Abstract
Acute meningoencephalitis syndrome surveillance was initiated in three medical college hospitals in Bangladesh in October 2007 to identify Japanese encephalitis (JE) cases. We estimated the population-based incidence of JE in the three hospitals' catchment areas by adjusting the hospital-based crude incidence of JE by the proportion of catchment area meningoencephalitis cases who were admitted to surveillance hospitals. Instead of a traditional house-to-house survey, which is expensive for a disease with low frequency, we attempted a novel approach to identify meningoencephalitis cases in the hospital catchment area through social networks among the community residents. The estimated JE incidence was 2.7/100,000 population in Rajshahi (95% confidence interval [CI] = 1.8–4.9), 1.4 in Khulna (95% CI = 0.9–4.1), and 0.6 in Chittagong (95% CI = 0.4–0.9). Bangladesh should consider a pilot project to introduce JE vaccine in high-incidence areas.
Introduction
Japanese encephalitis virus (JEV), a mosquito-borne virus, is the most common cause of vaccine-preventable viral encephalitis in Asia.1,2 Japanese encephalitis virus is transmitted in an enzootic cycle between animal hosts, mostly pigs and wading birds, and mosquito vectors; humans are incidental, dead-end hosts.3 Japanese encephalitis (JE) is endemic in most parts of Asia and the Western Pacific where approximately three billion people live.4,5 An estimated 30,000 to 50,000 human JE cases occur annually; the case fatality is 10–30% and 30–50% of survivors develop long-term neurologic and psychiatric sequelae.2,6
Bangladesh shares a similar ecology with its JE-endemic neighboring countries of India and Nepal, where several large outbreaks of JE have been identified in recent years.5,7 One confirmed JE outbreak was reported from Bangladesh in 1977, but there are no data on the incidence of JE cases because of limited diagnostic capacity and a lack of systematic disease surveillance.8 A hospital-based study conducted from 2003 to 2005 to assess the etiologies of encephalitis at four hospitals in Bangladesh found that among 492 patients with clinical encephalitis, 20 (4%) cases had laboratory evidence of recent JEV infection.9
The incidence of JE has been reduced dramatically in Japan, Korea, Taiwan, China, and Thailand since the introduction of a JE vaccine into the national immunization programs of these countries.10–14 Widely used live attenuated SA 14-14-12 JE vaccine is inexpensive and with a single dose provides 80–96% protection.6,15,16 The introduction of a life saving vaccine in a low income country such as Bangladesh depends on the cost-effectiveness, which in turn depends on the incidence of disease.
Since 2007, the Institute of Epidemiology, Disease Control and Research (IEDCR) of the Government of Bangladesh, in collaboration with other partners, has been conducting acute meningoencephalitis syndrome surveillance in three tertiary level hospitals where patients admitted with symptoms of meningoencephalitis were tested for JE. However, hospital-based surveillance data underestimates the true burden of disease because many ill persons do not seek treatment at surveillance hospitals.17–19 To adjust crude the estimates of hospital-based incidence, instead of using a traditional house-to-house survey, which is expensive for a disease with low frequency, we used a novel method to identify meningoencephalitis cases from the hospital catchment area. We leveraged the close social networks within Bangladeshi rural communities who actively discuss community events, such as family illness, and are generally able to report any serious health events experienced by their neighbors, to identify study participants in our community-based survey.
Because the population-based incidence of JE is of particular interest in Bangladesh, and vaccines are available and now being used in many countries, we estimated JE incidence in the three hospitals' catchment areas by adjusting the crude hospital-based JE incidence by the proportion of catchment area meningoencephalitis cases who were admitted to surveillance hospitals.
Materials and Methods
Hospital-based surveillance.
In October 2007, acute meningoencephalitis surveillance was initiated at Rajshahi Medical College Hospital, Khulna Medical College Hospital, and Chittagong Medical College Hospital, which are tertiary care referral hospitals in three different parts of the country (Figure 1). For the purposes of this surveillance, we defined a case of meningoencephalitis as a patient who had acute onset of fever with altered mental status, or new onset of seizures, or signs of meningeal irritation. Surveillance physicians collected clinical information from all patients identified with meningoencephalitis and coordinated the collection of cerebrospinal fluid (CSF) and two samples of serum; one at admission and another at discharge. The specimens were divided into aliquots at the study sites, stored at −20°C, and transported weekly to the Institute of Public Health, Dhaka for laboratory testing of JE and then preserved at −70°C. The CSF samples were tested for anti-JEV immunoglobulin M (IgM) antibodies using the XCyton enzyme-linked immunosorbent assay (ELISA) kit.20 Serum samples were tested for JEV and dengue virus IgM antibodies using the Panbio ELISA kit.21 A case of JE was defined as a patient with meningoencephalitis and anti-JEV IgM antibodies identified in a CSF or serum sample. Laboratory quality control was routinely assessed by proficiency panel samples testing and an onsite visit of a virologist from the World Health Organization (WHO), South-East Asia Regional Office, India. A subset of aliquots were also sent to the reference laboratory in Centers for Disease Control and Prevention (CDC) in Fort Collins, CO for quality assurance and further testing for potential causes of meningoencephalitis.
Figure 1.
