Abstract
Phytoplankton abundance is inversely related to sea surface temperature (SST). However, a positive relationship is observed between SST and phytoplankton abundance in coastal waters of Bay of Bengal. This has led to an assertion that in a warming climate, rise in SST may increase phytoplankton blooms and, therefore, cholera outbreaks. Here, we explain why a positive SST-phytoplankton relationship exists in the Bay of Bengal and the implications of such a relationship on cholera dynamics. We found clear evidence of two independent physical drivers for phytoplankton abundance. The first one is the widely accepted phytoplankton blooming produced by the upwelling of cold, nutrient-rich deep ocean waters. The second, which explains the Bay of Bengal findings, is coastal phytoplankton blooming during high river discharges with terrestrial nutrients. Causal mechanisms should be understood when associating SST with phytoplankton and subsequent cholera outbreaks in regions where freshwater discharge are a predominant mechanism for phytoplankton production.
Background
The causative agent of cholera, Vibrio cholerae, is endemic to brackish riverine, estuarine, and coastal waters. It is commensal with copepods that feed on phytoplankton.1,2 Therefore, it has been hypothesized that high levels of phytoplankton may lead to high numbers of cholera-containing copepods, increasing the likelihood of cholera epidemics in coastal human populations. There is an intense interest in the use of remote sensing satellite data for cholera outbreak prediction,3,4 because satellite remote sensing is an efficient and effective way to track the spatial and temporal concentrations of chlorophyll, a surrogate for phytoplankton, over large areas. Using chlorophyll, many rigorous studies show an inverse relationship between phytoplankton and sea surface temperature (SST).5–12 Puzzlingly, in the Bay of Bengal region (Figure 1), a positive relationship has been observed between phytoplankton and SST.3,13–17 This apparently contradictory relationship between Bay of Bengal SST and phytoplankton has led to the assertion that, in a warming climate scenario, increasing SST will lead to increasing phytoplankton and thus more cholera outbreaks globally.3 To address these contradictory viewpoints, we undertook an analysis of the role of nutrients carried by freshwater river discharge into the ocean in several major freshwater discharge ocean basins across the globe. This work seeks to: 1) explain why a positive SST-phytoplankton relationship exists in the Bay of Bengal and 2) understand the implications of such a relationship on cholera dynamics.
Figure 1.
Location of four major river basins: Ganges and Brahmaputra rivers discharge into the Bay of Bengal, Orinoco river drains into Atlantic Ocean, Congo River drains into the Atlantic Ocean, and Amazon River drains into the Atlantic Ocean.
Methods
Study design and dominant hypothesis.
We examined the role terrestrial nutrients—through fresh water discharge into the Bay of Bengal from the Ganges-Brahmaputra-Meghna (GBM) rivers—might play in causing phytoplankton and zooplankton blooms and their subsequent relationships with SST. The GBM river system in the Indian Subcontinent discharges ∼628 km3/year of freshwater into the Bay of Bengal,18 the third largest freshwater flow in the world behind the Amazon and the Congo. Increases in phytoplankton through freshwater nutrient discharge have been observed in the Amazon River19 Chesapeake Bay,20 and the Delaware,21 Po,22 Orinoco,23 and Mississippi rivers,24 implying that high discharge brings nutrients with it, that further aid in phytoplankton blooming. However, the effect of river discharge on the relationship between SST and satellite-derived phytoplankton abundance, through chlorophyll estimates, remains unexplored. We hypothesize that a large amount of terrestrial nutrients carried by the GBM rivers lead to a positive relationship between SST and phytoplankton abundance in the Bay of Bengal. We used correlation and wavelet analysis of appropriate time series to explore our hypothesis. We then validated the Bay of Bengal results by analyzing the SST and chlorophyll, a surrogate for phytoplankton abundance, relationships in three other major discharge regions around the globe (Figure 1: Amazon, Orinoco, and Congo), which are hydrologically similar to the GBM riverine system. Finally, we directly assessed the concurrent relationship between cholera incidence and coastal Bay of Bengal SST.
