Abstract
A growing body of research has demonstrated the importance of intergroup contact in reducing fear, threat and anxiety in intergroup domains. Here we focus on the regulatory benefits of intergroup contact. We hypothesized that past intergroup contact would facilitate recovery from a stressful intergroup evaluation. White and Black participants completed a stressful evaluative task in the presence of two White or two Black interviewers while autonomic nervous system and hormonal responses were assessed. When examining how participants recovered after the stressful task, intergroup contact predicted faster physiological recovery for both autonomic and neuroendocrine reactivity. The importance of recovery from stress for physiological resilience in diverse contexts is discussed.
Keywords: intergroup contact, recovery, stress, intergroup interaction, intergroup anxiety
A relatively extensive line of intergroup research has focused on intergroup anxiety: The propensity for social interactions with members from a different race category to evoke more anxiety, threat, and stress relative to social interactions between same race-group members (Paolini, Hewstone, Cairns, & Voci, 2004; W. G. Stephan & C. W. Stephan, 1985, 2000). Stress responses that are believed to be potentially harmful or maladaptive seem to be more likely to occur during intergroup interactions than same-race interactions (Blascovich, Mendes, Hunter, Lickel, & Kowai-Bell, 2001; Mendes, Blascovich, Lickel, & Hunter, 2002; Mendes, Gray, Mendoza-Denton, Major, & Epel, 2007; Page-Gould, Mendoza-Denton, & Tropp, 2008). Despite these findings, surprisingly little is known about what happens psychologically and physiologically once an intergroup stressor is over. The emotional and physiological residue of intergroup interactions might be especially important in initiating and shaping future interactions (Trawalter, Richeson, & Shelton, 2009). In the present research, we experimentally manipulate the ingroup or intergroup context and then examine physiological recovery from the interaction as a way to capture the psychological residue of the intergroup context.
Importantly, not all people respond to intergroup interactions with exacerbated distress and physiological reactivity. Arguably one of the most critical moderators in reducing, if not eliminating, intergroup threat is the history of intergroup contact. An original axiom of the contact hypothesis (Allport, 1954) was that contact with outgroup members increases outgroup familiarity (Pettigrew, 1998), thus any added uncertainty associated with intergroup interactions should be reduced among individuals who have intergroup interactions regularly. Recent work tested this axiom abstractly. Mendes and colleagues examined individuals’ responses during social interactions with partners who were counter-stereotypical, and hence less familiar, and observed greater distress and threat responses than social interactions with stereotypical partners who met expectations and were thus more familiar (Mendes, Blascovich, Hunter, Lickel, & Jost, 2007). More concretely, past intergroup contact can reduce distress, threat, or fear. In one study Blascovich and colleagues found that the greater the past interracial contact the lower the threat reactivity during interracial interactions (Blascovich et al., 2001). In another study, participants exposed to a fear-conditioning paradigm in which either same or different race-group faces were paired with electrical shocks showed different patterns of extinction based on their history of intergroup contact. In general, Black and White participants showed longer extinction to outgroup faces relative to participants exposed to ingroup faces during fear conditioning. However, participants who reported past romantic relationships with outgroup members showed extinction patterns similar to the same-race fear conditioning (Olsson, Ebert, Banaji, & Phelps, 2005). That is, in both studies participants with greater intergroup contact exhibited patterns of responses that mirrored same race outcomes.
However, an open question remains of whether intergroup contact facilitates adaptive coping once an intergroup stressor has occurred? We expected that individuals with more intergroup contact would perceive intergroup stressors as more predictable and controllable, thus facilitating coping. Research with humans and rats has consistently found that predictable and controllable stressors facilitate regulation of the neuroendocrine stress system (Stranahan, Lee, & Mattson, 2008). As such, we predicted that intergroup contact would facilitate physiological recovery, a key aspect of physiological regulation, following stressful intergroup interactions.
In the present research, we identified measures of recovery in two stress systems. As an index of recovery from ANS stress responses, we measured changes in respiratory sinus arrhythmia (RSA), a measure of parasympathetic activation that reflects the degree to which the heart rate accelerates and decelerates during the respiratory cycle. During stressful tasks that require attention and mental load, RSA typically decrease. Afterwards, RSA tends to overshoot baseline levels, referred to as vagal rebound. Lack of vagal rebound predicts deterioration of the left ventricular valve, which is a key factor in chronic hypertension (Mezzacappa, Kelsey, Katkin, & Sloan, 2001). Neuroendocrine recovery was measured by comparing cortisol levels during a stressor to post-stress cortisol. Quick neuroendocrine recovery is associated with increased immunity and psychological resilience (Epel, McEwen, & Ickovics, 1998). We hypothesized that, following a stressful intergroup interaction, past intergroup contact would predict greater vagal rebound and faster cortisol decline, indicating effective recovery from the stressor.
