Abstract
The objective of this study was to assess the prevalence of hepatitis B and hepatitis C coinfections in human immunodeficiency virus (HIV) -infected adults at an HIV center in Gaborone, Botswana. A retrospective review was performed of charts of currently active HIV-infected adult patients in the Family Model Clinic (FMC) of the Botswana-Baylor Children's Clinical Center of Excellence (BCOE) in Gaborone, Botswana, for the results of serum hepatitis B surface antigen (HBsAg) and antihepatitis C IgG tests performed between January 1, 2005 and December 15, 2009. Of 308 active FMC patients, 266 underwent HBsAg serology testing within the period of study. The HBsAg coinfection prevalence was 5.3% (14/266); 2 of 252 patients had at least one positive antihepatitis C IgG serology, a 0.8% prevalence. Hepatitis B coinfection is relatively common in HIV-infected adults at our center in Botswana, whereas hepatitis C coinfection is rare. In this setting, where the diagnosis of hepatitis B coinfection with HIV has implications for choice of first-line antiretroviral therapy and prevention of perinatal hepatitis B transmission, broader sampling to establish the true population prevalence of hepatitis B coinfection and the desirability of adding screening to HIV management should be considered. These findings provide little justification for adding hepatitis C coinfection screening to the management of HIV infection in Botswana.
Introduction
As antiretroviral therapy (ART) programs in resource-limited settings mature and people living with human immunodeficiency virus (HIV) survive longer, the morbidity and mortality associated with coinfections will become increasingly important. Although hepatitis B (HBV) and C (HCV) share risk factors for transmission with HIV and are important diseases among people living with HIV (PLWH) in industrialized settings, their demographics and impact remain less well-defined in resource-limited settings. Accordingly, the screening, monitoring, and treatment of HBV and HCV in PLWH present clinical dilemmas and challenges in such settings.1
Available data show widely variable rates of hepatitis B infection in both general and HIV-infected populations (1.3–49.2%).1–22 Geographically relevant to Botswana, general population studies in South Africa have shown an urban prevalence of HBV of 1.3% among pregnant women in Soweto and 7.4% in a Durban general clinic population.13,14 Additional southern African data include a 6% serum hepatitis B surface antigen (HBsAg)-positive rate for HIV-infected in-patients meeting acquired immunodeficiency syndrome (AIDS) criteria admitted to a public Johannesburg hospital, with an additional 3% positive for HBsAg, suggesting occult HBV infection,15 and a 4.8% HBsAg-positive rate in a Johannesburg outpatient HIV clinic population.16
Data from Botswana itself are sparse. Among 141 HIV-infected, antiretroviral-naive patients with a median CD4 of 104 cells/mL in the HIV clinic population of a major urban healthcare facility, a 10.6% HBsAg-positive rate was reported. Additionally, the HBsAg seroprevalence was 6% in 127 patients lacking grade II or higher transaminitis in a study of isoniazid-associated hepatitis in HIV patients in eight clinics located in two urban areas.17 Interestingly, in the latter study, the rate of positive hepatitis B surface antibody—indicating prior infection and immunity—was 47%, whereas in 13 patients with transaminitis, HBsAg seroprevalence was 0%, and surface antibody was 50%.18 In other Botswana studies, in 1985, an HBsAg seroprevalence of 47% (24/60) was reported among victims of a then-unidentified non-A, non-B hepatitis outbreak in northern Botswana.19 A serologic general population survey in the mid-1990s showed a 12% prevalence.20 Also, a 1973 study of the ethnic minority San population in the Kalahari found a male prevalence of 12% (N = 84, age > 16 years) and a female prevalence of 14% (N = 80, age > 16 years).21 Some data, including from South Africa, suggest that HBV prevalence may be higher in rural and pediatric populations.7,22
As defined by a positive test for hepatitis C antibodies (antihepatitis C IgG), the prevalence of hepatitis C in the general population in sub-Saharan Africa was recently estimated at approximately 3%.23 Data from HIV-infected individuals include a small Zimbabwean study showing a 0.6% rate of HCV coinfection.24 Studies from Nigeria and Tanzania in outpatient HIV clinics showed widely varying rates of 2.3% and 18.1%, respectively.11,12 Additionally, a Botswana study at a major urban hospital showed a HCV coinfection rate of 0%.17
The Botswana-Baylor Children's Clinical Center of Excellence (BCOE) is a national HIV/AIDS care and treatment facility that provides services in Gaborone, Botswana for HIV-infected children from around the country. The Family Model Clinic (FMC) operates within the BCOE and serves the primary care needs of many of the HIV-infected adult caregivers of these children.25
Given the paucity of Botswana-specific data, the aim of this study was to assess the prevalence of HIV coinfection with HBV and HCV in the FMC population and contribute to the emerging body of literature that may inform local guidance on chronic hepatitis screening in HIV-infected persons.