Data sources.
The coastal water zone of the Bay of Bengal is defined as the region between 21–22.5°N and 86–93°E based on bathymetry of the region.25 Our data products were Sea-viewing Wide Field-of-view Sensor (SeaWiFS) monthly chlorophyll data at 9-km resolution, obtained from the National Aeronautics and Space Administration (NASA)/Goddard Earth Sciences/Distributed Active Archive Center, for a 12-year period (1997–2009). More detailed descriptions about these products, sensors, estimation algorithms, and accuracy are available elsewhere.26,27 Prior work has demonstrated that the SeaWiFS sensor has been reasonably stable over the years of operation, the calibration approach provided consistent global water-leaving radiances, and the products meet the accuracy goals over a diverse set of open ocean validation sites.28–30 Monthly interpolated data31 for the concurrent time period were used for SST analysis. Daily Ganges and Brahmaputra river discharge data were obtained from the Bangladesh Water Development Board, and aggregated into a monthly time series for the analysis. The two river gauge stations are located in Bahadurabad and Paksey, where the Brahmaputra and the Ganges rivers enter Bangladesh from India, respectively. To determine total discharge into the Bay of Bengal, monthly river discharge data for the two rivers were added to obtain combined monthly discharge. Cholera incidence data from 1997 through 2009 were acquired from surveillance bulletins maintained by the International Center for Diarrheal Disease Research, Bangladesh. Cholera epidemiologic data from Bangladesh, perhaps one of the longest cholera data sets available,32 were averaged over sequential 3-month periods to obtain seasonal cholera incidence estimates.
Results
Relationship between SST, phytoplankton, and river discharge.
We calculated the correlation between SST and chlorophyll as a function of mean of three consecutive months for the entire year (e.g., January–February–March [JFM], February–March–April [FMA], March–April–March [MAM], etc.). A positive correlation in Figure 2 indicates the correlation coefficient between SST and chlorophyll is positive for a simultaneous 3-month seasonal period, and vice versa. Deep blue and red bars in Figure 2 are the statistically significant correlations (Kendall Tau test P < 0.05).
Figure 2.
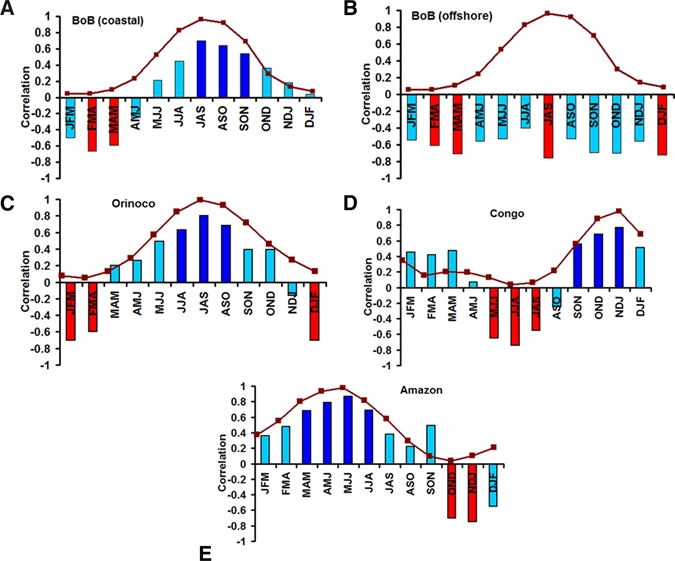
Seasonal correlation between sea surface temperature (SST) and chlorophyll from 1997 to 2010 (A) Bay of Bengal (coastal), (B) Bay of Bengal (offshore), (C) Orinoco, (D) Congo, and (E) Amazon. Deep blue and red bars represent statistically significant correlation (using Kendall Tau test P < 0.05). Brown line, superimposed on the correlation plots, is the seasonal river discharge, scaled between 0 and 1, from respective river basins. J, F, M …D represents January, February, March… December, respectively. JFM, FMA is the mean of three consecutive months.
