Abstract
Maternal dengue antibodies are important in determining the optimal age of dengue vaccination, but no study has quantified the heterogeneity of antibody decay and persistence in infants. We used longitudinal regression methods and survival analysis to measure decay and persistence times of serotype-specific neutralizing antibodies in 139 infants in Bangkok. A biphasic decay pattern was found with half-life times of 24–29 days between birth and 3 months and 44–150 days after 3 months. Atypical decay rates were found in 17% of infants for dengue virus-1 and -4. Median persistence times of plaque reduction neutralization tests > 10 ranged from 6 to 9 months. Persistence times for individuals could not be predicted based on antibody values at birth. Vaccination against dengue before 12 months of age would be ineffective if maternal antibodies at plaque reduction neutralization test levels below 80 interfere with vaccine uptake. Projections of average antibody persistence based on values at birth should be avoided in studies on dengue pathogenesis in infants.
Introduction
Dengue is a vector-borne disease caused by one of four dengue virus (DENV) serotypes (1, 2, 3, and 4) that all circulate in hyperendemic areas.1 Infection with one serotype provides short-term cross-immunity against the other serotypes but could increase the risk of severe disease in the longer term on secondary infection with a heterotypic serotype.1,2 Most DENV infections remain asymptomatic, but clinical disease can be caused by each of the DENV serotypes, ranging from mild febrile disease to plasma leakage and circulatory failure (dengue hemorrhagic fever [DHF] and dengue shock syndrome [DSS]).3 Severe dengue disease remains a leading cause of child hospitalization and death in several Asian countries, with case fatality rates over 1% in some of these areas.1
In the absence of a curative treatment and sustainable vector control, the best option for the reduction of dengue is a safe and efficacious vaccine.1 Multiple dengue vaccine candidates are currently being tested in clinical trials, with the chimeric tetravalent live attenuated YF-17D vaccine being the most advanced in phase IIb.4,5 Although infants have the highest risk of severe disease and death on DENV infection,3,6 this age group is currently not targeted in clinical vaccine trials. A vaccine may not be as immunogenic in infants compared with older children because of a reduced memory response and circulating maternal antibodies.7,8 Maternal antibodies have been associated with reduced efficacy of multiple vaccines such as live measles vaccine,9 oral poliomyelitis vaccine, pertussis, diphtheria and tetanus toxoids,10 Haemophilus influenzae conjugate vaccine, and hepatitis A vaccine.7
Maternal antibodies are also thought to contribute to the pathogenesis of severe dengue disease in infants because of antibody-dependent enhancement (ADE).2,11 In vitro studies have shown increased viral uptake by antibody-presenting cells in the presence of non-neutralizing levels of heterotypic antibodies.2,12 Recent studies have indicated that, in addition to ADE, T-cell activation by heterotypic serotypes may be another important factor in dengue pathogenesis.13,14 In infants, maternal dengue antibodies that have declined below neutralizing levels could enhance primary infection, explaining a peak of severe dengue disease between 6 and 8 months of age.11,15 It is generally agreed that a tetravalent dengue vaccine will be required to minimize the risk of ADE on DENV infection after vaccination.4,16
Multiple studies on maternal dengue antibodies have been conducted previously to assess (1) the association between maternal antibodies and severe dengue in infants13,17,18 and (2) the proportion of infants with detectable dengue antibody levels at different ages.19–23 Both are essential pieces of information for decisions regarding the optimal age of vaccination against dengue. These studies indicated that DHF occurred in most infants after maternal plaque reduction neutralization test (PRNT) declined to below 1:20 or 1:50 and that maternal PRNT levels at birth were associated with the age of infants at the time of presentation with DHF.13,17,18 In terms of persistence, these studies found that less than 50% of infants had detectable maternal antibodies against dengue at 6 months of age, and almost no infants had detectable antibodies at 12 months of age.19,21
Many of the above studies had to make projections of maternal antibody levels in infants based on rough assumptions of (monophasic) log-linear decay rates and persistence. It is unclear if these represent patterns in the majority of infants, because no study assessed the heterogeneity of decay rates between infants. Although the proportion of infants with detectable antibody levels at different ages is known, the underlying longitudinal antibody kinetics remain unknown. More insight in these kinetics will allow better assessment of the optimal age for dengue vaccination, particularly opportunities for infant vaccination.
We used data collected during a cohort study conducted by Mahidol University among infants in Bangkok to study maternal dengue antibody decay rates and persistence times. We found that PRNT levels in infants decayed in two phases and that PRNT levels at birth, although associated with decay rates, did not predict persistence times.
Materials and Methods
Data collection and serology.
Data collected previously in a cohort study of infants in Bangkok were used in this analysis. This study was not considered human subjects research, because pre-existing, deidentified data were used and no additional data were collected. Detailed information on data collection has been provided previously.21 In short, 219 mothers and their infants were enrolled at birth between November 2000 and March 2001 at the Rajavithi hospital in Bangkok. Of these, 139 infants were followed at 3-month intervals during the first year of life and at 18 and 24 months of age. A questionnaire was completed at birth and each visit, a general physical exam was conducted, and blood was drawn for serology. Serological testing was conducted at Mahidol University. Dengue immunoglobulin G (IgG) monoclonal indirect enzyme-linked immunosorbent assay (ELISA) and dengue IgM capture ELISA were modified from Shu and others24 using a protein A-based purified dengue complex monoclonal antibody (2H2) as coating material and flavivirus nonimmune serum as control.
