Abstract
Micronutrient deficiencies are associated with impaired growth and cognitive function. A school-based fortification program might benefit schoolchildren but a high prevalence of parasite infestation might affect effectiveness. A randomized, double-blind, placebo-controlled 2 × 2 factorial trial was conducted to assess the efficacy of multi-micronutrient fortified biscuits with or without de-worming on growth, cognitive function, and parasite load in Vietnamese schoolchildren. Schoolchildren (n = 510), 6–8 years of age were randomly allocated to receive albendazole or placebo at baseline and four months of multi-micronutrient fortified biscuits (FB) or non-fortified biscuits. Children receiving FB for four months scored higher on two cognitive tests: Raven's Colored Progressive Matrices and the Digit Span Forward test. Children receiving albendazole plus FB had the lowest parasite load after four months. In children receiving FB, mid-upper arm circumference was slightly improved (+0.082 cm) but there were no differences in other indexes of anthropometry. Combining multi-micronutrient fortified biscuits with de-worming is an effective strategy.
Introduction
Deficiencies of iron, iodine, zinc,and vitamin A are prevalent worldwide.1 Micronutrient deficiencies often coexist,2 and can result in delayed physical and cognitive development, preventing children from reaching their full potential3 because nutrients play an important role in cognitive and motor development in children.4 Children living in areas affected by severe iodine deficiency disorder may have an intelligence quotient > 12 points below that of non–iodine-deficient areas.5 Besides iodine, deficiencies of other micronutrients such as iron and zinc have also been associated with impaired psycho-motor development and cognitive function.6–8 Anemia in school age children may affect learning abilities,9 and zinc deficiency has been linked to low activity and depressed motor development among the most vulnerable children, although associations with cognitive development are less clear and may be limited to specific neuropsychological processes.4 Because deficiency of several micronutrients has been implicated in impaired cognitive and motor performance and development,10 correction of a single deficiency may not be enough to substantially improve cognitive performance.
Intestinal infections with Ascaris lumbricoides, Trichuris trichiura, and hookworm are associated with nutritional deficiencies, anemia, growth retardation, poor school attendance, and poor performance in cognitive tests.11–13 In addition, deficiencies of some nutrients such as vitamin A and zinc may affect immune function, and decrease the body's resistance to intestinal parasites, resulting in exacerbated worm burdens that in turn may affect growth.14 In areas where intestinal parasitic infection is endemic (prevalence > 50%), repeated anthelmintic treatment is recommended because as rapid re-infection can re-establish pre-treatment levels within 4–6 months.15 However, single-dose treatment with anti-helminth drugs such as albendazole have shown mixed results and low cure rates, especially for infection with T. Trichuria.16 Soil-transmitted helminthiasis is common in Vietnam, particularly in school children. In northern and central provinces, the infection rates are high, and a recent study in rural primary school children reported a prevalence of intestinal parasite infection of 92%.12
Micronutrient deficiencies and stunting still represent a major health burden in Vietnam, in spite of marked improvements caused by effective public health interventions.17–19 The national prevalence of stunting was 33.9% among children less than five years of age.20 Growth faltering is reported in populations with inadequate dietary intake of zinc, vitamin A, and iron, and supplementation with zinc has been shown to improve linear growth among children in deficient populations.21–23 A meta-analysis of multiple micronutrient supplementation showed a positive effect on child growth with effect sizes of 0.28 (95% confidence interval = 0.16–0.41) for height and 0.28 (95% confidence interval = –0.07 to 0.63) for weight.24 Multiple micronutrient fortification showed a positive effect on growth in some studies25,26 but not in other studies such as in school children in Thailand27 and South Africa.28
The present study investigated the impact of multi-micronutrient fortification in combination with de-worming in a school-based approach on growth, cognitive function, and parasite load among rural Vietnamese schoolchildren and whether combining the two interventions was more beneficial than either intervention alone.
Materials and Methods
Study area and population.
School children were recruited from two primary schools in Bai Say and Xuan Truc communes, Hung Yen province, Vietnam. Hung Yen is a province in the Red River Delta in northern Vietnam approximately 50 km east of Hanoi. Agriculture is predominant; rice farming is the main occupation and income source. Inclusion criteria were school children 6–8 years of age, visiting the selected schools, and written informed consent from parents/caregivers. Exclusion criteria were pupils with hemoglobin concentrations < 80 g/L (who were referred to the health center), chronic illness, congenital abnormalities, mental or severe physical handicap, severe malnutrition (Z-scores for weight-for-height < –3.0 SD), obesity (body mass index Z-scores for sex and age > 2 SD), or having received de-worming within the previous six months. Children who were excluded from data collection were fed with their classmates but given non-coded biscuits. The study was conducted during January–June 2007.
A sample size of 125 children per group was calculated for detecting a difference in weight of 0.3 kg with an estimated SD of 0.9 kg or a difference in Raven's scores of 0.5 SD, a significance level of 5% (two-tailed), and a power of 90% with an additional 15% to allow for dropout.
A total of 642 children 6–8 years of age in 20 classes were available, of which 550 children were randomly selected. Forty children did not meet the entering criteria (de-worming within the previous six months [n = 16], refused participation [n = 14], or excluded for other reasons [n = 10]) (Figure 1). Thus, 510 pupils were allocated to one of the four intervention groups on the basis of a computer-generated list, matched on age (12-month age groups), sex, and using a block size of 8 by one of the researchers not directly involved in the field work (FTW). The codes of fortified and non-fortified biscuits, albendazole, and placebo were kept by the manufacturers and by institute staff not directly involved in the study until the data analysis was finished. All investigators, field assistants, teachers, and children were blinded to the codes of the study groups. The multiple micronutrient-fortified and non-fortified biscuits were identical in taste, appearance, and smell and were distinguished by a color code on the package. Biscuits were packaged in aluminum foil with green and red marks. The codes of the groups were only made available after completion of the field work and sample analysis. The four intervention groups were 1) non-fortified biscuit plus de-worming-placebo (placebo); 2) multi-micronutrient–fortified biscuit plus de-worming-placebo (FB); 3) non-fortified biscuit plus de-worming (Alb); and 4) multi-micronutrient–fortified biscuit plus de-worming (FB + Alb).
Figure 1.
