Abstract
Bronchoconstriction has been reported in asthma and chronic obstructive pulmonary disease (COPD) patients after administration of some aqueous inhalation solutions. We investigated the incidence of this event during long-term clinical trials of tiotropium delivered via Respimat® Soft Mist™ Inhaler (SMI). We retrospectively analyzed pooled data from two identical Phase III clinical trials, in which 1990 patients with COPD received 48 weeks’ treatment with once-daily tiotropium (5 or 10 μg) or placebo inhaled via Respimat® SMI. We recorded the incidence of bronchospasm and of a range of respiratory events that could suggest bronchoconstriction during the first 30 minutes after inhalation of study treatment on each of the eight test days. No patients reported bronchospasm. Six patients (0.3%) reported a combination of at least two events suggestive of bronchoconstriction, and 21 (1.1%) reported either rescue medication use or a respiratory adverse event. Asymptomatic falls in forced expiratory volume in one second (FEV1) of ≥15% were recorded on all test days, with no change in incidence over time, and affected 8.2% of those in the tiotropium groups and 14.5% of those on placebo. In COPD patients receiving long-term treatment with tiotropium 5 or 10 μg via Respimat® SMI, no bronchospasm was recorded, and the number of events possibly indicative of paradoxical bronchoconstriction was very low.
Keywords: inhalation device, bronchoconstriction, COPD, tiotropium
Introduction
Aqueous aerosol delivery via nebulizers has been an important element in the treatment and prophylaxis of many obstructive lung diseases, particularly in emergency settings. More recently, the development of smaller portable inhaler devices that deliver small doses of aqueous aerosols has expanded the therapeutic options available for the everyday treatment of chronic obstructive pulmonary disease (COPD) and asthma. One such device is Respimat® Soft Mist™ Inhaler (SMI), a novel multidose metered-dose inhaler that uses mechanical energy instead of fluorocarbon propellants to deliver a fine and slow-moving aerosol spray.1,2 The device allows inhaled drug to be more efficiently delivered to the lungs than the pressurized metered-dose inhaler (pMDI),3 which enables a lower nominal dose of bronchodilator to be delivered.4,5 Currently, two bronchodilators are approved for marketing in the Respimat® SMI formulation, the long-acting anticholinergic tiotropium (Spiriva®) and a combination of ipratropium and fenoterol (Berodual®).
The purpose of the present analysis was to investigate whether inhalation of tiotropium aqueous solution via Respimat® SMI is associated with administration-related bronchoconstriction in patients with COPD. This phenomenon has been called paradoxical bronchoconstriction (or paradoxical bronchospasm),6 and describes respiratory events that happen shortly after administration of a bronchodilator drug, suggesting an effect opposite to what would be expected. The clearest manifestation of this phenomenon is defined as the presence of spontaneously reported respiratory symptoms classified according to World Health Organization (WHO) preferred terms as ‘bronchospasm’, ‘bronchospasm aggravated’ or ‘bronchospasm paradoxical’. Patients may also present with symptoms such as coughing, wheezing and dyspnea, possibly accompanied by the need for administration of rescue medication.7,8 Paradoxical bronchoconstriction may sometimes be suggested by a fall in forced expiratory volume in one second (FEV1) without any other symptoms.9 This phenomenon could have several potential causes including an adverse reaction either to the drug administered or to other constituents of the aerosol such as preservatives, surfactants, stabilizers or propellants, solution osmolarity, or pH.10 A fall in FEV1 without symptoms may also reflect air-flow turbulence, and some investigators have suggested that repeated spirometry efforts may trigger bronchoconstriction, as evidenced by a fall in FEV1 with successive attempts.11–13 In this context, a fall in FEV1 without symptoms likely does not reflect an adverse response to an inhaled substance, but rather is the result of the forced exhalation maneuver itself.