In the coastal Bay of Bengal region, Figure 2A, eight positive and four negative seasonal correlations were observed. Negative correlations were found for the following seasons: JFM, FMA, MAM, AMJ; and positive correlation for the rest of the seasons. A seasonal discharge curve, the brown line in Figure 2A, is superimposed on the correlation plot. As shown in Figure 2A, when the seasonal river discharge is high (JAS, ASO, and SON), the correlation between SST and chlorophyll is positive. In contrast, when river discharge is low (JFM, FMA, and MAM), the correlation between SST and chlorophyll is negative. There are two interesting observations in Figure 2A in coastal Bay of Bengal: 1) the highest statistically significant correlation value lies in the region of high (JAS: correlation between SST and chlorophyll = 0.70) and low (FMA: correlation between SST and chlorophyll = –0.66) discharge season and 2) the correlation value decreases outside two marked zone of interest (high and low discharge seasons). Correlation values decrease after the SON period and gradually become negative as flow decreases. Figure 3 provides seasonal values for coastal chlorophyll, SST, and river discharge for the Ganges and the Brahmaputra rivers. It shows that coastal Bay of Bengal SST has an annual bimodal peak, the first in the spring (MAM) season and the second in the fall (SON) season. If an increase in SST is related to the increase in phytoplankton and, subsequently, zooplankton, then a similar bimodal peak in coastal chlorophyll should be detected. However, seasonal chlorophyll shows only one peak similar to the peak observed in seasonal river discharge. As supporting evidence, correlation between the highest river discharge season (JAS: July–August–September) highest phytoplankton abundance (SON: September–October–November) season, in coastal Bay of Bengal, is 0.81 (P < 0.05). These relationships suggest that during time of high discharge, terrestrial nutrients are washed from land and deposited in the coastal Bay of Bengal, consequently increasing the concentration of chlorophyll (phytoplankton abundance). During low river discharge seasons, in contrast, the flow of terrestrial nutrients is limited and the correlation between SST and chlorophyll is negative, suggesting that the production of chlorophyll in the Bay of Bengal at these times of the year is controlled by processes other than river flow.
Figure 3.
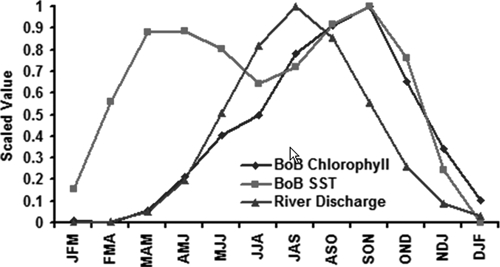
Three-month chlorophyll, sea surface temperature (SST), and river discharge at the mouth of the river for coastal Bay of Bengal. All values have been scaled from 0 to 1.
We then determined the statistical relationships between SST and chlorophyll in the offshore region of the Bay of Bengal. If high river discharge is the dominant mechanism for producing a positive relationship between SST and chlorophyll, then we would detect an inverse relationship between the two variables (SST, chlorophyll) in all the seasonal correlations. If such an inverse relationship is observed, it would imply that river discharge has little or no impact for offshore phytoplankton. The seasonal correlation between SST and chlorophyll, for the offshore region (17–18°N and 86–93°E), is indeed negative throughout the year (Figure 2B). Thus, river discharge appears to affect phytoplankton production only in the coastal zone of the Bay of Bengal during the high discharge season, and the expected inverse relationship between SST and chlorophyll re-emerged away from the coast.
As an aggregate metric, correlation can be deceptive as the only measure to ascertain causal relationships. To verify these results obtained using correlation analyses, we used wavelet analysis33 to decompose the coastal and offshore daily chlorophyll time series to determine if there are any discernable time period differences between the two time series. Daily chlorophyll time series show that coastal chlorophyll has a statistically significant 30–90 day peak (Figure 4A), whereas offshore chlorophyll has a peak at 3 years, which is absent in coastal waters (Figure 4B). This distinctive variation of time scales for coastal and offshore chlorophyll suggests that the physical processes and drivers for chlorophyll variability are indeed different in these two regions.