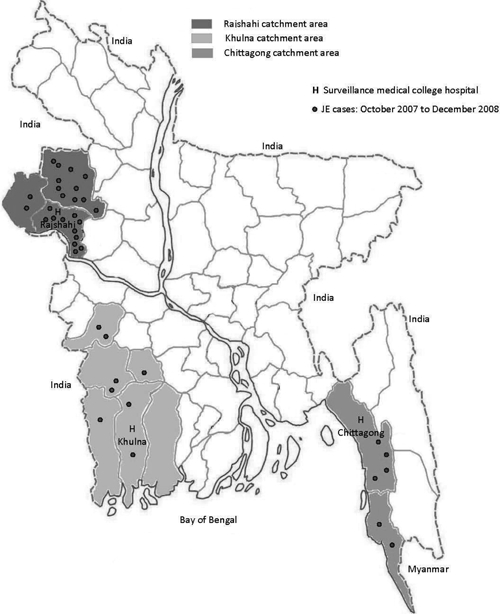
Map of Bangladesh showing surveillance hospitals and catchment areas.
Hospital catchment area assessment.
Catchment area and survey cluster selection.
We conducted a survey in the catchment area of surveillance hospitals to calculate the proportion of persons with symptoms of meningoencephalitis in the hospital catchment area who were admitted to the surveillance hospital. We defined the primary catchment area of a surveillance hospital as the districts where more than 50% of the admitted meningoencephalitis patients resided. Applying this criterion to the hospital records from November 2007 to April 2008, we selected Rajshahi, Naogaon, Chapai-Nawabgonj districts as the catchment area of Rajshahi Medical College Hospital; Khulna, Jessore, Jhenaidah, Narail, Satkhira, and Bagerhat districts as the catchment area of Khulna Medical College Hospital; and Chittagong and Cox's Bazar districts as the catchment area of Chittagong Medical College Hospital (Figure 1). For the community-based survey, we selected 20 unions, the lowest administrative area in Bangladesh, from each of these three hospital catchment areas using a probability proportional to size sampling approach. We first listed all the unions in a hospital catchment area by their population in a spreadsheet and calculated the sampling interval by dividing the total population by 20, and then selected a random number between one and the sampling interval. The union having the cumulative population that captured the random number was selected as the first union. To select the second union, the sampling interval was added to the random number and again the list was consulted to see which union included that number. This process was repeated until all 20 unions were indentified. The average population of each selected union was 27,000 and three were in urban metropolitan areas.
Study population.
In the community, we classified persons as having symptoms compatible with meningoencephalitis if they developed fever with altered mental status, or unconsciousness, or a new onset seizure within the last 12 months. To rule out epilepsy as the cause of disease, we excluded persons who had experienced multiple seizures within a 6-month interval during the previous 2 years.
Data collection technique.
In the selected rural unions of the hospital catchment area, the field team first approached key informants, including local health care providers, religious and community leaders, educational institutions and vendors, and customers in the village market. The team then walked through the village and met with some of the residents, especially women, in several informal courtyard gatherings, usually consisting of 6–7 households. The team explained the symptoms of meningoencephalitis in local terms and asked the people if they knew anybody in their community who had developed fever with unconsciousness or altered mental status or a new onset seizure in the previous one year. If the team received information about anyone with meningoencephalitis symptoms, they visited that household to confirm that the person's symptoms met our classification of symptoms compatible with meningoencephalitis. If the person's symptoms were compatible with meningoencephalitis, the study team administered a structured questionnaire to the affected person and/or his/her care givers during the illness that included questions on whether the person sought care at a surveillance hospital. The study team also documented the illness history and outcomes, along with demographic characteristics and healthcare seeking behavior.
In the selected urban clusters, the team began with the same community networking approach to identify meningoencephalitis cases. However, this approach failed in the urban communities because people in these areas did not know their neighbors well and was less aware of serious family events experienced by their neighbors. Therefore, house-to-house surveys were used instead of community networking in urban areas. Two field teams, each with three research assistants and one supervisor, worked from June 2008 to March 2009 to complete the survey in the three hospital catchment areas.
JE incidence calculation.
To focus on those persons having symptoms compatible with meningoencephalitis from the catchment area that most likely represented actual encephalitis, in our final incidence calculation we defined suspected meningoencephalitis as people who developed fever with at least 6 hours of altered mental status, or at least 1 hour of unconsciousness, or new onset seizure (jerking in body) within the last 12 months.
Because many meningoencephalitis cases in hospital catchment areas do not seek care at the surveillance hospitals, our premise was that if all of them did go to the surveillance hospitals we could then estimate the real population-based JE incidence. We first calculated the hospital-based JE incidence by dividing the number of confirmed JE cases admitted to study hospitals within the last 1 year from the catchment area by the total catchment population. We then estimated population-based JE incidence by adjusting this hospital rate by the proportion of suspected meningoencephalitis cases in the catchment area who were admitted to these surveillance hospital using the following formula:
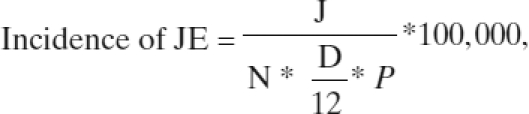 |
where
J = Number of JE cases identified in the surveillance hospital from within the catchment area;
N = Population of the hospital catchment area;
D = Duration of surveillance in months; and
P = Proportion of suspected meningoencephalitis cases in the catchment area who were admitted to surveillance hospital.
