using the PCR diagnostic, indicating either a failed sperm extraction or problems in the whole genome amplification step. In the two cross-mated Ae. albopictus females, the medium spermatheca also harbored the heterospecific sperm. However, in contrast to what was observed in Ae. aegypti females, in both Ae. albopictus females the Ae. aegypti DNA amplified from the sperm was mixed with Ae. albopictus DNA producing bands characteristic of both species on the agarose gel. In one of the two females the diagnostic failed for the largest spermatheca, whereas the second largest spermatheca contained conspecific sperm.
Frequency of insemination in MAG-injected females.
The potential asymmetry in the proportion of Ae. aegypti and Ae. albopictus females injected with conspecific and heterospecific MAG that subsequently mated when exposed to conspecific males was tested through logistic regression. Females of either species did not significantly differ in their likelihood of mating with conspecific males following MAG injection (Logistic regression–Likelihood-ratio test: χ2 = 2.45, df = 1, P = 0.117). The source of male accessory gland extracts (i.e., intraspecific or interspecific) injected in the thorax of virgin females from colonies of Ae. aegypti and Ae. albopicus significantly affected their likelihood to mate with males of their own species (Likelihood-ratio test: χ2 = 16.32, df = 1, P < 0.001). However, this effect strongly depended on the female species as evidenced by a strong interaction between female species and the source of MAG injected, thus supporting the mating interference asymmetry hypothesis (Likelihood-ratio test: χ2 = 61.51, df = 1, P < 0.001). The majority (83.7%) of Ae. albopictus females injected with Ae. aegypti MAG mated with conspecific males (Figure 2). This contrasted with Ae. albopictus and aegypti females injected with conspecific MAG (control groups) and with Ae. aegypti females injected with A. albopictus MAG, of which very few (0.01–12.3%) mated when exposed to conspecific males. Thus, the interspecific effects of MAG products on mating are highly asymmetric, promoting a satyrizing effect of Ae. albopictus males on Ae. aegypti females.
Figure 2.
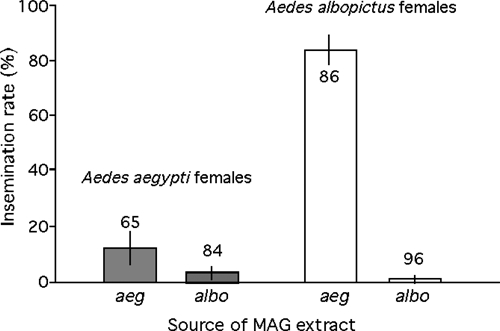
Percentage (±95% confidence intervals [CI]) of Aedes aegypti (grey bars) and Aedes albopictus (white bars) females injected with male accessory gland (MAG) from conspecific or heterospecific males that subsequently mated when exposed to conspecific males. Sample sizes are indicated.
Discussion
Asymmetric mating interference.
This is the first demonstration of interspecific mating in natural populations of these two important vector species. We found cross-inseminations at low rates (1.4–3.6%) in two auto salvage yards in south Florida, with no evidence of asymmetry, despite the numerical predominance of Ae. albopictus in aspirator collections at these sites (Table 1). These field data do not support earlier laboratory results, which showed higher heterospecific mating by Ae. albopictus compared with Ae. aegypti males,12,13 or suggestions that competitive reduction or exclusion of Ae. aegypti may be driven by the higher frequency of cross-mating secured by male Ae. albopictus.13
Our experimental MAG injections revealed a strong asymmetry in cross-reactivity to heterospecific male Acps between females of these species. MAG extracts from Ae. aegypti males failed to induce the switch to the mated state of Ae. albopictus females, whereas Ae. aegypti females injected with heterospecific Acps were rendered refractory to mating. Presently, we do not know whether females simply avoided mating with males or if some mating occurred but did not result in effective sperm transfer to the spermatheca. The observed difference in cross-reactivity is consistent with patterns of oviposition observed in cross-inseminated females of both species18 and females implanted with heterologous male accessory glands.19 Importantly, from an ecological point of view, the asymmetric effects of MAGs between species will lead to cross-mating favoring the satyrization of Ae. aegypti by Ae. albopictus.