A serotype-specific 50% PRNT (PRNT50) was done according to Russell and others.25 PRNT50 assays of all samples from each infant were done simultaneously to minimize variation within infants caused by the assay. Throughout this article, reciprocal PRNT titers will be reported. Dengue infections were defined as seroconversion (detection of IgM) or a greater than or equal to fourfold increase of PRNT50 values against any serotype.
Estimation of antibody decay rates.
The analysis of antibody decay for each DENV serotype was conducted using PRNT values from infants that had detectable values (PRNT ≥ 10) against a serotype at birth and no serologic evidence of dengue infection during the follow-up period. Infants were excluded from follow-up after PRNT declined to below 10.
All PRNT values were log-transformed as log10 (PRNT + 1). Because no exact values could be retrieved for PRNT < 10, these values were estimated by extrapolating the decay rate of the previous interval in the same infant through the last PRNT value that could be detected for that infant. This method of replacement affected the average decay rates the least (contrary to replacement by any single value). Negative estimates were replaced by zero. Projections ≥ 10 were replaced by 9.99, and values < 10 at 3 months of age were replaced by 5.
Antibody decay rates were estimated separately for each DENV serotype using a longitudinal model that included a linear model for the mean and a parametric model for the covariance structure.26 For the mean decay rate, a linear regression model was fitted using the log-transformed PRNT value as a dependent variable and age in months as a predictor variable.
The model was allowed to determine different decline rates for different age intervals by inclusion of linear splines as predictor variables. Various combinations of multiple linear splines that separated different age intervals were compared in an iterative process during which the best-fitting model for the mean was determined using Akaike's information criterion (AIC) and likelihood ratio testing. Spline variables were kept in the model only if they indicated a statistically significant difference in decline rate between two age intervals. This method was used to allow a completely data-driven determination of age intervals without any a priori assumptions. A linear model (instead of an exponential model) was selected to allow comparison of outcomes with other studies on dengue antibody decay.
Models for the covariance structure included a parameter to adjust for heteroscedasticity of residual variance over time and a parameter to adjust for the serial correlation between measurements within each infant. The best-fitting model for the covariance structure was determined using likelihood ratio testing, and the goodness of fit to the autocorrelation function and the empirical variogram was determined according to Diggle.26
Detection of atypical decay patterns.
The influence of each infant on the average decay rate was estimated by fitting the model excluding one infant at the time (leave one out validation) and measuring AIC and the sum of residuals. The decay pattern for an infant was considered atypical if the standardized residuals for that infant were not between −2 and 2 and if the change in AIC or the sum of residuals after leaving that infant out was lower than the 25th percentile minus 1.5 times the interquartile range (IQR) or higher than the 75th percentile plus 1.5 times the IQR of the distribution (outliers).
Estimation of persistence times.
The persistence of PRNT levels above four different cutoff values (10, 20, 50, and 80) was estimated using Kaplan–Meier survival analysis with log–log confidence intervals. Survival analysis allowed the use of information on persistence times from those infants that were lost to follow-up at some point during the study (right censoring).
For each cutoff, the median survival (persistence) time in months was estimated, and for those infants with events (PRNT decline under the cutoff value), the mean persistence time could be estimated. To obtain the most precise estimates, persistence times were measured using the exact age in months of each infant at different follow-up visits instead of the follow-up month itself. Finally, the association between PRNT values at birth and PRNT persistence times was studied using linear regression. The difference between the predicted and observed persistence times was calculated to estimate how well PRNT values at birth predicted persistence times.
Statistical analysis was done using the R system version 2.9.2 using the nlme package version 3.1-93 for longitudinal models and the survival package for survival analysis.
Results
One hundred thirty-nine infants were included at birth for follow-up until their PRNT values became undetectable. Forty-one infants (30%) were lost to follow-up during the 24-month study period. No differences in demographic indicators were found between infants that were lost to follow-up and those who remained in the study. Fourteen infants (10%) met the serologic criteria for DENV infection and were excluded from the analysis. Table 1 provides the number of infants in the cohort at each follow-up visit and the number with detectable PRNT values.
Table 1.
Summary measures for PRNT levels of infants in the study cohort
| Indicator | Birth | 3 months | 6 months | 9 months | 12 months | 18 months |
|---|---|---|---|---|---|---|
| Infants in cohort | 125 | 124 | 103 | 104 | 101 | 5 |
| DENV-1 | ||||||
| N ≥ 10 (%)* | 116 (100) | 107 (92) | 58 (50) | 17 (15) | 3 (3) | 0 (0) |
| GMT (95% CI)† | 968 (690–1,358) | 122 (94–159) | 35 (29–43) | 30 (19–46) | 32 (1–1,656) | 1 (–) |
| DENV-2 | ||||||
| N ≥ 10 (%)* | 116 (100) | 104 (90) | 41 (35) | 6 (5) | 2 (2) | 0 (0) |
| GMT (95% CI)† | 690 (508–939) | 96 (76–123) | 25 (21–31) | 18 (9–34) | 20 (1–446) | 1 (–) |
| DENV-3 | ||||||
| N ≥ 10 (%)* | 114 (100) | 103 (90) | 40 (35) | 4 (4) | 0 (0) | 0 (0) |
| GMT (95% CI)† | 528 (391–714) | 100 (78–127) | 35 (25–49) | 17 (5–61) | 1 (–) | – |
| DENV-4 | ||||||
| N ≥ 10 (%)* | 110 (100) | 69 (63) | 5 (5) | 0 (0) | – | – |
| GMT (95% CI)† | 109 (85–139) | 29 (24–35) | 17 (12–25) | 1 (–) | – | – |
Number of infants with detectable PRNT values (percent of the birth cohort).
GMT of log10 (PRNT + 1).