Methods
The charts of all currently active HIV-infected adult patients at the FMC were retrospectively reviewed to determine the prevalence of HBV and HCV infection in this population. Results of all HBsAg and antihepatitis C IgG tests performed between January 1, 2005 and December 15, 2009 were compiled. All tests were performed at the Botswana-Harvard HIV Reference Laboratory in Gaborone using Abbot Murex test kits (HBsAg version 3,: Abbot Murex, Dartford, United Kingdom. HCV IgG version 4 - Abbot Murex, Midrand, Gauteng, South Africa).
Currently active patients were defined as those patients either officially registered in the FMC or with hepatitis B or C serology ordered by FMC providers who had been seen within the past 180 days—the longest follow-up interval for stable HIV patients at the BCOE—and not classified otherwise as lost to follow-up, transferred out, or died in the BCOE's electronic medical record. Hepatitis B and C screening using HBsAg and antihepatitis C IgG markers, respectively, was part of routine baseline assessment for all new HIV-infected patients on enrollment into FMC, regardless of clinical suspicion for hepatitis, during the entire period of time reviewed in this study. However, in some cases, variations in provider practice, specimen quantity, labeling, and transport resulted in either multiple reorderings or missing data. Testing was generally performed at the time of enrollment in the FMC. Pre-existing patients who had enrolled into FMC before baseline testing for hepatitis B and C being implemented were also screened for hepatitis B and C regardless of clinical suspicion for hepatitis, generally at the point in time that it was noticed by a provider that their clinical chart did not contain documentation of hepatitis B and/or C status.
For patients who had serology results that changed over time, only the positive result was counted. Of patients who had more than one congruent result over time, only the first result was counted. Subgroups were analyzed by sex and age, with age ranges of 18–24 years versus 25–29, 30–34, 35–39, 40–44, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74, 75–79, and 80+ years. Females aged 18–44 years were also analyzed to estimate the prevalence of HBsAg among women of child-bearing age.
For patients with a positive HBsAg or anti-HCV IgG result, any transaminase values measured within a 3-month period before or after the diagnostic hepatitis serology were extracted and analyzed for prevalence of abnormalities, defined as alanine aminotranferase (ALT) or aspartate aminotransferase (AST) greater than one time the upper limit of normal (ULN). Normal laboratory values for ALT and AST are 11–41 units/L.
Results
Of 308 active FMC patients, 266 (86.4%) had at least one HBsAg serology documented within the period of study. Two patients had serology results that changed over time from negative to positive and vice versa. Because our interest was in the presence of HBsAg at any point in time, only positive results were recorded, as mentioned above. Overall, HBsAg-positive rate was found to be 5.3%; 252 patients had at least one anti-HCV IgG serology result, of which two were positive, yielding an overall HCV prevalence of 0.8%.
Patient age ranged from 18 to 80 years, with 43 males and 223 females evaluated with HBsAg serology and 40 males and 212 females documented with HCV serology. Demographic characteristics for HBsAg are available in Table 1. Of note, the HBsAg prevalence among women between the ages of 18 and 44 years was 4.6% in the subgroup analysis. Table 2 shows adult HBsAg prevalence by gender and age.
Table 1.