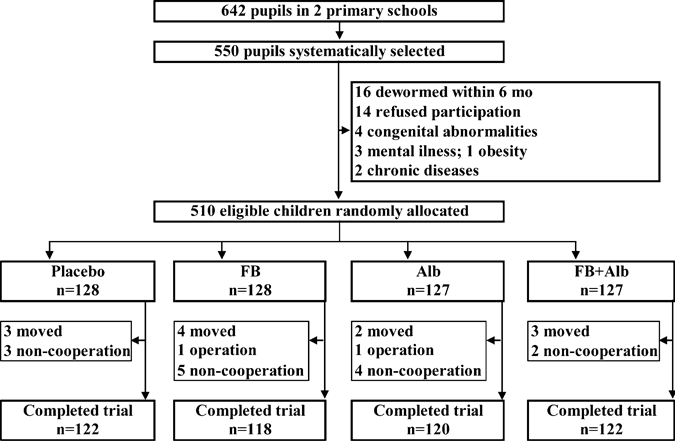
Trial profile. Placebo = placebo de-worming and non-fortified biscuit; FB = multi-micronutrient fortified biscuit and placebo de-worming; Alb = albendazole de-worming and non-fortified biscuits; FB + Alb = Multi-micronutrient fortified biscuit plus albendazole de-worming.
The study was reviewed and approved by the Ethical Committee on Human Research of the National Institute of Nutrition, Hanoi, Vietnam, and The Human Ethics Committees of Mahidol University, Bangkok, Thailand. Written informed consent was obtained from all parents/caregivers of pupils before enrollment in the study.
Intervention and monitoring.
After baseline data collection, a single dose of anthelmintic treatment as an orange-flavored chewable tablets containing 400 mg of albendazole (Vidoca, Thephaco, Vietnam) or identical placebo tablets was given. De-worming with albendazole was given to all children at the end of the study after final fecal sample collection. The composition of the multiple micronutrient fortified biscuit was similar to the non-fortified biscuit except for a premixture of vitamins and minerals (Table 1). Iron, zinc, iodine, and vitamin A composed 50%, 50%, 35%, and 60%, respectively, of the Recommended Nutrient Intake (RNI; Food and Agriculture Organization/World Health Organization, 2002). The B vitamins composed 60–110% of the RNI; vitamins E, K, and other nutrients included in the multiple micronutrient fortified biscuits met 10–40% of the RNI for children 7–9 years of age. Each serving consisted of 30-g biscuits and provided 556.5 kJ (133 kcal)/serving.
Table 1.
Composition of the fortified biscuits*
| Nutrients | Amount | Nutrients | Amount |
|---|---|---|---|
| Iron (ferrous fumarate), mg | 6 | Calcium (CaHPO4), mg | 150 |
| Zinc (zinc sulfate), mg | 5.6 | Cholecalciferol, IU | 74 |
| Iodine (potassium iodide), μg | 35 | Magnesium, mg | 40 |
| Vitamin A (retinyl acetate), μg RE | 300 | Selenium (sodium salt), μg | 6.8 |
| Thiamin (thiamin mononitrate), mg | 1 | Potassium (citrate), mg | 378 |
| Riboflavin, mg | 0.9 | Phosphorus, mg | 70 |
| Vitamin B6 (pyridoxine hydrochloride), mg | 1.1 | Pantothenic acid (calcium), mg | 3 |
| Niacin (niacinamide), mg NE | 10.5 | Vitamin E (α-tocopheryl acetate), μg | 2.8 |
| Vitamin B12, μg | 1.5 | Vitamin K (α-phylloquinone), μg | 10 |
| Folic acid, μg | 120 | Biotin (d-biotin), μg | 18 |
| Vitamin C, mg | 28.4 |
One serving size was 30 g and provided 556.5 kJ (133 kcal). RE = retinol equivalents; NE = niacin equivalents.
Biscuits were given at approximately 3:00 pm daily on 5 school days/week for 16 weeks. The teachers distributed one serving of biscuits each day to the pupils according to the assigned codes. Each pupil wore a colored name card that matched the color codes on the package, and teachers had a list of children assigned to every intervention group to ascertain the correct distribution of the biscuits. The biscuits not consumed by each pupil were given back to the teachers and recorded daily. The feeding session was strictly supervised by the research assistants, and the follow-up form was reviewed weekly.
Data collection.
Data concerning socioeconomic status was collected at baseline only. Anthropometric data, a blood sample, and an urine sample for all children was obtained at baseline and at end-point, whereas a fecal sample for parasite infestation was obtained at baseline, after two months, and at the end point to assess the dynamics of re-infestation. Details of assessments of biochemical micronutrient concentrations have been reported.29 Other measurements are described below.
Anthropometric measurements.
Anthropometry was performed by a team of experienced anthropometrists in which the same person performed the same measurement at baseline and at the end of intervention period. The height of the children was measured without shoes to the nearest 0.1 cm by using a wooden height board (United Nations Children's Fund, New York, NY). Child's weight was measured to the nearest 0.1 kg by using a portable battery powered digital scale (AND, Denmark) with children wearing light clothes according to standard procedures.29 Mid upper arm circumference (MUAC) was measured to the nearest 0.1 cm and biceps, triceps, sub-scapular, and suprailiac skin fold thickness were measured in triplicate by using a Harpenden caliper following standard methods.30 Each of these measurements was performed by the same experienced anthropometrist to avoid inter-observer variation. Z-scores for weight-for-age (WAZ), height-for-age (HAZ), and weight-for-height (WHZ) were calculated by using EpiInfo version 6.0 (Centers for Disease Control and Prevention, Atlanta, GA) and National Center for Health Statistics/World Health Organization growth reference data.
Fecal sample collection and parasite counts.
Specimen containers with detailed instructions for fecal sample collection were distributed to children one day before the survey and requested to be returned with fecal samples the next day. Fecal specimens were obtained at the school by laboratory technicians, preserved according to standard procedures of the World Health Organization, and brought to the Parasitic Laboratory on the same day of sample collection. Quantitative egg counts of Ascaris, Trichuris, and hookworm were performed by using the Kato-Katz technique at the Medicine Laboratory Technology Co., Ltd. (Hanoi, Vietnam).31 The egg output was expressed as mean eggs per gram feces. All laboratory personal were unaware of the subject's group assignment.
Cognitive function tests.