Per serotype, Table 1 lists the geometric mean titer (GMT) of detectable PRNT values at each follow-up visit; 116 infants had detectable PRNT values at birth against DENV-1, 116 infants had detectable PRNT values at birth against DENV-2, 114 infants had detectable PRNT values at birth against DENV-3, and 110 infants had detectable PRNT values at birth against DENV-4. At 6 months of age, 50% of the birth cohort had detectable PRNT values against DENV-1 with a GMT of 35 (95% confidence interval [CI] = 29–43). In comparison, 35% of infants had remaining detectable values against DENV-2, 35% of infants had remaining detectable values against DENV-3, and 5% of infants had remaining detectable values against DENV-4. Of a total of 119 infants with detectable PRNT values against any serotype at birth, 108 (91%) infants had antibodies against all four DENV serotypes, whereas 5 infants had antibodies against three serotypes, 3 infants had a bivalent antibody titer, and 3 infants had a monovalent antibody titer.
Antibody decay rates.
For each serotype, a longitudinal regression model was fitted to estimate the average decay rates while adjusting for the correlation between measurements within each infant. Infants with decay patterns that disproportionately influenced the average (atypical patterns) were identified by leave one out validation. For DENV-1 and -4, model fits (and average decay rates) for atypical patterns were statistically significantly different from non-atypical patterns. Figure 1 displays decay patterns for individual infants with and without atypical patterns. Table 2 provides model estimates. The decay rate between birth and 3 months was faster compared with rates at later ages for all serotypes. For DENV-1 and -2, an additional distinction was made between decay in the 3- to 9- and 9- to 18-month intervals. In the first interval after birth, decay rates ranged from −0.31 to −0.37 logs per month (half-life = 24–29 days). In the second age interval, decay rates were much slower at −0.19, −0.21, and −0.19 logs per month for DENV-1, -2, and -3, respectively, and −0.06 logs per month for DENV-4 (half-life times 48, 44, 48, and 150 days, respectively). In the last age interval, decay rates were not statistically significantly different from zero for DENV-1, -2, and -4.
Figure 1.
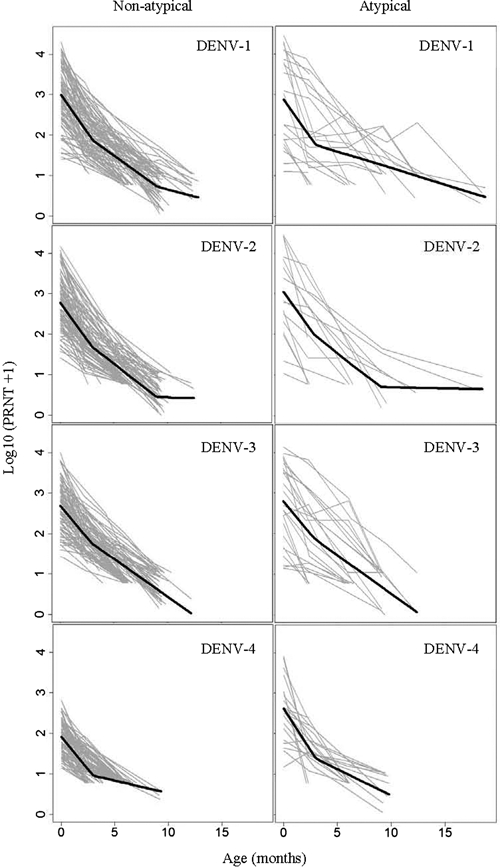
PRNT values by age and model fits for infants with and without atypical patterns for DENV-1, -2, -3, and -4. Average decay rates were statistically significantly different between atypical and non-atypical patterns for DENV-1 and -4. For all serotypes, PRNT values in atypical patterns were mostly higher than in non-atypical patterns. For DENV-1 and -3, examples of antibody increases can be seen that were too small to be considered serologic evidence of dengue infection but too large to be within range of the average variance.
Table 2.
Average decline rates per age interval
| Age (months) | Non-atypical patterns | Atypical patterns | |||||
|---|---|---|---|---|---|---|---|
| N | Δ/month* | 95% CI | N | Δ/month* | 95% CI | ||
| DENV-1 | |||||||
| 0–3 | 96 | −0.37 | −0.32 to −0.43 | 20 | −0.37 | −0.18 to −0.57 | |
| 3–9 | −0.19 | −0.07 to −0.31 | −0.08 | 0.36 to −0.52 | |||
| 9–18 | −0.07 | 0.14 to −0.28 | −0.08 | 0.56 to −0.72 | |||
| DENV-2† | |||||||
| 0–3 | 116 | −0.37 | −0.32 to −0.41 | – | – | – | |
| 3–9 | −0.21 | −0.11 to −0.30 | – | – | |||
| 9–18 | −0.01 | 0.14 to −0.15 | – | – | |||
| DENV-3† | |||||||
| 0–3 | 114 | −0.31 | −0.27 to −0.35 | – | – | – | |
| 3–12 | −0.19 | −0.09 to −0.28 | – | – | |||
| DENV-4 | |||||||
| 0–3 | 91 | −0.32 | −0.27 to −0.36 | 19 | −0.41 | −0.27 to −0.56 | |
| 3–9 | −0.06 | 0.05 to −0.17 | −0.13 | 0.22 to −0.48 | |||
Decline in log10 (PRNT + 1) per month.
No statistically significant difference in antibody decline rates between infants with and without atypical patterns.
Decay rates of PRNT against DENV-1 and -4 were most markedly different between atypical and non-atypical patterns in the 3- to 9-month age interval. These rates were two times as slow for DENV-1 (−0.08 versus −0.19 logs/month) and two times as fast for DENV-4 (−0.13 versus −0.06 logs/month). Although decay rates in this age interval were statistically significantly different between atypical and non-atypical patterns, they were not statistically significantly different from zero.
