Abstract
The extracellular matrix (ECM) of the developing heart contains numerous molecules that together form a dynamic environment that plays an active and crucial role in the regulation of cellular events. ECM molecules found in the heart include hyaluronan, fibronectin, fibrillin, proteoglycans, and collagens. Tight regulation of the spatiotemporal expression, and the proteolytic processing of ECM components by proteases including members of the ADAMTS family, is essential for normal cardiac development. Perturbation of expression of genes involved in matrix composition and remodeling can interfere with a myriad of events involved in the formation of the four-chambered heart and result in prenatal lethality and/or cardiac malformations as seen in humans with congenital heart disease (CHD). In this review we will briefly summarize what is known about the specific importance of some of the components of the ECM in relation to the cardiovascular development.
Introduction
While in the past the extracellular matrix (ECM) was commonly viewed as a rather inert scaffold, merely providing structural support for the cells embedded in its environment, it is now well recognized that the ECM forms in fact a very dynamic and plastic milieu which plays an active and crucial role in the regulation of a wide variety of cellular events. In recent years it has been convincingly demonstrated that the ECM is involved in cell-cell signaling, the regulation of cell proliferation, cell differentiation, and cell migration (Bowers and others, 2010; Daley and others, 2008; Frantz and others, 2010).
The ECM is of particular importance during embryogenesis, as the spatiotemporal regulation of cell migration, reorganization, and differentiation must be tightly orchestrated during the formation of the embryo and its respective complex anatomical structures such as the heart. There is a growing body of evidence that shows that perturbation of expression of ECM components, and/or alterations in the signaling pathways that regulate their production, can lead to cardiac malformations that in many cases resemble those found in patients with congenital heart disease (CHD).
In this review we will describe what is currently known regarding the role of the ECM in heart development, focusing on those molecules implicated in the pathogenesis of heart defects. Because of the wealth of information available, we will in this review focus on the role of the ECM in cardiac development in the mouse. In the first part of the paper we will briefly discuss a few key features of heart formation to set the stage for the discussion on how ECM components play a role in the respective steps involved in the morphogenesis of the 4-chambered heart. It is obviously beyond the scope of this review to discuss all aspects of cardiac development in detail. If and where needed we will elaborate on some of these aspects in the text below. In the second part of our review we will discuss a number of ECM molecules that have been demonstrated to play important roles in these developmental events. We acknowledge that this review does not provide a complete comprehensive summary of all the information available on the role of the ECM in heart development. In addition, we realize that some of the data presented in this review is open to alternative interpretations. However, we hope that we will able to convey the message that the ECM plays a very important and diverse role in the regulation of developmental events in the 4-chambered heart and that perturbation of expression and/or processing can lead to cardiac abnormalities as seen in patients suffering from congenital and acquired heart disease.
Heart Development; a short introduction
The Early Steps
During gastrulation, cardiac precursor cells emerge from the primitive streak and migrate laterally and cranially into the lateral plate mesoderm to form bilateral fields of cells that will eventually form the myocardial and endocardial layers of the primary heart tube. These cells are specified by endodermal cardiac-inducing signals and form the primary or first heart field (PHF or FHF). Anterior migration of these cells, combined with the fusion of the lateral walls of the developing embryo, will bring the two areas of cardiogenic cells together, resulting in the formation of a tubular heart (Abu-Issa and Kirby, 2007; Stainier, 2001; Yutzey and Kirby, 2002). At this point, the wall of the heart tube is composed of two layers of cells. The inner cell layer forms the endocardium which lines the lumen of the heart tube and the outer layer of cells forms the myocardium. The endocardium and myocardium are separated by an ECM-rich, acellular space which is commonly refered to as the cardiac jelly. This cardiac jelly is critically important for the proper formation of the endocardial cushions at the atrioventricular (AV) junction. The cells in the tubular heart at this stage are largely derived from the PHF and will primarily contribute to the developing atria, atrioventricular canal, and the left ventricle (Kelly and others, 2001; Meilhac and others, 2004; Zaffran and others, 2004).
After formation of the tubular heart, the newly formed organ hangs suspended in the future pericardial space, connected to the rest of the embryo by the dorsal mesocardium (Snarr and others, 2008). As the heart tube loops and expands, the dorsal mesocardium disintegrates, with the exception of its caudal-most part at the venous pole of the heart, by a yet unknown mechanism. This persisting dorsal mesocardium plays a crucial role in a number of events, including formation of the atrioventricular mesenchymal complex and the development of the pulmonary veins. (Snarr and others, 2008; Snarr and others, 2007a; Snarr and others, 2007b)
The expansion/elongation of the heart tube at this stage is a result of the addition, at either end of the heart, of a second wave of cardiac progenitor cells derived from the pharyngeal and splanchnic mesoderm. This population of highly proliferative cardiac precursor cells is commonly refered to as the second heart field (SHF)(Dyer and Kirby, 2009). At the arterial pole, these cells will ultimately give rise to the outflow tract (OFT), right ventricle, and the interventricular septum (Verzi and others, 2005). At the venous pole, the SHF adds cells to the developing atria and the atrial septum, but also forms a wedge of mesenchymal cells that protrude into the dorsal aspect of the atrial cavity using the dorsal mesocardium as its portal of entry. This mesenchymal cell mass is known as the dorsal mesenchymal protrusion (DMP) and will contribute to the formation of the AV mesenchymal complex (Snarr and others, 2008; Snarr and others, 2007a; Snarr and others, 2007b).
The Mesenchymal Tissues of the Heart
Extracellur matrix is found in all components of the heart. However, it is particularly abundant in the developing mesenchymal stuctures which play a crucial role in valvuloseptal morphogenesis. Cardiac mesenchyme derives from a variety of sources (Snarr and others, 2008). As will be demonstrated later in this review, the role of the ECM in the cardiac mesenchyme is of particular importance. Perturbation of the development of these structures often leads to defects in the derived valvuloseptal structures as seen in patients with congenital heart disease. Below, we provide a brief overview of the mesenchymal tissues seen in the developing heart.
The Atrioventricular Cushions
As mentioned earlier, the primary heart tube consists of an endocardial and myocardial layer in between which we find the cardiac jelly (Markwald and others, 1977). Accumulation of ECM in the region of the developing AV junction results in localized “swelling” of the cardiac jelly. This process is the first step in the formation of the AV cushions which will play an important role in AV septation and formation of the AV valves. Surprisingly little is known about the mechanisms that regulate this localized accumulation of ECM. The formation of these, initially acellular, AV cushions is followed by an epithelial-to-mesenchymal transformation (EMT) of the endocardial cells that line the cushions. The endocardially-derived mesenchymal cells (ENDCs) that result from this endocardial EMT populate the AV cushions (Eisenberg and Markwald, 1995; Markwald and others, 1996). Insight into the regulation of endocardial EMT has grown rapidly over the last few decennia. Thus, it is now well-established that EMT is critically dependent on proper expression of members of the family of growth factors, including BMP2 and TGFβ2 (Inai and others, 2008; Mercado-Pimentel and Runyan, 2007; Tian and others). It is also becoming increasingly clear that the ECM plays an important role in this event. In the second part of this review we will briefly address what is known about the involvement of ECM macromolecules in the regulation of EMT.
The Outflow Tract Cushions
The development of the OFT is a relatively late event in cardiac morphogenesis; the cells that form the OFT are largely derived from the SHF (Dyer and Kirby, 2009; Verzi and others, 2005). It is important to realize that the development and growth of the heart at the early stages is one continuous process (Moorman and others, 2007) and that, even though the cells of the SHF can be distinguished from the PHF-derived cells by a different molecular and “transgenic” signature, the SHF cells are gradualy added to the growing and remodeling heart and that there are no sharp anatomical/morphological boundaries between tissues derived from the PHF and SHF. As the SHF is added to the growing heart tube, the cardiac jelly at the luminal side of the OFT develops endocardial cushions which are, in many ways, reminiscent to those seen in the AV junction. In this review we will refer to these ECM-rich spaces as the OFT cushions, recognizing that these structures may be called endocardial OFT ridges, conal swellings and/or truncal ridges in other publications. Just like in the AV junction, endocardial EMT is playing a role in the generation of mesenchymal cells that populate the OFT cushions. Around ED 9.5–10, cardiac neural crest derived cells (NCDCs) start to migrate into the distal end of the OFT cushions and form two “prongs” of mesenchyme which eventually will occupy a significant portion of the cushions. The ENDC and NCDC populations do not intermingle and as a result they occupy distinct parts of the developing OFT cushions. This can be nicely demonstrated using cre-recombinase mice that allow the identification of ENDCs (e.g. Tie2-cre) and NCDCs (e.g. Wnt1-cre) in the developing heart (Snarr and others, 2008). In addition, the ECM in which the NCDCs are found and the ECM surrounding the ENDCs are characterized by the expression of different extracellular components.
The Dorsal Mesenchymal Protrusion
The dorsal mesenchymal protrusion (DMP) is a SHF-derived mesenchymal structure which, around ED 9.5–10.5, grows into the unseptated atrial cavity at the venous pole of the heart using the dorsal mesocardium as a portal of entry (Snarr and others, 2007a; Snarr and others, 2007b). It can be distinguished from the endocardially derived AV cushions by the expression of the SHF-specific transcription factor Isl1 (Snarr and others, 2007a), but it is also identified as a SHF derivative when using the SHF transgenic AHF-mef2C-cre mouse (Verzi and others, 2005) in combination with ROSA26 reporter mice (Goddeeris and others, 2008). The DMP develops in close association with the a mesenchymal cap on the leading edge of the developing primary atrial septum and fuses with the AV cushions to form the AV mesenchymal complex, an essential process in atrioventricular septation (Snarr and others, 2007a; Snarr and others, 2007b). A series of independent publications have demonstrated that perturbation of DMP development is associated with the pathogenesis of atrioventricular septal defects (Goddeeris and others, 2008; Snarr and others, 2007a; Tian and others, 2010; Wirrig and others, 2007)
The Epicardium
Another source of cardiac mesenchyme is the proepicardium which develops around ED9.5 in the mouse and Hamburger/Hamilton (H/H)16 in the chick. (Wessels and Perez-Pomares, 2004). Cells derived from the proepicardium attach to the outer surface of the myocardium and subsequently spread out over the heart to form the epicardial epithelium. Epicardial cells then undergo an epicardial EMT. In this process the Wilms’ tumor gene WT1 plays an important role through direct transcriptional regulation of Snail (Snai1) and E-cadherin (Cdh1) which are both mediators of EMT (Martinez-Estrada and others, 2010). Epicardial EMT is also regulated by TGFβ2, through the regulation of hyaluronan (Craig and others, 2010a). This epicardial EMT generates a population of epicardially-derived mesenchyme. A subset of the epicardially-derived cells (EPDCs) subsequently migrate into the ventricular myocardial walls where they either remain and reside as interstitial fibroblast, differentiate into coronary smooth muscle cells, or possibly, become coronary endothelium or cardiomyocytes (Cai and others, 2008; Perez-Pomares and others, 2002a; Perez-Pomares and others, 2002b; Zhou and others, 2008). In addition, relatively large numbers of EPDCs are also found within the developing endocardial cushions (Manner, 1999; Perez-Pomares and others, 2002b). The significance of EPDCs for valvuloseptal morphogenesis is illustrated by studies in which the development of the epicardium is perturbed, showing that this results in valvuloseptal defects (Wessels and Perez-Pomares, 2004).
The ECM and Cardiac Morphogenesis
As described above, respective cardiac tissues have different origins, functions, and fate. It is therefore not surprising that we see important spatial and temporal changes in the composition of the ECM in the individual structures of the heart as they develop, remodel, and mature. In the developing cardiac tissues we find representatives of all classes of ECM macromolecules. This includes hyaluronan, proteoglycans, collagens, elastin, fibrilin, tenascin, fibronectins, and laminins. While the specific roles of many of these molecules have not (yet) been fully elucidated, the importance of others has been revealed by genetic linkage to congenital disease in the human and/or by studies in animal models. Below we will, in the context of heart development and the etiology of cardiac malformations, discuss a number of these ECM molecules focusing on the mouse as the animal model of choice. An overview of the information provided is summarized in Table 1. It is important to note that while we can learn a lot from studying how genetic mutations lead to perturbation of heart development, the embryonic lethality often observed in animal models, and studied in developmental series in these models, may never manifest in humans as the severity of these defects may not be compatible with life and human embryos with the severe abnormalities seen in the animal models would not survive until birth.
Table 1. Animal models of extracellular matrix and cardiovascular development.