Figure 4.
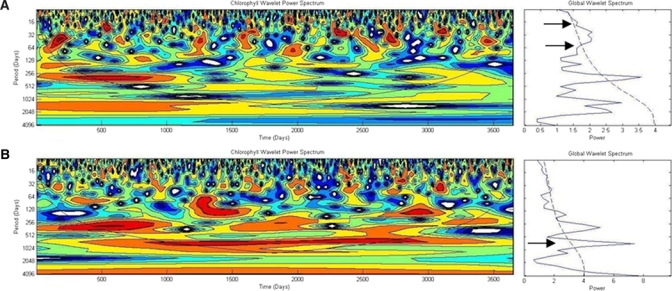
Wavelet power spectrum analysis for (A) daily coastal chlorophyll, and (B) offshore chlorophyll time series. A 5-day running mean was applied to the daily time series to remove missing values. Climatological daily values were inserted for missing chlorophyll values after a 5-day running mean was applied.
Relationship between SST and phytoplankton in Amazon, Orinoco, and Congo regions.
We conjectured that if high freshwater discharge in the coastal Bay of Bengal alters the more generally observed inverse relationship between chlorophyll and SST, this phenomenon should occur elsewhere. Thus, we should expect to see a positive association between SST and chlorophyll during high discharge and a negative relationship during low discharge months in other major freshwater basins globally. To explore this hypothesis, we calculated the correlation between SST and chlorophyll in three of the largest freshwater basins in the world (Figure 1)—the Amazon (discharge of 6,640 km3/year), Congo (1,308 km3/year), and Orinoco (1,129 km3/year). The results of these analyses are displayed adjacent to the Bay of Bengal data for ease of comparison. The Orinoco (Figure 2C) showed positive correlations (JJA: 0.63, JAS: 0.80, ASO: 0.68) during high discharge seasons and negative correlations (JFM: −0.70, FMA: −0.63 and DJF: −0.71) during low flow seasons. Similarly, high discharge seasons in the Congo (Figure 2C; OND: 0.68; NDJ: 0.77; DJF: 0.51) and Amazon (Figure 2D; MAM: 0.68; AMJ: 0.79: MJJ: 0.87: JJA: 0.69) rivers show positive correlations between SST and chlorophyll. During low discharge seasons, a negative correlation is observed in the Congo (Figure 2D) and Amazon (Figure 2E) rivers. The SST and chlorophyll relationships for the three large freshwater discharge regions are very similar to that for the Bay of Bengal, which is consistent with the hypothesis that river flow is a dominant driver for phytoplankton growth during the high river discharge season. For these three rivers, similar to coastal Bay of Bengal, we note that the highest statistically significant correlation values lie in the region of high (Orinoco JAS: 0.80; Congo NDJ: 0.77; Amazon MJJ: 0.87) and low (Orinoco JFM: −0.70; Congo JJA: −0.73; Amazon NDJ: −0.75) discharge seasons. This high degree of physical consistency between SST and chlorophyll variations in three other major freshwater discharge basins suggests that the positive correlation between SST and chlorophyll in major coastal zones around the world may result from similar dominant processes, such as terrestrial nutrients with high volume river discharge. The negative correlation pattern for these basins during low discharge seasons is also consistent with the hypothesis that during low flow, with limited terrestrial nutrient availability, non-riverine oceanic processes drive chlorophyll production.
Lack of a significant relationship between 3-month seasonal SST and cholera incidence.
Cholera remains endemic in the Bengal delta with typically two seasonal, spring (March–April–May) and autumn (September–October–November), outbreaks in a given year (Figure 5A). The majority of existing studies have suggested a positive association between these cholera outbreaks in Bangladesh and coastal Bay of Bengal SST.3,13–15 This observation is primarily based on a climatological understanding of SST and cholera incidence (Figure 5A), which shows that coastal SST and cholera incidence data both have concurrent bimodal peaks with high climatological correlation (r = 0.88; P < 0.01). If cholera outbreaks were causally associated with coastal SST, then we should expect to see a similar strong correlation between the two time series. However, we found the relationship to be weak (Figure 5B) with no statistically significant concurrent relationships between the two time series, or with any evidence of a bimodal distribution among correlation values.