The aqueous drug formulation delivered by Respimat® SMI contains two excipients, ethylene diamine tetra-acetic acid (EDTA) and benzalkonium chloride (BAC), which act as stabilizers and preservatives and have been reported to induce bronchoconstriction in patients when administered by nebulisers.6,14 In pooled analyses of studies in which a range of short-acting bronchodilators were given as single and repeated doses from Respimat® SMI to asthma and COPD patients, no bronchospasm was recorded and levels of paradoxical bronchoconstriction were very low.15,16 In asthma patients with documented hyperreactivity, four inhalations of a placebo equivalent of the tiotropium formulation (the usual dose for COPD is only two inhalations) did not produce paradoxical bronchoconstriction and was associated with less cough than a placebo chlorofluorocarbon (CFC)-pMDI,17 supporting results of an earlier study that showed no difference in the degree of fall in FEV1 between placebo aqueous or ethanolic inhalations and normal saline (all delivered via Respimat® SMI).18
This retrospective analysis used pooled data from nearly 2000 COPD patients who participated in two randomized, placebo-controlled, clinical trials that used identical protocols, and whose main objective was to assess the efficacy and safety of 48 weeks’ treatment with tiotropium delivered via Respimat® SMI. A pooled analysis of the primary outcomes of these two studies has already been reported.19 The aim of the study was to quantify paradoxical bronchoconstriction and grade adverse events that could indicate the occurrence of this phenomenon after administration of tiotropium via Respimat® SMI.
Materials and methods
Study details
The present study is a retrospective analysis of pooled data from two international clinical trials in which 1990 patients with COPD received 48 weeks’ treatment with once-daily tiotropium (5 or 10 μg; Boehringer Ingelheim, Ingelheim, Germany) or placebo, inhaled via Respimat® SMI (Boehringer Ingelheim, Ingelheim, Germany).19 Both active and placebo formulations contained EDTA and BAC. The two trials (NCT00168831 and 00168844) followed the same randomized, double-blind, parallel-group design, and their protocols were approved by the appropriate ethics review bodies. The three treatment arms were well balanced with respect to patient characteristics: the mean age of all patients was 65.0 years, they had a mean pre-bronchodilator FEV1 of 1.06 L (37.7% of predicted normal) and a mean ratio of pre-bronchodilator FEV1 to forced vital capacity (FVC) of 0.42 (Table 1).19
Table 1.
Baseline characteristics of the 1990 patients with COPD in the present study
| Characteristic | Tiotropium Respimat® 5 μg (n = 670) | Tiotropium Respimat® 10 μg (n = 667) | Placebo Respimat® (n = 653) |
|---|---|---|---|
| Number of men | 491 (73.3%) | 498 (74.7%) | 487 (74.6%) |
| Age (years) | 64.7 (8.6) | 65.1 (8.5) | 65.2 (8.7) |
| Duration of COPD (years) | 8.3 (6.4) | 9.0 (7.4) | 9.5 (7.5) |
| Pre-bronchodilator FEV1 (L) | 1.066 (0.398) | 1.065 (0.403) | 1.058 (0.388) |
| Pre-bronchodilator FEV1 (% predicted normal) | 38.0 (11.7) | 37.7 (11.7) | 37.5 (11.6) |
| FEV1/FVC ratio | 0.42 (0.11) | 0.42 (0.11) | 0.42 (0.11) |
| FEV1 reversibility to salbutamol (%)a | 19.9 (21.4) | 19.4 (18.1) | 20.8 (37.4) |
| Current smokers (%) | 37.9 | 34.8 | 36.1 |
Notes: Other than numbers and/or proportions, data shown are means (and standard deviation).
Value from screening visit.
Abbreviations: COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity.
Tolerability assessments
We recorded the incidence in the three treatment arms of pre-defined respiratory events that occurred during the 30 minutes immediately following inhalation of study treatment on each of the eight test days during the two trials (days 1, 15, 57, 113, 169, 225, 281, and 337). The events of interest were defined as follows:
Bronchospasm or aggravated bronchospasm, a spontaneously reported adverse event which was recorded by the investigator as “aggravated” or “paradoxical” bronchospasm.
Any respiratory adverse event other than bronchospasm that could indicate administration-related bronchoconstriction. These were recorded under a search category in MedDRA (Medical Dictionary for Regulatory Activities) that was defined specifically for the two trials and are referred to hereafter as administration-related bronchoconstriction indicators (ARBIs). These comprised 28 different MedDRA preferred terms such as dyspnea, wheezing, cough, and chest discomfort (Table 2).
Documented use of rescue medication (salbutamol pMDI 100 μg per inhalation).
A fall of ≥15% in FEV1 below test-day pre-dose value without other symptoms and without the need for rescue medication.
Table 2.
List of MedDRA preferred terms (other than bronchospasm and bronchospasm paradoxical) that could possibly indicate ARB
|
Abbreviations: ARB, administration-related bronchoconstriction; COPD, chronic obstructive pulmonary disease; MedDRA, Medical Dictionary for Regulatory Activities.