Numerous animal models have been described that have proven useful for the study of the role of extracellular matrix in congenital heart disease.
| Gene Affected | Animal Model | Normal Cardiovascular Expression | Lethality | Cardiovascular Phenotype | Reference |
|---|---|---|---|---|---|
| I. Hyaluronan, Proteoglycans, and Hyaluronan and Proteoglycan Link Proteins | |||||
| Aggrecan | Cartilage Matrix Deficiency (cmd) mouse | None | Perinatal | None reported | Wong and others, 1992 |
| Aggrecan | Nanomelic Chicken | Epicardium, mesenchyme of the OFT and AVC | Neonatal | None reported | Wong and others,1992; Zannin and others, 1999; Hinton and others, 2006 |
| Cartilage Link Protein (Hapln1) | Crtl1 Knockout | AV and OFT cushions, endocardium | Perinatal | ASD, VSD, AVSD, TMP | Watanabe and Yamada, 1999; Wirrig and others, 2007 |
| Glypican-3 | Gpc3 knock out | AV cushions | Perinatal | ASD, VSD, Coronary Artery Fistula, PDA, DORV | Ng and others, 2009 |
| Hyaluronan | Has2 Knockout | Myocardium and endocardium of the heart tube, endocardium and mesenchyme of the AV and OFT cushions, AV valves, CT | ED9.5-10 | Defects in vasculogenesis, fail to form AVC | Camenish and others, 2000 |
| Perlecan | Perlecan Knockout | BM, Cardiac Jelly, OFT Cushions, SMCs of Ao and PA | ED10.5-12 or perinataly | TMP, defects in OFT rotation | Costell and others, 2002 |
| Versican | Hdf | Cardiac jelly, AV and OFT cushions, mesenchyme of the PAS | ED10.5 | Fail to form RV, OFT, AVC, and TMP; Haploinsufficiency results in VSD | Yamamura and others, 1997; Mjaatvedt and others, 1998; Wirrig and others, 2007 |
| II. Collagens | |||||
| Collagen Type I | Mov13 mouse (insertional mutant) | AV valves, Vasculature | ED12-14 | Vascular Defects | Lohler and others, 1984; Lincoln and others, 2004; Kruithof and others, 2007 |
| Collagen Type I | Col1a1 Int1Δ | AV valves, Vasculature | Late Adulthood | Aortic dissection and rupture | Rahkonen and others, 2004; Lincoln and others, 2004 |
| Collagen Type II | Col2a1 Knockout | CT, AV valves | Perinatal | None reported | Metsaranta and others, 1992; Lincoln and others, 2004 |
| Collagen Type III | Col3a1 Knockout | AV valves, Vasculature | Late Adulthood | Blood Vessel Rupture | Liu and others,1997; Hinton and others, 2006; Kruithof and others, 2007 |
| Collagen Type IV | Col4a1/2 Knockout | BM, AV valves | ED10.5-11.5 | Vascular Defects | Poschl and others, 2004; Kruithof and others, 2007 |
| Collagen Type IV | Col4a1andCol4a2 missence mutations | BM, AV valves | ED15 | Vascular Defects | Favor and others, 2006; Kruithof and others, 2007 |
| Collagen Type V | Col5a1 Knockout | Ao, Anulous Fibrosus, Mural Leaflet | ED10 | Undescribed cardiovascular defects; Haploinsufficiency results in decreased Ao stiffness | Wenstrup and others, 2004; Roulet and others, 2006;Kruithof and others, 2007 |
| Collagen Type VI | Col6a1 Knockout | AV valves, myocardium, epicardium, Ao, and PA | None | None reported | Bonaldo and others, 1998; Klewer and others, 1998; Kruithof and others, 2007 |
| Collagen Type XV | Col15a1 Knockout | BM, Myocardial Capillaries | None | Vascular defects, cardiomyopathy | Eklund and others, 2001 Muona and others, 2002 |
| Collagen Type XVIII | Col18a1 Knockout | BM of myocardium and endocardium, Semilunar Valves | None | Broadened AV Valves | Carvalhaes and others, 2006; Utriainen and others, 2004 |
| III. Periostin | |||||
| Periostin | Periostin Knockout and PeriostinlacZ | Endothelium, OFT and AV cushions, Mitral and Tricuspid valves | Some neonatal lethality | Leaflet abnormalities and valve disease | Rios and others, 2005; Kii and others, 2006; Snider at al., 2008; Norris and others, 2008 |
| IV. Proteases | |||||
| ADAMTS1 | ADAMTS1 Knockout | Endocardium, myocardium, AV cushion | Embryonic Lethality in 50% | Hypertrabeculation | Thai and Irule-Arispe, 2002; Kern and others, 2006; Stakunas and others, 2008 |
| ADAMTS5 | ADAMTS5 Knockout | Endocardium, myocardium, pericardium, Ao, aortic valves | None | Normal | Majumdar and others, 2007; McCulloch and others, 2009 |
| ADAMTS9 | ADAMTS9 +/lacZ | Myocardium, right-sided OFT cushions, SLV, Ao, mitral valve | ED7.5 in Homozygous | Haploinsufficiency results in enlarged SLV, myxomatous mitral valve, “spongy” myocardium, cartilage nodules | Jungers and others, 2005; Kern and others, 2010 |
| V. Fibulins | |||||
| Fibulin 1 | Fibulin1 Knockout | AV and OFT cushions, CNDCs | Perinatal | ASD, TMP, abnormal AAA, DORV, overriding aorta, VSD, hemorrhage | Kern and others, 2006; Cooley and others, 2008 |
| VI. Fibronectin | |||||
| Fibronectin | Fibronectin Knockout | Embryonic Mesoderm, AVC, endocardium, dorsal Ao | ED8.5 – 10.5 | Failto form heart tube in most severely affected, vascular defects | Ffrench-Constant and Hynes, 1989; George and others, 1993; Georges-Labouesse and others, 1996 |
| FibronectinEIIIA andEIIIB | Fibronection EIIIA/EIIIB Knockout | Embryonic Mesoderm, AVC, endocardium, dorsal Ao, pharyngeal arch arteries | ED9.5 – 10.5 | Fail to form AVC, thin OFT, vascular hemorrhage | Tan and others, 2004; Astrof and others, 2007a,b |
| VII. Fibrillin | |||||
| Fibrillin 1 | Fibrillin 1 Knockout | Endocardium, AV and OFT cushions, AV valves, PA and Ao | Postnatal | Aortic Aneurism, MVP | Judge and others, 2004; Ng and others, 2004 |
AAA= Aortic Arch Artery
Ao= Aorta
ASD= Atrial sepatal defect
AV= atrioventricular
CNDCs= cardiac neural crest derived cells
CT= Chordea Tendineae
DORV= Double Outlet Right Ventricle
MVP= Mitral Valve Prolapse
OFT= Outflow Tract
PDA= Persistent ductus arteriosus
RV= Right Ventricle
SLV= Semi-lunar Valves
TMP= Thin Myocardium Phenotype
VSD= Ventricular septal defect
I: Hyaluronan, Proteoglycans, and Hyaluron and Proteoglycan Link Proteins
Hyaluronan
Glycosaminoglycans (GAGs) are polysaccharide chains consisting of repeating amino sugar and uronic acid disaccharide units. There are four classes of GAGs. The most abundant GAG in the developing heart is hyaluronan (HA) which consists of alternating UDP glucuronic acid and UDP-N-acetylglucosamine molecules which alternate to combine in a chain-like fashion (Toole, 2004). The enzymes UDP N-acetylglucosamine-1-phosphate uridyltransferase and UDP glucose dehydrogenase are necessary for the synthesis of glucuronic acid and N-acetylglucosamine, forming the building blocks of HA (Mengin-Lecreulx and van Heijenoort, 1994; Spicer and McDonald, 1998) Mutation of UDP glucose dehydrogenase in zebrafish results in malformation of the AV cushions, and gene patterning defects in the AV myocardium (Stainier, 2001; Walsh and Stainier, 2001). The other three classes of GAGs are chondroitin sulfate/dermatan sulfate (CS/DS), heparan sulfate/heparin (HS), and keratin sulfate (KS). These GAGs are found in the ECM of the heart linked to proteoglycan core proteins as discussed elsewhere in this review. Unlike the other GAGs, HA is synthesized at the plasma membrane and does not become linked to a core protein. Three different HA synthase enzymes (Has1, Has2, and Has3) are known to be involved in the synthesis of HA (Kramer and others, 2010). HA is part of a pericellular matrix that provides a hydrated environment that facilitates cellular proliferation and motility (Toole, 2001). In the developing heart, HA is found in the cardiac jelly and, as development progreses, in the derived AV and OFT cushion tissues and forming leaflets (Figures 1 and 2). In addition, HA is also a present in epicardial mesenchyme and in the interstitial space surrounding the cardiomyocytes of the myocardial structures in the heart (Figure 2).
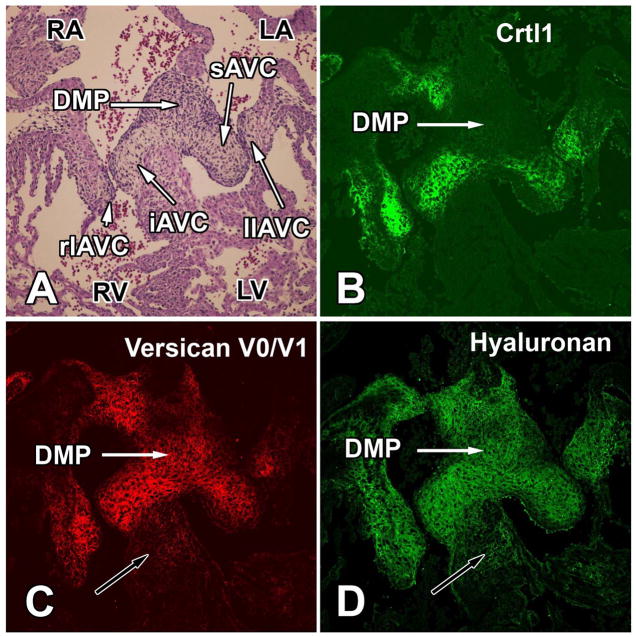
Figure 1. Expression of Crtl1, versican, and hyaluronan in the developing atrioventricular cushions of the mouse at ED 13.5.
This figure shows transverse serial sections through the AV junction of an ED13.5 wildtype mouse heart. The section in panel A is stained for hematoxylin-eosin. The immunofluorescent stainings in panels B–D demonstrate the overlapping expression of Crtl1 (B), versican (C), and hyaluronan (D) in the AV cushions but also the fact that the mesenchyme of the dorsal mesenchymal protrusion (white arrows) is expressing versican and hyaluronan but not Crtl1. The black arrows in panels C and D point to the muscular component of the interventricular septum in which expression of versican and hyaluronan is also, albeit it at a low level, expressed. iAVC=inferior AV cushion; sAVC=superior AV cushion; llAVC=left lateral AV cushion; rlAVC=right lateral AV cushion; DMP=dorsal mesenchymal protrusion; LA=left atrium; LV=left ventricle; RA=right atrium; RV=right ventricle.
Figure 2. Expression of versican, hyaluronan, and Crtl1 in the developing leaflets of the AV valves at ED 17.
This figure shows serial sections through the developing AV valves of a mouse heart at an ED 17 stained for versican (with an antibody recognizing the GAGβ epitope), hyaluronan (using an hyaluronan binding protein HABP approach) and Crtl1. The staining procedures used are essentially as described in Wirrig and others, 2007). Note that the staining for versican is more intense in the leaflets of the developing tricuspid valve when compared to the staining in the aortic and mural leaflet of the mitral valve (A). While versican (A,B) and hyaluronan (C) are still expressed at considerable levels in the tricuspid leaflets, the expression of Crtl1 has significantly decreased at this stage.
The importance of HA for proper heart development has been known for several decades. In 1979, Bernanke and Markwald showed that HA treatment of avian AV cushion explants resulted in an increase of mesenchymal cells invading a 3D collagen matrix (Bernanke and Markwald, 1979). Around the same time, Nakamura and Manasek showed the significance of HA in cardiac development by demonstrating that injection of hyaluronidase into the cardiac jelly of embryonic chick hearts caused severe reduction of cushion material and associated cardiac defects (Nakamura and Manasek, 1981). That HA is also important for the development of the mammalian heart was first demonstrated by Baldwin and colleagues who showed that treatment of cultured rat embryos with hyaluronidase, resulted in a loss of cardiac jelly, abnormal formation of the endocardial cushions, thinning of the ventricular myocardial wall, and altered ventricular performance (Baldwin and others, 1994). These results were similar to those found using the hyaluronan-synthase 2 (Has2) knock-out mouse (Camenisch and others, 2002; Camenisch and others, 2000). Has2 mRNA is normally expressed by the myocardial and endocardial cells at ED8, but as the AV junction develops, expression becomes virtually restricted to the endocardium and mesenchyme of the AV canal and OFT endocardial cushions. Has2 knockout mice are unable to produce HA which leads to embryonic lethality at approximately ED10.5. Has2 null embryos are characterized by severe cardiac malformations including thin myocardium and impaired AV cushion development (Camenisch and others, 2000). Using the 3D collagen gel assay, Camenisch and co-workers showed that the AV cushion defects are most likely caused by failure of the cushion endocardium to undergo EMT. They determined that the specific role of HA in this step of cushion development is to activate endocardial ErbB2-ErbB3 receptors and that the EMT defect can be rescued by either adding HA to the assay or by adding Heregulin (HRG) that activates the ErbB receptors (Camenisch and others, 2002; Schroeder and others, 2003).
There are also indications that HA plays a role in epicardial development. In an immortalized mouse epicardial cell line, stimulation with high molecular weight hyaluronan (HMW-HA) results in binding of MEKK1 to the CD44 receptor and in MEKK1-dependent activation of ERK1/2 and NFκB signaling pathways. Treatment of epicardial cells with HMW-HA also results in increased invasion in a collagen gel matrix assay, yet whether HA is able to affect epicardial cell migration in vivo has yet to be determined (Craig and others, 2010b).
The recent development of a Has2-flox mouse (Matsumoto and others, 2009), which in combination with tissue-specific cre mice will allow tissue-specific deletion of Has2, will undoubtedly be useful in unraveling in more detail the importance of HA in specific tissues and populations of cells within the developing heart.
Despite the fact that studies in the avian and murine heart clearly show the importance for HA in heart development, there are, to the best of our knowledge, no published studies that show that HA insufficiency is associated with congenital heart disease (CHD) in the human.
Proteoglycans
Proteoglycans are composed of a core protein and associated glycosaminoglycan (GAG) sidechains. The four classes of GAGs are hyaluronan (see above), chondroitin sulfate/dermatan sulfate (CS/DS), heparan sulfate/heparin (HS), and keratin sulfate (KS). During their synthesis, HS, CS/DS, and KS are covalently linked to the core proteins and sulfated before being secreted into the ECM as proteoglycans.