PRNT decay rates depended on values at birth.
We found a highly statistically significant association between PRNT values at birth and decay rates for all DENV serotypes. Infants were grouped in four quartiles of antibody levels at birth (quartile one for the lowest levels). Results are provided in Table 3. For each quartile increase, the decay rate increased with 0.12 logs per month for DENV-1 (95% CI = 0.08−0.15) and with 0.09 (95% CI = 0.06−0.11), 0.10 (95% CI = 0.07−0.13), and 0.12 (95% CI = 0.09−0.15) logs per month for DENV-2, -3, and -4, respectively (P < 0.0001).
Table 3.
Average change in decline rates of log10 (PRNT + 1) per quartile increases in PRNT levels at birth
| Age (months) | Change in decline rates per quartile increase (95% CI) | P value* |
|---|---|---|
| DENV-1 | ||
| 0–3 | −0.12 (−0.08 to −0.15) | < 0.0001 |
| 3–9 | −0.05 (0.03 to −0.13) | < 0.0001 |
| 9–18 | 0.01 (0.15 to −0.12) | 0.5780 |
| DENV-2 | ||
| 0–3 | −0.09 (−0.06 to −0.11) | < 0.0001 |
| 3–9 | −0.06 (0.02 to −0.13) | < 0.0001 |
| 9–18 | −0.01 (0.12 to −0.14) | 0.7431 |
| DENV-3 | ||
| 0–3 | −0.10 (−0.07 to −0.13) | < 0.0001 |
| 3–12 | −0.04 (0.03 to −0.07) | < 0.0001 |
| DENV-4 | ||
| 0–3 | −0.12 (−0.09 to −0.15) | < 0.0001 |
| 3–9 | −0.02 (0.05 to −0.09) | 0.0693 |
P value of F test for change in decline rate vs. no change in decline rate for an age interval.
Antibody persistence.
Although decay rates provide information on the heterogeneity of antibody kinetics within and between infants, they do not inform on persistence times, which is of particular use in determining the optimal age for vaccination. The time of PRNT persistence (survival) was estimated using a survival analysis in which PRNT decline below a cutoff was considered an event and the age of the infant was used to measure time. Persistence was estimated using various cutoff values: 10, 20, 50, and 80. Figure 2 provides the Kaplan−Meier survival curves for persistence of PRNT against each DENV serotype.
Figure 2.
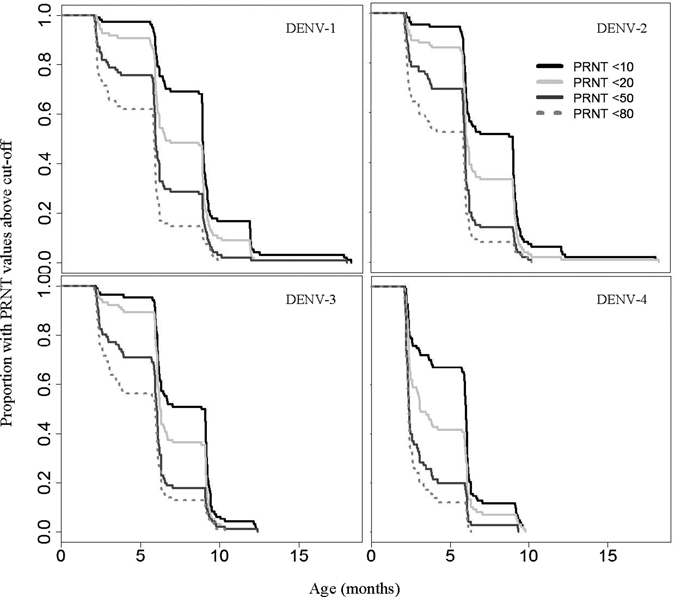
The proportion of infants with PRNT persistence beyond given time periods using four different cutoff values. This figure indicates a similar proportion of infants with persistence of PRNT beyond 3 months for DENV-1, -2, and -3 but persistence beyond 6 months for a higher proportion of infants for DENV-1 compared to the other serotypes. It also indicates that, if a slightly higher cutoff would be used (e.g., 50 or 80), a very low proportion of infants persists beyond 6 months.
The proportion of infants with PRNT against DENV-1 persisting above a cutoff value of 10 for longer than 3 months was 97%, and it was 70% for persistence beyond 7 months, 17% for persistence beyond 10 months, and 3% for persistence beyond 13 months. Persistence was shorter for PRNT against other DENV serotypes. For DENV-2 and -3, persistence over 7 months was found in 51% of infants, and for DENV-4, it was found in 12% of infants. Figure 2 indicates that PRNT values persisted markedly shorter for cutoff values of 20, 50, and 80. For example, PRNT values against DENV-1 persisted above a cutoff value of 50 for more than 7 months in 30% of infants and above a cutoff value of 80 in only 15% of infants.
Table 4 provides the median antibody persistence times for the entire cohort and the mean for those infants for whom an event was recorded. The median persistence time of PRNT above a cutoff of 10 or 50 for DENV-1, -2, and -3 was 9 and 6 months, respectively, and 6 and 2.4 months for DENV-4. The corresponding mean persistence times were 8.9 and 6.3 months for DENV-1, 7.8 and 5.5 months for DENV-2, 7.7 and 5.7 months for DENV-3, and 5.3 and 3.3 months for DENV-4.
Table 4.