FEV1 was measured as part of the planned spirometry assessments performed on each test day in the two trials. No bronchodilator (other than the study treatment) was administered during FEV1 measurements, which were performed 10 minutes before study treatment administration, then 5 and 30 minutes and 1, 2, and 3 hours afterwards.
As some of the above events are more suggestive of administration-related bronchoconstriction than others, four mutually exclusive categories (A–D) were defined (A = most suggestive, D = least suggestive) as follows.
Category A – Aggravated bronchospasm (with or without other events).
Category B – A combination of at least two of these three events: rescue medication use, ARBIs, and an asymptomatic fall in FEV1 of ≥15% from test-day pre-dose value.
Category C – Either rescue medication use or ARBIs (but not both).
Category D – Asymptomatic fall in FEV1 of ≥15% from test-day pre-dose value, not requiring rescue medication.
Statistical analysis
Data from the two trials were pooled and grouped by treatment arm. Within each of the four categories, the number of patients who experienced qualifying events on one or more test days was totaled. The number of patients reporting events on each test day was also recorded, to examine any temporal trend over the treatment period. A post-hoc analysis was performed to compare whether the overall severity of events differed between treatments, comparing all patients in the two tiotropium groups with patients in the placebo group by ordinal logistic regression.
Results
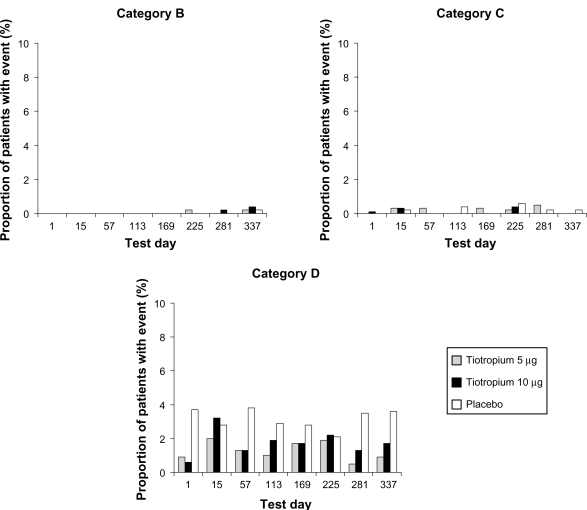
The number of patients who reported each category of event on one or more test days is shown in Table 3. (Note: Patients appear in one category only – the worst category of event experienced.) The number who reported Category B, C, and D events (as a proportion of all patients tested on each day) are shown in Figure 1. As patients withdrew from the study for various reasons during the 48-week treatment period, the numbers of patients tested on each test day gradually declined as the study progressed (Table 4).
Table 3.
Number of patients (and % of total) with respiratory events suggestive of administration-related bronchoconstriction during the 30 minutes immediately after inhalation of study treatment on at least one test day. Patients are grouped according to the worst category of event experienced. See text for detailed definition of categories
| Number of test days on which event occurred | Tiotropium Respimat® 5 μg (n = 670) | Tiotropium Respimat® 10 μg (n = 667) | Placebo Respimat® (n = 653) | |
|---|---|---|---|---|
| Number of patient tests (n) | 4850 | 4731 | 4232 | |
| Category A (aggravated bronchospasm) | At least 1 | 0 (0) | 0 (0) | 0 (0) |
| Category B (two or more of rescue use, ARBI, and 15% fall in FEV1) | At least 1 | 2 (0.3) | 3 (0.4) | 1 (0.2) |
| 1 | 2 | 3 | 1 | |
| Category C (either rescue use or ARBI) | At least 1 | 8 (1.2) | 5 (0.7) | 8 (1.2) |
| 1 | 7 | 5 | 8 | |
| 2 | 0 | 0 | 0 | |
| 3 | 1 | 0 | 0 | |
| Category D (15% fall in FEV1) | At least 1 | 45 (6.7) | 64 (9.6) | 95 (14.5) |
| 1 | 33 | 52 | 67 | |
| 2 | 8 | 8 | 20 | |
| 3 | 4 | 4 | 7 | |
| 4 | 0 | 0 | 1 | |
| None of the above | At least 1 | 615 (91.8) | 595 (89.2) | 549 (84.1) |
| 1 | 14 | 13 | 37 | |
| 2 | 23 | 30 | 53 | |
| 3 | 21 | 25 | 25 | |
| 4 | 11 | 17 | 22 | |
| 5 | 18 | 17 | 20 | |
| 6 | 1 | 12 | 19 | |
| 7 | 17 | 23 | 11 | |
| 8 | 501 | 458 | 362 |
Abbreviations: ARBI, administration-related bronchoconstriction indicator; FEV1, forced expiratory volume in one second.