Chondroitin Sulfate Proteoglycans
The chondroitin sulfate proteoglycans versican, aggrecan, and neurocan have each been identified as being expressed in the developing heart. However, only versican deficiency is associated with heart malformations in animal models. While versican is expressed in the avian, murine and human heart, its family members aggrecan and neurocan have only been observed in the avian heart. Interestingly, there are two reported human mutations in chondroitin sulfate proteoglycan processing enzymes that result in cardiovascular defects. Human patients carrying mutations in CHST3 (the gene encoding for the chondroitin 6-O-sulfotransferase-1 (C6ST-1)) suffer from ventricular septal defects while patients carrying a mutation in CHST14 (carbohydrate (N-acetylgalactosamine 4-0) sulfotransferase 14) have atrial septal defects (Dundar and others, 2009; Unger and others, 2010).
Versican
Versican is a chondroitin sulfate proteoglycan. Its core protein, a product of the CSPG2 gene, has four splice variants. The longest (full length) form of versican is named V0. It contains a G1 domain, which can bind HA and cartilage link protein (Crtl1), two GAG domains (GAG-alpha and GAG-beta) where the GAG chains attach, and a G3 domain, which is able to interact with other extracellular matrix proteins such as fibulin and ECM cell surface receptors such as integrins (Kern and others, 2007; Kern and others, 2006). The V1 splice variant of versican contains the G1, G3, and GAG-beta domain, the V2 isoform contains the G1, G3, and GAG-alpha, while the V3 isoform only contains the G1 and G3 domains (Kern and others, 2007; Kern and others, 2006; Sheng and others, 2005). The relative amounts of the respective splice variants can be analysed by reverse transcriptase-polymerase chain reaction (Kern and others, 2006) while, using antibodies that specifically recognize the GAG-alpha and GAG-beta domains, the spatial distributions of the versican variants can be determined by immunohistochemistry (Kern and others, 2006; Wirrig and others, 2007). Following these approaches, it has been established that the respective versican variants are expressed in very specific spatiotemporal patterns during heart development. The V0 and V1 isoforms are typically found in the cardiac jelly, in the AV and OFT cushions (Figure 1), in the developing leaflets of the AV (Figure 2) and semilunar valves, and OFTand in the mesenchymal cap of the primary atrial septum (Kern and others, 2007; Kern and others, 2006; Wirrig and others, 2007), whereas the V2 isoform is found in the myocardium and cardiac neural crest. As the AV valves mature, the expression of versican slowly decreases. It is interesting to note that this process occurs first in the leaflets of the mitral valve and only later in the leaflets of the developing tricuspid valve.
Versican can be cleaved by matrix metalloproteinases (MMPs) and members of the ADAMTS (“a disintegrin and metalloproteinase with thrombospondin motifs”) family (reviewed in (Kern and others, 2006). Cleavage by ADAMTS family takes place in the GAG-beta domain and the neoepitope sequence DPEAAE resulting from this process can be detected by specific antibodies that recognize the site of cleavage. Using these V0/V1-Neo antibodies in the developing mouse embryo, it has been determined that the versican cleavage products are primarily present in the densely packed mesenchyme adjacent to the endocardium of the AV cushions while intact V0 and/or V1 versican isoforms are predominantly observed in the “core” of the AV cushions where the EMT derived mesenchyme is loosely packed (Kern and others, 2006). The cleavage of versican is thought to play a significant role in maturation of the AV cushions and remodeling of the outflow tract (Kern and others, 2007; Kern and others, 2006). Specifically it has been suggested that this process facilitates the dense packing of the mesenchymal cells in the elongating valves (Kern and others, 2006). Furthermore, it has been hypothesized that in the outflow tract, cleavage of versican may promote the disaggregation of cardiomyocytes and a thinning of the outflow tract myocardium, thus promoting remodeling of the outflow tract (Barbosky and others, 2006; Kern and others, 2007; Sheng and others, 2005).
Additional insight into the role of versican in heart development comes from a number of studies using a versican mutant mouse (the hdf mutant) in which a lacZ transgene is inserted into the intronic sequences between exon 7 and exon 8 of the CSPG2 gene (Yamamura and others, 1997). Heterozygous hdf mice (hdf/+) are viable, although some display isolated ventricular septal defects (Wirrig and others, 2007). Embryos homozygous for the hdf insertional mutation (hdf/hdf) do not express versican and die between ED10.5 and 11.0 (Yamamura and others, 1997). Embryos isolated right before they die, are characterized by severe cardiac defects including an underdeveloped right ventricle and outflow tract, absent AV and OFT cushions, thinning of the ventricular myocardium, virtual absence of trabeculations, and a flattening of the endocardium against the myocardium (Mjaatvedt and others, 1998; Yamamura and others, 1997). It is interesting to note that these defects are very similar to those seen in mice that do not express HA, a binding partner of versican (Camenisch and others, 2000).
Aggrecan
Aggrecan is a chondroitin sulfate proteoglycan which, across species, is predominantly expressed in cartilaginous structures. Similar to versican, the aggrecan core protein is comprised of three globular domains, G1, G2, and G3 and two GAG attachment domains. Like the other chondroitin sulfate proteoglycan family members versican, neurocan, and brevican, aggrecan can interact with cartilage link protein (Crtl1) and HA to form large aggregates that contribute to the hydration of the extracellular matrix.
In the chick heart, aggrecan is expressed in the epicardium and the mesenchyme of the AV and OFT cushions (Zanin and others, 1999). As the AV cushions mature, aggrecan expression becomes restricted to the spongiosa and fibrosa layers (Hinton and others, 2006). Nanomelic chickens carry an autosomal recessive aggrecan gene mutation and display chondroplasia and are characterized by a parrot-like beak but cardiac abnormalities have not been documented (Wong and others, 1992).
While aggrecan is strongly expressed in the murine cartilage, it does, in contrast to what was reported in the avian heart, not appear to be expressed in the murine heart. Heterozygous aggrecan-deficient mice (cartilage matrix deficiency, cmd) are viable, but develop spinal abnormalities in adulthood (Watanabe and others, 1998). Homozygous cartilage matrix deficiency (cmd/cmd) mice die perinatally from respiratory failure and are characterized by dwarfism, short snout, and a cleft-palate. Heart defects have not been reported in the cmd mouse which is, given the expression pattern of aggrecan in the murine embryo, not surprising.
Heparin Sulfate Proteoglycans
Heparan sulfate proteoglycans (HSPGs) are found at the cell surface and in the ECM of most tissues. They play a crucial role in development and homeostasis, and are implicated in disease processes. Via their heparan sulfate chains they are able to bind to multiple growth factors and cytokines that have been demonstrated to be important for cardiac development, including VEGF, BMPs, FGFs, and TGFβs.
Perlecan
Perlecan is expressed in pericellular matrices and basement membranes during development (Handler and others, 1997) and is known to interact with other ECM proteins and ECM cell surface receptors, such as integrins and laminins, as well as with important signaling ligands such as fibroblast growth factor 2 (FGF2) and neuregulins (Handler and others, 1997; Li and Loeb, 2001). There are 16 different isoforms of laminin glycoproteins, which are derived from multiple forms of α, β, and γ chains, the three primary components of laminins (reviewed in Kruegel and Miosge, 2010). Laminins self assemble into ECM networks and form the major framework for basement membrane assembly (reviewed in Kruegel and Miosge, 2010). Laminins interact with perlecan via binding to the heparan sulfate chains (Battaglia and others, 1992; Sasaki and others, 1998).
Expression of perlecan is found in the cardiac jelly surrounding the developing trabeculae, in a diffuse staining pattern throughout the pericellular matrix of the ventricular myocardial cells, and, albeit at lower levels, in the AV cushions (Costell and others, 2002; Handler and others, 1997; Stankunas and others, 2008). Immunohistochemical staining for perlecan in the OFT indicates that the highest level of expression is in the distal part of the cushions, with lower levels of expression found in the proximal cushions (Costell and others, 2002). Perlecan is also expressed in the smooth muscle walls of the developing aorta and pulmonary artery (Handler and others, 1997).
Perlecan mutant mice show a spectrum of cardiac malformations, including defects in rotation of the outflow tract and abnormal development of the ventricular myocardial wall. Intriguingly, there are two developmental windows in which prenatal lethality is observed (Costell and others, 2002). Specimens with more severe phenotypes die between E10.5 and E12.0, while those with lesser malformations die perinatally. In the more severely affected specimens, hemorrhaging of blood into the pericardial cavity is observed, a defect caused by fenestrations in a thin and discontinuous ventricular myocardial wall, (Costell and others, 2002; Sasse and others, 2008). This hemorrhaging is associated with a virtual absence of the ECM basement membranes that typically surrounds the developing cardiomyocytes (Sasse and others, 2008). In perlecan mutants with a less severe phenotype, the primary defect is malrotation of the outflow tract. This appears to be caused by an overabundance of highly disorganized cardiac neural crest derived cells (CNDCs) that reach the proximal OFT cushions leading to abnormal development of these cushions and malrotation of the OFT (Costell and others, 2002). This remodeling defect results in complete transposition of the aorta and pulmonary artery, a situation in which the aorta connects to the right ventricle and the pulmonary trunk arises from the left ventricle (Costell and others, 2002). Thus, perlecan is important in the formation of the cardiomyocyte basement membrane, in maintaining the integrity of the ventricular wall, and in supporting the organized migration of cardiac neural crest into the OFT cushions (Costell and others, 2002; Sasse and others, 2008).
Agrin
Agrin is an ECM protein localized at the neuromuscular junction where it signals through MuSK, a muscle-specific receptor tyrosine kinase, to mediate the motor neuron-induced accumulation of acetylcholine receptors in the postsynaptic muscle fiber membrane (Ngo and others, 2007). Recent studies have shown that in the fetal and adult murine heart, agrin is expressed in the myocardium (Hilgenberg and others, 2009), where, by interacting with the α3 subunit of the Na, K-ATPase it modulates the basal frequency of myocyte contraction (Hilgenberg and others, 2009). Myocytes isolated from agrin mutant mice show a significantly higher spontaneous contraction frequency, a phenotype that is also achieved by treating wildtype cardiomyocytes with an agrin antagonist (Hilgenberg and others, 2009). The role of agrin in cardiac development has not been studied yet.
Glypicans
Of the 6 members in the Glypican family (Gpc1-6), Gpc3 is the most thoroughly studied. Gpc3 is a membrane-bound HSPG expressed during vertebrate development. In early studies of avian glypican it was demonstrated that it is expressed in the endocardial cushions at Hamburger/Hamilton stages 20–25 (Niu and others, 1998). In cultured rat neonatal cardiomyocytes, Gpc3 was reported to be expressed at the lateral regions of myocyte plasma membrane that contact the basement membrane, but also at sites of myocyte adhesion junctions on the cell surface (Asundi and others, 1997). In humans, mutations in in the GPC3 gene, localized at Xq26, are associated with Simpson–Golabi–Behmel syndrome (SGBS), a rare disorder associated with predisposition to certain childhood cancers, both pre- and postnatal overgrowth, and multiple congenital abnormalities including congenital heart disease, rhythm defects and cardiomyopathy (DeBaun and others, 2001; Lin and others, 1999; Neri and others, 1998). Gpc3 null mice have a high incidence of heart defects (69%) including ventricular septal defect (VSD), coronary artery fistula, double outlet right ventricle (DORV), atrioventricular septal defect (AVSD), and patent ductus arteriosus (PDA). Gpc3 deficient mice display a delayed development of the coronary vascular plexus and an increased number of coronary arteries relative to the number of veins (Ng and others, 2009).
Syndecans
Syndecans are type 1 transmembrane heparan sulfate proteoglycans (HSPGs) that play important roles during development and in events like wound healing and tumour progression. They are involved in controlling cell proliferation, differentiation, adhesion and migration. In mammals, four syndecan members can be distinguished (Syndecan1–4). The heparin sulfate (HS) chain on the extracellular domain of syndecan can interact with numerous ligands found in the ECM, including glycoproteins, collagens, and growthfactors. Proteolytic cleavage of the extracellular domain, including the HS chain, is know as shedding (Manon-Jensen and others, 2010). Enzymes that participate in this process are called sheddases and include members of the matrix metalloproteinases family (MMPs) and members of the ADAMTS family (disintegrin and metalloproteinase with thrombospondin motifs). Shedding serves two functions; on one hand, it may downregulate signal transduction, on the other, it converts the extracellular receptor domain into a soluble effector or antagonist. Studies on the developing rat heart (Asundi and others, 1997) show that high levels of syndecan 3, moderate levels of syndecan 2, and low levels of syndecan 1 are found in the adult heart. Furthermore, it was demonstrated using primary cultures of cardiomyocytes and non-cardiomyocytes, that syndecan 3 is not expressed by the myocardium but instead by the cardiac fibroblasts of the postnatal heart.
Hyaluronan and Proteoglycan Link Proteins
Hyaluronan and Proteoglycan Link Proteins (Haplns) form a family of ECM proteins consisting of four members; Hapln1 (better known as Cartilage link protein 1/Crtl1), Hapln2 (or Brain link protein 1), Hapln3 (or Lp3/Osteoblast-Habp), and Hapln4 (or Brain link protein 2) (Spicer and others, 2003). Each Hapln isoform contains two proteoglycan tandem repeats that are conserved among other hyaluronan (HA) binding proteins such as chondroitin sulfate proteoglycans (CSPGs). Interestingly, in the vertebrate genome every single HAPLN gene is found to be located immediately adjacent to one of the four CSPG genes, resulting in the following HAPLN/CSPG pairings: HAPLN 1/CSP G2(versican), HAPLN2/BCAN(brevican), HAPLN3/CSPG1(aggrecan), and HAPLN4/CSPG3(neurocan).
