Abstract
We report the first case of imported melioidosis in Spain from a diabetic immigrant who visited West Africa during the rainy season. Because of the unusual presentation of this disease in Africa, clinical and microbiological diagnosis of imported melioidosis from this continent can be very elusive.
Introduction
Burkholderia pseudomallei infections are an important cause of systemic pyogenic disease associated with high mortality1 in South East Asia and Northern Australia. In the last two decades, however, there have been increasing reports of confirmed autochthonous acquired cases and small outbreaks in America (especially Brazil)2,3 and some African countries.4 Melioidosis occasionally presents in case clusters related to extreme weather events or environmental contamination. In fact, the incidence of the disease increased after the 2004 tsunami in Indonesia because of the massive contact of humans with surface waters.1 Some experts suggest that climate change could modify the distribution and prevalence of this disease.3 We report here what seems to be the first case of imported melioidosis in Spain.
The clinical case
A 29-year-old Gambian man was attended in our hospital in October 2009 with a month-long history of fever and pain in the legs. The patient immigrated to Spain 4 years previously and in February 2009 was diagnosed with an autoimmune hepatitis that was treated with prednisone. The patient developed hyperglycemia requiring insulin.
From May to September 2009 he visited Gambia and during the month of June he traveled from there to Guinea-Bissau, also spending some days in Dakar (Senegal). In the weeks previous to the admission, he noted swelling and pain in both legs and developed fever and a productive cough. On examination, he appeared emaciated and his temperature was 38°C, and coarse crackles were heard in the left lower lobe of his chest. The patient's calves were swollen and painful and no signs of trauma, erythema, or warmth were present. No other physical signs or symptoms were observed. Laboratory evaluation showed a white blood cell count of 16,300 cells/mm3 (93% polymorphonuclear leukocytes). The biochemical tests, including liver enzymes, were normal except for hyperglycemia.
X-rays and computed tomography scans of chest and abdomen showed images consistent with septic embolism in the spleen, an infiltrate in the left lower lobe and an ipsilateral pleural effusion. A magnetic resonance imaging of both legs revealed images suggesting pyomyositis with abscess of 12 × 6 cm in the muscles of the right leg and 6 × 4 cm in the left (Figure 1). Aspiration and surgical drainage were performed the day of admission without complications.
Figure 1.
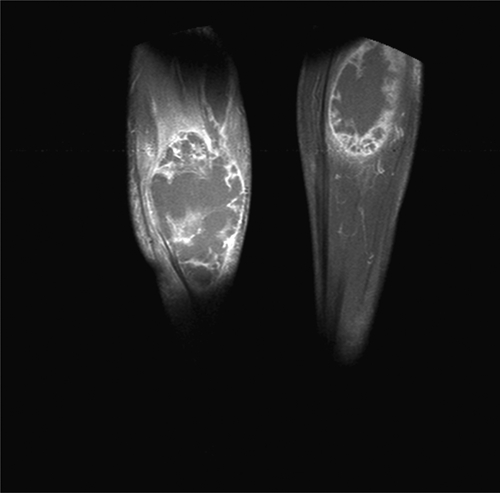
Bilateral calf abscess caused by Burkholderia pseudomallei (NMR).
The patient initially received ceftazidime, ciprofloxacin, and vancomycin and once the results of cultures and antibiotic susceptibility testing were available, the treatment was changed to ceftazidime 50 mg/kg/6 h and sulfamethoxazole-trimethoprim 1,600/320 mg/12 h. The microorganism was sensitive to ceftazidime, cefepime, amoxicillin/clavulanate, piperacillin, piperacillin/tazobactam, and chloramphenicol.
The intravenous treatment was administered for 5 weeks and was changed afterward to doxycycline and sulfamethoxazole-trimethoprim for 20 weeks. The clinical and radiological evolution was excellent and no residual signs were detected when the patient was discharged from the hospital
Isolation, identification, and characterization of B. pseudomallei
Culture from the pus aspirated from the calf and several sputums of the patient produced a pseudomonal-like microorganism that was biochemically identified initially by an automated system (VITEK II; bioMérieux, Marcy-l'Etoile, France) as Burkholderia cepacia. Because of the rarity of the finding in our context, further identification tests were performed (API 20 NE; bioMérieux), that indicated, in combination with the susceptibility pattern of the strain, that we had isolated a strain of B. pseudomallei (API 20 NE bioquemical test identification profile: 1156576, 99.9% of identification certainty). All mycobacterial and fungal cultures performed in the specimens were negative.