Aedes albopictus had co-existed sympatrically with Ae. aegypti in south Florida for 15–18 years before our 2005–2007 collections for this study.20,29,30 Therefore, we cannot discount the possibility that asymmetric interspecific mating might have been more common when the two species first met in the southern United States and was subsequently reduced in frequency by natural selection against the fitness costs of mating with the wrong species. The costs of an erroneous mating are much higher for Ae. aegypti than for Ae. albopictus females and, thus, one could expect females of the former species to have evolved behavior designed to escape satyrization by Ae. albopictus males. Variation in female cross-mating permissiveness among populations could explain the discrepancy between the Nasci and others15 study that suggested a potential strong role of satyrization and the results from this and laboratory studies that reported lower frequencies of interspecific mating.11,14 Although the costs associated with cross-mating in males of both species are unknown, males from both species should also evolve cross-mating avoidance so as to increase their chance of mating with conspecifics and maximize their reproductive success. In addition, females and males from both species should evolve cross-mating avoidance because of the costs in terms of time spent and risks of predation associated with any mating event.
Satyrization and competitive displacement.
Given the low rates of cross-insemination found in this study, satyrization alone may not account for the current competitive reduction or displacement of Ae. aegypti by invasive Ae. albopictus observed in many areas of sympatry. However, our results are compatible with the hypothesis that satyrization may have originally acted in concert with asymmetric larval competition4,31,32 to account for the rapid competitive reduction and sometimes displacement of Ae. aegypti by Ae. albopictus in the southeastern United States.15,29,33 As an example, Ae. aegypti disappeared from Lake Charles, LA, Mobile, LA, and southern Florida within, respectively, 9 months, < 3 years, and 1 to 2 years after the arrival and establishment of Ae. albopictus.15,30,33 A detailed study of the recent competitive displacement of Ae. aegypti by Ae. albopictusin Bermuda also concluded that larval competition alone was inadequate to explain the rapid disappearance of Ae. aegypti.34 Thus, satyrization could have played a role in recent competitive displacements where resident Ae. aegypti encountered the “moving fronts” of Ae. albopictus invasions, such as those observed in Brazil,35 West Africa,8 or Mayotte,36 and may still play a role in maintaining spatial segregation between these species in some areas of Florida and Brazil.37 In some other areas, prolonged contact between the two species may have led to the progressive evolution of resistance to satyrization by Ae. aegypti females and/or males from both species, eventually leading to low frequencies of interspecific mating such as those observed in this study. In this regard, it would be particularly interesting to test this scenario by comparing interspecific mating frequencies in new invasion fronts by Ae. albopictus to those reported in known areas of spatial segregation and areas of prolonged sympatry.
There is additional evidence suggesting that Ae. albopictus males may be particularly effective satyrs when exposed to other species of Aedes (Stegomyia). In cage experiments, Ae. albopictus males sterilized Aedes polynesiensis,38 and it has been interpreted that the arrival of Ae. albopictus on Guam after World War II may have led to observed competitive reductions of the endemic Aedes guamensis.39 Conceivably, the asymmetric effects of Acps from Ae. albopictus on females of closely related species may have been important effectors of these outcomes.
Physiological considerations.