Figure 1.
Proportion of patients (%) with respiratory events suggestive of administration-related bronchoconstriction during the 30 minutes immediately after inhalation of study treatment on each test day. Events are shown by treatment for Categories B–D (no Category A events occurred), and patients are grouped according to the worst category of event experienced. The number of patients tested on each day fell as the study progressed (see Table 4). See text for definition of categories.
Table 4.
Number of patients tested on each test day
| Test day | Tiotropium Respimat® 5 μg (n = 670) | Tiotropium Respimat® 10 μg (n = 667) | Placebo Respimat® (n = 653) |
|---|---|---|---|
| 1 | 670 | 667 | 653 |
| 15 | 649 | 647 | 604 |
| 57 | 628 | 620 | 549 |
| 113 | 608 | 590 | 524 |
| 169 | 593 | 572 | 496 |
| 225 | 576 | 554 | 476 |
| 281 | 564 | 539 | 458 |
| 337 | 562 | 542 | 472 |
No patients reported aggravated bronchospasm (Category A). Category B events were very uncommon, being experienced by six patients (0.3%) – two and three patients in the tiotropium 5 and 10 μg groups, respectively, and one in the placebo group. The events were recorded during the later stages of the two studies (days 225, 281, and 337). Despite being recorded throughout the studies, Category C events were also uncommon. They were experienced by no more than three patients in any treatment group on any test day (≤0.6%) and the total incidence in the two active treatment groups (13/1337; 1.0%) was very similar to that in the placebo group (8/653; 1.2%). There was no indication of a relationship between adverse event incidence and tiotropium dose. Category D events (asymptomatic falls in FEV1 of ≥15%) were experienced throughout the studies, with no evidence of a change in incidence over time. They affected 45/670 (6.7%) in the 5 μg group and 64/667 (9.6%) in the 10 μg group (total incidence in tiotropium groups, 8.2%) and 95/653 (14.5%) in the placebo group.
A post-hoc analysis showed that the odds of experiencing a less severe event were significantly higher in patients in the two tiotropium groups combined than in patients in the placebo group (odds ratio = 3.25, 95% confidence interval = 1.86–5.68; P < 0.0001).
Discussion
This retrospective analysis shows that events which are suggestive of administration-related bronchoconstriction are rare in COPD patients receiving tiotropium delivered via Respimat® SMI for 48 weeks in a clinical trial setting. No patients reported bronchospasm (Category A), and only six (0.3%) and 21 (1.1%) of 1990 patients, respectively, reported Category B and C events (which, after bronchospasm, were the next most important categories of events that could suggest administration-related bronchoconstriction). The most commonly occurring events were asymptomatic falls in FEV1 of ≥15%, the category which is the least suggestive of paradoxical bronchoconstriction. In the clinical trials included in this analysis, pulmonary function tests were performed in triplicate to establish reproducibility. Some studies have suggested that repeated spirometry efforts may act as a trigger for bronchoconstriction, as evidenced by a fall in FEV1 with successive attempts.11–13 Consequently, the incidence of Category D events reported in this analysis may not reflect administration-induced paradoxical bronchoconstriction alone, but may in part be due to repeated spirometric maneuvers.