Adult patient demographics by gender and age of those tested for hepatitis B surface antigen and/or antihepatitis C IgG in the FMC
| Patient characteristics | Number of active patients with hepatitis B serology evaluated | Number of active patients with hepatitis C serology evaluated |
|---|---|---|
| Sex | ||
| Male | 43 | 40 |
| Female | 223 | 212 |
| Age (years) | ||
| 18–24 | 12 | 12 |
| 25–29 | 19 | 19 |
| 30–34 | 68 | 62 |
| 35–39 | 73 | 75 |
| 40–44 | 45 | 40 |
| 45–49 | 30 | 25 |
| 50–54 | 13 | 13 |
| 55–59 | 2 | 2 |
| 60–64 | 0 | 0 |
| 65–69 | 1 | 1 |
| 70–74 | 0 | 0 |
| 75–79 | 0 | 0 |
| 80+ | 1 | 1 |
| Total patients | 266 | 252 |
Table 2.
Prevalence of positive hepatitis B surface antigen by gender and age in adult patients in the FMC
| Patient characteristics | Number of patients with a positive HBsAg serology |
|---|---|
| Sex | |
| Male | 2 (4.7%) |
| Female | 12 (5.4%) |
| Age (years) | |
| 18–24 | 0 |
| 25–29 | 0 |
| 30–34 | 4 (5.9%) |
| 35–39 | 2 (2.7%) |
| 40–44 | 4 (8.9%) |
| 45–49 | 2 (6.7%) |
| 50–54 | 1 (7.7%) |
| 55–59 | 1 (50%) |
| 60–80+ | 0 |
Transaminitis.
Of the 14 patients with an HBsAg-positive serology, 3 (21.4%) had elevated transaminases within 3 months before or after serology. All of those patients with elevated transaminases had paired AST/ALT elevations as shown in Figure 1.
Figure 1.
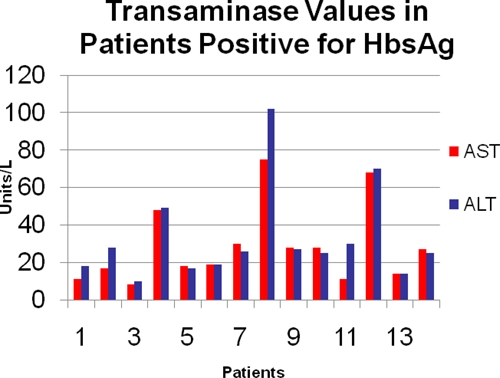
Baseline liver function tests in adult patients with positive HBsAg in the FMC.
Hepatitis C.
Of the 250 patients tested for hepatitis C, only 2 were positive. One patient was a 31-year-old female, and the other patient was a 49-year-old male. There were no transaminase elevations in either patient. No patients with a positive HCV result were also positive for HBsAg.
Discussion
The clinical impact of HBV and HCV coinfection for PLWH may be greater than for the general population.1,7 Although HBV and HCV seem to have little or no effect on HIV progression, HIV may profoundly affect the course of HBV and HCV.1 Coinfection is associated with greater risk of chronic hepatitis B and C after acute infection, lower rates of hepatitis virus clearance, higher risk of cirrhosis, increased incidence of hepatocellular carcinoma (HCC), and increased liver-related morbidity and mortality.1 Progression to liver failure also seems to occur sooner than when HIV is not present.1
Although screenings for HBV and HCV are routine elements of HIV primary care in industrialized settings,26–28 such screening is not part of the national HIV management guidelines in most resource-limited settings, including Botswana.29 Because options for first-line ART backbones in Botswana and many other resource-limited settings now include tenofovir-emtricitabine (TDF-FTC)—an antiretroviral combination also active against HBV—a diagnosis of concomitant HBV infection may affect the optimization of ART regimens for coinfected persons.29 Additionally, there are other implications to unappreciated HBV coinfection in HIV-infected patients, as discussed below. Given the non-availability of effective treatment of HCV in most resource-limited settings, the implications of diagnosis by screening are less clear but would include intensive lifestyle counseling, such as avoidance of factors that may exacerbate HCV progression, including alcohol abuse, and education on reducing the risk of transmission.
At 5.3%, the prevalence of positive HBsAg results in our clinic population is substantial. This may be an underestimate given our study's limitations, including defining hepatitis B coinfection based on a single positive HBsAg result as opposed to a more rigorous definition using sequential HBsAg results, HBV DNA testing, hepatitis B core antibodies, or a combination of these tests. Also, our population consisted of patients with a range of ARV exposure and degree of viral control; the even higher HBsAg prevalence in the other contemporary Gaborone-based study17 performed in ARV-naïve patients with a median CD4 count of 104 cells/mm3 is likely reflecting higher risk of active HBV in untreated severely immunosuppressed PLWH.
