Abstract
There has been a recent increase in reports of neurologic complications as major causes of morbidity and mortality in chikungunya virus infection. As a part of 2004–2009 global outbreaks, an unprecedented large chikungunya epidemic occurred in Southern Thailand during 2008–2009 in which 49,069 cases were reported. During this period, we encountered two patients with meningoencephalitis and another patient with myeloneuropathy among 1,018 cases diagnosed as chikungunya in our hospital. The clinical pictures are presented and the key points are used to recognize and differentiate chikungunya from Japanese encephalitis virus, dengue virus, and herpesvirus infections, which are more common causes of meningoencephalitis and myelitis in this region.
Chikungunya (CHIK) is an Aedes mosquito–transmitted infection caused by chikungunya virus (CHIKV), an RNA virus in the family Togaviridae.1 The disease is characteristically manifested as fever, arthralgia, and/or rash.2 In August 2008, CHIK emerged in the southern provinces of Thailand, and 49,069 cases reported by the end of 2009.3 We encountered three patients with neurologic complications among 1,018 patients given a diagnosis of CHIK in Songklanagarind Hospital in Songkla Province. Although neuro-chikungunya is relatively infrequent, there was a recent increase of reports of these complications because of the large number of persons infected in three continents during the 2004–2009 outbreaks. In addition, and more importantly, it is the major cause of death and disability in CHIK infection.4
Case reports
Patient 1.
In April 2009, high-grade fever with severe polyarthralgia developed in a 27-year-old woman. Two days later, a rash developed and the next day she became progressively stuporous. She was hospitalized on the eighth day after onset of symptoms and had alteration of consciousness. She appeared mute and drowsy with postinflammatory hyperpigmentation rash on her arms and abdomen and swelling of the interphalangeal joints. Neurologic examination showed normal cranial nerve signs without focal neurologic deficit or meningeal sign. Cranial magnetic resonance imaging (MRI) showed a hyperintense signal involving both temporal lobes and insular cortices without significant contrast enhancement (Figures 1 and 2).
Figure 1.

Axial cranial magnetic resonance imaging with fluid-attenuated inversion recovery of patient 1 showing hyper-intense signal involving both medial temporal lobes.
Figure 2.

Coronal cranial magnetic resonance imaging with fluid-attenuated inversion recovery of patient 1 showing hyper-intense signal involving both insular cortices.
Cerebrospinal fluid (CSF) obtained on the eighth day of illness contained 90 mononuclear leukocytes/mm3, and glucose and protein levels were 51 and 70 mg/dL, respectively. Cultures of CSF were negative for bacteria, fungi, and Mycobacterium. Results of a CSF–polymerase chain reaction (PCR) PCR test for herpes group viral DNA (herpesvirus 1, herpesvirus 2, cytomegalovirus, Epstein-Barr virus, and varicella zoster virus) and CSF-IgM assays for dengue virus and Japanese encephalitis virus were negative. However, IgM against CHIKV (Anti-chikungunya II FI; Euroimmun, Lübeck, Germany) was detectable in the CSF. Her serum contained high (1:640) CHIK hemagglutination-inhibition (HI) antibody titers in samples obtained on the eighth and fifteenth days of illness. Intravenous acyclovir treatment of presumed herpes encephalitis was given for one day but discontinued after reviewing the laboratory results. Her clinical condition rapidly improved within a few days without any sequelae upon follow-up six months later.
Patient 2.
In April 2009, fever, swelling, and intense pain in many joints developed in an 85-year-old man (religion teacher). He also became drowsy; and showed occasional, symmetrical jerky movements of the extremities on the third day post-onset of symptoms. An endotracheal tube was inserted at a local hospital, and he was transferred to Songklanagarind Hospital on the fifth day of illness. Physical examination showed a fever of 39.5°C, a Glasgow coma score of E1VtM4, and pinpoint pupils and symmetrical myoclonus upon stimulation. Cranial MRI showed diffused brain atrophy and confluent and large hyperintense signals of periventricular white matter area on T2-weighted and fluid-attenuated inversion recovery images (Figure 3).
Figure 3.