The amounts of BAC and EDTA present in aqueous solutions delivered by Respimat® SMI are much lower (by a factor of ≥500) than the doses reported to induce bronchoconstriction when inhaled from a nebulizer.6,14 The tiotropium solution in Respimat® SMI contains 100 μg/mL each of EDTA and BAC, which corresponds to about 1 μg of each excipient per actuation (volume per actuation = 11 μL). Thus, based on a lung deposition fraction of approximately 40% of the inhaled dose, as achieved in healthy volunteers who inhaled an aqueous formulation similar to that in tiotropium Respimat®,20 the approximate lung exposure of EDTA and BAC from a single actuation would be 0.40 μg each. Indeed, in single- and repeated-dose studies in which short-acting bronchodilators were inhaled from Respimat® SMI by patients with asthma or COPD, the incidence of adverse reactions was very similar to that recorded with pMDI formulations, which contain no BAC or EDTA, and the reactions were limited mainly to an asymptomatic decline in FEV1.15,16
Although the tiotropium solution in Respimat® SMI contains five times less EDTA than the corresponding ipratropium/fenoterol formulation, twice as much BAC (0.80 μg) is delivered at each dosing point with tiotropium as with ipratropium/fenoterol because the standard dose of tiotropium requires two actuations from Respimat® SMI, as opposed to only one actuation for the ipratropium/fenoterol formulation. This, and the fact that the tiotropium solution is more acidic than the ipratropium/fenoterol formulation (pH values of 2.9 and 3.4 respectively), could increase its potential to cause bronchial irritation. In spite of this, our analysis shows that administration-related bronchoconstriction is uncommon when tiotropium is administered via Respimat® SMI for 48 weeks in COPD patients. Our finding is consistent with results of studies in hyperreactive asthma patients who were given four inhalations of a placebo equivalent of the tiotropium formulation from Respimat® SMI (twice the usual tiotropium dose).17,18
In the context of our analysis, the placebo group from the clinical trial is not a true placebo group, as despite the absence of tiotropium, the formulation still contained the same amount of BAC and EDTA as in the two tiotropium inhalers. Nevertheless, the placebo data do provide useful insights. For example, the difference in incidence of asymptomatic FEV1 falls between tiotropium and placebo (8.2% and 14.5%, respectively), suggesting that the presence of the bronchodilator provides a measure of protection against any administration-related bronchoconstriction, in agreement with a previous retrospective analysis of clinical trials of ipratropium/fenoterol and ipratropium.16 When the average incidence of events over all test days in our analysis are calculated and compared, the frequency of asymptomatic falls in FEV1 (Category D) for tiotropium (1.5%) is very similar to values reported from studies in asthma and COPD patients that measured the incidence of falls in either FEV1 or peak expiratory flow soon after inhalation from pMDIs.21,22 Moreover, the values of 1.5% for tiotropium and 3.15% for placebo in our analysis are slightly lower than those previously reported for COPD patients (2% for active and 5.6% for placebo).16
These observations suggest that the incidence of events indicative of paradoxical bronchoconstriction in COPD patients who receive long-term treatment with tiotropium 5 or 10 μg via Respimat® SMI is very low.
Acknowledgments
Medical writing support for this article was provided by Roger Nutter at Lexeme, Chester, UK, and funding for this support was provided by Boehringer Ingelheim. This study was funded by Boehringer Ingelheim.
Footnotes
Clinical trial registration numbers
NCT00168831 and NCT00168844.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Dalby R, Spallek M, Voshaar T. A review of the development of Respimat® Soft Mist™ Inhaler. Int J Pharm. 2004;283:1–9. doi: 10.1016/j.ijpharm.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 2.Hochrainer D, Hölz H, Kreher C, Scaffidi L, Spallek M, Wachtel H. Comparison of the aerosol velocity and spray duration of Respimat® Soft Mist™ Inhaler and pressurized metered dose inhalers. J Aerosol Med. 2005;18:273–282. doi: 10.1089/jam.2005.18.273. [DOI] [PubMed] [Google Scholar]
- 3.Newman SP. Use of gamma scintigraphy to evaluate the performance of new inhalers. J Aerosol Med. 1999;12(Suppl 1):S25–S31. doi: 10.1089/jam.1999.12.suppl_1.s-25. [DOI] [PubMed] [Google Scholar]