As well, given the principally urban nature of our population, if the higher rural HBV prevalence noted elsewhere in southern Africa is true of Botswana, we may expect HBV coinfection among PLWH in rural Botswana to be higher than 5.3%.7 The subgroup prevalence of 4.6% among childbearing-aged females (ages 18–44) suggests that a substantial number of infants born to HIV-infected mothers in Botswana are at risk of perinatally transmitted HBV as well as, of course, HIV.
As noted elsewhere in southern Africa, hepatitis C screening results in the FMC population at the BCOE showed very low prevalence of coinfection in PLWH: less than 1%.
In Botswana, broader seroprevalence assessments may be feasible to inform policy recommendations; systematic laboratory screening of newly diagnosed HIV patients for other coinfections and comorbidities is already standard, and the addition of HBsAg screening in some ART sites may be possible. The availability of data from a wider variety of locales and populations in Botswana would make it possible to determine whether the addition of HBV screening to the national testing protocol for newly diagnosed PLWH would be appropriate.
There is considerable interest globally in the formulation of packages of services for HIV-infected persons in resource-limited settings, including the screening and management of coinfections.30,31 Botswana's national adult HIV management guidelines address some of these issues, although routine hepatitis B and C screenings are not included, and also, immunization against HBV is not included.29 (Although universal childhood immunization against HBV was introduced in 2000, most adults remain at risk for HBV acquisition and disease progression.) Current HBV-specific advice in Botswana's national adult HIV management guidelines recommends assessing HBV by HBsAg only if a hepatitis flare is suspected in patients who discontinue treatment with TDF or lamivudine (3TC/FTC)-containing regimens.29 Other approaches where routine baseline HBV assessment is not part of management for all HIV-infected adults include South Africa's current HIV Treatment Guidelines, wherein HBsAg is assessed only if initial ALT is elevated in patients eligible for ART.32 Given that only 21.4% of our patients with HBV coinfection had elevated transaminases, even this broader approach would have missed a substantial proportion of HBV coinfected patients.
In settings where routine HBV testing is not performed and tenofovir is not available, patients will be initiated on first-line regimens containing only one drug with antihepatitis B activity—lamivudine or emtricitabine. Hepatitis B has a well-known propensity to develop lamivudine resistance when lamivudine is used without a second drug with antihepatitis B activity.33 However, in settings where other antiretrovirals with antihepatitis B activity—such as tenofovir—are available as first-line regimens, opportunity exists to ensure that hepatitis B-coinfected PLWH who require ART are initiated on regimens using backbones of tenofovir plus lamivudine or emtricitabine, with which the risk of developing lamivudine-resistant hepatitis B is less.33 The availability of tenofovir is expanding in resource-limited settings, making this approach more relevant. Indeed, recently updated World Health Organization recommendations for ART in adolescents and adults support this approach as do national ART guidelines in most industrialized nations.26–28,30,34
Another clinical concern for PLWH on ART with undiagnosed HBV coinfection is the risk of precipitating a flare of underlying quiescent hepatitis, including in some cases, fulminant hepatic failure and death when ART containing lamivudine, emtricitabine, or tenofovir (i.e., antiretrovirals with antihepatitis B activity) is withdrawn suddenly.35 Studies show the prevalence of severe withdrawal hepatitis in coinfected patients to be as high as 50%.35 Almost all patients requiring ART in resource-limited settings are initiated on lamivudine-containing regimens.34 Suspensions and discontinuations of therapy—intentional and otherwise—occur during ART for a variety of reasons. In settings where hepatitis B is reasonably common but patients' hepatitis B status is not generally known, those patients at risk for hepatic complications if ART is suddenly withdrawn remain unrecognized.36
Another consideration highlighting the importance of HBV coinfection involves the perinatal transmission of hepatitis B. Much attention has been paid to the prevention of mother to child transmission (PMTCT) of HIV globally, and great success has been seen in this realm; Botswana, for example, is the first higher-prevalence country in sub-Saharan Africa to achieve a national MTCT rate of < 5%.37 However, PMTCT of hepatitis B occupies a much less clear position in the hierarchy of interventions offered through maternal–child health services in Botswana and many other resource-limited settings.