Method
We conducted a 2 (stressor context: ingroup or intergroup) × 2 (participant race: Black or White) × Continuous (past intergroup contact) experiment in which we measured physiological changes prior to, during, and following a stressful social interaction.
Participants
Participants were 125 Black and White adults (49.6% Black) recruited from the university study pool and surrounding communities. The sample was 54.4% female, and had a mean age of 28.1 years (range: 19–55; SD = 10.4). Just over half of the participants were college students (57.6%). The non-student sample was predominantly middle-class, with a mean annual household income of $50,000 to $60,000.
Materials and Procedure
Preexperiment
Participants completed a preexperiment survey through an online software service. The survey assessed demographics and intergroup contact. The intergroup contact measure was adapted for non-student samples from Islam and Hewstone’s (1993) scale, and was presented with either Black or White Americans as the target group, depending on the participant’s race. The intergroup contact scale assesses the quantity of contact across a number of situations (e.g., “How much contact have you had with African Americans as neighbors”), and is rated on a 1 (none at all) to 7 (a great deal) Likert scale (α = 0.89).
Laboratory session
Upon arrival at the lab, participants were interviewed for adherence to study guidelines that they should not exercise, eat foods with live cultures, or drink caffeine two hours prior to the laboratory session.
Physiological measures
After thirty minutes, participants provided the baseline saliva sample. Saliva was collected in 2-ml IBL cryovials. Three samples were collected: baseline, after the stressor, and 30 minutes following the stressor. Saliva samples were frozen in a −80°C freezer until they were shipped to be assayed for free salivary cortisol using commercial immunoassay kits (IBL, Hamburg, Germany). Intra- and interassay coefficients of variance were less than 10%.
To monitor autonomic nervous system changes, sensors were applied and participants rested quietly for a 5-minute baseline. Electrocardiograph was measured with two sensors placed on the right arm and left leg (limb lead II configuration) and impedance cardiograph was obtained with four band electrodes placed around the neck and torso. These measures allowed us to assess RSA, and sympathetic activity, pre-ejection period (PEP). PEP is a chronotropic measure of the time between the left ventricle contracting and the aortic valve opening. Biopac MP150 amplification hardware (Goleta, CA) was used to acquire signals at a frequency of 1000 Hz. RSA was calculated through a power spectral analysis of high frequency heart rate variability. All data were cleaned and scored in 1-minute intervals using the Mindware HRV and IMP modules (Gahanna, OH). RSA reactivity was calculated by subtracting baseline RSA from RSA during the TSST, and vagal rebound were calculated by subtracting baseline RSA from RSA during the recovery period.
TSST
To create a psychologically stressful task, we used the Trier social stress test (Kirschbaum, Pirke, & Hellhammer, 1993), which requires participants to prepare and then deliver a 5-minute speech to a panel of “evaluators.” Participants were randomly assigned to group context by nature of their evaluators who were both either Black or White (one man and one woman). During the speech, the evaluators sat ~4 feet away from the participant, and maintained neutral expressions with no behavioral feedback (e.g., no nodding). After the speech the evaluators described the second part of the TSST, which required counting backwards from a 3-digit number by steps of 7 for 5 minutes.
Recovery
After the math task, the evaluators left the room, and the participants rested for a 5-minute recovery period while autonomic responses were measured. Immediately following the 5-minute recovery period, participants provided a second saliva sample reflecting task cortisol. Twenty-five minutes later saliva was collected that served as recovery cortisol.
Results
Preliminary Analyses
Covariate analysis
The two continuous demographic variables (age, income) were significantly positively skewed, Shapiro-Wilkes Wage = 0.83, p < .0001, Shapiro-Wilkes Wincome = 0.84, p < .0001, so they were log-transformed. These two variables were included as covariates, because log age was significantly correlated with vagal rebound, r = .20, p = .03, and log income was significantly correlated with cortisol recovery, r = .17, p = .05. There were no significant differences in the dependent variables based on race or sex of the participants (all |ts| < 1.46). However, Black participants had significantly greater intergroup contact (M = 5.84, SD = 1.25) than White participants (M = 3.95, SD = 1.44), t(124) = 7.84, p < .001, so past contact was standardized within race so that the effects of contact by experimental condition could be compared between racial groups. There was no significant difference in past contact between the ingroup and intergroup conditions, F < 1.