Raven's Colored Progressive Matrices test was used to measure cognitive performance in the children. The test measures the ability to develop new insights and information from what is already perceived or known. In addition, a series of cognitive tests was selected from Wechsler's Intelligence Scale for Children III (WISC III), namely digit span backward and forward, coding, and block design. The digit span backward test assesses working memory for auditory information and the digit span forward assesses children's auditory attention span and the ability to focus on auditory information. Coding requires children to quickly pair either shapes or numbers with a symbol and measures the speed of information processing. Block design is a measure of problem solving to assess executive function short-term memory and attention span.32 These tests were selected on the basis of their wide use and validity.32
The cognitive test was conducted by a team of six technicians from the National Institute of Child Psychological Research and Mai Huong Day Care Mental Hospital. The technicians were trained on the test methods and use of published materials translated from the original test. The children were assigned randomly to the technician. The technician was not provided the information on the children's interventions and nutritional status. The technicians tested one by one for each child. Each child spent approximately 15–30 minutes taking the Raven's Colored Progressive Matrices test and approximately 20 minutes taking the four subtests of WISC III. Each child conducted the Raven's Colored Progressive Matrices test in one day and took the subtests of WISC III on the next day to avoid tiredness for the child. Similar procedures were conducted at baseline and endpoint survey. The standardized test protocols were translated into Vietnamese and have been used in Vietnam. Because the test has not been standardized locally, interpretations of the scores of cognitive test were used as raw scores. Furthermore, school grades of Vietnamese language and mathematics of each child were collected from the school records of the second semester of the academic year, which is the end of intervention.
Statistical analysis.
Data were analyzed by using SPSS version 13.0 software (SPSS, Inc., Chicago, IL). Normality of data was tested by using the Kolmogorov-Smirnov test. The difference of means within each group before and after the intervention was tested by using the paired t-test, and differences in prevalence from baseline were tested by using the McNemar test. Effects of deworming and fortified biscuits were tested by analysis of covariance (ANCOVA). When the F-test result was statistically significant, multiple comparisons were conducted by using the Bonferroni post-hoc test. The ANCOVA models were used to assess estimated effect sizes of fortification and deworming on the means of WAZ, HAZ, and WHZ scores, with baseline values, sex, and age as covariates. For the means of cognitive test scores, ANCOVA models were used to assess the estimated intervention effects adjusted for age and sex and family socioeconomic status and baseline outcome values as covariates. P values < 0.05 and < 0.1 were considered significant for main effects and interactions, respectively. If there was a significant interaction between de-worming and fortified biscuits, effects were analyzed group by group. Logistic regression controlling for age, sex, C-reactive protein concentrations, and baseline prevalence of the variables was performed to compare the effects of fortification and deworming on the prevalence of undernutrition. Values are reported as mean ± SD unless otherwise noted.
Results
Of 510 eligible children participating at baseline data collection, 28 children did not have end point measurements. Reasons for dropout included moving (n = 12), surgery (n = 2), and refusal to participate (n = 14) (Figure 1). The main characteristics of the dropouts did not differ from the remaining children. Mean consumption of biscuits was comparable among groups (94.2%, 94.8%, 95.7%, and 96.1% for the placebo, FB, Alb, and FB + Alb groups, respectively). A total of 92.1% of children ate all intended servings. There was no significant difference in the mean intakes of the biscuits among study groups. Of the 482 children with end point data, 469 (97%) completed the final cognitive tests (119, 114, 118, and 118 in the placebo, FB, Alb, and FB + Alb groups, respectively).
There were no statistically significant differences in baseline characteristics among the groups. The mean ± SD age of participants was 7.6 ± 0.9 years, and 47.6% were males (Table 2). The mean ± SD WAZ, HAZ, and WHZ scores at baseline were –1.53 ± 0.71, –1.43 ± 0.83, and –0.86 ± 0.77, respectively (Table 3), and the prevalence of underweight, stunting, and wasting was 28.2%, 25.5%, and 9.5%, respectively. Approximately 82% of the children were infected with at least one intestinal parasite, but there were no statistically significant differences among the groups. Prevalence of children infected with Ascaris, Trichuris, and hookworm was 65.4%, 55.8%, and 5.6%, respectively. Prevalence and intensity of parasite infections were similar across the intervention groups (Table 2). Most children infected with hookworm and Trichuris (92.6% and 88.5%, respectively) had a light intensity of infection, and 95.9% of the children with Ascaris infections had light-to-moderate intensity.
Table 2.
Baseline characteristics of school children who completed the study by intervention group*
| Outcomes | Placebo (n = 122) | Fortified biscuits (n = 118) | Albendazole (n = 120) | Fortified biscuits plus albendazole (n = 122) |
|---|---|---|---|---|
| Mean ± SD age (years) | 7.5 ± 0.9 | 7.6 ± 0.9 | 7.5 ± 0.9 | 7.6 ± 0.9 |
| Male (%) | 49.2 | 46.5 | 47.0 | 47.9 |
| Intestinal parasites (%) | 82.8 | 80.5 | 80.0 | 84.4 |
| Ascaris | 66.4 | 62.7 | 64.2 | 68.0 |
| Trichuris | 55.7 | 55.1 | 54.2 | 58.2 |
| Hookworm | 4.1 | 5.1 | 5.8 | 7.4 |
| Single infection | 40.2 | 38.1 | 37.5 | 36.1 |
| Double infections | 48.1 | 42.4 | 40.8 | 47.5 |
Placebo = placebo de-worming and non-fortified biscuits. No significant difference among groups by one-way analysis of variance for continuous variables or chi-square test for prevalence (P > 0.05).
Table 3.
Anthropometric outcomes of the school children before and after four months of the intervention*
| Outcome | Placebo (n = 122) | Fortified biscuits (n = 118) | Albendazole (n = 120) | Fortified biscuits plus albendazole (n = 122) | Estimated effect sizes† (95% CI), fortified biscuits plus albendazole | P for interaction fortification × deworming† | |
|---|---|---|---|---|---|---|---|
| WAZ-scores | |||||||
| Baseline | −1.56 ± 0.68 | −1.52 ± 0.74 | −1.47 ± 0.72 | −1.55 ± 0.68 | 0.02 | 0.01 | 0.667 |
| Endpoint‡ | −1.48 ± 0.70 | −1.42 ± 0.71 | −1.37 ± 0.73 | −1.44 ± 0.62 | −0.01 to 0.05 | −0.02 to 0.04 | |
| HAZ-scores | |||||||
| Baseline | −1.41 ± 0.86 | 1.47 ± 0.78 | −1.40 ± 0.82 | −1.44 ± 0.86 | 0.01 | 0.01 | 0.649 |
| Endpoint‡ | −1.34 ± 0.85 | −1.39 ± 0.79 | −1.33 ± 0.81 | −1.36 ± 0.85 | −0.001 to 0.03 | −0.01 to 0.02 | |
| WHZ-scores | |||||||
| Baseline | −0.94 ± 0.72 | −0.82 ± 0.78 | −0.79 ± 0.81 | −0.88 ± 0.78 | 0.03 | 0.01 | 0.626 |
| Endpoint§ | −0.86 ± 0.72 | −0.72 ± 0.76 | −0.70 ± 0.80 | −0.78 ± 0.76 | −0.02 to 0.08 | −0.04 to 0.06 | |
| MUAC (cm) | |||||||
| Baseline | 15.0 ± 1.1 | 15.1 ± 1.1 | 15.2 ± 1.2 | 15.0 ± 1.1 | 0.082¶ | 0.072¶ | 0.884 |
| End point | 15.3 ± 1.2 | 15.4 ± 1.1 | 15.6 ± 1.2 | 15.5 ± 1.1 | 0.02–0.15 | 0.01–0.13 | |
Values are mean ± SD. Placebo = placebo de-worming and non-fortified biscuits; CI = confidence interval; WAZ = weight for age Z score; HAZ = height for age Z score; MUAC = mid upper arm circumference.