Because most individuals infected with hepatitis B in resource-limited settings acquire their infection perinatally or in early childhood, preventing MTCT of HBV is a key public health intervention in settings of higher HBV prevalence.38 Because maternal HIV coinfection correlates with higher HBV viral load, which is also a major determinant of HBV MTCT risk, interventions to prevent MTCT of HBV may be of even greater impact in regions of higher HIV prevalence.39–41 Where such interventions are available, realization of HBV status through screening has substantial value.
A birth dose of HBV vaccine alone is 70–75% effective for prevention of MTCT of HBV, and the addition of a birth dose of hepatitis B immunoglobulin (HBIg) brings efficacy to 95%.38 The Botswana national healthcare system currently provides an at-birth HBV vaccine dose to all children born in healthcare facilities; although it does stock HBIg, it is rarely delivered at birth, because pregnant women are not routinely screened for HBV. Screening for HBV status would allow for the rational use of a birth dose of HBIg to decrease perinatal HBV transmission.
Although knowledge of PLWH's HCV coinfection status is helpful in terms of recommending avoidance of alcohol, hepatotoxic medications, and supplements, educating on preventing the risk of transmission, and encouraging appropriate screening for HCC and liver cirrhosis, hepatitis C management is both complex and expensive in the developing world. Standard treatment protocols present challenges in Botswana and other resource-limited settings, where HCV RNA assays are generally not available and the high costs of pegylated interferon and ribavirin and the necessary associated monitoring required during treatment prohibit standard HCV management. Given the very low HCV IgG rates found in this and other regional studies and the lack of available treatment in these contexts, there seems to be little justification for recommending the addition of routine hepatitis C screening to Botswana's HIV management guidelines or other regional guidelines.
Because the prevalence of occult hepatitis B infection with a negative HBsAg but detectable HBV DNA is higher in HIV-infected individuals, HBsAg testing alone can miss occult HBV infections.7 Accordingly, the additional prevalence and clinical significance of occult hepatitis B coinfection merits additional study. Other aspects of HBV and HCV coinfection with HIV in resource-limited settings that merit additional study include the effect of TDF-FTC–containing ART regimens in pregnancy on perinatal HBV transmission, the morbidity associated with reactivation hepatitis in the setting of intermittent poor adherence to TDF- and/or 3TC/FTC-containing ART, and the changes in HBV coinfection prevalence created by the recent adoption of universal infant HBV vaccination in some countries in many resource-limited settings.
ACKNOWLEDGMENTS
The authors would like to acknowledge Professors Gordon Schutze and Mark Kline, who gave valuable editorial input on the manuscript. We would also like to thank Dr. Jeff Zsohar and the other members of the Baylor College of Medicine International Pediatric AIDS Initiative and medical officer corps, who have overseen and continue to manage the Family Medical Clinic at the Botswana-Baylor Children's Clinical Centre of Excellence-Botswana. The authors thank the Centre's patients and clinical team as well as the Government of Botswana for its support of Baylor activities in Botswana. These findings were presented in abstract form at the XVIII International AIDS Conference in Vienna, Austria (July 18–23, 2010).
Footnotes
Authors' addresses: Premal Patel, Michael Tolle, Vincent Mabikwa, and Gabriel Anabwani, Botswana-Baylor Children's Clinical Centre of Excellence, Gaborone, Botswana, E-mails: premal33@gmail.com, tolle@bcm.edu, vmabikwa@baylorbotswana.org.bw and ganabwani@baylorbotswana.org.bw. Stephanie Davis, Johns Hopkins School of Public Health, Baltimore, MD, E-mail: sd135626@gmail.com.
Reprint requests: Michael Tolle, Botswana-Baylor Children's Clinical Centre of Excellence, Hospital Way, Plot 1836, Gaborone, Botswana, E-mail: tolle@bcm.edu.