Data analysis.
The population of each catchment area was projected for 2008 on the basis of the 2001 Bangladesh census considering the annual growth rate of 1.4%.22 The annual growth rate was estimated using crude birth rate, net external migration, and national crude death rate.23 We used a linear mixed effect model to adjust the cluster effects in calculating the proportion of meningoencephalitis cases who were admitted to surveillance hospitals and estimated the incidence of JE with 95% confidence intervals (CI) for each hospital catchment area. Most patients with JE have IgM antibodies detectable in convalescent CSF and serum specimens collected ≥ 7 days of onset of illness.24–26 Because we were unable to collect the convalescent specimens from all enrolled patients in the surveillance hospitals, we estimated the potential impact of the limited availability of convalescent specimens on the incidence of JE by applying the JE positivity rate of convalescent phase specimens to the acute phase specimens collected from the admitted patients. We performed a two-sample test of proportion to compare the demographic characteristics and clinical signs between cases admitted and not admitted to surveillance hospitals. We also performed the Wilcoxon rank-sum test to compare the distance between the surveillance hospital and villages of both admitted and not admitted cases.
Ethical concerns.
The field team obtained written informed consent from the identified meningoencephalitis cases or their guardians. Assent was taken from participants between 7 and 17 years of age. Because the hospital surveillance was a national surveillance activity under the Ministry of Health and Family Welfare, written informed consent was not required from the admitted meningoencephalitis cases. The study protocol was reviewed and approved by institutional review boards of the International Center for Diarrheal Disease Research, Bangladesh (ICDDR,B) and the CDC.
Results
Hospital-based surveillance.
In the hospital-based surveillance, 886 patients were enrolled from October 2007 through December 2008 and of these patients, 875 (99%) provided at least one serum or CSF specimen for JE testing. Among the 886 enrollees, 55 (6%) had laboratory evidence of recent JEV infection (Table 1). Overall, 705 (80%) of the 886 patients with meningoencephalitis and 38 (69%) of the 55 JE cases were admitted from the catchment districts of surveillance hospitals. Among the 38 JE cases from the catchment area, 68% were male and 45% were ≤ 15 years of age. They presented with fever (100%), altered mental status (95%), unconsciousness (38%), and new onset seizure (83%).
Table 1.
Laboratory-confirmed Japanese encephalitis cases by surveillance hospitals, October 2007–December 2008*
| Description | Surveillance Medical College Hospital | Total | ||
|---|---|---|---|---|
| Rajshahi | Khulna | Chittagong | ||
| Patients admitted with meningoencephalitis | 550 | 147 | 189 | 886 |
| At least one sample tested | 547 (99%) | 139 (95%) | 189 (100%) | 875 (99%) |
| Admission CSF samples tested | 342 (61%) | 106 (72%) | 176 (94%) | 624 (70%) |
| Admission serum samples tested | 425 (77%) | 138 (94%) | 179 (95%) | 742 (84%) |
| Discharge serum samples tested | 57 (10%) | 51 (35%) | 4 (2%) | 112 (13%) |
| JE positive cases | 33 (6.0%) | 10 (6.8%) | 12 (6.3%) | 55 (6.2%) |
CSF = cerebrospinal fluid.
Assessment of hospital catchment area.
From the community-based hospital catchment survey, the field team identified 1,161 people with symptoms compatible with meningoencephalitis within the preceding 12 months. Of these, 969 (83%) met the suspected meningoencephalitis case definition.
In the catchment area, 74% of suspected meningoencephalitis cases selected unqualified health care providers who had no institutional medical training qualifications, as their first choice in seeking health care. However, over their entire illness duration, 78 (8%) suspected meningoencephalitis cases were admitted to a surveillance hospital and 533 (55%) cases sought care from other qualified health care providers (Table 2). Distance was an important factor in seeking treatment at surveillance hospitals; the median distance between the surveillance hospital and the village of suspected meningoencephalitis case was 28 km for cases who were admitted to a surveillance hospital, but was 45 km for cases not admitted (P < 0.01).
Table 2.
Health care seeking behavior of suspect meningoencephalitis cases in the hospital catchment area (N = 969)
| Health care seeking | First visit n (%) | Second visit n (%) | Any visit n (%) |
|---|---|---|---|
| Unqualified practitioners* | 717 (74) | 186 (19) | 922 (95) |
| Surveillance hospitals | 9 (1) | 26 (3) | 78 (8) |
| Other qualified practitioners | 233 (24) | 226 (23) | 538 (55) |
| Health and Family Welfare Center | 18 (2) | 3 (0) | 22 (2) |
| Upazila Health Complex | 42 (4) | 59 (6) | 120 (12) |
| Private practitioner | 91 (10) | 86 (9) | 198 (20) |
| District hospital | 11 (1) | 21 (2) | 45 (5) |
| Private clinic | 33 (3) | 30 (3) | 82 (9) |
| Pediatric hospital | 38 (4) | 27 (3) | 71 (7) |
Having no medical training qualifications.