Figure 5.
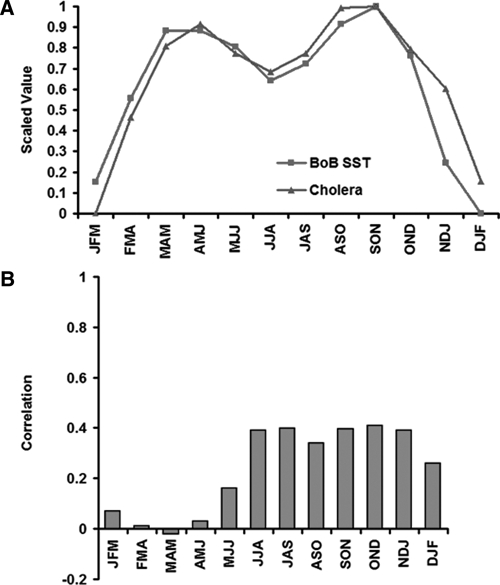
(A) Climatology of cholera and coastal Bay of Bengal sea surface temperature (SST). (B) Seasonal correlation of coastal Bay of Bengal SSTs with concurrent seasonal cholera outbreaks.
Discussion and Implications for Cholera Prediction Models
Our results suggest that it is the presence and dominance of high river discharge—not SST—that may account for the prior reports of a positive association between cholera and SST in the Bay of Bengal. If so, this may have important implications for the understanding of cholera transmission dynamics. If cholera outbreaks were associated with SST then we should observe strong correlations between the time series of cholera incidence and SST in coastal Bay of Bengal; however, no such relationship was found. The absence of a strong correlation is also an indication that cholera is not related to SST for concurrent seasons.
Consistent with our results, an asymmetric role for river discharge as a predictor of cholera outbreaks in the Bengal Delta has recently been reported.34,35 High and low river discharge conditions may differentially contribute to cholera transmission and outbreaks; for example, during low discharge periods, the population may be forced to ingest water already contaminated with cholera bacteria.35 Our analysis points to a similarly asymmetric influence of SST and chlorophyll for high and low discharge seasons (Figure 2). Consequently, any cholera prediction models need to carefully analyze and account for concurrent and lagged relationships among SST, chlorophyll, river discharge, and cholera incidences.
The relationship between coastal phytoplankton, SST, and river discharge may be critical to understanding the environmental conditions that lead to cholera epidemics in coastal regions of the Bay of Bengal. Here, we show that river discharge in major freshwater basins around the world affect the relationship between ocean temperature and coastal phytoplankton, both temporally and spatially. Using seasonal cross-correlation between SST and chlorophyll time series in the Bay of Bengal, these variables have been shown to be correlated positively during high discharge and negatively during low discharge. Furthermore, the SST and chlorophyll time series for coastal and offshore regions have been compared and shown that the usually observed inverse relationship between SST and chlorophyll, which exists for offshore region, does not hold true for coastal regions of major river basins (Figures 2). A plausible explanation for this is that the SST-chlorophyll relationship can be affected by nutrient influx during high river discharge into coastal regions. Our finding of a positive association between chlorophyll and SST during high flow months supports our hypothesis that chlorophyll productions in the coastal regions are dominated by river discharge through the influx of terrestrial nutrients.19,21,23
Our results are also supported by the data from coastal regions that do not have high freshwater input. Logically, if river discharge were indeed the dominant mechanism of phytoplankton production in the four major freshwater basins we examined; and if the positive SST-chlorophyll relationship during high discharge seasons were to be true; then, it is likely that a negative SST-chlorophyll relationship would be observed in coastal basins without significant terrestrial freshwater input. Indeed, several coastal regions without significant freshwater discharge show a consistently negative correlation between SST and chlorophyll, notably, the California coast,8,36 the Arabian Sea,16 and the Gulf of Cadiz along coastal Spain.37
In summary, we believe the observed positive correlation between SST and chlorophyll in the Bay of Bengal and other major freshwater basins globally are primarily caused by terrestrial nutrient inputs from river discharge. An important aspect of our study is that it provides a new and physically meaningful explanation as to why, despite higher SST, more phytoplankton is found in the coastal areas where freshwater discharge is high. Our results suggest that the observed positive correlation between SST and chlorophyll in the Bay of Bengal is in fact not causal, and should not form the basis to infer or construct prediction models for cholera outbreaks. Cholera prediction models may benefit from including data on terrestrial nutrient influx and subsequent phytoplankton and zooplankton blooms. These results will provide a mechanistic insight for constructing cholera prediction models based on environmental processes that influence the coastal regions of cholera endemic countries.