References
- 1.Modi AA, Feld JJ. Viral hepatitis and HIV in Africa. AIDS Rev. 2007;9:25–39. [PubMed] [Google Scholar]
- 2.Kiire CF. The epidemiology and prophylaxis of Hepatitis B in sub-Saharan Africa: a view from tropical and subtropical Africa. Gut. 1996;38((Suppl 2)):S5–S12. doi: 10.1136/gut.38.suppl_2.s5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Opaleye OO, Zakariyahu TO, Tijani BA, Bakarey AS. HBV, HCV co-infection among blood donors in Nigeria. Indian J Pathol Microbiol. 2010;53:182–183. doi: 10.4103/0377-4929.59229. [DOI] [PubMed] [Google Scholar]
- 4.Diarra A, Kouriba B, Baby M, Murphy E, Lefrere JJ. HIV, HCV, HBV and syphilis rate of positive donations among blood donations in Mali: lower rates among volunteer blood donors. Transfus Clin Biol. 2009;16:444–447. doi: 10.1016/j.tracli.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Bwogi J, Braka F, Makumbi I, Mishra V, Bakamutumaho B, Nanyunja M, Opio A, Downing R, Biryahwaho B, Lewis R. Hepatitis B infection is highly endemic in Uganda: findings from a national serosurvey. Afr Health Sci. 2009;9:98–108. [PMC free article] [PubMed] [Google Scholar]
- 6.Tagny C, Diarra A, Yahaya R, Hakizimana M, Nguessan A, Mbensa G, Nébié Y, Dahourou H, Mbanya D, Shiboski C, Murphy E, Lefrère J. Characteristics of blood donors and donated blood in sub-Saharan Francophone Africa. Transfusion. 2009;49:1592–1599. doi: 10.1111/j.1537-2995.2009.02137.x. [DOI] [PubMed] [Google Scholar]
- 7.Burnett R, Francois G, Kew M, Leroux-Roels G, Meheus A, Hoosen AA, Mphahlele MJ. Hepatitis B virus and human immunodeficiency virus co-infection in sub-Saharan Africa: a call for further investigation. Liver Int. 2005;25:201–213. doi: 10.1111/j.1478-3231.2005.01054.x. [DOI] [PubMed] [Google Scholar]
- 8.Nacro B, Dao B, Dahourour H. HBs antigen in children with suspicion of HIV infection. J Trop Pediatr. 2001;47:303–304. doi: 10.1093/tropej/47.5.303. [DOI] [PubMed] [Google Scholar]
- 9.Combe P, La Ruche G, Bonard D, Ouassa T, Faye-Kette H, Sylla-Koko F, Dabis F. Hepatitis B and C infections, human immunodeficiency virus and other sexually transmitted infections among women of childbearing age in Cote d'Ivoire, West Africa. Trans R Soc Trop Med Hyg. 2001;95:493–496. doi: 10.1016/s0035-9203(01)90015-x. [DOI] [PubMed] [Google Scholar]
- 10.Sutcliffe S, Taha TE, Kumwenda NI, Taylor E, Liomba GN. HIV-1 prevalence and herpes simplex virus 2, hepatitis C virus, and hepatitis B virus infections among male workers at a sugar estate in Malawi. J Acquir Immune Defic Syndr. 2002;95:493–496. doi: 10.1097/00126334-200209010-00012. [DOI] [PubMed] [Google Scholar]
- 11.Adewole OO, Anteyi E, Ajuwon Z, Wada I, Elegba F, Ahmed P, Betiku Y, Okpe A, Eze S, Ogbeche T, Erhabor GE. Hepatitis B and C virus co-infection in Nigerian patients with HIV infection. J Infect Dev Ctries. 2009;3:369–375. doi: 10.3855/jidc.245. [DOI] [PubMed] [Google Scholar]
- 12.Nagu TJ, Bakari M, Matee M. Hepatitis A, B and C viral co-infections among HIV-infected adults presenting for care and treatment at Muhimbili National Hospital in Dar es Salaam, Tanzania. BMC Public Health. 2008;19:416. doi: 10.1186/1471-2458-8-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kew MC, Kassianides C, Berger EL, Song E, Dusheiko GM. Prevalence of chronic hepatitis virus infection in pregnant women living in Soweto. J Med Virol. 1987;22:263–268. doi: 10.1002/jmv.1890220310. [DOI] [PubMed] [Google Scholar]
- 14.Vos GH, Rose EF, Marimuthu T. Hepatitis B antigen and antibodies in rural and urban southern African blacks. S Afr J Med. 1984;57:868–870. [PubMed] [Google Scholar]