Of the 969 suspected meningoencephalitis cases in the community-based survey, 60% were male. Children < 5 years of age accounted for 35% of suspected meningoencephalitis cases that were admitted to surveillance hospitals, but made up 69% of suspected cases who were not admitted to surveillance hospitals (P < 0.001). Of the reported clinical signs of the suspected meningoencephalitis cases, new onset of seizure was higher among suspected cases who were not admitted, whereas neck stiffness was higher among suspected cases who were admitted to surveillance hospitals. Although the proportion of altered mental status and unconsciousness was similar in both admitted and not admitted cases, the duration of illness was significantly higher among admitted cases (Table 3).
Table 3.
Demographic characteristics and reported clinical signs of suspect meningoencephalitis* cases in the hospital catchment area
| Characteristics | Admitted at study hospital (N = 78) n (%) | Not admitted at study hospital (N = 891) n (%) | P value† |
|---|---|---|---|
| Male | 46 (59) | 531 (60) | 0.86 |
| Age group | |||
| < 5 years | 27 (35) | 612 (69) | < 0.001 |
| 6–14 years | 18 (23) | 146 (16) | 0.11 |
| > 14 years | 33 (42) | 133 (15) | < 0.001 |
| Reported clinical signs | |||
| New onset seizure | 63 (81) | 828 (93) | < 0.001 |
| Stiff neck | 20 (26) | 70 (8) | < 0.001 |
| Altered mental status | 74 (95) | 819 (92) | 0.34 |
| > 6 hours | 59 (76) | 121 (14) | < 0.001 |
| Unconsciousness | 60 (77) | 750 (84) | 0.11 |
| > 1 hour | 54 (69) | 308 (35) | < 0.001 |
Suspect meningoencephalitis cases was defined as people who developed fever with at least 6 hours of altered mental status, or at least 1 hour of unconsciousness, or new onset seizure (jerking in body) within the last 12 months.
Two-sample proportion test was applied to compare the characteristics between admitted and not admitted cases.
Incidence estimation.
Among the 38 laboratory-confirmed JE cases admitted from the catchment areas of the three surveillance hospitals, 24 cases were from Rajshahi, 8 from Khulna, and 6 from Chittagong. Among the suspected meningoencephalitis cases in the hospital catchment area, a small proportion of cases visited the surveillance hospitals during their illness; 11% in Rajshahi, 4% in Khulna, and 9% in Chittagong (Table 4). Using the formula to measure the population-based incidence of JE through catchment area population, the catchment adjusted incidence of JE ranged from 0.6/100,000 population in Chittagong to 2.7 in Rajshahi (Table 4). The estimated incidence of JE was higher among children in all of the hospital catchment areas. Among the population < 15 years of age, the adjusted incidence was 4.7 in Rajshahi, 3.0 in Khulna, and 1.2 in Chittagong/100,000 population (Table 4).
Table 4.
Estimated incidence of Japanese encephalitis by area and age group
| Incidence calculation | Rajshahi | Khulna | Chittagong | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Age < 15 years | Age ≥ 15 years | All | Age < 15 years | Age ≥ 15 years | All | Age < 15 years | Age ≥ 15 years | All | |
| Survey findings* | |||||||||
| Suspect meningoencephalitis cases | 193 | 74 | 267 | 251 | 41 | 292 | 359 | 51 | 410 |
| Cases admitted to surveillance hospital | 17 | 12 | 29 | 5 | 6 | 11 | 23 | 15 | 38 |
| Proportion of cases admitted to surveillance hospital (P)† (CI, 95%) | 0.08 (0.02–0.14) | 0.15 (0.06–0.25) | 0.11 (0.06–0.16) | 0.02 (0.001–0.04) | 0.15 (0.03–0.27) | 0.04 (0.01–0.06) | 0.06 (0.03–0.09) | 0.29 (0.15–0.43) | 0.09 (0.06–0.12) |
| Incidence estimation | |||||||||
| Population of hospital catchment area (in millions)‡ | 2.3 | 4.4 | 6.7 | 3.9 | 7.7 | 11.6 | 3.1 | 6.1 | 9.2 |
| Identified JE cases from hospital catchment area§ | 11 | 13 | 24 | 3 | 5 | 8 | 3 | 3 | 6 |
| JE incidence per 100,000 population (CI, 95%) | 4.7 (2.7–16.6) | 1.5 (0.9–3.7) | 2.7 (1.8–4.9) | 3.0 (1.6–46.0) | 0.4 (0.2–1.9) | 1.4 (0.9–4.1) | 1.2 (0.9–2.3) | 0.1 (0.09–0.3) | 0.6 (0.4–0.9) |
Survey was conducted in 20 clusters randomly selected from each hospital catchment area and the average population of a cluster was about 27,000.
Adjusted for cluster effect using linear mixed effect model.
Population was projected for 2008 using census 2001.
Duration of surveillance was October 2007–December 2008.
Potential impact of limited availability of convalescent specimen.
Among the 705 enrolled patients admitted to the surveillance hospitals from the catchment areas (392 in Rajshahi, 144 in Khulna, and 169 in Chittagong), 319 (45%) had a serum or CSF sample that was collected ≥ 7 days after their onset of illness; of these, 26 (8.2%) were positive for anti-JEV IgM antibodies. In comparison, only 12 (3.1%) of the remaining 386 patients who had a sample collected < 7 days after onset of illness had evidence of anti-JEV IgM antibodies (P < 0.005). Applying the JE positivity rate for patients who had a specimen from ≥ 7 days after onset of illness available for testing to the meningoencephalitis patients who had only an acute specimen collected, we estimate that 20 additional JE cases would have been identified (12 in Rajshahi, 3 in Khulna, and 5 in Chittagong). Using these assumptions, the adjusted incidence of JE would be 4.0 (95% CI = 2.8–7.5) per 100,000 population in Rajshahi, 2.0 (95% CI = 1.2–5.6) in Khulna, and 1.0 (95% CI = 0.8–1.6) in Chittagong.