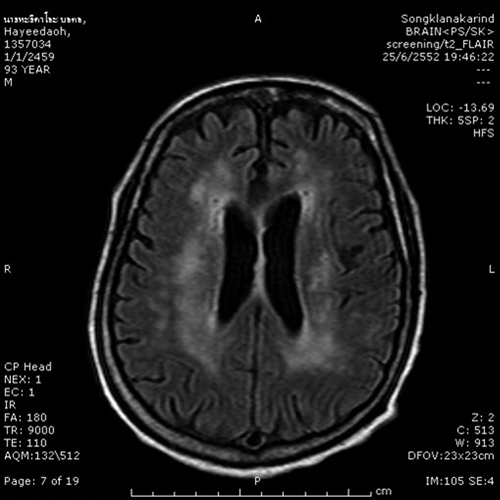
Axial cranial magnetic resonance imaging with fluid-attenuated inversion recovery of patient 2 showing confluent and large multiple hyper intense signals within both periventricular white matters.
Cerebrospinal fluid obtained on the fifth day of illness contained 47 mononuclear leukocytes/mm3 and protein and glucose levels were 90 and 70 mg/dL, respectively. Cultures of CSF were negative for bacteria, fungi, and Mycobacterium. Results of a CSF-PCR test for herpes group viruses and CSF dengue IgM and Japanese encephalitis virus antibody assays were negative. Results of acute-phase and convalescent-phase serum studies for human immunodeficiency virus, leptospirosis, and scrub and murine typhus antibodies were also negative. However, increases of his serum CHIK HI antibody titers from < 1:20 at admission to 1:2,560 13 days later were observed. His neurologic condition remained unchanged upon follow-up 12 months later.
Patient 3.
In June 2009, fever and polyarthralgia in the hands, wrists, and ankles associated with itching rash on the face, body, and extremities developed in a 44-year-old woman. All of these signs subsided within two weeks. However, progressive weakness developed, followed by dysphonia, difficulty in swallowing, and frequent aspiration that necessitated insertion of an endotrachial tube on the twentieth day of illness. Neurologic examination showed a conscious woman with facial diplegia and an areflexic grade 2/5 motor power on proximal muscles of the lower and upper extremities. The pain and propioceptive sensation were intact. The extraocular muscles and pupils were normal. Cranial MRI showed a tiny hyperintense signal on T2-weighted and fluid-attenuated inversion recovery images at the right occipital lobe. However, cervico-thoracic MRI showed only an equivocal hyperintense signal at the C4-5 spinal cord.
Electromyography and nerve conduction velocity studies showed no sensorineural action potential and delayed distal motor latencies of right median and ulnar nerve with small compound muscle action, suggestive of acute motor and sensory peripheral neuropathy. Lumbar puncture done on the twentieth day of illness showed acellular CSF with a protein and glucose levels of 260 and 85 mg/dL, respectively. Serologic test results for human immunodeficiency virus infection, antinuclear antibodies, syphilis, leptospirosis, scrub typhus, and murine typhus were negative. Tests results of CSF for IgM to dengue virus and Japanese encephalitis virus viruses were also negative. However, CHIK IgM was detected in the CSF, and the result of a reverse transcription–PCR for CHIKV RNA was negative. After four days of treatment with intravenous immunoglobulin (IVIG), rapid improvement was observed with complete recovery upon follow-up six months later.
Discussion
Our patients were confirmed as having CHIKV infection on the basis of detection of CHIK IgM in the CSF of patients 1 and 35,6 and by a four-fold increase in serum CHIK HI antibody titer in patient 2.7 The negative result of the CSF reverse transcription–PCR assay for CHIKV RNA for patient 3 was not surprising given the late (twentieth day after symptom onset) presentation of the patient5 and the usual negative finding of CHIKV genomic products in patients who were positive for CHIK IgM in CSF.8 All of our patients had fever, polyarthralgia, and/or rash typical of CHIK infection. Patients 1 and 2 had meningoencephalitis, and patient 3 had myeloneuropathy. These neurologic complications of CHIKV infection are infrequent; there were only two previous case reports of encephalitis among the estimated 44,000 cases during the 1962–1964 outbreaks in Bangkok.7,9,10 Chikungunya virus was isolated for the first time from the brain from one of these patients.9
Despite these uncommon findings, there has been a recent increase in reports describing neurologic manifestations of CHIK infections, mainly from Reunion Island and India, where a massive epidemic of CHIK involving millions of patients occurred.5,6,8,11 Although this 2004–2009 CHIKV epidemic was caused by a variant virus of the central/eastern African genotype,12,13 there has been no study to see whether this recent CHIKV strain is more virulent than the previous CHIKV of Asian lineages14 Island-wide surveillance in Reunion Island identified 23 (1.4 per 1,000) adult patients with neurologic involvement among 16,050 laboratory-confirmed cases of CHIK infections.15 The proportion of our neurologic cases (3 per 1,018) was slightly higher than that in Reunion Island but indicated that this complication was relatively infrequent, and the increased reports of neuro-chikungunya might be attributed to the exceptionally large number of patients involved during these outbreaks.