In all analyses, to account for physiological reactivity during the stressor – differences in recovery could be driven by differences in stress reactivity—we included stress reactivity as a covariate. Indeed, stress reactivity was correlated with recovery levels—cortisol during the tasks was significantly negatively correlated with recovery from the tasks, r = −.23, p = .01, and RSA during tasks was significantly positively correlated with vagal rebound, r = .38, p < .0001. We controlled for respiration rate since RSA varies as a function of pulmonary activity.
Task reactivity
Paired t-tests revealed that the TSST activated the sympathetic nervous system as evidenced by significant decreases in PEP from baseline (M = −9.24, SD = 11.85), t(108) = −8.10, p < .001, but sympathetic activation was not moderated by any of the independent variables, all Fs < 1. Participants also showed an overall increase in cortisol (M = 1.38, SD = 5.68), t(123) = 2.69, p = .008, and a decrease in RSA (M = −0.27; SD = 1.36), t(123) = −2.22, p = .029, however we did not observe any effects of the independent variables, all Fs < 1.37.
Primary Analyses
To account for possible shared variance between our dependent variables, analyses were conducted as an omnibus multivariate regression to reduce the likelihood of Type I error, even though the correlation between the dependent variables was low, r(125) = −.013, p = .88. Vagal rebound and cortisol recovery were simultaneously regressed on evaluation condition (ingroup = −1, intergroup = 1), participant race, centered past contact, and their interaction, controlling for the covariates. Participant race did not moderate any of the effects reported below (all Fs < 1).
Neuroendocrine recovery
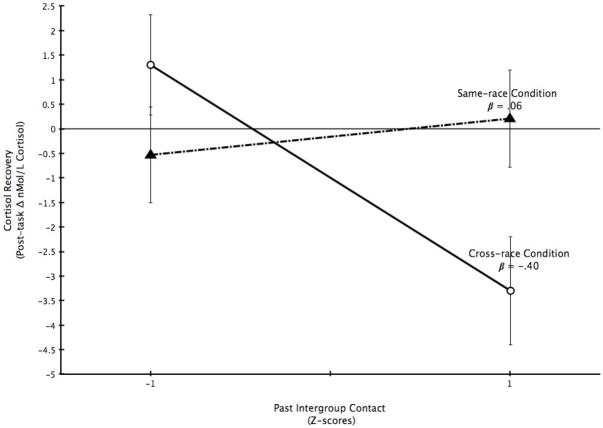
We hypothesized that intergroup contact would predict successful regulation of HPA responses after the stressful interview. There was no main effect of condition, F < 1, and a marginal main effect of contact, F(1, 116) = 3.51, p = .07. This effect was qualified by a significant condition by contact interaction, F(1, 116) = 6.76, p = .01 (Figure 1). Contact predicted significantly steeper cortisol decline from the intergroup stressor, β = −.40, t(116) = −2.83, p = .01, but was unrelated to cortisol recovery after an ingroup stressor, β = .06, t(116) = .59, p = .56. Thus, contact predicted faster recovery of cortisol responses following an intergroup stressor.
Figure 1.
Cortisol recovery. Estimated marginal means of recovery of cortisol from stress tasks in the same-race and cross-race evaluation conditions are plotted at one standard deviation above and below the mean of past contact.