Discussion
This is the first study in Bangladesh to estimate the population-based incidence of JE. The estimated incidence varied in different parts of the country, with the highest in the Rajshahi area, in northwestern Bangladesh (2.7/100,000 population). This rate is similar to the JE incidence of some other JE endemic countries before the introduction of a JE vaccine into the national immunization programs; in Taiwan the incidence was 2.1 and in Thailand the incidence was 3–5/100,000 population.27,28 Nepal has recently focused on a mass immunization program of JE in their 20 endemic and four hyperendemic districts where the incidence of JE was 1.6/100,000 population in JE-endemic districts.29–31
Identifying the highest JE incidence in the Rajshahi Medical College Hospital catchment area is consistent with the hospital-based JE study conducted in 2003–2005, where over half of the cases (11 of 20) were identified from this hospital among four study hospitals.9 A higher number of JE cases in Rajshahi may result from the local ecology. The mosquito vector of JE breeds prolifically in wet fields, especially in paddy fields.32 The Rajshahi area is famous for rice and other agricultural products and the proportion of people directly involved in agriculture is higher than any other area of the country.33 Although pig raising is not common in Bangladesh's predominately Muslim communities, indigenous and tribal people commonly raise pigs, including the Santal, the most populous indigenous community living in northwestern Bangladesh.34 The actual pig density is not known, but a recent pig census conducted in three catchment districts of Rajshahi Medical College Hospital indentified over 11,000 pigs, which were well distributed throughout the districts (Khan SU, personal communication).
The vast majority of JEV infections are asymptomatic and the clinical features of JE have a broad spectrum, ranging from a mild flu-type illness to a severe meningoencephalitis.6 The case definition of suspect meningoencephalitis in the catchment survey included similar clinical signs to those used for the case definition in surveillance hospitals to enroll the patients for JE testing. However, the presenting clinical signs, in terms of duration of altered mental status and unconsciousness, were more severe among admitted cases in surveillance hospitals than those of cases who were not admitted. The severity of illness of these admitted patients could be higher because surveillance at the tertiary level hospital captures patients with more severe illnesses.35,36 In Bangladesh, people initially seek care from local health care providers, either qualified or unqualified. Of the identified suspected meningoencephalitis cases, only 1% of cases visited a surveillance medical college hospital as their first visit to any health care provider even though many of these people lived quite close to that surveillance hospital.
Although only 45% of hospital-confirmed JE cases were < 15 years of age, in the catchment area of all study hospitals the estimated incidence of JE was higher among children compared with adults, which is similar to other studies conducted in JE-endemic countries.30,37,38 In the catchment area more than two-thirds of the suspected meningoencephalitis cases were found in children < 5 years of age, but these cases were less likely to be admitted to the surveillance hospitals. For tertiary level health care, people preferred pediatric hospitals for the treatment of their children instead of medical college hospitals (Table 2). However, as no pediatric hospital is available in the Rajshahi area, all children admitted to pediatric hospitals were identified from the Chittagong and Khulna area.
The approach used in this study, to estimate the population-based incidence of JE by adjusting crude incidence based on healthcare facility usage, builds on approaches used by other investigators in other settings to estimate the incidence of other diseases of public health importance.35,39,40 Incidence estimation of a disease with low frequency requires a large sample. The average population of our survey area (20 unions) in each hospital catchment area was more than 500,000. Rather than traditional door-to-door visits, which would be relatively expensive, we deployed a novel low-cost practical approach leveraging the strong social networks of rural Bangladeshi communities to identify persons with symptoms compatible with meningoencephalitis in the rural study areas. A typical village in Bangladesh is made up of a number of neighborhoods (paras) that are composed of a group of homesteads (baris).41 A bari usually consists of 6–7 households around a single courtyard and generally it's members are patrilineally related; in a para people are generally involved in the same occupation and are closely related to each other.41,42 Hence, because of deep kinship prevailing in rural Bangladesh, residents are quite knowledgeable of major events that occurred in the other's households. As the field team visited the village doctors, educational institutions, and various groups in the village market, in addition to courtyard gathering, they usually received information of a suspected meningoencephalitis case from multiple sources. Although this community networking approach did not work in the three urban clusters because of weaker social networks among the urban dwellers, the approach may be efficient in identifying persons with severe diseases in other areas where strong social networking exist.