Adjusted for sex, age, baseline outcome values, and C-reactive protein levels.
P < 0.001, by paired t-test.
P < 0.01, by paired t-test.
P < 0.05, by analysis of covariance.
Anthropometry.
Fortification and de-worming significantly increased MUAC (0.082 cm, 95% confidence interval [CI] = 0.02–0.15 cm, P < 0.01, and 0.072 cm, 95% CI = 0.01–0.13 cm, P < 0.05, respectively) (Table 3). There was no statistically significant effect of de-worming or fortification on weight, height, HAZ scores, WAZ scores, or WHZ scores, although there was a tendency for higher HAZ-scores in children receiving fortified biscuits (0.014 Z-score higher, 95% CI = –0.001 to 0.029, P = 0.06). There were no statistically significant differences in skin fold thickness after four months of intervention. Fortification and de-worming had no effect on the prevalence of underweight and wasting or on stunting (P > 0.05, by binary logistic regression analysis adjusted for sex, age, and baseline outcome values) Multiple stepwise regression analysis showed that baseline MUAC, age, fortification, de-worming, and high end point plasma zinc concentration were predictors of end point MUAC.
Effects on cognitive function.
For children receiving fortified biscuits, Raven's scores were significantly higher than in children receiving non-fortified biscuits (0.86 score higher; P = 0.035, by ANCOVA controlling for sex, age, mother's education, family socioeconomic status, baseline Raven's score, and anemia) (Table 4). However, there was a highly significant interaction with baseline anemia and end-point Raven's scores (P = 0.019). In children anemic at baseline (n = 118), those receiving FB biscuits scored 1.86 points higher on the Raven's test (P = 0.01) than those receiving non-fortified biscuits. In children not anemic at baseline, there was no statistically significant effect of consumption of fortified biscuits on Raven's score.
Table 4.
Cognitive outcomes (in raw scores) of the school children before and after four months of the intervention*
| Outcome | Placebo (n = 119) | Fortified biscuits (n = 114) | Albendazole (n = 118) | Fortified biscuits plus albendazole (n = 118) | Estimated effect sizes† (95% CI), Fortified biscuits plus albendazole | P for interaction | |
|---|---|---|---|---|---|---|---|
| Raven's Colored Matrices | |||||||
| All children | |||||||
| Baseline | 16.4 ± 5.6 | 16.5 ± 5.0 | 16.4 ± 5.3 | 16.5 ± 4.9 | 0.86 | −0.18 | 0.486 |
| End point | 19.2 ± 5.8 | 20.1 ± 4.9 | 19.2 ± 5.4 | 19.4 ± 5.0 | 0.06–1.7 | −0.97 to 0.62 | |
| Anemic children‡ | |||||||
| Baseline | 14.9 ± 5.1 | 15.6 ± 3.8 | 15.3 ± 5.6 | 15.4 ± 4.3 | 1.86§ | 0.10 | 0.971 |
| End point | 18.0 ± 5.1 | 20.6 ± 4.4 | 18.2 ± 5.8 | 20.8 ± 4.2 | 0.46–3.3 | −1.29 to 1.50 | |
| Digit span forward | |||||||
| Baseline | 7.1 ± 1.4 | 6.9 ± 1.2 | 6.9 ± 1.5 | 7.1 ± 1.2 | 0.34¶ | 0.07 | 0.310 |
| End point | 7.1 ± 1.4 | 7.5 ± 1.1 | 7.1 ± 1.3 | 7.4 ± 1.1 | 0.11–0.56 | −0.15 to 0.30 | |
| Digit span backward | |||||||
| Baseline | 2.9 ± 0.9 | 2.9 ± 1.0 | 2.8 ± 1.0 | 2.9 ± 0.8 | −0.07 | −0.03 | 0.701 |
| End point | 3.1 ± 0.8 | 3.1 ± 0.9 | 3.1 ± 0.8 | 3.0 ± 0.9 | −0.25 to 0.10 | −0.21 to 0.14 | |
| Block design | |||||||
| Baseline | 11.8 ± 8.5 | 12.5 ± 8.7 | 11.1 ± 8.4 | 12.8 ± 7.4 | −1.12 | 0.42 | 0.726 |
| End point | 16.6 ± 9.0 | 16.5 ± 8.6 | 16.9 ± 9.6 | 17.0 ± 9.1 | −2.48 to 0.23 | −0.94 to 1.77 | |
| Coding | |||||||
| Baseline | 31.9 ± 10.0 | 30.8 ± 8.4 | 32.2 ± 9.4 | 30.8 ± 8.6 | 0.50 | 0.54 | 0.603 |
| End point | 39.6 ± 8.0 | 39.5 ± 7.0 | 39.6 ± 7.8 | 39.5 ± 8.5 | −0.95 to 1.94 | −0. 90 to 1.99 | |
Values are mean ± SD. Placebo = placebo de-worming and non-fortified biscuits; CI = confidence interval. There were no significant differences between groups at baseline.
Adjusted for age, family socioeconomic status, mother's education, and baseline outcome values,
Sample sizes for placebo, fortified biscuits, albendazole, and fortified biscuits plus albendazole were 29, 30, 28, and 31, respectively.
P < 0.05, by analysis of covariance.
P < 0.01, by analysis of covariance.