ANS recovery
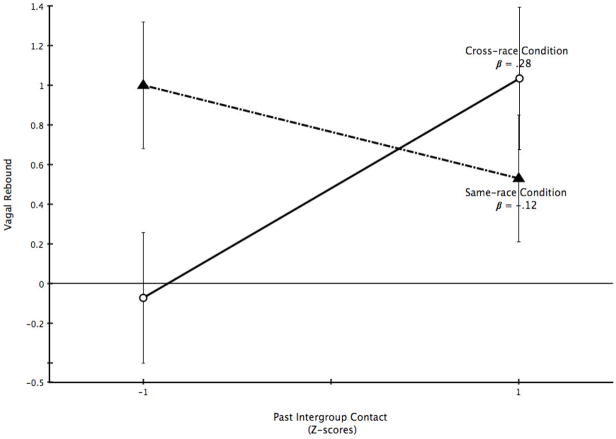
Next, we examined the trajectory of vagal reactivity after the task with the interpretation that efficient vagal recovery is characterized by an over-shoot relative to baseline of RSA after a stressor. There were no significant main effects of condition, F < 1, nor contact, F < 1, but there was a significant interaction of condition and contact, F(1, 116) = 5.62, p = .019 (Figure 2). Contact predicted significantly greater vagal rebound in the intergroup condition, β = .28, t(116) = 2.10, p = .04, but was unrelated to vagal rebound in the ingroup condition, β = −.12, t(116) = −1.15, p = .25. The greater the intergroup contact the greater the vagal rebound after the stressor, but only following intergroup interactions. By comparison, participants did not show differences in PEP recovery by experimental condition, contact, or the interaction (all Fs < 1).
Figure 2.
Vagal rebound. Estimated marginal means of vagal rebound in the same-race and cross-race evaluation conditions are plotted at one standard deviation above and below the mean of past contact.
Discussion
We hypothesized that prior intergroup contact would predict faster physiological recovery after a stressful intergroup interaction. We found that relatively high degree of previous experience with outgroup members was associated with faster recovery following an intergroup stressor. This adaptive recovery was observed across two independent physiological systems. Surprisingly, the race of the participant did not moderate these findings, implying that one’s degree of intergroup contact relative to other ingroup members predicts similar processes in intergroup interactions for members of both minority and majority groups.
Importance of Recovery
We used a strong situation to evoke stress responses and, in general, all participants showed heightened physiological responses during the TSST regardless of past contact or evaluator race. However, a key contribution of this work is that individual differences in contact and the intergroup context differentially predicted recovery from social stress. Although much research has studied individual differences in who becomes stressed, we manipulated stress so that we could examine who successfully copes with stress once the stressor is over. Thus, this research represents one empirical step toward understanding intergroup interactions from a stress and coping framework (Trawalter et al., 2009).
Dienstbier’s (1989) theory on physiological toughening suggests stress toughens an organism as long as there is sufficient recovery following stressors. Moreover, effective recovery from intermittent stressors is thought to reflect a resilient system that can flexibly respond to environmental demands (Epel et al., 1998). When considered in the context of diverse societies in which intergroup interaction may be a daily occurrence, the ability to physiologically recover from stressful intergroup experiences may be key to thriving in diverse contexts (Page-Gould, in press).
Limitations
A key limitation of this study is the reliance on the quasi-experimental variable of past intergroup contact. From these data, it is not clear whether contact directly facilitates recovery following intergroup stressors, or if those who recover quickly from stressful intergroup interactions seek more intergroup contact. However, by manipulating the race of the evaluators we can say that the ingroup compared to the intergroup context evokes different rates of physiological recovery as a function of intergroup contact. If the effects we observed were explained solely by an underlying trait like sociability or extraversion, intergroup contact would have predicted recovery in the ingroup as well as the intergroup context. Nevertheless, there remains an open question on directional causality between intergroup contact and the socioemotional benefits described here.
Conclusion
This work demonstrates physiological benefits of intergroup contact following stressful interactions with unfamiliar outgroup members. Our dependent measures represented recovery across two stress systems to provide convergent evidence within a single study. It appears that past experience with people of other groups predicts physiological regulation, which in turn may facilitate smoother interactions with unfamiliar outgroup members. Altogether, these findings suggest that intergroup experience promotes adaptive coping to intergroup stress.
Acknowledgments
The research was funded by the National Institute of Heart Lung and Blood grant (RO1 HL079383) awarded to the second and third authors, and a Harvard Mind/Brain/Behavior postdoctoral fellowship awarded to the first author.
Footnotes
Analyses of other variables from a White participant subset of these data were published in a previous manuscript (Mendes et al., 2007).
We reran the analyses controlling for prejudice and contact quality to ensure that these two related constructs did not explain our results, and the pattern of results remained the same, F(1, 99) = 4.36, p = 0.04.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Elizabeth Page-Gould, Email: elizabeth.page-gould@utsc.utoronto.ca, Department of Psychology, University of Toronto Scarborough.
Wendy Berry Mendes, Department of Psychology, Harvard University.
Brenda Major, Department of Psychology, UC Santa Barbara.