We note certain study limitations. First, the adjusted incidence assumes that meningoencephalitis patients who were admitted to the study hospital had a similar likelihood of having JEV infection as patients who received care elsewhere. However, the cases who were admitted to the study hospital were older, sicker, and lived closer to the hospital than the other meningoencephalitis cases. However, whether these differences would result in an increased or decreased proportion of the hospitalized cases being caused by JE is unknown and, therefore, could not be accounted for in the analysis. Second, in the surveillance hospitals only 45% of admitted patients had a CSF or serum specimen collected ≥ 7 days of onset of illness. This limited availability of convalescent specimens would underestimate the incidence of JE, so we made an additional estimate of incidence that accounted for this underestimation. Our estimate would be more precise if we were actually able to test the convalescent sera of all patients. Third, we collected information on persons who had symptoms compatible with meningoencephalitis using a community-based survey, usually collected months after the acute illness. However, as our trained field team explained the symptoms of meningoencephalitis to the villagers in local terms, we believe that the reports of seizures and altered consciousness, especially coma, were reliably reported by lay persons and therefore provide a reasonable estimate of the proportion of persons with serious central nervous system infections living in hospital catchment areas.
The data obtained from this study provides a credible estimate of the JE incidence in three wide geographic areas in Bangladesh that can be used as a rationale for policymakers to take effective control measures. Vaccination is the most effective means to control the incidence of JE.43 Current JE vaccines are safe and inexpensive3; the neighboring countries of Bangladesh, such as India, Nepal, and Sri Lanka, have recently introduced JE vaccine into their JE endemic regions. Bangladesh should consider a vaccine demonstration project in areas where the incidence is high, especially in Rajshahi, and should assess the cost-effectiveness of vaccine introduction in the routine childhood immunization program.
ACKNOWLEDGMENTS
We acknowledge the efforts of the collaborating partners, specifically the Institute of Epidemiology, Disease Control and Research of the Government of the People's Republic of Bangladesh. We thank the field staff for their hard work in this study and express gratitude to study participants and the villagers of study areas for their spontaneous participation to find out the persons having symptoms compatible with meningoencephalitis. We acknowledge the administrative and logistic support of Milton Quiah and Mustak Ahmed. We are thankful to Dorothy Southern for her critical review of the manuscript.
Footnotes
Financial support: This study was funded by the Centers for Disease Control and Prevention, Atlanta, GA (CDC), cooperative award number I-U01-C1000298, and South-East Asia Regional Office (SEARO), World Health Organization (WHO), allotment no. SE BAN AAD 210 XC 08 8. ICDDR,B acknowledges with gratitude the commitment of the CDC and WHO to the ICDDR,B's research efforts.
Authors' addresses: Repon C. Paul, Emily S. Gurley, and Stephen P. Luby, Programme on Infectious Diseases and Vaccine Sciences, ICDDR,B, Mohakhali, Dhaka, Bangladesh, E-mails: repon@icddrb.org, egurley@icddrb.org, and sluby@icddrb.org. Mahmudur Rahman, Sultana S. Banu, and ASM Alamgir, Institute of Epidemiology Disease Control and Research (IEDCR), Mohakhali, Dhaka, Bangladesh, E-mails: mrahman@citeecho.net, sultana_aus@yahoo.com, and aalamgir@gmail.com. M. Jahangir Hossain, Clinical Sciences Division, ICDDR,B, Mohakhali, Dhaka, Bangladesh, E-mail: jhossain@icddrb.org. Serguei Diorditsa, World Health Organization, Dhaka, Bangladesh, E-mail: diorditsas@searo.who.int. ASM Mainul Hasan, World Health Organization, Bangladesh, Dhaka, Bangladesh, E-mail: hasana@searo.who.int. Muhammad Aziz Rahman, Discipline of Public Health, School of Population Health and Clinical Practice, The University of Adelaide, Adelaide, Australia, E-mail: drazizdmc@yahoo.com. Hardeep Sandhu, Global Immunization Division, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: hjs3@cdc.gov. Marc Fischer, Surveillance and Epidemiology Activity, Arboviral Diseases Branch, DVBID, NCZVED, CDC, E-mail: mfischer@cdc.gov.
References
- 1.Tsai TF. In: Japanese encephalitis vaccines. Vaccines. Plotkin SA, Mortimer E, editors. Philadelphia, PA: W.B. Saunders; 1994. pp. 671–713. [Google Scholar]