A multiplex polymerase chain reaction (PCR) combined with reverse line blot was designed for Burkholderia species-detection using specific targets previously described for B. pseudomallei (orf11), B. mallei (bimA), and Burkholderia thailandensis (BpSCU2).5 Primers and probes used in this assay are shown in Table 1. The method was capable of detecting up to 10 genome equivalents of B. pseudomallei (Figure 2, panel A, lanes 1–4) and differentiated this species from B. mallei and B. thailandensis (Figure 2, panel A, lanes 5–6). Moreover, the method showed a good specificity as no hybridization signals were obtained with close species like Burkholderia cepacia and Burkholderia vietnamiensis. The strain isolated from the patient (BpSp1) was confirmed as B. pseudomallei by specific hybridization with the probe P-Bps (Figure 2, panel A, lanes 13–14). The blood sample from the patient, however, was negative (Figure 2, panel A, lane 11), indicating no circulation of this pathogen in the blood at that stage of the disease.
Table 1.
Primers and probes used in the Burkholderia multiplex polymerase chain reaction (PCR) combined with reverse line blot (RLB)
| Target* | Oligo | Sequence† | Reference |
|---|---|---|---|
| Orf11 B. pseudomallei | HORF11FBIO | 5′-bio-ATCGCCAAATGCCGGGTTTCGGC | This study |
| HORF11RBIO | 5′-bio-CAAATGGCCATCGTGATGTTCGG | This study | |
| P-Bps | 5′-a- TCGGCGAACGCGATTTGATCGTTC | 5 | |
| bimA B. mallei | BIO-BimA68 | 5′-bio-GCGGAGCGATGGACGAAC | This study |
| BIO-BimA216 | 5′-bio-CGCCAGTTGATTCTCCCACC | This study | |
| P-Bma | 5′-a-CATACGGATGTATAGAACCAAT | 11 | |
| BpSCU2 B. thailandensis | HSCU2FBIO | 5′-bio-CTCGAGCTCGTGAAGATGATCG | This study |
| HSCU2RBIO | 5′-bio-ACGCGTGCGATCTTGTAATCGG | This study | |
| P-Bth | 5′-a-CTGTTTCCGCTCGTCGTCG | This study |
Specific target for each pathogen. BpSCU2 is present in the three species; the probe P-Bth is specific for Burkholderia thailandensis.
Primers were modified with biotin (bio) and probes with amino (a) at the 5′.
Figure 2.
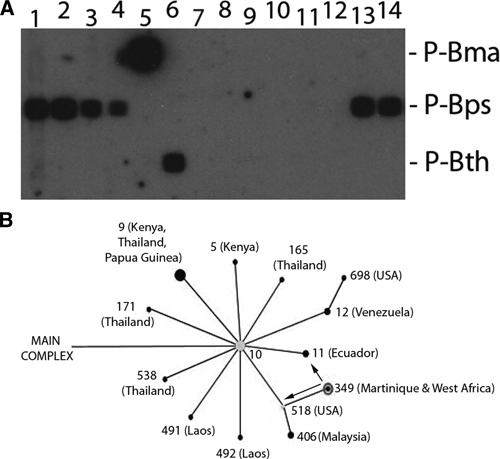
Panel A: Burkholderia multiplex polymerase chain reaction (PCR) combined with reverse line blot (RLB). Lane 1–4: Burkholderia pseudomallei strain NCTC 11642 104, 103, 102, and 10 genomic equivalent (GE), respectively; 5: Burkholderia mallei NCTC 10245 104 GE; 6: Burkholderia thailandensis CCUG 48851 104 GE; 7: Burkholderia vietnamiensis CCUG 34170 104 GE; 8–9: Burkholderia cepacia 1,614/09 and 1,675/09 (Spanish patients) 104 GE, respectively; 10: DNA Extraction control; 11: 100 ng of blood sample DNA from the patient; 12: PCR negative control (water); 13–14: strain BpSp1 106 and 104 GE. Probes are indicated on the left. P-Bma: probe specific for B. mallei, P-Bps: specific for B. pseudomallei, and P-Bth: specific for B. thailandensis. Panel B: Fragment of the main complex of B. pseudomallei, obtained with the eBURST software, showing the subgroup founder ST 10 (Thailand) and its relationships with other STs. Geographic regions where strains of these ST were isolated are indicated between brackets. Arrows show single locus variants (only one locus different in the MLST pattern) of ST 349. The size of the dots represents the number of isolates of each ST available in the MLST database.