Linear regression analysis showed that baseline factors predicting end point Raven's score were baseline Raven's score (P < 0.001), age (P < 0.001), and anemia (P = 0.008). The only two end point variables that significantly contributed to the model of end-point Raven's score were vitamin A deficiency (P = 0.12) and zinc deficiency (P = 0.056). There was also a statistically significant intervention effect for the digits span forward test among children receiving fortification, who recalled on average 0.34 (95% CI = 0.11–0.56) more items than those in the non-fortified group. There were no statistically significant differences among groups for the digits span backward, coding, or block design tests (Table 4). De-worming had no significant effect on any of the cognitive tests. There were no statistically significant differences among groups for the average grade for mathematics or Vietnamese before and after the intervention.
Effects on parasite infestation and parasite load.
As expected, de-worming significantly decreased the prevalence of Ascaris and Trichuris infection at end point. However, because there was a statistically significant interaction between consumption of fortified biscuits and de-worming on these infections at end point (P < 0.05), results were analyzed group by group. This analysis showed that for children receiving only albendazole, the prevalence of Ascaris and Trichuris infection was only slight reduced from baseline to four months later (from 65% to 56% and from 53% to 49%, respectively), whereas in children receiving albendazole and fortified biscuits, the reductions were much more marked (from 68% to 43% and from 58% to 39%) (Table 5).
Table 5.
Parasite egg counts at baseline, two months, and four months per supplementation group*
| Parasite | No. | Placebo | No. | Fortified biscuits | No. | Albendazole | No. | Fortified biscuits plus albendazole |
|---|---|---|---|---|---|---|---|---|
| Ascaris e/g T0 | 79 | 8,736 (4,080–17,376) | 73 | 9,840 (4,896–19,944) | 75 | 10,008 (4,344–19,272) | 80 | 10,032 (4,158–16,272) |
| Ascaris e/g T2† | 78 | 8,448 (4,044–14,478 | 80 | 7,860 (4,284–15,498) | 46 | 960‡ (600–2,256) | 12 | 864‡ (252–2,796) |
| Ascaris e/g T4† | 85 | 7,536 (4,380–12,420 | 80 | 7,512 (380–413) | 66 | 5,352§ (3,456–9,264) | 51 | 2,304‡¶ (960–3,792) |
| Trichuris e/g T0 | 65 | 264 (120–516) | 64 | 288 (198–522) | 65 | 312 (204–696) | 69 | 264 (156–480) |
| Trichuris e/g T2† | 63 | 288 (168–528) | 62 | 288 (168–462) | 33 | 168# (84–288) | 23 | 144‡ (72–288) |
| Trichuris e/g T4 | 74 | 276 (138–558) | 69 | 288 (144–468) | 58 | 264 (144–486) | 46 | 192‡ (144–384) |
| Hookworm e/g T0 | 5 | 816 (504–924) | 6 | 912 (384–1,110) | 7 | 864 (456–1,152) | 8 | 852 (630–1,182) |
| Hookworm e/g T2 | 6 | 552 (420–1,176) | 6 | 648 (522–1,098) | 4 | 144‡ (84–690) | 3 | |
| Hookworm e/g T4 | 6 | 672 (408–2,178) | 6 | 756 (258–1,080) | 7 | 312 (24–1,680) | 6 | 180 (96–1,032) |
Values are median and 25th–75th percentiles among infected children. Placebo = placebo de-worming and non-fortified biscuits; e/g = eggs per gram (of feces); T0 = baseline; T2 = two months; T4 = four months.
P < 0.001 among groups, by Kruskal-Wallis test.
P < 0.001 vs. placebo and fortified biscuits group, by Mann-Whitney U test.
P < 0.05 vs. placebo group, by Mann-Whitney U test.
P < 0.001 vs. albendazole group, by Mann-Whitney U test.
P < 0.01 vs. placebo and fortified biscuits group, by Mann-Whitney U test.
Because the prevalence of Ascaris and Trichuris infection was much lower two months after de-worming in the Alb and FB + Alb groups than after four months, we hypothesized that the interaction between de-worming and fortified biscuits was caused by differences in re-infection rates. Therefore, we investigated infection rates in the subgroup of children without Ascaris (n = 170) or Trichuris (n = 219) infection at baseline. After four months, 41% and 49% of the children in the placebo and Alb groups, respectively, were infected with Ascaris. In contrast, only 23% and 15% of the children in the FB and FB + Alb groups, respectively, were infected with Ascaris (P = 0.003). Therefore, the relative risk for Ascaris infection during the four-month study was 0.30 (95% CI = 0.15–0.59, P < 0.001) for children receiving fortified biscuits. For Trichuris infection, a similar pattern was found (with infection rates of 28%, 30%, 18%, and 8% for the placebo, Alb, FB, and FB + Alb groups, respectively), resulting in a relative risk for Trichuris infection during the study of 0.36 (95% CI = 0.18–0.73, P = 0.004). Moreover, relative risk for Ascaris and Trichuris infection in the first two months of the study and the last two months of the study for children consuming fortified biscuits were similar, indicating the protective effect of FB was constant over the study period.
In addition to an effect on the prevalence of Ascaris and Trichuris infection, there was also an effect of fortified biscuits on the parasite load of Ascaris. Infected children in the FB + Alb group had the lowest parasite load after four months (Supplemental Table).
Discussion
This study shows that consumption of multi-micronutrient fortified biscuits provided as a snack at schools for four months had only modest effects on anthropometry, but resulted in improvements in the Raven's test score, especially in children anemic at baseline, who showed a large improvement, and in the digits span forward test result. Moreover, consumption of fortified biscuits resulted in lower infection rates for Ascaris and Trichuris.