References
- Allport GW. The nature of prejudice. Reading, MA, US: Addison-Wesley; 1954. [Google Scholar]
- Blascovich J, Mendes WB, Hunter SB, Lickel B, Kowai-Bell N. Perceiver threat in social interactions with stigmatized others. Journal of Personality and Social Psychology. 2001;80:253–267. doi: 10.1037/0022-3514.80.2.253. [DOI] [PubMed] [Google Scholar]
- Dienstbier RA. Arousal and physiological toughness: Implications for mental and physical health. Psychological Review. 1989;96:84–100. doi: 10.1037/0033-295x.96.1.84. [DOI] [PubMed] [Google Scholar]
- Epel ES, McEwen BS, Ickovics JR. Embodying psychological thriving: physical thriving in response to stress. Journal of Social Issues. 1998;54:301–322. [Google Scholar]
- Islam MR, Hewstone M. Dimensions of contact as predictors of intergroup anxiety, perceived out-group variability, and out-group attitude: An integrative model. Personality and Social Psychology Bulletin. 1993;19:700–710. [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’ — a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Mendes WB, Blascovich J, Hunter SB, Lickel B, Jost JT. Threatened by the unexpected: Physiological responses during social interactions with expectancy-violating partners. Journal of Personality and Social Psychology. 2007;92:698–716. doi: 10.1037/0022-3514.92.4.698. [DOI] [PubMed] [Google Scholar]
- Mendes WB, Blascovich J, Lickel B, Hunter S. Challenge and threat during social interactions with White and Black men. Personality and Social Psychology Bulletin. 2002;28:939–952. [Google Scholar]
- Mendes WB, Gray HM, Mendoza-Denton R, Major B, Epel ES. Why egalitarianism might be good for your health: Physiological thriving during stressful intergroup encounters. Psychological Science. 2007;18:991–998. doi: 10.1111/j.1467-9280.2007.02014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzacappa ES, Kelsey RM, Katkin ES, Sloan RP. Vagal rebound and recovery from psychological stress. Psychosomatic Medicine. 2001;63:650–657. doi: 10.1097/00006842-200107000-00018. [DOI] [PubMed] [Google Scholar]
- Olsson A, Ebert JP, Banaji MR, Phelps EA. The role of social groups in the persistence of learned fear. Science. 2005;309:785–787. doi: 10.1126/science.1113551. [DOI] [PubMed] [Google Scholar]
- Page-Gould E. The Unhealthy Racist. In: Marsh JH, Mendoza-Denton R, Smith JA, editors. Are We Born Racist? New Insights from Neuroscience and Positive Psychology. Boston, MA, US: Beacon Press; (In Press) [Google Scholar]
- Page-Gould E, Mendoza-Denton R, Tropp LR. With a little help from my cross-group friend: Reducing anxiety in intergroup contexts through cross-group friendship. Journal of Personality and Social Psychology. 2008;95:1080–1094. doi: 10.1037/0022-3514.95.5.1080. [DOI] [PubMed] [Google Scholar]
- Paolini S, Hewstone M, Cairns E, Voci A. Effects of direct and indirect cross-group friendships on judgments of Catholics and Protestants in Northern Ireland: The mediating role of an anxiety-reduction mechanism. Personality and Social Psychology Bulletin. 2004;30:770–786. doi: 10.1177/0146167203262848. [DOI] [PubMed] [Google Scholar]
- Pettigrew TF. Intergroup contact theory. Annual review of psychology. 1998;49:65–85. doi: 10.1146/annurev.psych.49.1.65. [DOI] [PubMed] [Google Scholar]
- Stephan WG, Stephan CW. Intergroup anxiety. Journal of Social Issues. 1985;41:157–175. [Google Scholar]
- Stephan WG, Stephan CW. An integrated threat theory of prejudice. In: Oskamp S, editor. Reducing prejudice and discrimination. The Claremont Symposium on Applied Social Psychology. Mahwah, NJ: Lawrence Erlbaum Associates; 2000. pp. 23–45. [Google Scholar]
- Stranahan AM, Lee K, Mattson MP. Central mechanisms of HPA axis regulation by voluntary exercise. NeuroMolecular Medicine. 2008;10:118–127. doi: 10.1007/s12017-008-8027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trawalter S, Richeson JA, Shelton JN. Predicting behavior during interracial interactions: A stress and coping approach. Personality and Social Psychology Review. 2009;13:243–268. doi: 10.1177/1088868309345850. [DOI] [PubMed] [Google Scholar]