Median and mean PRNT persistence times using different cutoffs
| Serotype | Indicator | PRNT persistence in months (95% CI) | |||
|---|---|---|---|---|---|
| PRNT ≥ 10 | PRNT ≥ 20 | PRNT ≥ 50 | PRNT ≥ 80 | ||
| DENV-1 | Median | 9.1 (9.1–9.2) | 6.7 (6.3–9.1) | 6.1 (6.1–6.3) | 6.0 (5.9–6.1) |
| DENV-1 | Mean* | 8.9 (8.3–9.4) | 7.7 (7.1–8.2) | 6.3 (5.7–6.8) | 5.2 (4.7–5.7) |
| DENV-2 | Median | 9.1 (6.2–9.1) | 6.1 (6.1–6.3) | 6.0 (5.9–6.1) | 5.9 (3.1–6.0) |
| DENV-2 | Mean* | 7.8 (7.3–8.3) | 6.8 (6.3–7.3) | 5.5 (5.1–5.9) | 4.7 (4.2–5.1) |
| DENV-3 | Median | 8.9 (6.3–9.1) | 6.2 (6.1–6.7) | 6.0 (5.9–6.1) | 5.9 (3.7–6.0) |
| DENV-3 | Mean* | 7.7 (7.2–8.1) | 6.9 (6.5–7.4) | 5.7 (5.2–6.2) | 5.0 (4.5–5.5) |
| DENV-4 | Median | 6.0 (5.9–6.1) | 3.1 (2.6–5.9) | 2.4 (2.3–2.6) | 2.3 (2.3–2.4) |
| DENV-4 | Mean* | 5.3 (4.9–5.7) | 4.3 (3.8–4.7) | 3.3 (2.9–3.7) | 2.9 (2.6–3.2) |
Mean survival time for infants that experienced an event (PRNT decline under cutoff).
PRNT values at birth did not predict persistence times.
We also investigated the dependence of antibody persistence times on PRNT values at birth using a linear regression. For each log increase of PRNT values against DENV-1 at birth, persistence of these antibody levels increased with 1.6 months (95% CI = 0.9–2.3 months). For each log increase of PRNT against DENV-2, -3, and -4 at birth, persistence increased with 1.9 months (95% CI = 1.3–2.5 months), 1.3 months (95% CI = 0.8–1.9 months), and 0.6 months (95% CI = −0.2 to 1.4 months), respectively.
Although PRNT values against DENV-1, -2, and -3 at birth were statistically significantly associated with persistence times, prediction of persistence based on values at birth failed because of large variance between infants. In Figure 3, the model estimates and 95% CIs of persistence times based on the PRNT values at birth are compared with the actual observed persistence times. For DENV-1, only 28% of the observed values fell within the confidence limits of predicted persistence times compared with 28%, 21%, and 11% for DENV-2, -3, and -4, respectively.
Figure 3.
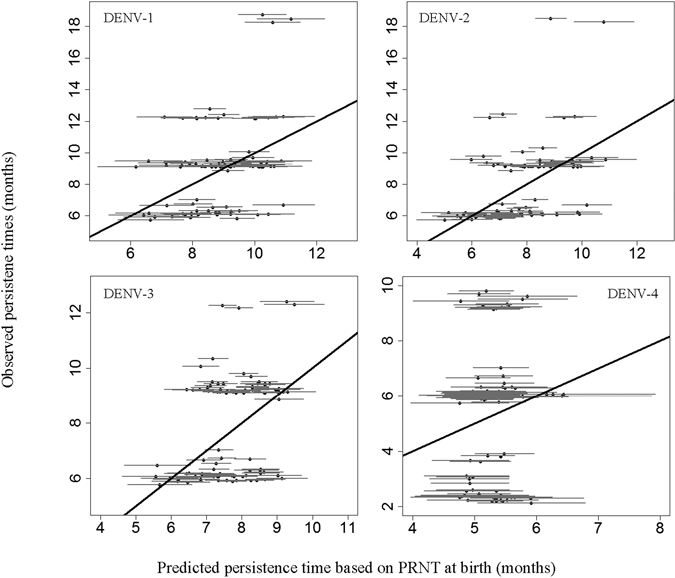
Predicted persistence times with 95% CIs versus observed persistence times for DENV-1, -2, -3, and -4. Persistence times that were predicted based on PRNT values at birth did not correspond well with observed persistence times. Only between 11% and 20% of the observed persistence times were captured by the 95% CIs of predicted persistence times.
Discussion
Dengue vaccine candidates are in an advanced stage of development, but because of a potential reduction of vaccine efficacy by maternal antibodies in infants and a reduced memory response, this age group is currently not targeted for vaccination. Maternal dengue antibodies are also thought to play a significant role in dengue pathogenesis among infants. The objective of this study was to provide more insight in maternal dengue antibody kinetics among infants and implications for the age of vaccination and studies on dengue pathogenesis in infants.