The improvement in Raven's scores found in anemic children in our study is consistent with studies in Filipino school children and Tanzanian preschool children. A beverage fortified with multiple micronutrients supplied for 16 weeks had a positive effects on cognitive performance among anemic and mildly iodine deficient Filipino school children.33 In preschool children (6–59 months of age) in Tanzania, iron supplementation for 12 months improved language and motor development in children.34 Improvements in the digits span forward test were also reported in South African primary school children biscuits fortified with iron, iodine, and beta-carotene.28 Thus, vitamin and mineral deficiencies in children may limit the child's optimal ability in cognition and intelligence. In this study, baseline anemia and total body iron, and end point vitamin A and zinc status predicted Raven's score, whereas end point iodine status predicted the digits forward test, demonstrating that single micronutrient interventions will not be enough to improve the cognitive development of school children. The failure of the intervention to improve all cognitive test results might be caused by differences in sensitivity of different aspects of cognition to a short-term intervention and also to the complex nature of cognition, which also requires environmental factors such as stimuli and learning inputs. Similar observations were also reported in a recent study in Australian and Indonesian school children receiving a drink fortified with vitamins and minerals for 12 months.35
There have been several trials of the effect of de-worming on tests of cognitive function or educational achievement, but the results have been inconsistent. Thus, there is no clear evidence of an effect of worm treatment on cognitive function and education achievement.36 Most trials showed no treatment effect by single dose or multiple dose de-worming on cognition in preschool or school children or on academic achievement, even in children with high worm loads. Additional education programs may also be needed complementary to nutrition and other health interventions.37
We reported that multi-micronutrient fortified biscuits significantly reduced the prevalence of anemia and increased micronutrient status.29 In this study, in-depth analysis of growth outcomes show that multi-micronutrient fortified biscuits only significantly improved MUAC but had no effect on growth in weight or height and four points of skin fold thickness (biceps, triceps, sub-scapular, and suprailiac). The positive intervention effect of fortified biscuits on MUAC compared with non-fortified biscuits is consistent with micronutrient-fortified supplementary feeding studies in school children in Botswana38 and adolescent girls in Bangladesh.26 Interestingly, the improvement in MUAC and no change in biceps and triceps skinfold thickness suggests an increase in lean arm mass. This effect may be explained in part by the additional zinc and energy in the biscuits.39 The lack of effect of the fortification and de-worming on physical growth are in contrast with results of some studies and with findings of two recent meta-analysis of multiple micronutrient fortification and de-worming on child growth.13,24,38 Possible explanations for the lack of a significant effect of multiple micronutrient fortification and de-worming on children's growth in the present study are the relatively limited duration of the intervention of four months or macronutrient deficiencies in the diet of the children.
Although we did not assess dietary intake of the children during study, a one-day 24-hour recall interview in a sub-sample of children (n = 207) at baseline showed a lack of approximately 35% energy and 40% fat compared with the Vietnamese recommended daily allowance for children in this age group. Interesting in this context is the increase in weight from baseline among the children in all four groups, which could be caused by provision of an additional 130 kcal/day from the biscuits during the intervention.
In the present study, consumption of multi-micronutrient–fortified biscuits resulted in significantly lower re-infection rates for Ascaris and Trichuris and a lower parasite load for Ascaris at the end point. Effects of micronutrients on parasite infestation have been reported. Iron supplementation, but not multiple micronutrient supplementation, reduced Ascaris infection intensity in Zambian children.40 Possible mechanisms in which micronutrients might interact with re-infection rates include the integrity from the mucosal epithelium and changes in immune reactivity. Both vitamin A and zinc have been shown to be essential for maintaining epithelium integrity,41 although in an animal model, vitamin A-deficient pigs had a significant lower infection rate for T. suis than vitamin A-sufficient pigs.42 Vitamin A deficiency has been shown to polarize the immune response towards a Th1 response, whereas vitamin A supplementation benefits a Th2-mediated immune response.43
In this study, we did not have data on immune responses. However, in a subsample of infants (n = 207), intestinal absorption of two sugars (lactulose and mannitol) was measured as measure of gut integrity. There were no significant differences among the groups at the end of the study in urinary lactulose:mannitol ratios, indicating that effects on immune responses might have been more important than gut integrity in the present study. The lower parasite load for Ascaris in the children receiving FB also supports this hypothesis. The effect could be caused by better iron status because Olson and others found that iron supplementation significantly reduced the reinfection rates from Ascaris and Trichuris in Kenyan adults, although they observed no effect in children.44 More research into the underlying mechanisms is warranted, but combining de-worming with improvements in micronutrient status appear to increase the effectiveness of de-worming.
This study showed that significant improvements in cognitive test results can be achieved even with an intervention with a relatively short duration of four months. Thus, multi-micronutrient–fortified snack foods distributed in a school feeding program offer an excellent opportunity to supply micronutrients needed for school children at risk for micronutrient deficiency and to improve cognitive function, especially in anemic children.
Supplementary Material
ACKNOWLEDGMENTS
We thank all parents and children, health personnel, and teachers of Hung Yen Province for their full participation in this study; the National Institute of Nutrition team for their dedication and support in data collection and field monitoring; and the psychologist team at the National Institute of Child Psychological Research and Mai Huong Day Care Mental Hospital for performing cognitive tests and providing advice on cognitive assessment.
Note: Supplemental table appears at www.ajtmh.org.
Footnotes
Financial support: This study was supported by the Neys-van Hoogstraten Foundation, The Netherlands, and the Ellison Medical Foundation.
Disclosure: None of the authors had any conflicts of interest.
Authors' addresses: Tran T. Nga and Nguyen C. Khan, Department of Micronutrient Research and Application, National Institute of Nutrition, Hanoi, Vietnam, E-mails: thuynga672000@yahoo.co.uk and nguyencongkhan@vfa.gov.vn. Pattanee Winichagoon and Emorn Wasantwisut, Institute of Nutrition, Mahidol University, Nakhon Pathom, Thailand, E-mails: nupwn@mahidol.ac.th and numdk@mahidol.ac.th. Marjoleine A. Dijkhuizen, Department of Human Nutrition, Copenhagen University, Copenhagen, Denmark, E-mail: madijkhuizen@gmail.com. Frank T. Wieringa, Unite Medicale Researche, 204 Prevention des Malnutritions, Institut de Recherche pour le Développement, Montpellier, France, E-mail: franck.wieringa@ird.fr.