Of the four Hapln isoforms, only Crtl1 (Hapln1) and Hapln3 have been reported to be expressed in the developing heart (Lincoln and others, 2007; Ogawa and others, 2004; Wirrig and others, 2007). Moreover, only Crtl1 has been demonstrated to play an essential role in cardiac development (Wirrig and others, 2007). Crtl1 is an ECM protein that functions to stabilize the interaction between hyaluronan and proteoglycans such as aggrecan, versican, and neurocan (Binette and others, 1994; Matsumoto and others, 2003; Rauch and others, 2004; Shi and others, 2004). While Crtl1 is best known for its role in cartilage formation, where it stabilizes aggregates of aggrecan and hyaluronan to give cartilage its tensile strength and elasticity (Hardingham, 1979), it also mediates and stabilizes the binding between versican and HA (Matsumoto and others, 2003; Shi and others, 2004).
In the developing heart, Crtl1 is expression is first seen in the mesenchyme of the AV and OFT cushions and in the endocardial lining of the developing heart between ED9.5 and ED10.5 where it is co-expressed with its putative cardiac binding partners hyaluronan and versican (Wirrig and others, 2007). By ED13, the expression of Crtl1 becomes largely restricted to the endocardially-derived mesenchymal tissues of the AV complex (Figure 1), the endocardially-derived cells in the distal-most part of the OFT cushions, and to the endocardially-derived cells in the most proximal part of the OFT cushions. At late fetal stages, Crtl1 expression is virtually restricted to the leaflets of tricuspid valve (Figure 2). The leaflets of the mitral valve show, however, very little expression and virtually no expression is seen in any other part of the heart (Wirrig and others, 2007).
The importance of Crtl1 in development was initially demonstrated in publications focusing on the role of Crtl1 in cartilage and bone formation. In these papers it was demonstrated that homozygous Crtl1 knock-out animals are characterized by dwarfism and craniofacial abnormalities. Reportedly, most homozyotes that survive the pre-natal period die shortly after birth (Czipri and others, 2003; Watanabe and Yamada, 1999).
Analysis of the cardiac phenotype of Crtl1-deficient mouse embryos revealed a spectrum of cardiac malformations, including atrial and atrioventricular septal defects due to hypoplasia of the mesenchymal-derived structures. Myocardial defects, including thinning of the ventricular walls and septum and muscular ventricular septal defects, were also observed (Wirrig and others, 2007).
While the abnormalities observed in the endocardially-derived cushion tissues are probably resulting from perturbation of one of the processes involved in cushion formation (e.g. EMT, migration, proliferation, and differentiation), the myocardial proliferation-defects found in the ventricular myocardial walls of the Crtl1 knockout mouse are probably associated with the absence of expression of Crtl1 in the endocardium lining of the developing chamber myocardium at early stages of development. This suggests that Crtl1, in all likelihood through interactions with its putative cardiac binding partners HA and versican, is involved in the regulation of myocardial proliferation.
II: Collagens
In vertebrates, there are at least 28 different types of collagen (Gordon and Hahn, 2010) and in the developing heart several of them are expressed. Collagen types I, III, V, and VI are found in the ventricular myocardium, the developing AV valves and the chordae tendineae. In contrast, collagen II, IV, XI, and XIII appear to be essentially restricted to the developing valve structures and the tendonous apparatus (Favor and others, 2007; Hinton and others, 2006; Lincoln and others, 2004; Lincoln and others, 2006; Liu and others, 1997; Metsaranta and others, 1992; Poschl and others, 2004). Collagen is primarily produced by the developing mesenchyme and fibroblasts. Within the AV valves, collagens form a dense, ordered fibrous network on the ventricularis side of the leaflet, while on the atrialis side of the leaflet, collagens are more loosely packed, creating a spongy ECM (Icardo and Colvee, 1995). In addition, two non-fibrillar collagens, types XV and XVIII, are expressed in the developing and adult heart (Halfter and others, 1998; Saarela and others, 1998). Below we have summarized some of the heart development-related data published on collagen as relate to cardiac development. It is noteworthy that relatively little work has been done to elucidate the roles of collagens in early stages of development.
Collagen type I, III, V (fibrillar collagens)
In the mouse, collagen type I is initially expressed throughout the developing leaflets of the AV valves, gradually becoming restricted to the ventricular side of the leaflets after birth (Kruithof and others, 2007; Lincoln and others, 2004). Collagen I is also expressed in the aortic wall and important for maintanance of this tissue’s elasticity and integrity. Mice that lack a large portion intron 1 of the Col1a1 gene survive to adulthood but are more susceptible to aortic dissection and rupture. Mice with an insertional mutation in intron 1, Mov13 mice, are embryonic lethal at E12-14 due to rupturing of developing vessels (Lohler and others, 1984; Rahkonen and others, 2004).
In the chick, collagen type III is co-expressed with aggrecan in the fibrosa layer of the maturing cushion until the juvenile stage (Hinton and others, 2006). Collagen type III is also expressed in the vasculature of mice. The Col3a1 knockout mouse shows a phenotype similar to that seen in the Col1a1 Intron 1 deletion mouse, as they die late in adult life from rupturing of blood vessels (Liu and others, 1997).
Collagen type V is seen throughout the mural leaflet at E18.5 but becomes restricted to the ventricular side of the leaflet by 8 weeks of age (Kruithof and others, 2007). At E18.5, Collagen V is one of the first collagens to appear in the annulus fibrosus and it remains expressed within this tissue until adulthood (Kruithof and others, 2007). Col5a1 knockout mice have significantly decreased collagen fibril formation and are embryonic lethal at ED10 due to cardiovascular insufficiency (Wenstrup and others, 2004).
Collagen type VI (a network-forming collagen)
Collagen type VI expression has been documented in the developing AV cushions and the adult AV valves of a number of species (Bonaldo and others, 1998; Gittenberger-de Groot and others, 2003; Klewer and others, 1998; Kruithof and others, 2007). In addition, collagen type VI is seen in the myocardium and epicardium of the developing heart as well as in the aorta and pulmonary vasculature where its expression is thought to be necessary for providing tensile strength to the surrounding tissue (Klewer and others, 1998). It is noteworthy that the genes for the alpha 1 and alpha 2 chains of collagen type VI are located on chromosome 21 within a region associated with Down Syndrome related CHD. Although there is increased expression of collagen type VI within the AV valves in the hearts of human fetuses with Down Syndrome, there is no evidence for the involvement of collagen type VI in the pathogenesis of the congenital defects found in Down Syndrome patients (Gittenberger-de Groot and others, 2003).
Collagen types XV and XVIII (MULTIPLEXIN collagens)
Type XV and XVIII collagens are nonfibrillar collagens and belong to the MULTIPLEXIN (“multiple triple-helix domains with interruptions”) subgroup of the collagen family that is unique in its multi-domain structure. Both collagen XV and XVIII have associated GAG chains (which also classifies them as proteoglycans), C-terminal regions that can be cleaved to generate endostatin or restin, and are localized in basement membranes.
Collagen XV is expressed in basement membranes and the capillaries of the myocardium during development and adulthood. Collagen XV deficient mice have defects in vessel architecture and are more susceptible to cardiomyopathy under conditions of increased cardiac load (Eklund and others, 2001; Muona and others, 2002; Rasi and others).
Collagen XVIII can be alternatively spliced to form multiple variants. It has a c-terminal non-collagenous domain that can undergo proteolytic cleavage to produce the biologically active fragment endostatin (Saarela and others, 1998). Collagen XVIII is expressed in the endocardial and myocardial basement membrane during early AV cushion development and later becomes localized to the AV and semilunar valves (Carvalhaes and others, 2006). Col18a1 knockout mice are characterized by a thickening of the endothelial basement membrane surrounding the AV valves (Utriainen and others, 2004).
Collagen type XI
Collagen XI is thought to be involved in the regulation of collagen fibril initiation and assembly. In the developing heart it is expressed in the developing AV valves as well as in the myocardial component of the trabeculae of the ventricles. It is, however, not found in the compact layer of the ventricular myocardium (Lincoln and others, 2006). Collagen XI deficient mice die at birth, presumably resulting from respiratory failure. Analysis of late fetal collagen XI null specimens reveals an increase in the thickness of the interventricular septum, thickening of the AV valve leaflets, and a pronounced change in the overall shape of the heart. The thickened leaflets show a prominent increase in the expression of collagen I, pointing to a compensatory mechanism in collagen production in the hearts of these animals (Lincoln and others, 2006).
III. Periostin
Periostin is a 90kDa secreted ECM protein belonging to the fascilin gene family (Horiuchi 1999). Periostin can interact with fibronectin, tenascin-C, collagen I, collagen V, and itself (Conway and Molkentin, 2008; Kii and others, 2006; Norris and others, 2007). In the chick, periostin expression is first observed at stage 17 in the endothelial lining of the right ventricle and then extends to the OFT cushions at stage 18. By stage 21, the periostin expression domain includes the endothelium of the trabeculae, the AV and the OFT cushions. As the chick heart matures, periostin expression is observed in the left AV valve, the chordae tendineae, and the semilunar valves but not in the right AV valve (Norris and others, 2004). It is noteworthy to mention that in the chick the right AV valve is predominantly muscular in nature. The function of periostin, a TGFbeta-3 responsive gene, appears to be to mediate the maturation of the AV valve in the chick by promoting mesenchymal-to-fibroblast differentiation while at the same time blocking the differentiation of other cell types, in particular cardiomyocytes (Norris and others, 2009). The expression pattern of periostin in the mouse is reminiscent to that seen in the chick. Initially, periostin is observed in the AV and OFT cushions, in the endocardium, and in the epicardium. As the heart matures, periostin becomes localized to both the mitral and tricuspid valves, the chordae tendineae, and the semilunar valves (Kruzynska-Frejtag and others, 2001; Norris and others, 2008).
Periostin knockout mice (Kii and others, 2006) and PerilacZ-null mice (Rios and others, 2005) exhibit shortened and thickened mitral and tricuspid leaflets (Norris and others, 2008; Snider and others, 2008). The leaflets show reduced collagen expression (Snider and others, 2008) and ectopically express cardiac muscle markers indicating abnormal muscularization of the valvular tissues (Norris and others, 2008; Snider and others, 2008). The chordae tendineae of periostin null mice are also shorter and thicker than that of wildtype mice and, in addition, some periostin knockout mice exhibit atrial septal defects (Norris and others, 2008).
IV. Proteases
ADAMTS isoforms
The ADAMTS (a disintegrin and metalloprotease with thrombospondin repeats) family of ECM metalloproteases consists of 19 related genes which play important roles in the regulation of many different processes in cells and tissues. Three family members, ADAMTS1, ADAMTS5, and ADAMTS9 have been reported to be expressed in the developing heart, each having a unique expression domain. Degradation of ECM molecules by proteases, including members of the ADAMTS family, has been demonstrated to be necessary for maturation of the AV cushions, trabeculation, and outflow tract remodeling (Kern and others, 2007; Kern and others, 2006; Stankunas and others, 2008).
ADAMTS1
Cleavage of versican by ADAMTS1 has been demonstrated to be necessary for AV cushion, OFT, and trabecular development (Kern and others, 2007; Kern and others, 2006; Stankunas and others, 2008). Cardiac expression of ADAMTS1 is found as early as ED9.5. The expression of ADAMTS1 is initially observed in the AV endocardium and AV cushion mesenchyme where it overlaps with that of its co-factor Fibulin1, its substrate versican V0/V1, and its versican cleavage product (Kern and others, 2006). Additionally, ADAMTS1 is expressed at relatively low levels in the endocardium and myocardium of the ventricles up to ED12.5-14.5, when it becomes robustly expressed in the ventricular endocardium (Stankunas and others, 2008). At later stages, ADAMTS1 is also found in the smooth muscle cells of the tunica media of the dorsal aortae and the pulmonary artery (Thai and Iruela-Arispe, 2002). Based on a series of observations it has been suggested that proteolytic cleavage of versican by ADAMTS1 during AV cushion development may reduce cell-cell adhesion of the AV endocardium and newly formed mesenchyme and promote proliferation of the AV mesenchymal cells (Kern and others, 2006). Proteolytic cleavage of intact versican V1 isoform by ADAMTS1 is also thought to be responsible for the loss of cell-cell association of the outflow tract cardiomyoctyes, resulting in a thinning of the OFT myocardium, and allowing for the normal process of OFT remodeling to occur (Kern and others, 2007). In the developing ventricles, ADAMTS1 transcription is believed to be mediated by Brg1, which represses ADAMTS1 expression in the ventricular endocardium until approximately ED12.5-14.5 when repression is lifted (Stankunas and others, 2008). High levels of ADAMTS1 expression in the ventricular endocardium between ED12.5-14.5 are thought to contribute to cessation of trabeculation and compaction of the ventricular myocardium through degradation of versican in the ventricular cardiac jelly (Stankunas and others, 2008). In fact, abnormalities in myocardial trabeculation are observed in ADAMTS1 knockout mice. The ventricular chambers of these mice are characterized by hypertrabeculation. It is suggested that this is the result of persisting expression of versican in the ventricular cardiac jelly surrounding the trabeculae (Stankunas and others, 2008).