In this study, a longitudinal model was used to assess the heterogeneity of dengue antibody decay patterns within and between infants. Antibody persistence times were estimated by survival analysis. Most infants had neutralizing antibodies against all four serotypes at birth, with similar GMT for DENV-1, -2, and 3. DENV-4 antibody levels at birth were lower, reflecting the lower level of circulation of this serotype in the years before the study.6
For all DENV serotypes, antibody decay rates were statistically significantly faster between birth and 3 months of age compared with the next age interval. Half-life times for DENV serotypes ranged between 24 and 29 days in the first phase compared with 44–150 days in the second phase. In a previous study among 2,716 infants in the Philippines, the half-life time of PRNT50 against DENV-3 was estimated at 38 days (95% CI = 34–42 days).13 A cohort study in Thailand determined a half-life time of 41.2 days for hemagglutination inhibition (HI) titers against DENV, and another study in Vietnam found a half-life of 42 days for DENV IgG.18,19 Decay rates between birth and 3 months of age found in our study were much higher than those described above, and they were somewhat slower in the second phase. It is commonly known that antibody titers decay exponentially, with decay rates decreasing over time. After log transformation, however, a linear pattern would be expected. Monophasic linear decay rates of log-transformed antibody levels have been used widely to assess the role of maternal antibodies on dengue pathogenesis, for example, by projecting antibody levels from values at birth to titers at the time of infection.11,13,17,18
Even after log transformation, a biphasic PRNT decay as well as a statistically significant association between decay rates and PRNT levels at birth found in our study could both be explained by faster decay rates at higher PRNT levels. Log transformation did not entirely normalize the distribution of PRNT values caused by extreme skewness in the non–log-transformed values. This finding may be a particular characteristic of antibody levels measured by PRNT versus other assays. More detailed longitudinal analysis on such patterns in decay of IgG and HI titers would be required to ensure that valid projections and assumptions will be made in future studies. Use of monophasic decay rates could lead to overestimates of antibody values at specific ages (at 3 months in our study). Biphasic decay rates should be considered in studies that project antibody levels in infants.
We detected a large heterogeneity of PRNT decay patterns between infants illustrated by atypical patterns found for DENV-1 and -4 in 17% of the sample. Because the average decay in these infants was statistically significantly different from other infants, these deviations cannot be solely explained by variation in the assay. It is unknown what caused the atypical patterns in almost one-fifth of the sample. Figure 1 indicates that these patterns included many instances of antibody increase. Although these values were smaller than fourfold and no IgM was detected, these increases could have been caused by infant antibody production in response to asymptomatic flavivirus infections. Large amounts of maternal antibody in young infants may not entirely prevent viral uptake by antigen-presenting cells, resulting in a limited amount of infant antibody production. This finding would also explain some very long persistence times of antibody (> 12 months) found in this study. Some infants may already have acquired some immunological memory at a very young age, which may lead to a boosted instead of reduced vaccine response.
In addition to decay rates, we also estimated antibody persistence times, which are more informative on the optimal age of vaccination. Because the exact age of infants was used to measure persistence times compared with the month of follow-up, the proportion of infants persisting beyond a certain month can be different from the percentage of infants with PRNT above a cutoff as presented cross-sectionally in Table 1. For example, an infant of 10 months at the 9-month follow-up visit could have PRNT < 10 at follow-up month 9 but actually have PRNT persistence beyond 9 months of age (time of event is 10 months). Because a few months can make a substantial difference in estimating the optimal age of vaccination in infants, we preferred to use of the actual age versus the follow-up month itself. In addition, survival analysis was used to adjust for infants lost to follow-up by excluding censored observations from the denominator after censoring, whereas in Table 1, the entire birth cohort was consistently used as the denominator.
We found that persistence times of PRNT levels were greatly dependent on the cutoff value used and that they cannot be predicted based on values at birth because of the large amount of heterogeneity. PRNT levels against DENV-1 persisted above a cutoff value of 10 for more than 7 months in 70% of infants compared with 15% for a cutoff value of 80. These persistence times were much longer than those reported, for example, by Chau and others20 on a cohort in Vietnam. The PRNT values at birth were much lower in that cohort, indicating that comparisons can only be made between cohorts with similar antibody levels at birth given the dependency of persistence times on PRNT values at birth.
It is unclear what value of PRNT would reduce vaccine efficacy. Some studies suggested that antibodies would be neutralizing above PRNTs of 20, 50, or 80.13,18,27 In our study, persistence above a cutoff value of 10 for longer than 12 months was estimated in 3% of infants for PRNT against DENV-1. If maternal antibodies would reduce vaccine efficacy at PRNT above 10, dengue vaccine should be given after the first year of life for optimal efficacy. If vaccine efficacy would only depend on maternal antibodies and if these antibodies would only affect a vaccine above PRNT of 50 or 80, full efficacy could be expected in 50% of infants at 6 months and 75% or 85% at 9 months, respectively.
We found statistically significant associations between PRNT values at birth and persistence times for DENV-1, -2, and -3. However, because of the large heterogeneity, PRNT values at birth could not be used for prediction of PRNT persistence times. Only 25% or less of the observed values was captured by the predicted persistence times and their 95% CIs. This heterogeneity was also illustrated by the atypical decay patterns described above. Projections of average persistence times (similar to projections of the age at which certain PRNT levels would occur) should be avoided in studies on dengue pathogenesis. Estimates or measurements for individual infants should be used instead.
Our estimates of antibody decay and persistence were constrained by the measurement of PRNT levels at follow-up visits only, providing snapshots of a continuous process. For that reason, persistence times all hover around 3, 6, 9, and 12 months. This finding would occur in any study of a continuous process using such snapshots. More closely spaced observations would have provided more detailed information but would also pose major logistical challenges.
We considered 3-month follow-up visits sufficient for meaningful inference on antibody decay and persistence.
A total of 41 infants were lost to follow-up in the 24-month study period (30%). Because demographic indicators, including age, were not different in these infants compared with the rest of the cohort, no bias was introduced in our study results. PRNT results have been recognized to vary significantly depending on the conditions of the assay.28 In our study, PRNT assays of all samples from each infant were conducted in the same run to minimize variability within infants. Analytical techniques were used to separate random variation from statistically significant differences. Although comparability with other studies using PRNT may be limited because of assay variation, the qualitative conclusions would be expected to remain unchanged. Furthermore, neutralizing antibody titers are determined by more factors than antibody concentration only, such as avidity or affinity. Because PRNT values are still considered the best measure of dengue immunity, we used this assay to allow inference on the decay and persistence of immunity as well as on an approximation of antibody concentration assuming that other determinants of PRNT remain relatively constant over time.