References
- 1.Ramakrishnan U. Prevalence of micronutrient malnutrition worldwide. Nutr Rev. 2002;60:S46–S52. doi: 10.1301/00296640260130731. [DOI] [PubMed] [Google Scholar]
- 2.Dijkhuizen MA, Wieringa FT, West CE, Muherdiyantiningsih , Muhilal Concurrent micronutrient deficiencies in lactating mothers and their infants in Indonesia. Am J Clin Nutr. 2001;73:786–791. doi: 10.1093/ajcn/73.4.786. [DOI] [PubMed] [Google Scholar]
- 3.Demment MW, Young MM, Sensenig RL. Providing micronutrients through food-based solutions: a key to human and national development. J Nutr. 2003;133:3879S–3885S. doi: 10.1093/jn/133.11.3879S. [DOI] [PubMed] [Google Scholar]
- 4.Black M. The evidence linking zinc deficiency with children's cognitive and motor functioning. J Nutr. 2003;133:1473S–1476S. doi: 10.1093/jn/133.5.1473S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qian M, Wang D, Watkins WE, Gebski V, Yan YQ, Li M, Chen ZP. The effects of iodine on intelligence in children: a meta-analysis of studies conducted in China. Asia Pac J Clin Nutr. 2005;14:32–42. [PubMed] [Google Scholar]
- 6.Grantham-McGregor SM, Ani C. A review of studies on the effect of iron deficiency on cognitive development in children. J Nutr. 2001;131:649S–666S. doi: 10.1093/jn/131.2.649S. [DOI] [PubMed] [Google Scholar]
- 7.Grantham-McGregor S. Early child development in developing countries. Lancet. 2007;369:824. doi: 10.1016/S0140-6736(07)60404-8. [DOI] [PubMed] [Google Scholar]
- 8.Pollitt E, Jahari A, Husaini M, Kariger P, Saco-Pollitt C. Developmental trajectories of poorly nourished toddlers that received a micronutrient supplement with and without energy. J Nutr. 2002;132:2617–2625. doi: 10.1093/jn/132.9.2617. [DOI] [PubMed] [Google Scholar]
- 9.Sanghvi T, Van Ameringen M, Baker J, Fiedler J, Borwankar R, Phillips M, Houston R, Ross J, Heymann H, Dary O. Vitamin and mineral deficiencies technical situation analysis: a report for the ten year strategy for the reduction of vitamin and mineral deficiencies. Food Nutr Bull. 2007;28:S160–S219. [PubMed] [Google Scholar]
- 10.Black MM, Baqui AH, Zaman K, Ake Persson L, El Arifeen S, Le K, McNary SW, Parveen M, Hamadani JD, Black RE. Iron and zinc supplementation promote motor development and exploratory behavior among Bangladeshi infants. Am J Clin Nutr. 2004;80:903–910. doi: 10.1093/ajcn/80.4.903. [DOI] [PubMed] [Google Scholar]
- 11.Jalal F, Nesheim MC, Agus Z, Sanjur D, Habicht JP. Serum retinol concentrations in children are affected by food sources of beta-carotene, fat intake, and anthelmintic drug treatment. Am J Clin Nutr. 1998;68:623–629. doi: 10.1093/ajcn/68.3.623. [DOI] [PubMed] [Google Scholar]
- 12.Le HT, Brouwer ID, Verhoef H, Nguyen KC, Kok FJ. Anemia and intestinal parasite infection in school children in rural Vietnam. Asia Pac J Clin Nutr. 2007;16:716–723. [PubMed] [Google Scholar]
- 13.Hall A, Hewitt G, Tuffrey V, de Silva N. A review and meta-analysis of the impact of intestinal worms on child growth and nutrition. Matern Child Nutr. 2008;4((Suppl 1)):118–236. doi: 10.1111/j.1740-8709.2007.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes S, Kelly P. Interactions of malnutrition and immune impairment, with specific reference to immunity against parasites. Parasite Immunol. 2006;28:577–588. doi: 10.1111/j.1365-3024.2006.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization . Preventive Chemotherapy in Human Helminthiasis: Coordinated Use of Anthelminthic Drugs in Control Interventions: A Manual for Health Professionals and Programme Managers. Geneva: World Health Organization; 2006. [Google Scholar]
- 16.Keiser J, Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. JAMA. 2008;299:1937–1948. doi: 10.1001/jama.299.16.1937. [DOI] [PubMed] [Google Scholar]
- 17.Hop le T, Berger J. Multiple micronutrient supplementation improves anemia, micronutrient nutrient status, and growth of Vietnamese infants: double-blind, randomized, placebo-controlled trial. J Nutr. 2005;135:660S–665S. doi: 10.1093/jn/135.3.660S. [DOI] [PubMed] [Google Scholar]
- 18.National Institute of Nutrition, Ministry of Health . Report on Anemia and Sub-Clinical Vitamin A Deficiency in Some Provinces in Vietnam. Hanoi: National Institute of Nutrition, Ministry of Health; 2006. [Google Scholar]
- 19.Van Thuy P, Berger J, Nakanishi Y, Khan NC, Lynch S, Dixon P. The use of NaFeEDTA-fortified fish sauce is an effective tool for controlling iron deficiency in women of childbearing age in rural Vietnam. J Nutr. 2005;135:2596–2601. doi: 10.1093/jn/135.11.2596. [DOI] [PubMed] [Google Scholar]
- 20.National Institute of Nutrition . National Nutrition Survey 2009. Hanoi: National Institute of Nutrition; 2009. [Google Scholar]
- 21.Rivera JA, Hotz C, Gonzalez-Cossio T, Neufeld L, Garcia-Guerra A. The effect of micronutrient deficiencies on child growth: a review of results from community-based supplementation trials. J Nutr. 2003;133:4010S–4020S. doi: 10.1093/jn/133.11.4010S. [DOI] [PubMed] [Google Scholar]
- 22.Bhandari N, Bahl R, Taneja S. Effect of micronutrient supplementation on linear growth of children. Br J Nutr. 2001;85((Suppl 2)):S131–S137. [PubMed] [Google Scholar]
- 23.Brown KH, Peerson JM, Rivera J, Allen LH. Effect of supplemental zinc on the growth and serum zinc concentrations of prepubertal children: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2002;75:1062–1071. doi: 10.1093/ajcn/75.6.1062. [DOI] [PubMed] [Google Scholar]
- 24.Ramakrishnan U, Aburto N, McCabe G, Martorell R. Multimicronutrient interventions but not vitamin A or iron interventions alone improve child growth: results of 3 meta-analyses. J Nutr. 2004;134:2592–2602. doi: 10.1093/jn/134.10.2592. [DOI] [PubMed] [Google Scholar]