The strain was further characterized by multilocus sequence typing (MLST) as described previously6 and the obtained sequence type (ST) was compared with the public database (http://bpseudomallei.mlst.net/). The identified ST was 349 (1-1-13-2-6-1-1). Interestingly, only one strain, isolated in Martinique, shared this ST in the database. The analysis by eBURST (hosted at the Imperial College, London, UK) grouped this ST in the main clonal complex of B. pseudomallei into the subgroup founder 10 (Figure 2, panel B). Moreover, the ST 349 showed only two single locus variants [ST 12 (Ecuador) and ST 518 (USA)] indicating a closer relationship with these American STs rather than with others from Africa.
Discussion
Although sporadic cases of melioidosis have been reported in West and East Africa and, more recently, in Madagascar and Mauritius,5 the extent and relevance of this disease in the continent remains largely unknown, due probably to the absence of bacteriological diagnostic resources,7 the overlapping of the clinical disease caused by B. pseudomallei with other pyogenic infections, such as tuberculosis,3 and a lack of awareness of the disease among clinicians. From Gambia, only one previous case report has been published until now.8 Our patient traveled from Gambia to Guinea-Bissau for a month and also spent a few days in Dakar (Senegal); however, he was in Gambia (Sambaya) when the rainy season started and was probably infected there, because it is widely recognized that many patients in endemic countries are infected in the first week of the rainy season.7
The identification of B. pseudomallei can be elusive, even in developed countries, as widely used automated identification systems, such as (Phoenix; BD Diagnostic Systems, Sparks, MD) or VITEK 2,1 normally confound this bacterium with B. cepacia, a common respiratory pathogen commonly found in cystic fibrosis patients and lung nosocomial infections. The API 20 NE system is usually more accurate in the bacterial identification,9 and in one study 35 from 58 isolates were correctly identified.1 One clear problem is the lack of awareness of this disease in patients coming from non-endemic areas. In such cases, only a high index of clinical and microbiological suspicion can help in identifying such an isolate. For instance, the identification of a Burkholderia spp. with an unusual pattern of antibiotic susceptibility (sensitive to amoxicillin/clavulanate) or a characteristic fenotipe (wrinkled lactose-positive colonies) of old cultures in MacConkey agar9 could indicate that we are actually dealing with a B. pseudomallei isolate.
In our case, the presence of a bilateral calf abscess in a diabetic patient coming from tropical Africa raised the diagnostic possibility of a tropical pyomyositis produced by Staphylococcus aureus; however, Gram stain results were unclear and an empirical therapy covering both gram-positive and gram-negative bacteria was started while waiting for the culture results. After detecting with image techniques pleural and splenic microabscesses and isolating a Burkholderia from the pus of the calf, the possibility of a meliodiosis was first raised, regardless of the travel history of the patient.
Pneumonia is the most common clinical presentation of melioidosis, accounting for up to 50% of cases in most studies, followed by skin and soft tissue infections (13% to 24%), acute suppurative parotitis (representing in Thailand up to 40% of pediatric cases), and genitourinary infections (18% of males with prostatitis in Australia).3 Pyomyositis is a rare early clinical presentation in melioidosis; in a recent study from Thailand pyomyositis was detected in only 12 of 679 patients with confirmed infection.10
In relation to the molecular study, it is difficult to determine a geographical distribution and relationship among different ST considering the data available in the MLST database, as there are only a few isolates from America and Africa. However, the presence of ST 349 in both the Caribbean and West Africa is intriguing. It could be hypothesized that this distribution is related to the established trading posts in the French colonies during the 17th–19th centuries, although more data would be needed to corroborate it.