ADAMTS5
A detailed analysis of ADAMTS5 expression in the developing heart has to date not been reported. However, published data using an ADAMTS5lacZ mouses show that at 16.5ED ADAMTS5 is expressed in the aortic wall of the OFT as well as in the the developing semilunar valve leaflets. Furthermore, expression is also seen in the endocardial lining of the cardiac chambers and in scattered cells throughout the myocardium (McCulloch and others, 2009). Despite this cardiac expression profile ADAMTS5 knockout mice are reportedly phenotypically normal (Majumdar and others, 2007; McCulloch and others, 2009).
ADAMTS9
ADAMTS9 is expressed as early as ED9.5 in the developing heart tube where it is expressed in SHF-derived cells of the developing OFT (Kern and others, 2010). At ED11.5 ADAMTS9 is found in the mesenchyme surrounding the dorsal aortae and in the myocardium and right-sided endocardial cushion of the OFT (Jungers and others, 2005; Kern and others). As cardiac development progresses ADAMTS9 continues to be expressed in the aortic wall and is in the leaflets of the aortic valve, aortic valvulosinus, interstitial cells of the mitral valve, and the mitral valve hinge region that all develop as a result of OFT remodeling. In these tissues, ADAMTS9 is co-expressed with the cleaved versican isoform recognized by the anti-DPEAAE antibody, indicating that ADAMTS9 is playing an active role in remodeling of these tissues. To study the importance of ADAMTS9 for heart development a mouse model carrying a lacZ insertion in exon 12 was used. Mice homozygous for this null allele, ADAMTS9lacZ/lacZ die by ED7.5. Haploinsufficient animals, however, survive into adulthood but show a spectrum of cardiac anomalies believed to be related to an accumulation of versican within the myocardium and valves. The valvular abnormalities include cartilage-like nodules within the valvular sinus, malformed semi-lunar valves, enlarged aortic leaflets, and myxomatous mitral valves. The myocardium of ADAMTS9+/lacZ mice exhibits defects in compaction. This results in a “spongy” myocardium that features abnormal projections into the ventricular space (Kern and others, 2010). This “spongy myocardium” is phenotypically reminiscent of a condition known in humans as “left ventricular noncompaction LVNC” which is a rare and unclassified cardiomyopathy (Sarma and others, 2010).
V: Fibulins
The fibulin gene family consists of 7 members. Each fibulin molecule has a common structural signature consisting of a series of repeated EGFlike domains followed by a C-terminal element referred to as a ‘fibulin module’ (Argraves and others, 2003). During murine cardiac development In mouse embryos, both bulin-1and fibulin-2 are expressed at sites of EMT and cell migration.
Fibulin 1
Fibulin-1 can bind a variety of ECM molecules, including aggrecan and versican, which are known targets for cleavage by ECM metalloproteases (Cooley and others, 2008; Kern and others, 2006; Lee and others, 2005). The alleged role for Fibulin 1 is to simultaneously interact with the carboxy-terminal of ADAMTS1 as well as with ADAMTS1 substrates, thereby bringing the molecules closer together and enhancing proteolytic cleavage of the substrate (Cooley and others, 2008; Kern and others, 2006; Lee and others, 2005). At ED9.0-9.5 in the mouse, fibulin 1 is expressed in the dorsal neural tube and associated with the developing cardiac neural crest cells. By ED10.5, fibulin 1 is found in pharyngeal arches 3–4 and the distal OFT cushions (Cooley and others, 2008; Spence and others, 1992). Fibulin 1 is also expressed throughout the extracellular matrix of the AV cushions (ED9.5-ED14.5), eventually becoming restricted to the elongating portions of the AV valves (Kern and others, 2006). Fibulin 1 has been implicated in the regulation of cell migration of the AV mesenchyme. Addition of fibulin 1 to collagen gels containing fibronectin resulted in a significant reduction in the number of invading mesenchymal cells and the distance over which the mesenchymal cells migrated (Twal and others, 2001). It is believed that this effect of fibulin 1 on the migratory response of AV mesenchymal cells is related to its role, as a co-factor to ADAMTS1, in the cleavage of ECM molecules known to promote migration, such as versican V0/V1 (Kern and others, 2006; Twal and others, 2001). Additional evidence for the role of Fibulin 1 in heart development comes from studies on the Fibulin 1 knockout mouse (Cooley and others, 2008). Fibulin deficient mice die perinatally and are characterized by a number of cardiac malformations; approximately half of fibulin 1 knockout animals present with atrial septal defects and a thinning of the ventricular myocardium. Additionally, there is a high incidence of abnormalities typically associated with defects in cardiac neural crest, such as abnormal remodeling of the aortic arch arteries, double outlet right ventricle, overriding aorta, and ventricular septal defects (Cooley and others, 2008). In most cases, the ventricular septal defects are secondary to malrotation of the outflow tract as both outflow vessels are connected to the right ventricle, thus preventing blood flow from the left ventricle except through an abnormal opening in the interventricular septum. These data demonstrate that fibulin 1 is essential for migration of the cardiac neural crest into the aortic arches and the ouflow tract cushions, for proper rotation of the great vessels, and for promoting versican cleavage during AV cushion morphogenesis by acting as a cofactor to ADAMTS1 (Cooley and others, 2008; Kern and others, 2006; Twal and others, 2001).
VI: Fibronectin
Fibronectin is a multi-domain ECM protein that interacts with multiple integrins, heparan sulfate proteoglycans, collagens, and fibrins to mediate cellular behaviors (Pankov and Yamada, 2002). Fibronectin can be alternatively spliced at its EIIIA, EIIIB, and V exons to yield a multitude of splice variants that are differentially expressed in development and adulthood. The EIIIA and EIIIB exons are especially significant in development as they are included in the majority of embryonic fibronectin mRNAs, but have been detected in very few adult tissues (ffrench-Constant, 1995). Fibronectin is expressed early in embryonic development in the mesoderm and in the space between the embryonic germ layers (Ffrench-Constant and Hynes, 1989; George and others, 1993). As cardiac development progresses, EIIIA- and EIIIB-containing fibronectins are expressed in the dorsal aortae, pharyngeal arch arteries and the endocardium, (Astrof and others, 2007b; Ffrench-Constant and Hynes, 1989). Fibronectin is also expressed in the mesenchyme of the endocardial cushions where it is required for EMT-mediated development (Icardo and others, 1992; Mjaatvedt and others, 1987).
In fibronectin deficient embryos, developmental abnormalities are first seen at ED8.0 with the most severely affected mutants failing to undergo fusion of the PHF into a primitive heart tube. Embryos that do form a heart tube have a thickened myocardium, lack of cardiac jelly, and abnormal endocardium. Fibronectin deficient embryos do not survive beyond ED10 due to cardiovascular and vascular defects (George and others, 1993; Georges-Labouesse and others, 1996). Because of the importance of alternative splicing of fibronectin during development, mice lacking both the fibronectin EIIIA and EIIIB exons were generated. While individually fibronectin EIIIA and fibronectin EIIIB nulls are phenotypically normal, the combined fibronectin EIIIA/EIIIB knockout mouse is embryonic lethal at ED9.5-10.5 (Astrof and others, 2004; Tan and others, 2004). Cardiovascular defects become apparent in fibronectin EIIIA/EIIIB double knockouts where the embryos fail to form normal endocardial cushions, have a thin outflow tract, and are prone to vascular hemorrhage (Astrof and others, 2007a).
VII: Fibrillin
Fibrillin is an elastic-type ECM molecule which forms microfibrils along with other ECM molecules such as elastin (Hurle and others, 1994; Sakai and others, 1986; Visconti and others, 2003). Fibrillin is expressed in the developing heart, associated with the basal surface of the early endocardium of both the AV and OFT cushions. Prior to EMT, fibrillin appears to be concentrated under the endocardial surface and, subsequent to EMT, fine fibrillar tracts can be found associated with the migrating cells (Hurle and others, 1994). Fibrillin expression persists in the AV valve leaflets until late stages of development (Hurle and others, 1994). Following septation of the OFT, intense fibrillin staining is observed in the walls of both the pulmonary artery and the aorta (Hurle and others, 1994; Sakai and others, 1986).
Mutations in fibrillin 1 gene (FBN1) are associated with a systemic disorder of connective tissue known as Marfan’s syndrome (Judge and others, 2004). The incidence of Marfan’s is about 2–3 per 10000 individuals (Judge and Dietz, 2005). The cardiac abnormality found most frequently in Marfan patients is thickening of the AV valves which is often associated with mitral and/or tricuspid valve prolapse. Aortic aneurysm and dissection is the most serious and life-threatening cardiovascular abnormality in Marfan’s syndrome. Mutant mice harboring a missense mutation in the fibrillin 1 gene are characterized by a phenotype that recapitulates the Marfan syndrome (Judge and others, 2004; Ng and others, 2004). Fibrillin 1 knockout mice die shortly after birth due to rupture (dissection) of aortic aneurysms caused by a severe weakening of the aortic wall (Ng and others, 2004). Heterozygote fibrillin 1 mutants have reduced microfibrils in the aortic wall, which leads to the disorganization of the aortic smooth muscle cells and an abnormal deposition of extracellular matrix molecules (Judge and others, 2004). Both Fibrillin 1 knockout and heterozygote specimens are characterized by mitral valve prolapse (Ng and others, 2004). Histologically, the mitral valves are thickened and elongated in comparison to wild type valves (Ng and others, 2004). There is a high degree of homology between fibrillin 1 and latent transforming growth factor β (TGFβ) binding proteins (Pereira and others, 1993). Based on this homology and on some of the histological features of the Fibrillin deficient mouse, the hypothesis was developed that dysregulation of TGFβ signaling could be involved in the etiology of the abnormalities seen in the knockout mouse and in patients with Marfan’s syndrome. Indeed, in a series of studies on the fibrillin 1 knockout mouse it was found that in the abnormally developing lungs and valves of this mouse, TGFβ signaling was upregulated and that this dysmorphogenesis could be rescued with TGFβ-neutralising antibodies (Neptune and others, 2003; Ng and others, 2004). In addition, it was demonstrated that systematic administration of TGFβ neutralizing antibody also prevented the pathological changes in the aortic root leading to aortic dilatation (Habashi and others, 2006). Based on these results, studies were initiated with the angiotensin II type1 receptor blocker, losartan, known to antagonize TGFβ signaling. Using a heterozygous fibrillin-1 mutant mouse, Fbn1C1039G/+, which develops aortic dilatation reminiscent of those seen in human Marfan patients, it was demonstrated that administration of losartan resulted in normalization of aortic growth in the mutant mouse (Habashi and others, 2006). These and other results have prompted trials to study the effect of losartan in the treatment of patients with Marfan’s syndrome (Detaint and others, 2010; Moberg and others, 2011; Radonic and others, 2010).
Conclusion and Perspectives
The extracellular matrix (ECM) is involved in all aspects of heart development and, although not emphasized in this review, plays a critical role in important post-natal remodeling events in health and disease. The mechanisms through which ECM molecules regulate cardiac morphogenesis are in many cases still poorly understood. Increasing our fundamental knowledge of ECM-controled steps in early heart development may be very valuable in elucidating pathways and mechanisms involved in the pathology of the hearts of patients suffering from cardiac disease.
Acknowledgments
This work was supported by NIH grants C06 RR018823 and C06 RR015455, NIH grant T-32 HL07260 (to M.L), NCRR grant P20-RR016434 (to A.W.), NHLBI grant R01HL084285 (to A.W.), R01HL094319 (to E.W.) and American Heart Association 09GRNT2060075 (to A.W.).