- 2.Halstead SB, Jacobson J. Japanese encephalitis. Adv Virus Res. 2003;61:103–138. doi: 10.1016/s0065-3527(03)61003-1. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization Japanese encephalitis vaccines. Wkly Epidemiol Rec. 1998;73:337–344. [PubMed] [Google Scholar]
- 4.Solomon T. Control of Japanese encephalitis–within our grasp? N Engl J Med. 2006;355:869–871. doi: 10.1056/NEJMp058263. [DOI] [PubMed] [Google Scholar]
- 5.Kabilan L, Rajendran R, Arunachalam N, Ramesh S, Srinivasan S, Philip SP, Dash AP. Japanese encephalitis in India: an overview. Indian J Pediatr. 2004;71:609–615. doi: 10.1007/BF02724120. [DOI] [PubMed] [Google Scholar]
- 6.Solomon T, Dung NM, Kneen R, Gainsborough M, Vaughn DW, Khanh VT. Japanese encephalitis. J Neurol Neurosurg Psychiatry. 2000;68:405–415. doi: 10.1136/jnnp.68.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bista MB, Banerjee MK, Shin SH, Tandan JB, Kim MH, Sohn YM, Ohrr HC, Tang JL, Halstead SB. Efficacy of single-dose SA 14-14-2 vaccine against Japanese encephalitis: a case control study. Lancet. 2001;358:791–795. doi: 10.1016/s0140-6736(01)05967-0. [DOI] [PubMed] [Google Scholar]
- 8.Khan AM, Khan AQ, Dobrzynski L, Joshi GP, Myat A. A Japanese encephalitis focus in Bangladesh. J Trop Med Hyg. 1981;84:41–44. [PubMed] [Google Scholar]
- 9.Hossain MJ, Gurley ES, Montgomery S, Petersen L, Sejvar J, Fischer M, Panella A, Powers AM, Nahar N, Uddin AK, Rahman ME, Ekram AR, Luby SP, Breiman RF. Hospital-based surveillance for Japanese encephalitis at four sites in Bangladesh, 2003–2005. Am J Trop Med Hyg. 2010;82:344–349. doi: 10.4269/ajtmh.2010.09-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.IASR Japanese encephalitis, Japan, 1999–2002. Infectious Agents Surveillance Report. 2003:149–150. [Google Scholar]
- 11.Sohn YM, Park MS, Rho HO, Chandler LJ, Shope RE, Tsai TF. Primary and booster immune responses to SA14-14-2 Japanese encephalitis vaccine in Korean infants. Vaccine. 1999;17:2259–2264. doi: 10.1016/s0264-410x(99)00006-7. [DOI] [PubMed] [Google Scholar]
- 12.Sohn YM. Japanese encephalitis immunization in South Korea: past, present, and future. Emerg Infect Dis. 2000;6:17–24. doi: 10.3201/eid0601.000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tseng HF, Tan HF, Chang CK, Huang WL, Ho WC. Seroepidemiology study of Japanese encephalitis neutralizing antibodies in southern Taiwan: a comparative study between urban city and country townships. Am J Infect Control. 2003;31:435–440. doi: 10.1067/mic.2003.73. [DOI] [PubMed] [Google Scholar]
- 14.Liu W, Clemens JD, Yang JY, Xu ZY. Immunization against Japanese encephalitis in China: a policy analysis. Vaccine. 2006;24:5178–5182. doi: 10.1016/j.vaccine.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Xin YY, Ming ZG, Peng GY, Jian A, Min LH. Safety of a live-attenuated Japanese encephalitis virus vaccine (SA14-14-2) for children. Am J Trop Med Hyg. 1988;39:214–217. doi: 10.4269/ajtmh.1988.39.214. [DOI] [PubMed] [Google Scholar]
- 16.Tandan JB, Ohrr H, Sohn YM, Yoksan S, Ji M, Nam CM, Halstead SB. Single dose of SA 14-14-2 vaccine provides long-term protection against Japanese encephalitis: a case-control study in Nepalese children 5 years after immunization. Vaccine. 2007;25:5041–5045. doi: 10.1016/j.vaccine.2007.04.052. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed S, Sobhan F, Islam A, Barkat-e-Khuda Neonatal morbidity and care-seeking behavior in rural Bangladesh. J Trop Pediatr. 2001;47:98–105. doi: 10.1093/tropej/47.2.98. [DOI] [PubMed] [Google Scholar]
- 18.Syed U, Khadka N, Khan A, Wall S. Care-seeking practices in South Asia: using formative research to design program interventions to save newborn lives. J Perinatol. 2008;28((Suppl 2)):S9–S13. doi: 10.1038/jp.2008.165. [DOI] [PubMed] [Google Scholar]
- 19.Killewo J, Anwar I, Bashir I, Yunus M, Chakraborty J. Perceived delay in healthcare-seeking for episodes of serious illness and its implications for safe motherhood interventions in rural Bangladesh. J Health Popul Nutr. 2006;24:403–412. [PMC free article] [PubMed] [Google Scholar]
- 20.Ravi V, Robinson JS, Russell BJ, Desai A, Ramamurty N, Featherstone D, Johnson BW. Evaluation of IgM antibody capture enzyme-linked immunosorbent assay kits for detection of IgM against Japanese encephalitis virus in cerebrospinal fluid samples. Am J Trop Med Hyg. 2009;81:1144–1150. doi: 10.4269/ajtmh.2009.09-0144. [DOI] [PubMed] [Google Scholar]
- 21.Jacobson JA, Hills SL, Winkler JL, Mammen M, Thaisomboonsuk B, Marfin AA, Gibbons RV. Evaluation of three immunoglobulin M antibody capture enzyme-linked immunosorbent assays for diagnosis of Japanese encephalitis. Am J Trop Med Hyg. 2007;77:164–168. [PubMed] [Google Scholar]
- 22.Bangladesh Bureau of Statistics . Key Indicators on Report of Sample Vital Registration System 2008. 2008. [Google Scholar]