Footnotes
Authors' addresses: Juan Cuadros, Clinical Microbiology and Parasitology, Hospital Príncipe de Asturias, Alcalá de Henares, Madrid, Spain, E-mail: jcuadros.hupa@salud.madrid.org. Horacio Gil, Special Pathogens and Spirochetes Branch, Nacional Center for Microbiology, Majadahonda, Madrid, Spain, E-mail: hgil@isciii.es. Julio de Miguel, Internal Medicine, Hospital Príncipe de Asturias, Alcalá de Henares, Madrid, Spain, E-mail: juliodemiguel51@gmail.com. Graciela Marabé, Internal Medicine, Hospital Príncipe de Asturias, Alcalá de Henares, Madrid, Spain, E-mail: grassiella@hotmail.com. Teresa Arroyo, Clinical Microbiology and Parasitology, Hospital Príncipe de Asturias, Alcalá de Henares, Madrid, Spain, E-mail: tarroyo26@hotmail.com. Peña Gómez-Herruz, Clinical Microbiology and Parasitology, Hospital Príncipe de Asturias, Alcalá de Henares, Madrid, Spain, E-mail: pgherruz@gmail.com. Bruno Lobo, Special Pathogens and Spirochetes Branch, Nacional Center for Microbiology, Majadahonda, Madrid, Spain, E-mail: blobo@isciii.es. Ruth Marcos, General Surgery Hospital Príncipe de Asturias, Alcalá de Henares, Madrid, Spain, E-mail: rmarcosh@telefonica.net. Pedro Anda, Nacional Center for Microbiology, Majadahonda, Madrid, Spain, E-mail: panda@isciii.es.
References
- 1.Peacock SJ. Melioidosis. Curr Opin Infect Dis. 2006;19:421–428. doi: 10.1097/01.qco.0000244046.31135.b3. [DOI] [PubMed] [Google Scholar]
- 2.Inglis TJ, Rolim DB, Sousa Ade Q. Melioidosis in the Americas. Am J Trop Med Hyg. 2006;75:947–954. [PubMed] [Google Scholar]
- 3.Cheng AC, Currie BJ. Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev. 2005;18:383–416. doi: 10.1128/CMR.18.2.383-416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Currie BJ, Dance DA, Cheng AC. The global distribution of Burkholderia pseudomallei and melioidosis: an update. Trans R Soc Trop Med Hyg. 2008;102((Suppl 1)):S1–S4. doi: 10.1016/S0035-9203(08)70002-6. [DOI] [PubMed] [Google Scholar]
- 5.Thibault FM, Valade E, Vidal DR. Identification and discrimination of Burkholderia pseudomallei, B. mallei, and B. thailandensis by real-time PCR targeting type III secretion system genes. J Clin Microbiol. 2004;42:5871–5874. doi: 10.1128/JCM.42.12.5871-5874.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Godoy D, Randle G, Simpson AJ, Aanensen DM, Pitt TL, Kinoshita R, Spratt BG. Multilocus sequence typing and evolutionary relationships among the causative agents of melioidosis and glanders, Burkholderia pseudomallei and Burkholderia mallei. J Clin Microbiol. 2003;41:2068–2079. doi: 10.1128/JCM.41.5.2068-2079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inglis TJ, Sousa AQ. The public health implications of melioidosis. Braz J Infect Dis. 2009;13:59–66. doi: 10.1590/s1413-86702009000100013. [DOI] [PubMed] [Google Scholar]
- 8.Wall RA, Mabey DC, Corrah PT, Peters L. A case of melioidosis in West Africa. J Infect Dis. 1985;152:424–425. doi: 10.1093/infdis/152.2.424a. [DOI] [PubMed] [Google Scholar]
- 9.Dance DA, Wuthiekanun V, Naigowit P, White NJ. Identification of Pseudomonas pseudomallei in clinical practice: use of simple screening tests and API 20NE. J Clin Pathol. 1989;42:645–648. doi: 10.1136/jcp.42.6.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teparrakkul P, Tsai JJ, Chierakul W, Gerstenmaier JF, Wacharaprechasgu T, Piyaphanee W, Limmathurotsakul D, Chaowaqul W, Day NP, Peacock SJ. Rheumatological manifestations in patients with melioidosis. Southeast Asian J Trop Med Public Health. 2008;39:649–655. [PubMed] [Google Scholar]
- 11.Ulrich MP, Norwood DA, Christensen DR, Ulrich RL. Using real-time PCR to specifically detect. Burkholderia mallei. J Med Microbiol. 2006;55:551–559. doi: 10.1099/jmm.0.46350-0. [DOI] [PubMed] [Google Scholar]