References
- Abu-Issa R, Kirby ML. Heart field: from mesoderm to heart tube. Annu Rev Cell Dev Biol. 2007;23:45–68. doi: 10.1146/annurev.cellbio.23.090506.123331. [DOI] [PubMed] [Google Scholar]
- Argraves WS, Greene LM, Cooley MA, Gallagher WM. Fibulins: physiological and disease perspectives. EMBO Rep. 2003;4(12):1127–1131. doi: 10.1038/sj.embor.7400033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrof S, Crowley D, George EL, Fukuda T, Sekiguchi K, Hanahan D, Hynes RO. Direct test of potential roles of EIIIA and EIIIB alternatively spliced segments of fibronectin in physiological and tumor angiogenesis. Mol Cell Biol. 2004;24(19):8662–8670. doi: 10.1128/MCB.24.19.8662-8670.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrof S, Crowley D, Hynes RO. Multiple cardiovascular defects caused by the absence of alternatively spliced segments of fibronectin. Dev Biol. 2007a;311(1):11–24. doi: 10.1016/j.ydbio.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrof S, Kirby A, Lindblad-Toh K, Daly M, Hynes RO. Heart development in fibronectin-null mice is governed by a genetic modifier on chromosome four. Mech Dev. 2007b;124(7–8):551–558. doi: 10.1016/j.mod.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Asundi VK, Keister BF, Stahl RC, Carey DJ. Developmental and cell-type-specific expression of cell surface heparan sulfate proteoglycans in the rat heart. Exp Cell Res. 1997;230(1):145–153. doi: 10.1006/excr.1996.3400. [DOI] [PubMed] [Google Scholar]
- Baldwin HS, Lloyd TR, Solursh M. Hyaluronate degradation affects ventricular function of the early postlooped embryonic rat heart in situ. Circ Res. 1994;74(2):244–252. doi: 10.1161/01.res.74.2.244. [DOI] [PubMed] [Google Scholar]
- Barbosky L, Lawrence DK, Karunamuni G, Wikenheiser JC, Doughman YQ, Visconti RP, Burch JB, Watanabe M. Apoptosis in the developing mouse heart. Dev Dyn. 2006;235(9):2592–2602. doi: 10.1002/dvdy.20885. [DOI] [PubMed] [Google Scholar]
- Battaglia C, Mayer U, Aumailley M, Timpl R. Basement-membrane heparan sulfate proteoglycan binds to laminin by its heparan sulfate chains and to nidogen by sites in the protein core. Eur J Biochem. 1992;208(2):359–366. doi: 10.1111/j.1432-1033.1992.tb17195.x. [DOI] [PubMed] [Google Scholar]
- Bernanke DH, Markwald RR. Effects of hyaluronic acid on cardiac cushion tissue cells in collagen matrix cultures. Tex Rep Biol Med. 1979;39:271–285. [PubMed] [Google Scholar]
- Binette F, Cravens J, Kahoussi B, Haudenschild DR, Goetinck PF. Link protein is ubiquitously expressed in non-cartilaginous tissues where it enhances and stabilizes the interaction of proteoglycans with hyaluronic acid. J Biol Chem. 1994;269(29):19116–19122. [PubMed] [Google Scholar]
- Bonaldo P, Braghetta P, Zanetti M, Piccolo S, Volpin D, Bressan GM. Collagen VI deficiency induces early onset myopathy in the mouse: an animal model for Bethlem myopathy. Hum Mol Genet. 1998;7(13):2135–2140. doi: 10.1093/hmg/7.13.2135. [DOI] [PubMed] [Google Scholar]
- Bowers SL, Banerjee I, Baudino TA. The extracellular matrix: at the center of it all. J Mol Cell Cardiol. 2010;48(3):474–482. doi: 10.1016/j.yjmcc.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai CL, Martin JC, Sun Y, Cui L, Wang L, Ouyang K, Yang L, Bu L, Liang X, Zhang X, Stallcup WB, Denton CP, McCulloch A, Chen J, Evans SM. A myocardial lineage derives from Tbx18 epicardial cells. Nature. 2008;454(7200):104–108. doi: 10.1038/nature06969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camenisch TD, Schroeder JA, Bradley J, Klewer SE, McDonald JA. Heart-valve mesenchyme formation is dependent on hyaluronan-augmented activation of ErbB2-ErbB3 receptors. Nat Med. 2002;8(8):850–855. doi: 10.1038/nm742. [DOI] [PubMed] [Google Scholar]
- Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A, Jr, Kubalak S, Klewer SE, McDonald JA. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000;106(3):349–360. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalhaes LS, Gervasio OL, Guatimosim C, Heljasvaara R, Sormunen R, Pihlajaniemi T, Kitten GT. Collagen XVIII/endostatin is associated with the epithelial-mesenchymal transformation in the atrioventricular valves during cardiac development. Dev Dyn. 2006;235(1):132–142. doi: 10.1002/dvdy.20556. [DOI] [PubMed] [Google Scholar]
- Conway SJ, Molkentin JD. Periostin as a heterofunctional regulator of cardiac development and disease. Curr Genomics. 2008;9(8):548–555. doi: 10.2174/138920208786847917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley MA, Kern CB, Fresco VM, Wessels A, Thompson RP, McQuinn TC, Twal WO, Mjaatvedt CH, Drake CJ, Argraves WS. Fibulin-1 is required for morphogenesis of neural crest-derived structures. Developmental biology. 2008;319(2):336–345. doi: 10.1016/j.ydbio.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costell M, Carmona R, Gustafsson E, Gonzalez-Iriarte M, Fassler R, Munoz-Chapuli R. Hyperplastic conotruncal endocardial cushions and transposition of great arteries in perlecan-null mice. Circulation research. 2002;91(2):158–164. doi: 10.1161/01.res.0000026056.81424.da. [DOI] [PubMed] [Google Scholar]
- Craig EA, Austin AF, Vaillancourt RR, Barnett JV, Camenisch TD. TGFbeta2-mediated production of hyaluronan is important for the induction of epicardial cell differentiation and invasion. Exp Cell Res. 2010a;316(20):3397–3405. doi: 10.1016/j.yexcr.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig EA, Parker P, Austin AF, Barnett JV, Camenisch TD. Involvement of the MEKK1 signaling pathway in the regulation of epicardial cell behavior by hyaluronan. Cell Signal. 2010b;22(6):968–976. doi: 10.1016/j.cellsig.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czipri M, Otto JM, Cs-Szabo G, Kamath RV, Vermes C, Firneisz G, Kolman KJ, Watanabe H, Li Y, Roughley PJ, Yamada Y, Olsen BR, Glant TT. Genetic rescue of chondrodysplasia and the perinatal lethal effect of cartilage link protein deficiency. J Biol Chem. 2003;278(40):39214–39223. doi: 10.1074/jbc.M303329200. [DOI] [PubMed] [Google Scholar]
- Daley WP, Peters SB, Larsen M. Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci. 2008;121(Pt 3):255–264. doi: 10.1242/jcs.006064. [DOI] [PubMed] [Google Scholar]
- DeBaun MR, Ess J, Saunders S. Simpson Golabi Behmel syndrome: progress toward understanding the molecular basis for overgrowth, malformation, and cancer predisposition. Mol Genet Metab. 2001;72(4):279–286. doi: 10.1006/mgme.2001.3150. [DOI] [PubMed] [Google Scholar]
- Detaint D, Aegerter P, Tubach F, Hoffman I, Plauchu H, Dulac Y, Faivre LO, Delrue MA, Collignon P, Odent S, Tchitchinadze M, Bouffard C, Arnoult F, Gautier M, Boileau C, Jondeau G. Rationale and design of a randomized clinical trial (Marfan Sartan) of angiotensin II receptor blocker therapy versus placebo in individuals with Marfan syndrome. Arch Cardiovasc Dis. 2010;103(5):317–325. doi: 10.1016/j.acvd.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Dundar M, Muller T, Zhang Q, Pan J, Steinmann B, Vodopiutz J, Gruber R, Sonoda T, Krabichler B, Utermann G, Baenziger JU, Zhang L, Janecke AR. Loss of dermatan-4-sulfotransferase 1 function results in adducted thumb-clubfoot syndrome. Am J Hum Genet. 2009;85(6):873–882. doi: 10.1016/j.ajhg.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer LA, Kirby ML. The role of secondary heart field in cardiac development. Dev Biol. 2009;336(2):137–144. doi: 10.1016/j.ydbio.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg LM, Markwald RR. Molecular regulation of atrioventricular valvuloseptal morphogenesis. Circ Res. 1995;77(1):1–6. doi: 10.1161/01.res.77.1.1. [DOI] [PubMed] [Google Scholar]
- Eklund L, Piuhola J, Komulainen J, Sormunen R, Ongvarrasopone C, Fassler R, Muona A, Ilves M, Ruskoaho H, Takala TE, Pihlajaniemi T. Lack of type XV collagen causes a skeletal myopathy and cardiovascular defects in mice. Proc Natl Acad Sci U S A. 2001;98(3):1194–1199. doi: 10.1073/pnas.031444798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favor J, Gloeckner CJ, Janik D, Klempt M, Neuhauser-Klaus A, Pretsch W, Schmahl W, Quintanilla-Fend L. Type IV procollagen missense mutations associated with defects of the eye, vascular stability, the brain, kidney function and embryonic or postnatal viability in the mouse, Mus musculus: an extension of the Col4a1 allelic series and the identification of the first two Col4a2 mutant alleles. Genetics. 2007;175(2):725–736. doi: 10.1534/genetics.106.064733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ffrench-Constant C. Alternative splicing of fibronectin--many different proteins but few different functions. Exp Cell Res. 1995;221(2):261–271. doi: 10.1006/excr.1995.1374. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant C, Hynes RO. Alternative splicing of fibronectin is temporally and spatially regulated in the chicken embryo. Development. 1989;106(2):375–388. doi: 10.1242/dev.106.2.375. [DOI] [PubMed] [Google Scholar]
- Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123(Pt 24):4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119(4):1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- Georges-Labouesse EN, George EL, Rayburn H, Hynes RO. Mesodermal development in mouse embryos mutant for fibronectin. Dev Dyn. 1996;207(2):145–156. doi: 10.1002/(SICI)1097-0177(199610)207:2<145::AID-AJA3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Gittenberger-de Groot AC, Bartram U, Oosthoek PW, Bartelings MM, Hogers B, Poelmann RE, Jongewaard IN, Klewer SE. Collagen type VI expression during cardiac development and in human fetuses with trisomy 21. Anat Rec A Discov Mol Cell Evol Biol. 2003;275(2):1109–1116. doi: 10.1002/ar.a.10126. [DOI] [PubMed] [Google Scholar]
- Goddeeris MM, Rho S, Petiet A, Davenport CL, Johnson GA, Meyers EN, Klingensmith J. Intracardiac septation requires hedgehog-dependent cellular contributions from outside the heart. Development. 2008;135(10):1887–1895. doi: 10.1242/dev.016147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MK, Hahn RA. Collagens. Cell Tissue Res. 2010;339(1):247–257. doi: 10.1007/s00441-009-0844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, Podowski M, Neptune ER, Halushka MK, Bedja D, Gabrielson K, Rifkin DB, Carta L, Ramirez F, Huso DL, Dietz HC. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312(5770):117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfter W, Dong S, Schurer B, Cole GJ. Collagen XVIII is a basement membrane heparan sulfate proteoglycan. J Biol Chem. 1998;273(39):25404–25412. doi: 10.1074/jbc.273.39.25404. [DOI] [PubMed] [Google Scholar]
- Handler M, Yurchenco PD, Iozzo RV. Developmental expression of perlecan during murine embryogenesis. Dev Dyn. 1997;210(2):130–145. doi: 10.1002/(SICI)1097-0177(199710)210:2<130::AID-AJA6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Hardingham TE. The role of link-protein in the structure of cartilage proteoglycan aggregates. Biochem J. 1979;177(1):237–247. doi: 10.1042/bj1770237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgenberg LG, Pham B, Ortega M, Walid S, Kemmerly T, O’Dowd DK, Smith MA. Agrin regulation of alpha3 sodium-potassium ATPase activity modulates cardiac myocyte contraction. J Biol Chem. 2009;284(25):16956–16965. doi: 10.1074/jbc.M806855200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton RB, Jr, Lincoln J, Deutsch GH, Osinska H, Manning PB, Benson DW, Yutzey KE. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ Res. 2006;98(11):1431–1438. doi: 10.1161/01.RES.0000224114.65109.4e. [DOI] [PubMed] [Google Scholar]
- Hurle JM, Kitten GT, Sakai LY, Volpin D, Solursh M. Elastic extracellular matrix of the embryonic chick heart: an immunohistological study using laser confocal microscopy. Dev Dyn. 1994;200(4):321–332. doi: 10.1002/aja.1002000407. [DOI] [PubMed] [Google Scholar]
- Icardo JM, Colvee E. Atrioventricular valves of the mouse: III. Collagenous skeleton and myotendinous junction. Anat Rec. 1995;243(3):367–375. doi: 10.1002/ar.1092430311. [DOI] [PubMed] [Google Scholar]
- Icardo JM, Nakamura A, Fernandez-Teran MA, Manasek FJ. Effects of injecting fibronectin and antifibronectin antibodies on cushion mesenchyme formation in the chick. An in vivo study. Anat Embryol (Berl) 1992;185(3):239–247. doi: 10.1007/BF00211822. [DOI] [PubMed] [Google Scholar]
- Inai K, Norris RA, Hoffman S, Markwald RR, Sugi Y. BMP-2 induces cell migration and periostin expression during atrioventricular valvulogenesis. Dev Biol. 2008;315(2):383–396. doi: 10.1016/j.ydbio.2007.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge DP, Biery NJ, Keene DR, Geubtner J, Myers L, Huso DL, Sakai LY, Dietz HC. Evidence for a critical contribution of haploinsufficiency in the complex pathogenesis of Marfan syndrome. J Clin Invest. 2004;114(2):172–181. doi: 10.1172/JCI20641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge DP, Dietz HC. Marfan’s syndrome. Lancet. 2005;366(9501):1965–1976. doi: 10.1016/S0140-6736(05)67789-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungers KA, Le Goff C, Somerville RP, Apte SS. Adamts9 is widely expressed during mouse embryo development. Gene Expr Patterns. 2005;5(5):609–617. doi: 10.1016/j.modgep.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Kelly RG, Brown NA, Buckingham ME. The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev Cell. 2001;1(3):435–440. doi: 10.1016/s1534-5807(01)00040-5. [DOI] [PubMed] [Google Scholar]