- 15.Lodenyo H, Schoup B, Ally R, Kairu S, Segal I. Hepatitis B and C virus infections and liver function in AIDS patients at Chris Hani Baragwanath Hospital, Johannesburg. East Afr Med J. 2000;77:13–15. doi: 10.4314/eamj.v77i1.46369. [DOI] [PubMed] [Google Scholar]
- 16.Firnhaber CS, Reyneke A, Schulze D, Malope B, Maskew M, Macphail P, Sanne I, Biscerglie A. The prevalence of hepatitis B co-infection in a South African urban government HIV clinic. S Afr Med J. 2008;98:541–544. [PMC free article] [PubMed] [Google Scholar]
- 17.Wester W, Bussmann H, Moyo S, Avalos A, Gaolathe T, Ndwapi N, Essex M, MacGregor RR, Marlink R. Serological evidence of HIV-associated infection among HIV-1–infected adults in Botswana. Clin Infect Dis. 2006;14:1612–1615. doi: 10.1086/508865. [DOI] [PubMed] [Google Scholar]
- 18.Tedla Z, Nyirenda S, Peeler C, Agizew T, Sibanda T, Motsamai O, Vernon A, Wells CD, Samandari T. Isoniazid-associated hepatitis and antiretroviral drugs during tuberculosis prophylaxis in HIV-infected adults in Botswana. Am J Respir Crit Care Med. 2010;182:278–285. doi: 10.1164/rccm.200911-1783OC. [DOI] [PubMed] [Google Scholar]
- 19.Byskov AJS, Wouters M, Sathekge TJ, Swanepoel R. An outbreak of suspected water-borne epidemic non-A non-B hepatitis in northern Botswana with a high prevalence of hepatitis B carriers and hepatitis delta markers among patients. Trans R Soc Trop Med Hyg. 1989;83:110–116. doi: 10.1016/0035-9203(89)90731-1. [DOI] [PubMed] [Google Scholar]
- 20.Mulwa JKM. Hepatitis B situation in Botswana. J Prev Med Hyg. 1994;35:67. [Google Scholar]
- 21.Nurse GT, Tanaka N, MacNab G, Jenkins T. Non-venereal syphilis and Australia antigen among the G/Wi and G-Ana San of the Central Kalahari Reserve, Botswana. Cent Afr J Med. 1973;19:10. [PubMed] [Google Scholar]
- 22.Dibisceglie AM, Kew MC, Dusheiko GM, Berger EL, Song E, Paterson AC, Hodkinson HJ. Prevalence of hepatitis B virus infection among black children in Soweto. Br Med J (Clin Res Ed) 1986;292:1440–1442. doi: 10.1136/bmj.292.6533.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madhava V, Burgess C, Drucker E. Epidemiology of chronic hepatitis C virus infection in sub-Saharan Africa. Lancet Infect Dis. 2002;2:293–302. doi: 10.1016/s1473-3099(02)00264-5. [DOI] [PubMed] [Google Scholar]
- 24.Kallestrup P, Zinyama R, Gomo E. Low prevalence of HCV antibodies in HIV-endemic area of Zimbabwe support sexual transmission as the major route of HIV transmission in Africa. AIDS. 2003;17:1400–1402. doi: 10.1097/00002030-200306130-00019. [DOI] [PubMed] [Google Scholar]
- 25.Patel P, Davis S, Tolle M, Anabwani G. The Family Medical Clinic (FMC) at the Botswana-Baylor Children's Clinical Centre of Excellence (BBCCCOE) in Gaborone, Botswana—A model of care. Vienna, Austria: 2010. Proceedings of the 19th International AIDS Conference, July 18–23, 2010. [Google Scholar]
- 26.Aberg J, Kaplan J, Libman H, Emmanuel P, Anderson J, Stone V, Oleske J, Currier J, Gallant J. IDSA guidelines: primary care guidelines for the management of persons infected with human immunodeficiency virus: 2009 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:659. doi: 10.1086/605292. [DOI] [PubMed] [Google Scholar]
- 27.Department of Health and Human Services Panel on Antiretroviral Guidelines for Adults and Adolescents: Guidelines for the Use of Antiretroviral Agents in HIV-1–Infected Adults and Adolescents. 2009. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf Available at. Accessed September 6, 2010.