Among our field-collected specimens, the smallest spermatheca of Ae. aegypti and Ae. albopictus rarely contained sperm (Table 1). The failure of most inseminations to fill the third spermatheca is thus consistent with earlier laboratory investigations of mating in Ae. aegypti.40
Two of three Ae. aegypti females detected with heterospecific sperm were also found to have mated with conspecific males. In both cases, conspecific sperm was detected in the large spermatheca and heterospecific sperm in the medium one. Amplification of maternal DNA can occasionally occur if the sperm ball is lost during its transfer to the whole genome amplification tube, but it is very unlikely that this occurred by chance in two of three cases of cross-mating. Our results thus suggest that interspecific mating could often be associated with multiple insemination. Despite the efficacy of Acps in making Ae. aegypti females refractory to subsequent mating (Reference 16 and this study), multiple insemination is known to occur occasionally when first and second mating are separated by < 5 hours.41 It has also been reported that MAG effects on refractoriness to further mating by Ae. aegypti may wear off after one gonotrophic cycle.42 Because nothing is known of the relationship between multiple-mating, spermathecal usage, and sperm precedence in aedine mosquitoes, it is difficult to assess whether the conspecific or heretospecific mating would have occurred first. Interestingly, a very similar pattern was reported in An. gambiae sensu stricto, in which cross-mating between two molecular forms of this species were found to be rare (1.2%) but often associated with double-mating. This was true despite very low frequencies of multiple-insemination within molecular forms.22 Given that anophelines possess a single spermatheca, here again, mating precedence could not be inferred when the organ contained sperm from both molecular forms; however in that study, the sperm was genotyped, thereby ruling out contamination with female DNA.22 Taken together, these results suggest that in both mosquito species some females may be more likely to cross-mate and multiply mate potentially because of some form of reproductive dysfunction affecting the switch to the mated state.
Another explanation for the co-occurrence of cross-mating and multiple inseminations may be that heterospecific sperm survival in females may be lower than that of conspecific sperm leading to a higher probability of remating at a later stage and a loss of the effect of satyrization over time. Nasci and others15 in their study of cross-mating in field released males and females of both species found dead sperm in a high proportion of cross-mated Ae. aegypti female thereby lending support to the hypothesis that sperm survival could be linked to the pattern of remating observed here. Interspecific matings between Ae. albopictus and Ae. aegypti encounter many barriers,11 and efficient sperm transfer to the spermathecae is also dependent on Acps.43 It may thus also be that Ae. albopictus Acps successfully induce temporary refractoriness but might not induce sperm transfer and retention in the spermathecae as effectively as conspecific Acps. The long-lasting effects of sperm presence and Acps on female refractoriness could possibly also wear out faster in the case of cross-mating.
Further studies are clearly needed to determine to what extent such effects may lessen the fitness costs of satyrization to Ae. aegypti females and the overall competitive advantage for Ae. albopictus in sympatric populations. To do so, a much better understanding of the relationship between multiple-mating, spermathecal usage, and sperm precedence is needed. In that regard, the recent description of MAG proteins in the transcriptional profiles of Ae. aegypti44 and recent functional studies of female post-mating changes in An. gambiae,45,46 suggest that some of those exciting aspects of mosquito biology may soon be unraveled.
Two cross-inseminated Ae. albopictus females in this study were also found to have mated twice, their medium spermatheca containing a mixture of hetero- and conspecific sperm and the largest spermatheca conspecific sperm (data available for one female only). This is the expected pattern in cross-inseminated Ae. albopictus females because Ae. aegypti Acps fail to induce refractoriness to further mating in this species. Thus, one can assume that conspecific inseminations were secondary because they would have precluded further mating had they happened first. It would thus appear that the sperm from a conspecific secondary mating replaced heterospecific sperm in the large spermatheca but just part of it in the medium one. Here again further studies on sperm displacement in this species might shed light on the exact process involved and their fitness consequences in these important disease vector species.
ACKNOWLEDGMENTS
We thank K. Greene, H. Robinson, T. Stenn, and C. Westbrook for field collecting, R. Escher and C Westbrook for colony maintenance, D. Borovsky for advice and loaning equipment, and G. O'Meara, J. Rey, and C. Westbrook for comments on an earlier version of this report.
Footnotes
Financial support: This research was supported by QR funding to FT from Keele University and a NIH grant 2R01 AI044973 to LPL.
Authors' addresses: Frederic Tripet, Dannielle Robbins, and Jenny Moran, Center for Applied Entomology and Parasitology, School of Life Sciences, Keele University, Keele, Staffordshire, UK, E-mails: f.tripet@biol.keele.ac.uk, dannii_robbins@hotmail.com, and moranjenny@aol.com. L. Philip Lounibos, Naoya Nishimura, and Erik M. Blosser, Florida Medical Entomology Laboratory, University of Florida, Vero Beach, FL, E-mails: lounibos@ufl.edu, nishimur@ufl.edu, and eblosser@ufl.edu.