- Kern CB, Norris RA, Thompson RP, Argraves WS, Fairey SE, Reyes L, Hoffman S, Markwald RR, Mjaatvedt CH. Versican proteolysis mediates myocardial regression during outflow tract development. Dev Dyn. 2007;236(3):671–683. doi: 10.1002/dvdy.21059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern CB, Twal WO, Mjaatvedt CH, Fairey SE, Toole BP, Iruela-Arispe ML, Argraves WS. Proteolytic cleavage of versican during cardiac cushion morphogenesis. Dev Dyn. 2006;235(8):2238–2247. doi: 10.1002/dvdy.20838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern CB, Wessels A, McGarity J, Dixon LJ, Alston E, Argraves WS, Geeting D, Nelson CM, Menick DR, Apte SS. Reduced versican cleavage due to Adamts9 haploinsufficiency is associated with cardiac and aortic anomalies. Matrix Biol. 29(4):304–316. doi: 10.1016/j.matbio.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern CB, Wessels A, McGarity J, Dixon LJ, Alston E, Argraves WS, Geeting D, Nelson CM, Menick DR, Apte SS. Reduced versican cleavage due to Adamts9 haploinsufficiency is associated with cardiac and aortic anomalies. Matrix Biol. 2010;29(4):304–316. doi: 10.1016/j.matbio.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kii I, Amizuka N, Minqi L, Kitajima S, Saga Y, Kudo A. Periostin is an extracellular matrix protein required for eruption of incisors in mice. Biochem Biophys Res Commun. 2006;342(3):766–772. doi: 10.1016/j.bbrc.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Klewer SE, Krob SL, Kolker SJ, Kitten GT. Expression of type VI collagen in the developing mouse heart. Dev Dyn. 1998;211(3):248–255. doi: 10.1002/(SICI)1097-0177(199803)211:3<248::AID-AJA6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Kramer MW, Escudero DO, Lokeshwar SD, Golshani R, Ekwenna OO, Acosta K, Merseburger AS, Soloway M, Lokeshwar VB. Association of hyaluronic acid family members (HAS1, HAS2, and HYAL-1) with bladder cancer diagnosis and prognosis. Cancer. 2010 doi: 10.1002/cncr.25565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruithof BP, Krawitz SA, Gaussin V. Atrioventricular valve development during late embryonic and postnatal stages involves condensation and extracellular matrix remodeling. Dev Biol. 2007;302(1):208–217. doi: 10.1016/j.ydbio.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Kruzynska-Frejtag A, Machnicki M, Rogers R, Markwald RR, Conway SJ. Periostin (an osteoblast-specific factor) is expressed within the embryonic mouse heart during valve formation. Mech Dev. 2001;103(1–2):183–188. doi: 10.1016/s0925-4773(01)00356-2. [DOI] [PubMed] [Google Scholar]
- Lee NV, Rodriguez-Manzaneque JC, Thai SN, Twal WO, Luque A, Lyons KM, Argraves WS, Iruela-Arispe ML. Fibulin-1 acts as a cofactor for the matrix metalloprotease ADAMTS-1. The Journal of biological chemistry. 2005;280(41):34796–34804. doi: 10.1074/jbc.M506980200. [DOI] [PubMed] [Google Scholar]
- Li Q, Loeb JA. Neuregulin-heparan-sulfate proteoglycan interactions produce sustained erbB receptor activation required for the induction of acetylcholine receptors in muscle. The Journal of biological chemistry. 2001;276(41):38068–38075. doi: 10.1074/jbc.M104485200. [DOI] [PubMed] [Google Scholar]
- Lin AE, Neri G, Hughes-Benzie R, Weksberg R. Cardiac anomalies in the Simpson-Golabi-Behmel syndrome. Am J Med Genet. 1999;83(5):378–381. [PubMed] [Google Scholar]
- Lincoln J, Alfieri CM, Yutzey KE. Development of heart valve leaflets and supporting apparatus in chicken and mouse embryos. Dev Dyn. 2004;230(2):239–250. doi: 10.1002/dvdy.20051. [DOI] [PubMed] [Google Scholar]
- Lincoln J, Florer JB, Deutsch GH, Wenstrup RJ, Yutzey KE. ColVa1 and ColXIa1 are required for myocardial morphogenesis and heart valve development. Dev Dyn. 2006;235(12):3295–3305. doi: 10.1002/dvdy.20980. [DOI] [PubMed] [Google Scholar]
- Lincoln J, Kist R, Scherer G, Yutzey KE. Sox9 is required for precursor cell expansion and extracellular matrix organization during mouse heart valve development. Dev Biol. 2007;305(1):120–132. doi: 10.1016/j.ydbio.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wu H, Byrne M, Krane S, Jaenisch R. Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. Proc Natl Acad Sci U S A. 1997;94(5):1852–1856. doi: 10.1073/pnas.94.5.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohler J, Timpl R, Jaenisch R. Embryonic lethal mutation in mouse collagen I gene causes rupture of blood vessels and is associated with erythropoietic and mesenchymal cell death. Cell. 1984;38(2):597–607. doi: 10.1016/0092-8674(84)90514-2. [DOI] [PubMed] [Google Scholar]
- Majumdar MK, Askew R, Schelling S, Stedman N, Blanchet T, Hopkins B, Morris EA, Glasson SS. Double-knockout of ADAMTS-4 and ADAMTS-5 in mice results in physiologically normal animals and prevents the progression of osteoarthritis. Arthritis Rheum. 2007;56(11):3670–3674. doi: 10.1002/art.23027. [DOI] [PubMed] [Google Scholar]
- Manner J. Does the subepicardial mesenchyme contribute myocardioblasts to the myocardium of the chick embryo heart? A quail-chick chimera study tracing the fate of the epicardial primordium. Anat Rec. 1999;255(2):212–226. doi: 10.1002/(sici)1097-0185(19990601)255:2<212::aid-ar11>3.3.co;2-o. [DOI] [PubMed] [Google Scholar]
- Manon-Jensen T, Itoh Y, Couchman JR. Proteoglycans in health and disease: the multiple roles of syndecan shedding. FEBS J. 2010 doi: 10.1111/j.1742-4658.2010.07798.x. [DOI] [PubMed] [Google Scholar]
- Markwald R, Eisenberg C, Eisenberg L, Trusk T, Sugi Y. Epithelial-mesenchymal transformations in early avian heart development. Acta Anat (Basel) 1996;156(3):173–186. doi: 10.1159/000147845. [DOI] [PubMed] [Google Scholar]
- Markwald RR, Fitzharris TP, Manasek FJ. Structural development of endocardial cushions. Am J Anat. 1977;148(1):85–119. doi: 10.1002/aja.1001480108. [DOI] [PubMed] [Google Scholar]
- Martinez-Estrada OM, Lettice LA, Essafi A, Guadix JA, Slight J, Velecela V, Hall E, Reichmann J, Devenney PS, Hohenstein P, Hosen N, Hill RE, Munoz-Chapuli R, Hastie ND. Wt1 is required for cardiovascular progenitor cell formation through transcriptional control of Snail and E-cadherin. Nat Genet. 2010;42(1):89–93. doi: 10.1038/ng.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Li Y, Jakuba C, Sugiyama Y, Sayo T, Okuno M, Dealy CN, Toole BP, Takeda J, Yamaguchi Y, Kosher RA. Conditional inactivation of Has2 reveals a crucial role for hyaluronan in skeletal growth, patterning, chondrocyte maturation and joint formation in the developing limb. Development. 2009;136(16):2825–2835. doi: 10.1242/dev.038505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Shionyu M, Go M, Shimizu K, Shinomura T, Kimata K, Watanabe H. Distinct interaction of versican/PG-M with hyaluronan and link protein. J Biol Chem. 2003;278(42):41205–41212. doi: 10.1074/jbc.M305060200. [DOI] [PubMed] [Google Scholar]
- McCulloch DR, Goff CL, Bhatt S, Dixon LJ, Sandy JD, Apte SS. Adamts5, the gene encoding a proteoglycan-degrading metalloprotease, is expressed by specific cell lineages during mouse embryonic development and in adult tissues. Gene Expr Patterns. 2009 doi: 10.1016/j.gep.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meilhac SM, Esner M, Kelly RG, Nicolas JF, Buckingham ME. The clonal origin of myocardial cells in different regions of the embryonic mouse heart. Dev Cell. 2004;6(5):685–698. doi: 10.1016/s1534-5807(04)00133-9. [DOI] [PubMed] [Google Scholar]
- Mengin-Lecreulx D, van Heijenoort J. Copurification of glucosamine-1-phosphate acetyltransferase and N-acetylglucosamine-1-phosphate uridyltransferase activities of Escherichia coli: characterization of the glmU gene product as a bifunctional enzyme catalyzing two subsequent steps in the pathway for UDP-N-acetylglucosamine synthesis. J Bacteriol. 1994;176(18):5788–5795. doi: 10.1128/jb.176.18.5788-5795.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado-Pimentel ME, Runyan RB. Multiple transforming growth factor-beta isoforms and receptors function during epithelial-mesenchymal cell transformation in the embryonic heart. Cells Tissues Organs. 2007;185(1–3):146–156. doi: 10.1159/000101315. [DOI] [PubMed] [Google Scholar]
- Metsaranta M, Garofalo S, Decker G, Rintala M, de Crombrugghe B, Vuorio E. Chondrodysplasia in transgenic mice harboring a 15-amino acid deletion in the triple helical domain of pro alpha 1(II) collagen chain. J Cell Biol. 1992;118(1):203–212. doi: 10.1083/jcb.118.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mjaatvedt CH, Lepera RC, Markwald RR. Myocardial specificity for initiating endothelial-mesenchymal cell transition in embryonic chick heart correlates with a particulate distribution of fibronectin. Dev Biol. 1987;119(1):59–67. doi: 10.1016/0012-1606(87)90206-5. [DOI] [PubMed] [Google Scholar]
- Mjaatvedt CH, Yamamura H, Capehart AA, Turner D, Markwald RR. The Cspg2 gene, disrupted in the hdf mutant, is required for right cardiac chamber and endocardial cushion formation. Dev Biol. 1998;202(1):56–66. doi: 10.1006/dbio.1998.9001. [DOI] [PubMed] [Google Scholar]
- Moberg K, De Nobele S, Devos D, Goetghebeur E, Segers P, Trachet B, Vervaet C, Renard M, Coucke P, Loeys B, De Paepe A, De Backer J. The Ghent Marfan Trial -A randomized, double-blind placebo controlled trial with losartan in Marfan patients treated with beta-blockers. Int J Cardiol. 2011 doi: 10.1016/j.ijcard.2010.12.070. [DOI] [PubMed] [Google Scholar]
- Moorman AF, Christoffels VM, Anderson RH, van den Hoff MJ. The heart-forming fields: one or multiple? Philos Trans R Soc Lond B Biol Sci. 2007;362(1484):1257–1265. doi: 10.1098/rstb.2007.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muona A, Eklund L, Vaisanen T, Pihlajaniemi T. Developmentally regulated expression of type XV collagen correlates with abnormalities in Col15a1(−/−) mice. Matrix Biol. 2002;21(1):89–102. doi: 10.1016/s0945-053x(01)00187-1. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Manasek FJ. An experimental study of the relation of cardiac jelly to the shape of the early chick embryonic heart. J Embryol Exp Morphol. 1981;65:235–256. [PubMed] [Google Scholar]
- Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet. 2003;33(3):407–411. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- Neri G, Gurrieri F, Zanni G, Lin A. Clinical and molecular aspects of the Simpson-Golabi-Behmel syndrome. Am J Med Genet. 1998;79(4):279–283. doi: 10.1002/(sici)1096-8628(19981002)79:4<279::aid-ajmg9>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Ng A, Wong M, Viviano B, Erlich JM, Alba G, Pflederer C, Jay PY, Saunders S. Loss of glypican-3 function causes growth factor-dependent defects in cardiac and coronary vascular development. Dev Biol. 2009;335(1):208–215. doi: 10.1016/j.ydbio.2009.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng CM, Cheng A, Myers LA, Martinez-Murillo F, Jie C, Bedja D, Gabrielson KL, Hausladen JM, Mecham RP, Judge DP, Dietz HC. TGF-beta-dependent pathogenesis of mitral valve prolapse in a mouse model of Marfan syndrome. The Journal of clinical investigation. 2004;114(11):1586–1592. doi: 10.1172/JCI22715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo ST, Noakes PG, Phillips WD. Neural agrin: a synaptic stabiliser. Int J Biochem Cell Biol. 2007;39(5):863–867. doi: 10.1016/j.biocel.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Niu S, Bahl JJ, Adamson C, Morkin E. Structure, regulation and function of avian glypican. J Mol Cell Cardiol. 1998;30(3):537–550. doi: 10.1006/jmcc.1997.0618. [DOI] [PubMed] [Google Scholar]
- Norris RA, Damon B, Mironov V, Kasyanov V, Ramamurthi A, Moreno-Rodriguez R, Trusk T, Potts JD, Goodwin RL, Davis J, Hoffman S, Wen X, Sugi Y, Kern CB, Mjaatvedt CH, Turner DK, Oka T, Conway SJ, Molkentin JD, Forgacs G, Markwald RR. Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J Cell Biochem. 2007;101(3):695–711. doi: 10.1002/jcb.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris RA, Kern CB, Wessels A, Moralez EI, Markwald RR, Mjaatvedt CH. Identification and detection of the periostin gene in cardiac development. Anat Rec A Discov Mol Cell Evol Biol. 2004;281(2):1227–1233. doi: 10.1002/ar.a.20135. [DOI] [PubMed] [Google Scholar]