- 25.Latham MC, Ash DM, Makola D, Tatala SR, Ndossi GD, Mehansho H. Efficacy trials of a micronutrient dietary supplement in schoolchildren and pregnant women in Tanzania. Food Nutr Bull. 2003;24:S120–S128. doi: 10.1177/15648265030244S209. [DOI] [PubMed] [Google Scholar]
- 26.Hyder SM, Haseen F, Khan M, Schaetzel T, Jalal CS, Rahman M, Lonnerdal B, Mannar V, Mehansho H. A multiple-micronutrient-fortified beverage affects hemoglobin, iron, and vitamin A status and growth in adolescent girls in rural Bangladesh. J Nutr. 2007;137:2147–2153. doi: 10.1093/jn/137.9.2147. [DOI] [PubMed] [Google Scholar]
- 27.Manger MS, McKenzie JE, Winichagoon P, Gray A, Chavasit V, Pongcharoen T, Gowachirapant S, Ryan B, Wasantwisut E, Gibson RS. A micronutrient-fortified seasoning powder reduces morbidity and improves short-term cognitive function, but has no effect on anthropometric measures in primary school children in northeast Thailand: a randomized controlled trial. Am J Clin Nutr. 2008;87:1715–1722. doi: 10.1093/ajcn/87.6.1715. [DOI] [PubMed] [Google Scholar]
- 28.van Stuijvenberg ME, Kvalsvig JD, Faber M, Kruger M, Kenoyer DG, Benade AJ. Effect of iron-, iodine-, and beta-carotene-fortified biscuits on the micronutrient status of primary school children: a randomized controlled trial. Am J Clin Nutr. 1999;69:497–503. doi: 10.1093/ajcn/69.3.497. [DOI] [PubMed] [Google Scholar]
- 29.Nga TT, Winichagoon P, Dijkhuizen MA, Khan NC, Wasantwisut E, Furr H, Wieringa FT. Multi-micronutrient-fortified biscuits decreased prevalence of anemia and improved micronutrient status and effectiveness of deworming in rural Vietnamese school children. J Nutr. 2009;139:1013–1021. doi: 10.3945/jn.108.099754. [DOI] [PubMed] [Google Scholar]
- 30.Gibson RS. Principles of Nutritional Assessment. Oxford: Oxford University Press; 1990. [Google Scholar]
- 31.World Health Organization . Training Manual on Diagnosis of Intestinal Parasites. Geneva: World Health Organization; 1998. [Google Scholar]
- 32.Hughes D, Bryan J. The assessment of cognitive performance in children: considerations for detecting nutritional influences. Nutr Rev. 2003;61:413–422. doi: 10.1301/nr.2003.dec.413-422. [DOI] [PubMed] [Google Scholar]
- 33.Solon FS, Sarol JN, Jr, Bernardo AB, Solon JA, Mehansho H, Sanchez-Fermin LE, Wambangco LS, Juhlin KD. Effect of a multiple-micronutrient-fortified fruit powder beverage on the nutrition status, physical fitness, and cognitive performance of schoolchildren in the Philippines. Food Nutr Bull. 2003;24:S129–S140. doi: 10.1177/15648265030244S210. [DOI] [PubMed] [Google Scholar]
- 34.Olney DK, Pollitt E, Kariger PK, Khalfan SS, Ali NS, Tielsch JM, Sazawal S, Black R, Allen LH, Stoltzfus RJ. Combined iron and folic acid supplementation with or without zinc reduces time to walking unassisted among Zanzibari infants 5- to 11-mo old. J Nutr. 2006;136:2427–2434. doi: 10.1093/jn/136.9.2427. [DOI] [PubMed] [Google Scholar]
- 35.Osendarp SJ, Baghurst KI, Bryan J, Calvaresi E, Hughes D, Hussaini M, Karyadi SJ, van Klinken BJ, van der Knaap HC, Lukito W, Mikarsa W, Transler C, Wilson C. Effect of a 12-mo micronutrient intervention on learning and memory in well-nourished and marginally nourished school-aged children: 2 parallel, randomized, placebo-controlled studies in Australia and Indonesia. Am J Clin Nutr. 2007;86:1082–1093. doi: 10.1093/ajcn/86.4.1082. [DOI] [PubMed] [Google Scholar]
- 36.Taylor-Robinson DC, Jones AP, Garner P. Deworming drugs for treating soil-transmitted intestinal worms in children: effects on growth and school performance. Cochrane Database Syst Rev. 2007:CD000371. doi: 10.1002/14651858.CD000371.pub3. [DOI] [PubMed] [Google Scholar]
- 37.Hall A. Micronutrient supplements for children after deworming. Lancet Infect Dis. 2007;7:297–302. doi: 10.1016/S1473-3099(07)70084-1. [DOI] [PubMed] [Google Scholar]
- 38.Abrams SA, Mushi A, Hilmers DC, Griffin IJ, Davila P, Allen L. A multinutrient-fortified beverage enhances the nutritional status of children in Botswana. J Nutr. 2003;133:1834–1840. doi: 10.1093/jn/133.6.1834. [DOI] [PubMed] [Google Scholar]
- 39.Golden BE, Golden MH. Effect of zinc on lean tissue synthesis during recovery from malnutrition. Eur J Clin Nutr. 1992;46:697–706. [PubMed] [Google Scholar]
- 40.Nchito M, Geissler PW, Mubila L, Friis H, Olsen A. The effect of iron and multi-micronutrient supplementation on Ascaris lumbricoides reinfection among Zambian schoolchildren. Trans R Soc Trop Med Hyg. 2009;103:229–236. doi: 10.1016/j.trstmh.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 41.Udomkesmalee E, Dhanamitta S, Sirisinha S, Charoenkiatkul S, Tuntipopipat S, Banjong O, Rojroongwasinkul N, Kramer TR, Smith JC., Jr Effect of vitamin A and zinc supplementation on the nutrition of children in northeast Thailand. Am J Clin Nutr. 1992;56:50–57. doi: 10.1093/ajcn/56.1.50. [DOI] [PubMed] [Google Scholar]
- 42.Pedersen SE, Saeed I, Jensen SK, Michaelsen KF, Friis H. Marginal vitamin A deficiency in pigs experimentally infected with Trichuris suis: a model for vitamin A inadequacy in children. Trans R Soc Trop Med Hyg. 2001;95:557–565. doi: 10.1016/s0035-9203(01)90040-9. [DOI] [PubMed] [Google Scholar]
- 43.Stephensen CB, Rasooly R, Jiang XW, Ceddia MA, Weaver CT, Chandraratna RAS, Bucy RP. Vitamin A enhances in vitro Th2 development via retinoid X receptor pathway. J Immunol. 2002;168:4495–4503. doi: 10.4049/jimmunol.168.9.4495. [DOI] [PubMed] [Google Scholar]
- 44.Olsen A, Thiong'o FW, Ouma JH, Mwaniki D, Magnussen P, Michaelsen KF, Friis H, Geissler PW. Effects of multimicronutrient supplementation on helminth reinfection: a randomized, controlled trial in Kenyan schoolchildren. Trans R Soc Trop Med Hyg. 2003;97:109–114. doi: 10.1016/s0035-9203(03)90042-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