Based on this in-depth assessment of longitudinal antibody kinetics in infants, there seems to be little opportunity for an immunogenic vaccine response among infants before the peak of severe disease at 6–8 months. Such opportunity would only exist if maternal antibodies would start affecting vaccine immunogenicity above PRNTs of 80. Additional work will be required using longitudinal methods to explain the large heterogeneity in antibody decay rates and persistence, particularly the atypical patterns.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Sutee Yoksan for conducting the laboratory work of which the results were used in this analysis, Dr. Lawrence Moulton from the Johns Hopkins Bloomberg School of Public Health for his advice on the statistical analysis, and Dr. Bruno Guy from Sanofi Pasteur for using his expertise in reviewing the manuscript.
Disclaimer: Christine Luxemburger and Jean Lang are employees of Sanofi Pasteur. No conflicts of interest are declared by the other authors.
Footnotes
Financial support: The original study that generated the data used in this analysis was funded by Sanofi Pasteur. No funding was used to conduct the analysis for this manuscript.
Authors' addresses: Willem G. van Panhuis, Department of Epidemiology, University of Pittsburgh Graduate School of Public Health, Pittsburgh PA, E-mail: wav10@pitt.edu. Christine Luxemburger, Sanofi Pasteur, Lyon, France, E-mail: christine.luxemburger@sanofipasteur.com. Krisana Pengsaa, Kriengsak Limkittikul, and Arunee Sabchareon, Department of Tropical Pediatrics, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand, E-mails: tmkps@mahidol.ac.th, tmklk@mahidol.ac.th, and tmasc@mahidol.ac.th. Jean Lang, Sanofi Pasteur, Marcy l'Etoile, France, E-mail: jean.lang@sanofipasteur.com. Anna P. Durbin, Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, E-mail: adurbin@jhsph.edu. Derek A. T. Cummings, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, E-mail: dcumming@jhsph.edu.
Reprint requests: Willem G. van Panhuis, Department of Epidemiology, University of Pittsburgh Graduate School of Public Health, 130 DeSoto Street, 704 Parran Hall, Pittsburgh PA 15213, E-mail: wav10@pitt.edu.
References
- 1.Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, Hunsperger E, Kroeger A, Margolis HS, Martinez E, Nathan MB, Pelegrino JL, Simmons C, Yoksan S, Peeling RW. Dengue: a continuing global threat. Nat Rev Microbiol. 2010;8:S7–S16. doi: 10.1038/nrmicro2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halstead SB. Neutralization and antibody-dependent enhancement of dengue viruses. Adv Virus Res. 2003;60:421–467. doi: 10.1016/s0065-3527(03)60011-4. [DOI] [PubMed] [Google Scholar]
- 3.Hammond SN, Balmaseda A, Perez L, Tellez Y, Saborio SI, Mercado JC, Videa E, Rodriguez Y, Perez MA, Cuadra R, Solano S, Rocha J, Idiaquez W, Gonzalez A, Harris E. Differences in dengue severity in infants, children, and adults in a 3-year hospital-based study in Nicaragua. Am J Trop Med Hyg. 2005;73:1063–1070. [PubMed] [Google Scholar]
- 4.Trent D, Shin J, Hombach J, Knezevic I, Minor P. WHO Working Group on technical specifications for manufacture and evaluation of dengue vaccines, Geneva, Switzerland, 11–12 May 2009. Vaccine. 2010;28:8246–8255. doi: 10.1016/j.vaccine.2010.10.043. [DOI] [PubMed] [Google Scholar]
- 5.Guy B, Saville M, Lang J. Development of Sanofi Pasteur tetravalent dengue vaccine. Hum Vaccin. 2010;6((9)) doi: 10.4161/hv.6.9.12739. Sept 16. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Nisalak A, Endy TP, Nimmannitya S, Kalayanarooj S, Thisayakorn U, Scott RM, Burke DS, Hoke CH, Innis BL, Vaughn DW. Serotype-specific dengue virus circulation and dengue disease in Bangkok, Thailand from 1973 to 1999. Am J Trop Med Hyg. 2003;68:191–202. [PubMed] [Google Scholar]
- 7.Siegrist CA. Neonatal and early life vaccinology. Vaccine. 2001;19:3331–3346. doi: 10.1016/s0264-410x(01)00028-7. [DOI] [PubMed] [Google Scholar]
- 8.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 9.Gans H, Yasukawa L, Rinki M, DeHovitz R, Forghani B, Beeler J, Audet S, Maldonado Y, Arvin AM. Immune responses to measles and mumps vaccination of infants at 6, 9, and 12 months. J Infect Dis. 2001;184:817–826. doi: 10.1086/323346. [DOI] [PubMed] [Google Scholar]
- 10.Siegrist CA. Mechanisms by which maternal antibodies influence infant vaccine responses: review of hypotheses and definition of main determinants. Vaccine. 2003;21:3406–3412. doi: 10.1016/s0264-410x(03)00342-6. [DOI] [PubMed] [Google Scholar]
- 11.Halstead SB, Lan NT, Myint TT, Shwe TN, Nisalak A, Kalyanarooj S, Nimmannitya S, Soegijanto S, Vaughn DW, Endy TP. Dengue hemorrhagic fever in infants: research opportunities ignored. Emerg Infect Dis. 2002;8:1474–1479. doi: 10.3201/eid0812.020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halstead SB, O'Rourke EJ. Antibody-enhanced dengue virus infection in primate leukocytes. Nature. 1977;265:739–741. doi: 10.1038/265739a0. [DOI] [PubMed] [Google Scholar]