Patients 1 and 2 had encephalitis, the most common form of neuro-chikungunya.4,8,11,16 A broad range of neurologic manifestations has been reported for this syndrome, including altered mental status, seizures, speech disturbances, motor dysfunction, and sensorial disorders.4,8 Patient 2 had myoclonus. Involuntary movements and extrapyramidal signs were seen in one-fifth of patients with CHIK encephalitis in India.11,16 However, all these encephalitic symptoms and signs were not pathognomonic of neuro-chikungunya and have been described in Japanese encephalitis and dengue encephalitis, the more common encephalitides in this region.17,18
Although we could not differentiate CHIK from other common encephalitides on neurologic grounds alone, the presence of erythematous or maculopapular rashes, which was found in 25–39% of patients with neuro-chikungunya8,16 might be more helpful because they are not features of Japanese encephalitis or herpes simplex encephalitis.17 However, dengue encephalitis may be confused with CHIKV infection because it can also show a rash, and concurrent outbreaks of CHIK and dengue encephalitis have occurred.7,19 However, arthralgia was observed more frequently in CHIK than in dengue, and leukopenia < 3,000 cells/mm3 and petechial rash, which might be associated with dengue, was uncommon in CHIK infection.7,20
There have been few reports of detailed neuroimaging findings for neuro-chikungunya.21,22 For patient 2, brain MRI abnormalities were exclusively located in the subcortical white matter, and multiple tiny hyperintense dots were observed in T2-weighted and fluid-attenuated inversion recovery images (Figure 2). Such findings were rather unique and were also reported in patients with CHIK in India.21,22 A pathologic correlation with autopsy specimens suggested that these findings were caused by the subcortical demyelination process.22 Although such findings have been described in Nipah virus encephalopathy, they were quite distinct from Japanese encephalitis or herpes virus encephalitis, which affect only the gray matter,23 are patchy lesions, and are usually involve the thalamus.24
Patient 3 had acute progressive quadriparesis and myeloneuropathy confirmed by MRI and electromyography, which showed acute sensorimotor peripheral neuropathy typical for demyelinating disease. Two types of neuropathy in CHIK have been described in CHIK infection.21 The early-onset type, which occurs within a few days of illness and usually with signs and symptoms of additional encephalitis, is more severe and shows a poor response to plasmapheresis or IVIG treatment. Conversely, the late-onset type, which occurs 1–3 weeks after fever, arthritis, and/or rash,5 is more typical of Guillain-Barré syndrome and shows a favorable response to plasmapheresis/IVIG treatment.5,21 Patient 3 was an example of the latter type.
The pathogenesis of neuro-chikungunya is not well understood because few autopsies with full virologic studies have been reported.8 The pathologic changes were more those of non-specific encephalitis, and no definite viral inclusions were seen.9,22 However, direct invasion of the central nervous system by the virus probably accounts for the encephalitic form because neurologic manifestations arise at the acute viremic stage of infection6 and CHIKV has been isolated from brain9 and CSF of patients.4,21 In addition, the presence of IgM against CHIKV in the CSF, such as that found in patient 1, also supports the theory of neuroinvasion. Conversely, the pathophysiology underlying the Guillain-Barré–like neuropathy remains unknown. We are not aware of any report showing isolation of CHIKV from the CSF or any autopsy report of such a patient. The late onset of neurologic manifestations beyond the viremic phase and the response to IVIG treatment were suggestive of the immunopathologic process rather than direct viral invasion.