- 4.Kilfeather SA, Ponitz HH, Beck E, Schmidt P, Lee A, Bowen I, et al. Improved delivery of ipratropium bromide/fenoterol from Respimat® Soft Mist™ Inhaler in patients with COPD. Respir Med. 2004;98:387–397. doi: 10.1016/j.rmed.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Von Berg A, Jeena PM, Soemantri PA, et al. Efficacy and safety of ipratropium bromide plus fenoterol inhaled via Respimat® Soft Mist™ inhaler versus a conventional metered dose inhaler plus spacer in children with asthma. Pediatr Pulmonol. 2004;37:264–272. doi: 10.1002/ppul.10428. [DOI] [PubMed] [Google Scholar]
- 6.Beasley CRW, Rafferty P, Holgate ST. Bronchoconstrictor properties of preservatives in ipratropium bromide (Atrovent) nebuliser solution [letter] BMJ. 1987;294:1197–1198. doi: 10.1136/bmj.294.6581.1197-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cocchetto DM, Sykes RS, Spector S. Paradoxical bronchoconstriction after use of inhalation aerosols: a review of the literature. J Asthma. 1991;28:49–53. doi: 10.3109/02770909109073370. [DOI] [PubMed] [Google Scholar]
- 8.Nicklas RA. Paradoxical bronchospasm associated with the use of inhaled beta agonists. J Allergy Clin Immunol. 1990;85:959–964. doi: 10.1016/0091-6749(90)90084-h. [DOI] [PubMed] [Google Scholar]
- 9.Yarbrough J, Mansfield LE, Ting S. Metered-dose inhaler induced bronchospasm in asthmatic patients. Ann Allergy. 1985;55:25–27. [PubMed] [Google Scholar]
- 10.Pavia D, Moonen D. Preliminary data from phase III studies with Respimat, a propellant-free soft mist inhaler. J Aerosol Med. 1999;12(Suppl 1):S33–S39. doi: 10.1089/jam.1999.12.suppl_1.s-33. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki S, Miyashita A, Matsumoto Y, Okubo T. Bronchoconstriction induced by spirometric manoeuvres in patients with bronchial asthma. Ann Allergy. 1990;65:315–320. [PubMed] [Google Scholar]
- 12.Mackay AD, Mustchin CP, Sterling GM. The response of asthmatic patients and normal subjects to maximum respiratory manoeuvres. Spirometry-induced bronchoconstriction. Eur J Respir Dis Suppl. 1980;106:35–40. [PubMed] [Google Scholar]
- 13.Roncoroni AJ, Goldman E, Puy RJ, Mancino M. Bronchoconstriction induced by repeated forced vital capacity manoeuvres. Acta Allergol. 1975;30:375–389. doi: 10.1111/j.1398-9995.1975.tb01673.x. [DOI] [PubMed] [Google Scholar]
- 14.Rafferty P, Beasley R, Holgate ST. Comparison of the efficacy of preservative free ipratropium bromide and Atrovent nebuliser solution. Thorax. 1988;43:446–450. doi: 10.1136/thx.43.6.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koehler D, Pavia D, Dewberry H, Hodder R. Low incidence of paradoxical bronchoconstriction with bronchodilator drugs administered by Respimat® Soft Mist™ Inhaler: results of Phase II single-dose crossover studies. Respiration. 2004;71:469–476. doi: 10.1159/000080631. [DOI] [PubMed] [Google Scholar]
- 16.Hodder R, Pavia D, Dewberry H, et al. Low incidence of paradoxical bronchoconstriction in asthma and COPD patients during chronic use of Respimat® Soft Mist™ Inhaler. Respir Med. 2005;99:1087–1095. doi: 10.1016/j.rmed.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 17.Leclerc V, Lafferre M, Pavia D. Acute local tolerability of acidic aqueous vehicles delivered via Respimat® Soft Mist™ Inhaler in hyperreactive asthma patients. Respiration. 2007;74:691–696. doi: 10.1159/000107739. [DOI] [PubMed] [Google Scholar]
- 18.Patel KR, Pavia D, Lowe L, Spiteri M. Inhaled ethanolic and aqueous solutions via Respimat Soft Mist Inhaler are well-tolerated in asthma patients. Respiration. 2006;73:434–440. doi: 10.1159/000089426. [DOI] [PubMed] [Google Scholar]
- 19.Bateman E, Singh D, Smith D, et al. Efficacy and safety of tiotropium Respimat® SMI in COPD in two 1-year randomised studies. Int J Chron Obstruct Pulmon Dis. 2010;5:197–208. [PMC free article] [PubMed] [Google Scholar]
- 20.Newman SP, Brown J, Steed KP, Reader SJ, Kladders H. Lung deposition of fenoterol and flunisolide delivered using a novel device for inhaled medicines: comparison of Respimat with conventional metered-dose inhalers with and without spacer devices. Chest. 1998;113:957–963. doi: 10.1378/chest.113.4.957. [DOI] [PubMed] [Google Scholar]
- 21.Shaheen MZ, Ayres JG, Benincasa C. Incidences of acute decreases in peak expiratory flow following the use of metered-dose inhalers in asthmatic patients. Eur Respir J. 1994;7:2160–2164. doi: 10.1183/09031936.94.07122160. [DOI] [PubMed] [Google Scholar]
- 22.Huchon G, Hofbauer P, Cannizzaro G, Iacono P, Wald F. Comparison of the safety of drug delivery via HFA- and CFC-metered dose inhalers in CAO. Eur Respir J. 2000;15:663–669. doi: 10.1034/j.1399-3003.2000.15d07.x. [DOI] [PubMed] [Google Scholar]