ACKNOWLEDGEMENTS
This research was supported by a Research Challenge Grant from the National Institutes of Health (1RC1TW008587-01) under the American Recovery and Reinvestment Act of 2009.
Footnotes
Authors' addresses: Antarpreet S. Jutla, Water and Environmental Research, Education and Actionable Solutions Network (WE REASoN), Department of Civil and Environmental Engineering, Tufts University, Medford, MA, E-mail: antarpreet.jutla@tufts.edu. Ali S. Akanda, Water and Environmental Research, Education and Actionable Solutions Network (WE REASoN), Department of Civil and Environmental Engineering, Tufts University, Medford, MA, E-mail: ali.akanda@tufts.edu. Jeffrey K. Griffiths, Director, Global Health, Public Health and Professional Degree Programs, Tufts University School of Medicine, Associate Professor of Public Health and of Medicine, Department of Public Health and Community Medicine, Tufts University School of Medicine, Adjunct Associate Professor, Friedman School of Nutrition Science and Policy, Adjunct Associate Professor, School of Engineering, Adjunct Associate Professor, Cummings School of Veterinary Medicine at Tufts University, Medford, MA, E-mail: jeffrey.griffiths@tufts.edu. Rita Colwell, Institute for Advanced Computer Studies, University of Maryland, College Park, MD, E-mail: rcolwell@umiacs.umd.edu. Shafiqul Islam, Water and Environmental Research, Education and Actionable Solutions Network (WE REASoN), The Fletcher School of Law and Diplomacy, Department of Civil and Environmental Engineering, Tufts University, Medford, MA 02155, E-mail: Shafiqul.islam@tufts.edu.
References
- 1.Colwell RR, Huq A. Marine ecosystems and cholera. Hydrobiologia. 2001;460:141–145. [Google Scholar]
- 2.Reidl J, Klose KE. Vibrio cholerae and cholera: out of the water and into the host. FEMS Microbiol Rev. 2002;26:125–139. doi: 10.1111/j.1574-6976.2002.tb00605.x. [DOI] [PubMed] [Google Scholar]
- 3.Lobitz BM, Beck LR, Huq A, Wood B, Fuchs G, Faruque ASG, Colwell RR. Climate and infectious disease: use of remote sensing for detection of Vibrio cholerae by indirect measurement. Proc Natl Acad Sci USA. 2000;97:1438–1443. doi: 10.1073/pnas.97.4.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jutla AS, Akanda AS, Islam S. Tracking cholera in coastal regions using satellite observations. J Am Water Resour Assoc. 2010;46:651–662. doi: 10.1111/j.1752-1688.2010.00448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solanki HU, Dwivedi RM, Nayark SR. Synergistic analysis of SeaWiFS chlorophyll concentration and NOAA-AVHRR SST feature for exploring marine living resources. Int J Remote Sens. 2001;22:3877–3882. [Google Scholar]
- 6.Davenport R, Neuer S, Helmke P, Perez-Marrero J, Linas O. Primary productivity in the northern Canary Islands region as inferred from SeaWiFS imagery. Deep-Sea Res. 2002;49:3481–3496. [Google Scholar]
- 7.Acha EM, Mianzan HW, Guerrero RA, Favero M, Bava J. Marine fronts at the continental shelves of austral South America: physical and ecological processes. J Mar Syst. 2004;44:83–105. [Google Scholar]
- 8.Legaard KL, Thomas AC. Spatial patterns in seasonal and interannual variability of chlorophyll and sea surface temperature in the California Current. J Geophys Res. 2006;111:C06032. [Google Scholar]