The outcomes of our patients were satisfactory except for patient 2, who was 85 years old and had permanent neurologic sequelae. Studies from India and Reunion Island have shown that age was an independent risk factor for neurologic complications and mortality for CHIK.4,15 Because CHIK is a threat to many countries, physicians are expected to see more cases of neuro-chikungunya in the future. A preceding history of fever, polyarthralgia, and/or rash in patients with neurologic symptoms may be the first clues to neuro-chikungunya. Clinical findings or neuroimaging suggestive of demyelination process are also helpful and may lead to proper selection of appropriate serodiagnostic tests.
ACKNOWLEDGMENT
We thank Dr. Alan F. Geator for reviewing the manuscript.
Footnotes
Authors' addresses: Sarunyou Chusri, Pisud Siripaitoon, and Khachornsakdi Silpapojakul, Department of Medicine, Faculty of Medicine, Prince of Songkla University, Hat Yai, Songkhla 90110 Thailand. Siriporn Hirunpat, Department of Radiology, Faculty of Medicine, Prince of Songkla University, Hat Yai, Songkhla 90110 Thailand.
References
- 1.Palaux G, Gaüzere BA, Jaureguiberry S, Strobel M. Chikungunya, an epidemic arbovirosis. Lancet Infect Dis. 2007;7:319–217. doi: 10.1016/S1473-3099(07)70107-X. [DOI] [PubMed] [Google Scholar]
- 2.Borgherini G, Poubeau P, Staikowsky F, Lory M, Le Moullec N, Becquart JP, Wengling C, Michault A, Paganin F. Outbreak of chikungunya on Reunion Island: early clinical and laboratory features in 157 adult patients. Clin Infect Dis. 2007;44:1401–1407. doi: 10.1086/517537. [DOI] [PubMed] [Google Scholar]
- 3.Sawongtho P, Thavara U. Situation of chikungunya. Weekly Epidemiological Surveillance Report. 2009;40:878. [Google Scholar]
- 4.Tandale BV, Sathe PS, Arankalle VA, Wadia RS, Kulkarni R, Shah SV, Shah SK, Sheth JK, Sudeep AB, Tripathy AS, Mishra AC. Systemic involvements and fatalities during chikungunya epidemic in India, 2006. J Clin Virol. 2009;46:145–149. doi: 10.1016/j.jcv.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 5.Lebrun G, Chadda K, Reboux A, Gaüzère B. Guillain-Barré syndrome after chikungunya infection. Emerg Infect Dis. 2009;15:495–496. doi: 10.3201/eid1503.071482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robin S, Ramful D, Le Seach' F, Jaffar-Bandjee MC, Rigou G, Alessandri JL. Neurologic manifestations of pediatric chikungunya infection. J Child Neurol. 2008;23:1028–1035. doi: 10.1177/0883073808314151. [DOI] [PubMed] [Google Scholar]
- 7.Nimmannitya S, Halstead SB, Cohen SN, Margiotta MR. Dengue and chikungunya virus infection in man in Thailand, 1962–1964. Observations on hospitalized patients with hemorrhagic fever. Am J Trop Med Hyg. 1969;18:954–971. doi: 10.4269/ajtmh.1969.18.954. [DOI] [PubMed] [Google Scholar]
- 8.Tournebize P, Charlin C, Lagrange M. Neurological manifestations in chikungunya: about 23 cases collected in Reunion Island. Rev Neurol (Paris) 2009;165:48–51. doi: 10.1016/j.neurol.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Bhamarapravati N, Boonyaratvej S, Russell PK. Encephalitis and pneumonitis due to chikungunya virus: report of a fatal case. J Med Assoc Thai. 1966;49:627–632. [Google Scholar]
- 10.Halstead S. Epidemiological studies of Thai haemorrhagic fever, 1962–64. Bull World Health Organ. 1966;35:80–81. [PMC free article] [PubMed] [Google Scholar]