- 28.European AIDS Clinical Society . Guidelines Clinical Management and Treatment of Chronic Hepatitis B and Hepatitis C Coinfection in HIV-Infected Adults. 2010. http://www.europeanaidsclinicalsociety.org/guidelinespdf/1_Treatment_of_HIV_Infected_Adults.pdf Available at. Accessed September 6, 2010. [Google Scholar]
- 29.Ministry of Health Department of HIV/AIDS Prevention and Care . Botswana National HIV/AIDS Treatment Guidelines, 2008 Version. 2008. http://www.aidstarone.com/botswana_national_hivaids_treatment_guidelines_2008_version Available at. Accessed September 6, 2010. [Google Scholar]
- 30.World Health Organization Essential Prevention and Care Interventions for Adults and Adolescents Living with HIV in Resource-Limited Settings. 2008. http://www.who.int/hiv/pub/toolkits/Essential%20Prevention%20and%20Care%20interventioni%20Jan%2008.pdf Available at. Accessed September 6, 2010.
- 31.Tolle MA. A package of primary health care services for comprehensive family-centred HIV/AIDS care and treatment programs in low-income settings. Trop Med Int Health. 2009;14:663–672. doi: 10.1111/j.1365-3156.2009.02282.x. [DOI] [PubMed] [Google Scholar]
- 32.Department of Health Republic of South Africa The South African Antiretroviral Treatment Guidelines 2010. 2010. http://www.sanac.org.za/documents/2010%20ART%20Guideline-Short.pdf Available at. Accessed August 10, 2010.
- 33.Firnhaber CS, Ive P. Hepatitis B and HIV co-infection in South Africa: just treat it. Southern African Journal of HIV Medicine. 2009;10:10–14. [Google Scholar]
- 34.World Health Organization Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for a Public Health Approach, 2010 revision. 2010. http://whqlibdoc.who.int/publications/2010/9789241599764_eng.pdf Available at. Accessed September 6, 2010. [PubMed]
- 35.Thio CL, Locarnini S. Treatment of HIV/HBV coinfection: clinical and virologic issues. AIDS Rev. 2007;9:40–53. [PubMed] [Google Scholar]
- 36.Nuesch R, Ananworanich J, Srasuebkul P, Klinbuayam W, Mahanontharit A, Jupimai T, Ruxrungtham K, Hirshcel B. Interruptions of tenofovir/emtricitabine-based antiretroviral therapy in patients with HIV/hepatitis B virus co-infection. AIDS. 2008;22:152–154. doi: 10.1097/QAD.0b013e3282f303bf. [DOI] [PubMed] [Google Scholar]
- 37.Baleta A. Botswana reduces mother-to-child transmission of HIV. Lancet. 2010;375:1954. doi: 10.1016/s0140-6736(10)60909-9. [DOI] [PubMed] [Google Scholar]
- 38.Read JS, Cannon MJ, Stanberry LR, Schuval S. Prevention of mother-to-child transmission of viral infections. Curr Probl Pediatr Adolesc Health Care. 2008;38:274–297. doi: 10.1016/j.cppeds.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 39.Puoti M, Torti C, Bruno R, Filice G, Carosi G. Natural history of chronic hepatitis B in co-infected patients. J Hepatol. 2006;44((Suppl 1)):S65–S70. doi: 10.1016/j.jhep.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 40.Wang Z, Zhang J, Yang H, Li X, Wen S, Guo Y, Sun J, Hou J. Quantitative analysis of HBV DNA level and HBeAg titer in Hepatitis B surface antigen positive mothers and their babies: HBeAg passage through the placenta and the rate of decay in babies. J Med Virol. 2003;71:360–366. doi: 10.1002/jmv.10493. [DOI] [PubMed] [Google Scholar]
- 41.Sangaré L, Sombié R, Combasséré AW, Kouanda A, Kania D, Zerbo O, Lankoandé J. Antenatal transmission of hepatitis B virus in an area of HIV moderate prevalence, Burkina Faso. Bull Soc Pathol Exot. 2009;102:226–229. [PubMed] [Google Scholar]