- 9.Perez V, Fernandez E, Maranon E, Serret P, Garcia-Soto C. Seasonal and interannual variability of chlorophyll a and primary production in the Equatorial Atlantic: in situ and remote sensing observations. J Plankton Res. 2005;27:189–197. [Google Scholar]
- 10.Jolliff JK, Kindle JC, Penta B, Helber R, Lee Z, Shulman I, Arnone R, Rowley CD. On the relationship between satellite-estimated bio-optical and thermal properties in the Gulf of Mexico. J Geol Res. 2008;113:G01024. [Google Scholar]
- 11.Smyth TJ, Miller PI, Groom SB, Lavender SJ. Remote sensing of sea surface temperature and chlorophyll during Lagrangian experiments at the Iberian margin. Prog Oceanogr. 2001;51:269–281. [Google Scholar]
- 12.Shen S, Leptoukh GG, Acker JG, Yu Z, Kempler SJ. Seasonal variations of chlorophyll a concentration in the northern South China Sea. IEEE Geosci Remote Sens Lett. 2008;5:315–319. [Google Scholar]
- 13.Emch M, Feldacker C, Yunus M, Streatfield PK, Thiem V, Canh D, Mohammad A. Local environmental predictors of cholera in Bangladesh and Vietnam. Am J Trop Med Hyg. 2008;78:823–832. [PubMed] [Google Scholar]
- 14.Magny G, Murtugudde R, Sapianob M, Nizam A, Brown C, Busalacchi A, Yunus M, Nair G, Gil A, Calkins J, Manna B, Rajendran K, Bhattacharya M, Huq A, Sack B, Colwell RR. Environmental signatures associated with cholera epidemics. Proc Natl Acad Sci USA. 2008;105:17676–17681. doi: 10.1073/pnas.0809654105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colwell RR. Global climate and infectious disease: the cholera paradigm. Science. 1996;274:2025–2031. doi: 10.1126/science.274.5295.2025. [DOI] [PubMed] [Google Scholar]
- 16.Chaturvedi N. Variability of chlorophyll concentration in the Arabian Sea and Bay of Bengal as observed from SeaWiFS data from 1997–2000 and its interrelationship with Sea Surface Temperature (SST) derived from NOAA AVHRR. Int J Remote Sens. 2005;26:3695–3706. [Google Scholar]
- 17.Kumari B, Babu KN. Provincial nature of chlorophyll and sea surface temperature observed by satellite. Int J Remote Sens. 2009;30:1091–1097. [Google Scholar]
- 18.Dai A, Trenberth KE. Estimates of freshwater discharge from continents: latitudinal and seasonal variations. J Hydrometeorol. 2002;3:660–687. [Google Scholar]
- 19.Smith WO, Jr, Demaster DJ. Phytoplankton biomass and productivity in the Amazon river plume: correlation with seasonal river discharge. Cont Shelf Res. 1996;16:291–319. [Google Scholar]
- 20.Acker J.G., Harding L.W., Leptoukh G., Zhu T., Shen S. Remotely-sensed chl a at the Chesapeake Bay mouth is correlated with annual freshwater flow to Chesapeake Bay. Geophys. Res. Lett. 2005;32:L05601. [Google Scholar]
- 21.Pennock JR, Sharp JH. Phytoplankton production in the Delaware Estuary: temporal and spatial variability. Estuar Coast Shelf Sci. 1985;21:711–725. [Google Scholar]
- 22.Revelante N, Gilmartin M. The effect of Po River discharge on phytoplankton dynamics in the Northern Adriatic Sea. Mar Biol. 1976;34:259–271. [Google Scholar]
- 23.Bidigare RR, Ondrusek M, Brooks J. Influence of the Orinoco river outflow on distributions of algal pigments in the Caribbean Sea. J Geophys Res. 1993;98:2259–2269. [Google Scholar]