Reprint requests: Frederic Tripet, Center for Applied Entomology and Parasitology, School of Life Sciences, Keele University, Staffordshire, ST5 5BG, UK, E-mail: f.tripet@biol.keele.ac.uk.
References
- 1.Gröning J, Hochkirch A. Reproductive interference between animal species. Q Rev Biol. 2008;83:257–282. doi: 10.1086/590510. [DOI] [PubMed] [Google Scholar]
- 2.Ribeiro JM. Can satyrs control pests and vectors? J Med Entomol. 1988;25:431–440. doi: 10.1093/jmedent/25.6.431. [DOI] [PubMed] [Google Scholar]
- 3.Ribeiro JM, Spielman A. The satyr effect: a model predicting parapatry and species extinction. Am Nat. 1986;128:513–528. [Google Scholar]
- 4.Lounibos LP. (Competitive displacement and reduction).Floore TG, editor. Biorational Control of Mosquitoes. Am Mosq Control Assoc Bull No. 2007;7:276–282. doi: 10.2987/8756-971x(2007)23[276:cdar]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang Y-M. Contributions to the mosquito fauna of Southeast Asia. XIV. The subgenus Stegomyia of Aedes in Southeast Asia. 1. The scutellaris group of species (Diptera: Culicidae) Contrib Am Entomol Inst. 1972;9:1–109. [Google Scholar]
- 6.Huang Y-M. The subgenus Stegomyia of Aedes of the Afrotropical Region with keys to the species (Diptera: Culicidae) Zootaxa. 2004;700:1–120. [Google Scholar]
- 7.Lounibos LP. Invasions by insect vectors of human disease. Annu Rev Entomol. 2002;47:233–266. doi: 10.1146/annurev.ento.47.091201.145206. [DOI] [PubMed] [Google Scholar]
- 8.Paupy C, Ollomo B, Kamgang B, Moutailler S, Rousset D, Demanou M, Hervé JP, Leroy E, Simard F. Comparative role of Aedes albopictus and Aedes aegypti in the emergence of dengue and chikungunya in Central Africa. Vector Borne Zoonotic Dis. 2010;10:259–267. doi: 10.1089/vbz.2009.0005. [DOI] [PubMed] [Google Scholar]
- 9.Hartberg WK. Observations on the mating behavior of Aedes aegypti in nature. Bull World Health Organ. 1970;45:847–850. [PMC free article] [PubMed] [Google Scholar]
- 10.Gubler DJ, Bhattacharya NC. Swarming and mating of Aedes (S.) albopictus in nature. Mosq News. 1972;32:219–223. [Google Scholar]
- 11.Leahy MG, Craig GB. Barriers to hybridization between Aedes aegypti and Aedes albopictus. Evolution. 1967;21:41–58. doi: 10.1111/j.1558-5646.1967.tb00129.x. [DOI] [PubMed] [Google Scholar]
- 12.Nijhout HF, Craig GB. Reproductive isolation in Stegomyia mosquitoes. III Evidence for a sexual pheromone. Entomol Exp Appl. 1971;14:399–412. [Google Scholar]
- 13.Brogdon WG. Measurement of flight tone differences between female Aedes aegypti and A. albopictus (Diptera: Culicidae) J Med Entomol. 1994;31:700–703. doi: 10.1093/jmedent/31.5.700. [DOI] [PubMed] [Google Scholar]
- 14.Harper JP, Paulson SL. Reproductive isolation between Florida strains of Aedes aegypti and Aedes albopictus. J Am Mosq Control Assoc. 1994;10:88–92. [PubMed] [Google Scholar]
- 15.Nasci RS, Hare CG, Willis FS. Interspecific mating between Louisiana strains of Aedes albopictus and Aedes aegypti in the field and the laboratory. J Am Mosq Control Assoc. 1989;5:1–5. [PubMed] [Google Scholar]
- 16.Craig GB. In: Biological Pollution: The Control and Impact of Invasive Exotic Species. McKnight BN, editor. Indianapolis, IN: Indiana Academy of Sciences; 1993. pp. 101–120. (The diaspora of the Asian Tiger Mosquito). [Google Scholar]