- 13.Libraty DH, Acosta LP, Tallo V, Segubre-Mercado E, Bautista A, Potts JA, Jarman RG, Yoon IK, Gibbons RV, Brion JD, Capeding RZ. A prospective nested case-control study of Dengue in infants: rethinking and refining the antibody-dependent enhancement dengue hemorrhagic fever model. PLoS Med. 2009;6:e1000171. doi: 10.1371/journal.pmed.1000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mongkolsapaya J, Dejnirattisai W, Xu XN, Vasanawathana S, Tangthawornchaikul N, Chairunsri A, Sawasdivorn S, Duangchinda T, Dong T, Rowland-Jones S, Yenchitsomanus PT, McMichael A, Malasit P, Screaton G. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat Med. 2003;9:921–927. doi: 10.1038/nm887. [DOI] [PubMed] [Google Scholar]
- 15.Capeding RZ, Brion JD, Caponpon MM, Gibbons RV, Jarman RG, Yoon IK, Libraty DH. The incidence, characteristics, and presentation of dengue virus infections during infancy. Am J Trop Med Hyg. 2010;82:330–336. doi: 10.4269/ajtmh.2010.09-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Webster DP, Farrar J, Rowland-Jones S. Progress towards a dengue vaccine. Lancet Infect Dis. 2009;9:678–687. doi: 10.1016/S1473-3099(09)70254-3. [DOI] [PubMed] [Google Scholar]
- 17.Kliks SC, Nimmannitya S, Nisalak A, Burke DS. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am J Trop Med Hyg. 1988;38:411–419. doi: 10.4269/ajtmh.1988.38.411. [DOI] [PubMed] [Google Scholar]
- 18.Simmons CP, Chau TN, Thuy TT, Tuan NM, Hoang DM, Thien NT, Lien le B, Quy NT, Hieu NT, Hien TT, McElnea C, Young P, Whitehead S, Hung NT, Farrar J. Maternal antibody and viral factors in the pathogenesis of dengue virus in infants. J Infect Dis. 2007;196:416–424. doi: 10.1086/519170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watanaveeradej V, Endy TP, Samakoses R, Kerdpanich A, Simasathien S, Polprasert N, Aree C, Vaughn DW, Ho C, Nisalak A. Transplacentally transferred maternal-infant antibodies to dengue virus. Am J Trop Med Hyg. 2003;69:123–128. [PubMed] [Google Scholar]
- 20.Chau TN, Hieu NT, Anders KL, Wolbers M, Lien le B, Hieu LT, Hien TT, Hung NT, Farrar J, Whitehead S, Simmons CP. Dengue virus infections and maternal antibody decay in a prospective birth cohort study of Vietnamese infants. J Infect Dis. 2009;200:1893–1900. doi: 10.1086/648407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pengsaa K, Luxemburger C, Sabchareon A, Limkittikul K, Yoksan S, Chambonneau L, Chaovarind U, Sirivichayakul C, Lapphra K, Chanthavanich P, Lang J. Dengue virus infections in the first 2 years of life and the kinetics of transplacentally transferred dengue neutralizing antibodies in thai children. J Infect Dis. 2006;194:1570–1576. doi: 10.1086/508492. [DOI] [PubMed] [Google Scholar]
- 22.Pengsaa K, Yoksan S, Limkittikul K, Wisetsing P, Sirivichayakul C, Hutacharoen P, Chanthavanich P, Sabchareon A. Maternally transferred neutralising dengue antibodies in Thai infants: a pilot study. Ann Trop Paediatr. 2003;23:159–165. doi: 10.1179/027249303322296466. [DOI] [PubMed] [Google Scholar]
- 23.Pengsaa K, Limkittikul K, Luxemburger C, Yoksan S, Chambonneau L, Ariyasriwatana C, Lapphra K, Chanthavanich P, Lang J, Sabchareon A. Age-specific prevalence of dengue antibodies in Bangkok infants and children. Pediatr Infect Dis J. 2008;27:461–463. doi: 10.1097/INF.0b013e3181646d45. [DOI] [PubMed] [Google Scholar]
- 24.Shu PY, Chen LK, Chang SF, Yueh YY, Chow L, Chien LJ, Chin C, Lin TH, Huang JH. Dengue NS1-specific antibody responses: isotype distribution and serotyping in patients with Dengue fever and Dengue hemorrhagic fever. J Med Virol. 2000;62:224–232. doi: 10.1002/1096-9071(200010)62:2<224::aid-jmv14>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 25.Russell PK, Nisalak A, Sukhavachana P, Vivona S. A plaque reduction test for dengue virus neutralization antibodies. J Immunol. 1967;99:285–290. [PubMed] [Google Scholar]
- 26.Diggle P. Analysis of Longitudinal Data. New York, NY: Oxford University Press; 2004. [Google Scholar]
- 27.Burke DS, Nisalak A, Johnson DE, Scott RM. A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg. 1988;38:172–180. doi: 10.4269/ajtmh.1988.38.172. [DOI] [PubMed] [Google Scholar]
- 28.Thomas SJ, Nisalak A, Anderson KB, Libraty DH, Kalayanarooj S, Vaughn DW, Putnak R, Gibbons RV, Jarman R, Endy TP. Dengue plaque reduction neutralization test (PRNT) in primary and secondary dengue virus infections: how alterations in assay conditions impact performance. Am J Trop Med Hyg. 2009;81:825–833. doi: 10.4269/ajtmh.2009.08-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]