- 24.Lohrenz SE, Dagg MJ, Whitledge TE. Enhanced primary production at the plume/oceanic interface of the Mississippi River. Cont Shelf Res. 1990;10:639–664. [Google Scholar]
- 25.Barua DK, Kuehl SA, Miller RL, Moore WS. Suspended sediment distribution and residual transport in the coastal ocean off of the Ganges Brahmaputra river mouth. Mar Geol. 1994;120:41–61. [Google Scholar]
- 26.Martin S. An Introduction to Ocean Remote Sensing. Cambridge, UK: Cambridge University Press; 2004. [Google Scholar]
- 27.Uz BM, Yoder JA. High frequency and mesoscale variability in SeaWiFS chlorophyll imagery and its relation to other remotely sensed oceanographic variables. Deep Sea Res Part II Top Stud Oceanogr. 2004;51:1001–1071. [Google Scholar]
- 28.McClain CR, Cleave ML, Feldman GC, Gregg WW, Hooker SB, Kurin N. Science quality SeaWiFS data for global biosphere research. Sea Technol. 1998;39:10–16. [Google Scholar]
- 29.O'Reilly JE, Maritorena S, Siegel DA, O'Brien MC, Toole D, Chavez FP, Strutton P, Cota GF, Hooker SB, McClain CR, Carder KL, Muller-Karger F, Harding L, Magnuson A, Phinney D, Moore GF, Aiken J, Arrigo KR, Letelier R, Culver M. In: SeaWiFS Postlaunch Calibration and Validation Analyses, Part 3. O'Reilly J.E., editor. Volume 11. NASA Tech; 2000. pp. 9–19. (Ocean chlorophyll a algorithms for SeaWiFS, OC2, and OC4: Version 4). and 24 Coauthors. Memo. 2000-206892. [Google Scholar]
- 30.Gregg WW, Casey NW. Global and regional evaluation of the SeaWiFS chlorophyll data set. Remote Sens Environ. 2004;93:463–479. [Google Scholar]
- 31.Reynolds RW, Smith TM. Improved global sea surface temperature analyses using optimum interpolation. J Clim. 1994;7:929–948. [Google Scholar]
- 32.Longini IM, Jr, Yunus M, Zaman K, Siddique AK, Sack RB, Nizam A. Epidemic and endemic cholera trends over a 33-year period in Bangladesh. J Infect Dis. 2002;186:246–251. doi: 10.1086/341206. [DOI] [PubMed] [Google Scholar]
- 33.Najafi B, Aminian K, Paraschiv-Ionescu A, Loew F, Büla CJ, Robert P. Ambulatory system for human motion analysis using a kinematic sensor: monitoring of daily physical activity in the elderly. IEEE Trans Biomed Eng. 2003;50:711–723. doi: 10.1109/TBME.2003.812189. [DOI] [PubMed] [Google Scholar]
- 34.Akanda AS, Jutla AS, Siddique AK, Alam M, Sack R, Huq A, Colwell R, Islam S. Hydroclimatic influences on seasonal and spatial cholera transmission cycles: implications for public health intervention in the Bengal Delta. Water Resources Research. 2011;47:W00H07. doi:10.1029/2010WR009914. [Google Scholar]
- 35.Akanda AS, Jutla AS, Islam S. Dual peak cholera transmission in Bengal Delta: a hydroclimatological explanation. Geophys Res Lett. 2009;36:L19401. [Google Scholar]
- 36.Nezlin NP, Li BL. Time-series analysis of remote-sensed chlorophyll and environmental factors in the Santa Monica-San Pedro Basin off Southern California. J Mar Syst. 2003;39:185–202. [Google Scholar]
- 37.Navarro G, Ruiz J. Spatial and temporal variability of phytoplankton in the Gulf of Cadiz through remote sensing images. Deep Sea Res Part II Top Stud Oceanogr. 2006;53:1241–1260. [Google Scholar]