- 17.Klowden MJ. The check is in the male: male mosquitoes affect female physiology and behavior. J Am Mosq Control Assoc. 1999;15:213–220. [PubMed] [Google Scholar]
- 18.De Buck A. Kreuzungsversuche mit Stegomyia fasciatus Fabricius and S. albopicta Skuse. Zeitsch Angew Entomol. 1942;19:309–312. [Google Scholar]
- 19.Leahy MG, Craig GB. Accessory gland substance as a stimulant for oviposition in Aedes aegypti and Ae. albopictus. Mosq News. 1965;21:448–452. [Google Scholar]
- 20.Lounibos LP, O'Meara GF, Juliano SA, Nishimura N, Escher RL, Reiskind MH, Cutwa M, Greene K. Differential survivorship of invasive mosquito species in south Florida cemeteries: do site-specific microclimates explain patterns of coexistence and exclusion? Ann Entomol Soc Am. 2010;103:757–770. doi: 10.1603/AN09142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tripet F, Touré Y, Taylor CE, Norris DE, Dolo G, Lanzaro GC. DNA analysis of transfered sperm reveals significant levels of gene flow between molecular forms of the Anopheles gambiae complex. Mol Ecol. 2001;10:1725–1732. doi: 10.1046/j.0962-1083.2001.01301.x. [DOI] [PubMed] [Google Scholar]
- 22.Tripet F, Touré Y, Dolo G, Lanzaro GC. Frequency of multiple inseminations in field-collected Anopheles gambiae females revealed by DNA analysis of transfered sperm. Am J Trop Med Hyg. 2003;68:1–5. [PubMed] [Google Scholar]
- 23.Tripet F, Thielman T, Lanzaro GC. The effect of seminal fluids on mating between the Anopheles gambiae M and S forms. J Med Entomol. 2005;42:596–603. doi: 10.1093/jmedent/42.4.596. [DOI] [PubMed] [Google Scholar]
- 24.Craig GB. Mosquitoes: female monogamy induced by male accessory gland substance. Science. 1967;156:1499–1501. doi: 10.1126/science.156.3781.1499. [DOI] [PubMed] [Google Scholar]
- 25.Wu CC, Fallon AM. Analysis of a ribosomal DNA intergenic spacer region from the yellow fever mosquito, Aedes aegypti. Insect Mol Biol. 1998;7:19–29. doi: 10.1046/j.1365-2583.1998.71194.x. [DOI] [PubMed] [Google Scholar]
- 26.Shutt B, Stables L, Aboagye-Antwi F, Moran J, Tripet F. Male accessory gland proteins induce female monogamy in anopheline mosquitoes. Med Vet Entomol. 2010;24:91–94. doi: 10.1111/j.1365-2915.2009.00849.x. [DOI] [PubMed] [Google Scholar]
- 27.Hayes RO. Determination of a physiological saline solution for Aedes aegypti (L.) J Econ Entomol. 1953;10:88–92. [Google Scholar]
- 28.SAS Institute Inc . JMP Statistics and Graphics Guide. Cary, NC: 2007. [Google Scholar]
- 29.O'Meara GF, Gettman AD, Evans LF, Curtis GA. The spread of Aedes albopictus in Florida. Am Entomol. 1993;39:163–172. [Google Scholar]
- 30.O'Meara GF, Evans LF, Gettman AD, Cuda JP. Spread of Aedes albopictus and decline of Ae. aegypti (Diptera: Culicidae) in Florida. J Med Entomol. 1995;32:554–562. doi: 10.1093/jmedent/32.4.554. [DOI] [PubMed] [Google Scholar]
- 31.Juliano SA. Species introduction and replacement among mosquitoes: interspecific resource competition or apparent competition? Ecology. 1998;79:255–268. [Google Scholar]