- 23.Siegel JS, Swanson DA. The Methods and Materials of Demography. San Diego, CA: Elsevier Academic Press; 2004. [Google Scholar]
- 24.Burke DS, Nisalak A, Ussery MA, Laorakpongse T, Chantavibul S. Kinetics of IgM and IgG responses to Japanese encephalitis virus in human serum and cerebrospinal fluid. J Infect Dis. 1985;151:1093–1099. doi: 10.1093/infdis/151.6.1093. [DOI] [PubMed] [Google Scholar]
- 25.Solomon T, Thao LT, Dung NM, Kneen R, Hung NT, Nisalak A, Vaughn DW, Farrar J, Hien TT, White NJ, Cardosa MJ. Rapid diagnosis of Japanese encephalitis by using an immunoglobulin M dot enzyme immunoassay. J Clin Microbiol. 1998;36:2030–2034. doi: 10.1128/jcm.36.7.2030-2034.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chanama S, Sukprasert W, Sa-ngasang A, An A, Sangkitporn S, Kurane I, Anantapreecha S. Detection of Japanese encephalitis (JE) virus-specific IgM in cerebrospinal fluid and serum samples from JE patients. Jpn J Infect Dis. 2005;58:294–296. [PubMed] [Google Scholar]
- 27.Wu YC, Huang YS, Chien LJ, Lin TL, Yueh YY, Tseng WL, Chang KJ, Wang GR. The epidemiology of Japanese encephalitis on Taiwan during 1966–1997. Am J Trop Med Hyg. 1999;61:78–84. doi: 10.4269/ajtmh.1999.61.78. [DOI] [PubMed] [Google Scholar]
- 28.Endy TP, Nisalak A. Japanese encephalitis virus: ecology and epidemiology. Curr Top Microbiol Immunol. 2002;267:11–48. doi: 10.1007/978-3-642-59403-8_2. [DOI] [PubMed] [Google Scholar]
- 29.Partridge J, Ghimire P, Sedai T, Bista MB, Banerjee M. Endemic Japanese encephalitis in the Kathmandu valley, Nepal. Am J Trop Med Hyg. 2007;77:1146–1149. [PubMed] [Google Scholar]
- 30.Wierzba TF, Ghimire P, Malla S, Banerjee MK, Shrestha S, Khanal B, Sedai TR, Gibbons RV. Laboratory-based Japanese encephalitis surveillance in Nepal and the implications for a national immunization strategy. Am J Trop Med Hyg. 2008;78:1002–1006. [PubMed] [Google Scholar]
- 31.Bhattachan A, Amatya S, Sedai TR, Upreti SR, Partridge J. Japanese encephalitis in hill and mountain districts, Nepal. Emerg Infect Dis. 2009;15:1691–1692. doi: 10.3201/eid1510.081641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Innis BL. In: Exotic Viral Infection. Porterfield JS, editor. London: Chapman and Hall; 1996. pp. 147–173. (Japanese encephalitis). [Google Scholar]
- 33.Bangladesh Bureau of Statistics Preliminary Report of Agri Census. 2008.
- 34.Minority Rights Group International World Directory of Minorities and Indigenous Peoples—Bangladesh: Adivasis. 2008.
- 35.Crump JA, Youssef FG, Luby SP, Wasfy MO, Rangel JM, Taalat M, Oun SA, Mahoney FJ. Estimating the incidence of typhoid fever and other febrile illnesses in developing countries. Emerg Infect Dis. 2003;9:539–544. doi: 10.3201/eid0905.020428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murdoch DR, Woods CW, Zimmerman MD, Dull PM, Belbase RH, Keenan AJ, Scott RM, Basnyat B, Archibald LK, Reller LB. The etiology of febrile illness in adults presenting to Patan hospital in Kathmandu, Nepal. Am J Trop Med Hyg. 2004;70:670–675. [PubMed] [Google Scholar]
- 37.Vaughn DW, Hoke CH., Jr The epidemiology of Japanese encephalitis: prospects for prevention. Epidemiol Rev. 1992;14:197–221. doi: 10.1093/oxfordjournals.epirev.a036087. [DOI] [PubMed] [Google Scholar]
- 38.Kari K, Liu W, Gautama K, Mammen MP, Jr, Clemens JD, Nisalak A, Subrata K, Kim HK, Xu ZY. A hospital-based surveillance for Japanese encephalitis in Bali, Indonesia. BMC Med. 2006;4:8. doi: 10.1186/1741-7015-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gargouri N, Walke H, Belbeisi A, Hadadin A, Salah S, Ellis A, Braam HP, Angulo FJ. Estimated burden of human Salmonella, Shigella, and Brucella infections in Jordan, 2003–2004. Foodborne Pathog Dis. 2009;6:481–486. doi: 10.1089/fpd.2008.0192. [DOI] [PubMed] [Google Scholar]
- 40.Chompook P, Samosornsuk S, von Seidlein L, Jitsanguansuk S, Sirima N, Sudjai S, Mangjit P, Kim DR, Wheeler JG, Todd J, Lee H, Ali M, Clemens J, Tapchaisri P, Chaicumpa W. Estimating the burden of shigellosis in Thailand: 36-month population-based surveillance study. Bull World Health Organ. 2005;83:739–746. [PMC free article] [PubMed] [Google Scholar]
- 41.Marty C. Poverty, gender, and work in Bangladesh. Econ Polit Wkly. 1986;21:217–222. [Google Scholar]
- 42.Aziz KMA. Kinship in Bangladesh. Dhaka, Bangladesh: International Center for Diarrhoeal Disease Research; 1979. [Google Scholar]
- 43.Lowry PW, Truong DH, Hinh LD, Ladinsky JL, Karabatsos N, Cropp CB, Martin D, Gubler DJ. Japanese encephalitis among hospitalized pediatric and adult patients with acute encephalitis syndrome in Hanoi, Vietnam 1995. Am J Trop Med Hyg. 1998;58:324–329. doi: 10.4269/ajtmh.1998.58.324. [DOI] [PubMed] [Google Scholar]