- 11.Chandak NH, Kashyap RS, Kabra D, Karandikar P, Saha SS, Morey SH, Purohit HJ, Taori GM, Daginawal HF. Neurological complications of chikungunya virus infection. Neurol India. 2009;57:177–180. doi: 10.4103/0028-3886.51289. [DOI] [PubMed] [Google Scholar]
- 12.Schuffenecker I, Iteman I, Michault A, Murri S, Frangeul L, Vaney MC, Lavenir R, Pardigon N, Reynes JM, Pettinelli F, Biscornet L, Diancourt L, Michel S, Duquerroy S, Guigon G, Frenkiel MP, Bréhin AC, Cubito N, Desprès P, Kunst F, Rey FA, Zeller H, Brisse S. Genome microevolution of chikungunya viruses causing the Indian Ocean outbreak. PLoS Med. 2006;3:e263. doi: 10.1371/journal.pmed.0030263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Theamboonlers A, Rianthavorn P, Praianantathavorn K, Wuttirattanakowit N, Poovorawan Y. Clinical and molecular characterization of chikungunya virus in south Thailand. Jpn J Infect Dis. 2009;62:303. [PubMed] [Google Scholar]
- 14.Simon F, Savini H, Parola P. Chikungunya: a paradigm of emergence and globalization of vector-borne diseases. Med Clin North Am. 2008;92:1323–1343. doi: 10.1016/j.mcna.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Renault P, Solet JL, Sissoko D, Balleydier E, Larrieu S, Filleul L, Lassalle C, Thiria J, Rachou E, de Valk H, Ilef D, Ledrans M, Quatresous I, Quenel P, Pierre V. A major epidemic of chikungunya virus infection on Reunion Island, France, 2005–2006. Am J Trop Med Hyg. 2007;77:727–731. [PubMed] [Google Scholar]
- 16.Rampal , Sharda M, Meena H. Neurological complications in chikungunya fever. J Assoc Physicians India. 2007;55:765–769. [PubMed] [Google Scholar]
- 17.Solomon T, Dung NM, Kneen R, Gainsborough M, Vaughn DW, Khanh VT. Japanese encephalitis. J Neurol Neurosurg Psychiatry. 2000;68:405–415. doi: 10.1136/jnnp.68.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solomon T, Dung NM, Vaughn DW, Kneen R, Thao LT, Raengsakulrach B, Loan HT, Day NP, Farrar J, Myint KS, Warrell MJ, James WS, Nisalak A, White NJ. Neurological manifestations of dengue infection. Lancet. 2000;25:1053–1059. doi: 10.1016/S0140-6736(00)02036-5. [DOI] [PubMed] [Google Scholar]
- 19.Ratsitorahina M, Harisoa J, Ratovonjato J, Biacabe S, Reynes JM, Zeller H, Raoelina Y, Talarmin A, Richard V, Louis Soares J. Outbreak of dengue and chikungunya fevers, Toamasina, Madagascar, 2006. Emerg Infect Dis. 2008;14:1135–1137. doi: 10.3201/eid1407.071521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hochedez P, Canestri A, Guihot A, Brichler S, Bricaire F, Caumes E. Management of travelers with fever and exanthema, notably dengue and chikungunya infections. Am J Trop Med Hyg. 2008;78:710–713. [PubMed] [Google Scholar]
- 21.Wadia RS. A neurotropic virus (chikungunya) and a neuropathic amino acid (homocysteine) Ann Indian Acad Neurol. 2007;10:198–213. [Google Scholar]
- 22.Ganesan K, Diwan A, Shankar SA, Desai SB, Sainani GS, Katrak SM. Chikungunya encephalomyeloradiculitis: report of 2 cases with neuroimaging and 1 case with autopsy findings. AJNR Am J Neuroradiol. 2008;29:1636–1637. doi: 10.3174/ajnr.A1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim CC, Sitoh YY, Hui F, Lee KE, Ang BS, Lim E, Lim WE, Oh HM, Tambyah PA, Wong JS, Tan CB, Chee TS. Nipah viral encephalitis or Japanese encephalitis? MR findings in a new zoonotic disease. AJNR Am J Neuroradiol. 2000;21:455–461. [PMC free article] [PubMed] [Google Scholar]
- 24.Kalita J, Misra UK. Comparison of CT scan and MRI findings in the diagnosis of Japanese encephalitis. J Neurol Sci. 2000;174:3–8. doi: 10.1016/s0022-510x(99)00318-4. [DOI] [PubMed] [Google Scholar]