- 32.Juliano SA, Lounibos LP. Ecology of invasive mosquitoes: effects on resident species and on human health. Ecol Lett. 2005;8:558–574. doi: 10.1111/j.1461-0248.2005.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hobbes JH, Hughes EA, Eichold BH. Replacement of Aedes aegypti by Aedes albopictus in Mobile, Alabama. J Am Mosq Control Assoc. 1991;7:488–489. [PubMed] [Google Scholar]
- 34.Kaplan L, Kendell D, Robertson D, Livdahl T, Katchikian C. Aedes aegypti and Aedes albopictus in Bermuda: extinction, invasion, invasion and extinction. Biol Invas. 2010;12:3277–3288. [Google Scholar]
- 35.Braks MA, Honório NA, Lounibos LP, Lourenco-De-Oliveira R, Juliano SA. Interspecific competition between two invasive species of container mosquitoes, Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in Brazil. Ann Entomol Soc Am. 2004;97:130–139. [Google Scholar]
- 36.Bagny L, DeLatte H, Elissa N, Quilici S, Fontenille D. Aedes (Diptera: Culicidae) vectors of arboviruses in Mayotte (Indian Ocean): distribution area and larval habitats. J Med Entomol. 2009;46:198–207. doi: 10.1603/033.046.0204. [DOI] [PubMed] [Google Scholar]
- 37.Braks MA, Honório NA, Lourenço-de-Oliveira R, Juliano SA, Lounibos LP. Convergent habitat segregation of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in southeastern Brazil and Florida, USA. J Med Entomol. 2003;40:785–794. doi: 10.1603/0022-2585-40.6.785. [DOI] [PubMed] [Google Scholar]
- 38.Gubler DJ. Induced sterility in Aedes (Stegomyia) polynesiensis marks by cross-inseminating with Aedes (Stegomyia) albopictus Skuse. J Med Entomol. 1970;7:65–70. doi: 10.1093/jmedent/7.1.65. [DOI] [PubMed] [Google Scholar]
- 39.Rozeboom LE, Bridges JR. Relative population densities of Aedes albopictus and A. guamensis on Guam. Bull World Health Organ. 1972;46:477–483. [PMC free article] [PubMed] [Google Scholar]
- 40.Jones JC. The sexual life of a mosquito. Sci Am. 1968;218:108–116. doi: 10.1038/scientificamerican0468-108. [DOI] [PubMed] [Google Scholar]
- 41.Spielman A, Leahy MG, Skaff V. Seminal loss in repeatedly mated female Aedes aegypti. Biol Bull. 1967;132:404–412. [Google Scholar]
- 42.Young AD, Downe AE. Renewal of sexual receptivity in mated female mosquitoes, Aedes aegypti. Physiol Entomol. 1982;7:467–471. [Google Scholar]
- 43.Spielman A. The mechanics of copulation in Aedes aegypti. Biol Bull. 1964;127:324–344. [Google Scholar]
- 44.Sirot LK, Poulson RL, Caitlin McKenna M, Girnary H, Wolfner MF, Harrington LC. Identity and transfer of male reproductive gland proteins of the dengue vector mosquito, Aedes aegypti: potential tools for control of female feeding and reproduction. Insect Biochem Mol Biol. 2008;38:176–189. doi: 10.1016/j.ibmb.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rogers DW, Whitten MM, Thailayil J, Soichot J, Levashina EA, Catteruccia F. Molecular and cellular components of the mating machinery in Anopheles gambiae females. Proc Natl Acad Sci USA. 2008;105:19390–19395. doi: 10.1073/pnas.0809723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pondeville E, Maria A, Jacques JC, Bourgouin C, Dauphin-Villemant C. Anopheles gambiae males produce and transfer the vitellogenic steroid hormone 20-hydroxyecdysone to females during mating. Proc Natl Acad Sci USA. 2008;105:19631–19636. doi: 10.1073/pnas.0809264105. [DOI] [PMC free article] [PubMed] [Google Scholar]