- Norris RA, Moreno-Rodriguez RA, Sugi Y, Hoffman S, Amos J, Hart MM, Potts JD, Goodwin RL, Markwald RR. Periostin regulates atrioventricular valve maturation. Dev Biol. 2008;316(2):200–213. doi: 10.1016/j.ydbio.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris RA, Potts JD, Yost MJ, Junor L, Brooks T, Tan H, Hoffman S, Hart MM, Kern MJ, Damon B, Markwald RR, Goodwin RL. Periostin promotes a fibroblastic lineage pathway in atrioventricular valve progenitor cells. Dev Dyn. 2009;238(5):1052–1063. doi: 10.1002/dvdy.21933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa H, Oohashi T, Sata M, Bekku Y, Hirohata S, Nakamura K, Yonezawa T, Kusachi S, Shiratori Y, Ninomiya Y. Lp3/Hapln3, a novel link protein that co-localizes with versican and is coordinately up-regulated by platelet-derived growth factor in arterial smooth muscle cells. Matrix Biol. 2004;23(5):287–298. doi: 10.1016/j.matbio.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci. 2002;115(Pt 20):3861–3863. doi: 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- Pereira L, D’Alessio M, Ramirez F, Lynch JR, Sykes B, Pangilinan T, Bonadio J. Genomic organization of the sequence coding for fibrillin, the defective gene product in Marfan syndrome. Hum Mol Genet. 1993;2(7):961–968. doi: 10.1093/hmg/2.7.961. [DOI] [PubMed] [Google Scholar]
- Perez-Pomares JM, Carmona R, Gonzalez-Iriarte M, Atencia G, Wessels A, Munoz-Chapuli R. Origin of coronary endothelial cells from epicardial mesothelium in avian embryos. Int J Dev Biol. 2002a;46(8):1005–1013. [PubMed] [Google Scholar]
- Perez-Pomares JM, Phelps A, Sedmerova M, Carmona R, Gonzalez-Iriarte M, Munoz-Chapuli R, Wessels A. Experimental studies on the spatiotemporal expression of WT1 and RALDH2 in the embryonic avian heart: a model for the regulation of myocardial and valvuloseptal development by epicardially derived cells (EPDCs) Dev Biol. 2002b;247(2):307–326. doi: 10.1006/dbio.2002.0706. [DOI] [PubMed] [Google Scholar]
- Poschl E, Schlotzer-Schrehardt U, Brachvogel B, Saito K, Ninomiya Y, Mayer U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development. 2004;131(7):1619–1628. doi: 10.1242/dev.01037. [DOI] [PubMed] [Google Scholar]
- Radonic T, de Witte P, Baars MJ, Zwinderman AH, Mulder BJ, Groenink M. Losartan therapy in adults with Marfan syndrome: study protocol of the multi-center randomized controlled COMPARE trial. Trials. 2010;11:3. doi: 10.1186/1745-6215-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahkonen O, Su M, Hakovirta H, Koskivirta I, Hormuzdi SG, Vuorio E, Bornstein P, Penttinen R. Mice with a deletion in the first intron of the Col1a1 gene develop age-dependent aortic dissection and rupture. Circ Res. 2004;94(1):83–90. doi: 10.1161/01.RES.0000108263.74520.15. [DOI] [PubMed] [Google Scholar]
- Rasi K, Piuhola J, Czabanka M, Sormunen R, Ilves M, Leskinen H, Rysa J, Kerkela R, Janmey P, Heljasvaara R, Peuhkurinen K, Vuolteenaho O, Ruskoaho H, Vajkoczy P, Pihlajaniemi T, Eklund L. Collagen XV is necessary for modeling of the extracellular matrix and its deficiency predisposes to cardiomyopathy. Circ Res. 107(10):1241–1252. doi: 10.1161/CIRCRESAHA.110.222133. [DOI] [PubMed] [Google Scholar]
- Rauch U, Hirakawa S, Oohashi T, Kappler J, Roos G. Cartilage link protein interacts with neurocan, which shows hyaluronan binding characteristics different from CD44 and TSG-6. Matrix Biol. 2004;22(8):629–639. doi: 10.1016/j.matbio.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Rios H, Koushik SV, Wang H, Wang J, Zhou HM, Lindsley A, Rogers R, Chen Z, Maeda M, Kruzynska-Frejtag A, Feng JQ, Conway SJ. periostin null mice exhibit dwarfism, incisor enamel defects, and an early-onset periodontal disease-like phenotype. Mol Cell Biol. 2005;25(24):11131–11144. doi: 10.1128/MCB.25.24.11131-11144.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarela J, Rehn M, Oikarinen A, Autio-Harmainen H, Pihlajaniemi T. The short and long forms of type XVIII collagen show clear tissue specificities in their expression and location in basement membrane zones in humans. Am J Pathol. 1998;153(2):611–626. doi: 10.1016/S0002-9440(10)65603-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai LY, Keene DR, Engvall E. Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils. The Journal of cell biology. 1986;103(6 Pt 1):2499–2509. doi: 10.1083/jcb.103.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma RJ, Chana A, Elkayam U. Left ventricular noncompaction. Prog Cardiovasc Dis. 2010;52(4):264–273. doi: 10.1016/j.pcad.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Costell M, Mann K, Timpl R. Inhibition of glycosaminoglycan modification of perlecan domain I by site-directed mutagenesis changes protease sensitivity and laminin-1 binding activity. FEBS Lett. 1998;435(2–3):169–172. doi: 10.1016/s0014-5793(98)01063-1. [DOI] [PubMed] [Google Scholar]
- Sasse P, Malan D, Fleischmann M, Roell W, Gustafsson E, Bostani T, Fan Y, Kolbe T, Breitbach M, Addicks K, Welz A, Brem G, Hescheler J, Aszodi A, Costell M, Bloch W, Fleischmann BK. Perlecan is critical for heart stability. Cardiovascular research. 2008;80(3):435–444. doi: 10.1093/cvr/cvn225. [DOI] [PubMed] [Google Scholar]
- Schroeder JA, Jackson LF, Lee DC, Camenisch TD. Form and function of developing heart valves: coordination by extracellular matrix and growth factor signaling. J Mol Med. 2003;81(7):392–403. doi: 10.1007/s00109-003-0456-5. [DOI] [PubMed] [Google Scholar]
- Sheng W, Wang G, Wang Y, Liang J, Wen J, Zheng PS, Wu Y, Lee V, Slingerland J, Dumont D, Yang BB. The roles of versican V1 and V2 isoforms in cell proliferation and apoptosis. Mol Biol Cell. 2005;16(3):1330–1340. doi: 10.1091/mbc.E04-04-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Grothe S, Zhang Y, O’Connor-McCourt MD, Poole AR, Roughley PJ, Mort JS. Link protein has greater affinity for versican than aggrecan. J Biol Chem. 2004;279(13):12060–12066. doi: 10.1074/jbc.M310091200. [DOI] [PubMed] [Google Scholar]
- Snarr BS, Kern CB, Wessels A. Origin and fate of cardiac mesenchyme. Dev Dyn. 2008;237(10):2804–2819. doi: 10.1002/dvdy.21725. [DOI] [PubMed] [Google Scholar]
- Snarr BS, O’Neal JL, Chintalapudi MR, Wirrig EE, Phelps AL, Kubalak SW, Wessels A. Isl1 expression at the venous pole identifies a novel role for the second heart field in cardiac development. Circ Res. 2007a;101(10):971–974. doi: 10.1161/CIRCRESAHA.107.162206. [DOI] [PubMed] [Google Scholar]
- Snarr BS, Wirrig EE, Phelps AL, Trusk TC, Wessels A. A spatiotemporal evaluation of the contribution of the dorsal mesenchymal protrusion to cardiac development. Dev Dyn. 2007b;236(5):1287–1294. doi: 10.1002/dvdy.21074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider P, Hinton RB, Moreno-Rodriguez RA, Wang J, Rogers R, Lindsley A, Li F, Ingram DA, Menick D, Field L, Firulli AB, Molkentin JD, Markwald R, Conway SJ. Periostin is required for maturation and extracellular matrix stabilization of noncardiomyocyte lineages of the heart. Circ Res. 2008;102(7):752–760. doi: 10.1161/CIRCRESAHA.107.159517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence SG, Argraves WS, Walters L, Hungerford JE, Little CD. Fibulin is localized at sites of epithelial-mesenchymal transitions in the early avian embryo. Developmental biology. 1992;151(2):473–484. doi: 10.1016/0012-1606(92)90186-k. [DOI] [PubMed] [Google Scholar]
- Spicer AP, Joo A, Bowling RA., Jr A hyaluronan binding link protein gene family whose members are physically linked adjacent to chondroitin sulfate proteoglycan core protein genes: the missing links. The Journal of biological chemistry. 2003;278(23):21083–21091. doi: 10.1074/jbc.M213100200. [DOI] [PubMed] [Google Scholar]
- Spicer AP, McDonald JA. Characterization and molecular evolution of a vertebrate hyaluronan synthase gene family. J Biol Chem. 1998;273(4):1923–1932. doi: 10.1074/jbc.273.4.1923. [DOI] [PubMed] [Google Scholar]
- Stainier DY. Zebrafish genetics and vertebrate heart formation. Nat Rev Genet. 2001;2(1):39–48. doi: 10.1038/35047564. [DOI] [PubMed] [Google Scholar]
- Stankunas K, Hang CT, Tsun ZY, Chen H, Lee NV, Wu JI, Shang C, Bayle JH, Shou W, Iruela-Arispe ML, Chang CP. Endocardial Brg1 represses ADAMTS1 to maintain the microenvironment for myocardial morphogenesis. Developmental cell. 2008;14(2):298–311. doi: 10.1016/j.devcel.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan MH, Sun Z, Opitz SL, Schmidt TE, Peters JH, George EL. Deletion of the alternatively spliced fibronectin EIIIA domain in mice reduces atherosclerosis. Blood. 2004;104(1):11–18. doi: 10.1182/blood-2003-09-3363. [DOI] [PubMed] [Google Scholar]
- Thai SN, Iruela-Arispe ML. Expression of ADAMTS1 during murine development. Mechanisms of development. 2002;115(1–2):181–185. doi: 10.1016/s0925-4773(02)00115-6. [DOI] [PubMed] [Google Scholar]
- Tian Y, Yuan L, Goss AM, Wang T, Yang J, Lepore JJ, Zhou D, Schwartz RJ, Patel V, Cohen ED, Morrisey EE. Characterization and in vivo pharmacological rescue of a Wnt2-Gata6 pathway required for cardiac inflow tract development. Dev Cell. 18(2):275–287. doi: 10.1016/j.devcel.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Yuan L, Goss AM, Wang T, Yang J, Lepore JJ, Zhou D, Schwartz RJ, Patel V, Cohen ED, Morrisey EE. Characterization and in vivo pharmacological rescue of a Wnt2-Gata6 pathway required for cardiac inflow tract development. Dev Cell. 2010;18(2):275–287. doi: 10.1016/j.devcel.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole BP. Hyaluronan in morphogenesis. Semin Cell Dev Biol. 2001;12(2):79–87. doi: 10.1006/scdb.2000.0244. [DOI] [PubMed] [Google Scholar]
- Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4(7):528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- Twal WO, Czirok A, Hegedus B, Knaak C, Chintalapudi MR, Okagawa H, Sugi Y, Argraves WS. Fibulin-1 suppression of fibronectin-regulated cell adhesion and motility. Journal of cell science. 2001;114(Pt 24):4587–4598. doi: 10.1242/jcs.114.24.4587. [DOI] [PubMed] [Google Scholar]
- Unger S, Lausch E, Rossi A, Megarbane A, Sillence D, Alcausin M, Aytes A, Mendoza-Londono R, Nampoothiri S, Afroze B, Hall B, Lo IF, Lam ST, Hoefele J, Rost I, Wakeling E, Mangold E, Godbole K, Vatanavicharn N, Franco LM, Chandler K, Hollander S, Velten T, Reicherter K, Spranger J, Robertson S, Bonafe L, Zabel B, Superti-Furga A. Phenotypic features of carbohydrate sulfotransferase 3 (CHST3) deficiency in 24 patients: congenital dislocations and vertebral changes as principal diagnostic features. Am J Med Genet A. 2010;152A(10):2543–2549. doi: 10.1002/ajmg.a.33641. [DOI] [PubMed] [Google Scholar]
- Utriainen A, Sormunen R, Kettunen M, Carvalhaes LS, Sajanti E, Eklund L, Kauppinen R, Kitten GT, Pihlajaniemi T. Structurally altered basement membranes and hydrocephalus in a type XVIII collagen deficient mouse line. Hum Mol Genet. 2004;13(18):2089–2099. doi: 10.1093/hmg/ddh213. [DOI] [PubMed] [Google Scholar]
- Verzi MP, McCulley DJ, De Val S, Dodou E, Black BL. The right ventricle, outflow tract, and ventricular septum comprise a restricted expression domain within the secondary/anterior heart field. Dev Biol. 2005;287(1):134–145. doi: 10.1016/j.ydbio.2005.08.041. [DOI] [PubMed] [Google Scholar]
- Visconti RP, Barth JL, Keeley FW, Little CD. Codistribution analysis of elastin and related fibrillar proteins in early vertebrate development. Matrix Biol. 2003;22(2):109–121. doi: 10.1016/s0945-053x(03)00014-3. [DOI] [PubMed] [Google Scholar]
- Walsh EC, Stainier DY. UDP-glucose dehydrogenase required for cardiac valve formation in zebrafish. Science. 2001;293(5535):1670–1673. doi: 10.1126/science.293.5535.1670. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Yamada Y. Mice lacking link protein develop dwarfism and craniofacial abnormalities. Nat Genet. 1999;21(2):225–229. doi: 10.1038/6016. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Yamada Y, Kimata K. Roles of aggrecan, a large chondroitin sulfate proteoglycan, in cartilage structure and function. J Biochem. 1998;124(4):687–693. doi: 10.1093/oxfordjournals.jbchem.a022166. [DOI] [PubMed] [Google Scholar]
- Wenstrup RJ, Florer JB, Brunskill EW, Bell SM, Chervoneva I, Birk DE. Type V collagen controls the initiation of collagen fibril assembly. J Biol Chem. 2004;279(51):53331–53337. doi: 10.1074/jbc.M409622200. [DOI] [PubMed] [Google Scholar]
- Wessels A, Perez-Pomares JM. The epicardium and epicardially derived cells (EPDCs) as cardiac stem cells. Anat Rec A Discov Mol Cell Evol Biol. 2004;276(1):43–57. doi: 10.1002/ar.a.10129. [DOI] [PubMed] [Google Scholar]
- Wirrig EE, Snarr BS, Chintalapudi MR, O’Neal JL, Phelps AL, Barth JL, Fresco VM, Kern CB, Mjaatvedt CH, Toole BP, Hoffman S, Trusk TC, Argraves WS, Wessels A. Cartilage link protein 1 (Crtl1), an extracellular matrix component playing an important role in heart development. Dev Biol. 2007;310(2):291–303. doi: 10.1016/j.ydbio.2007.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M, Lawton T, Goetinck PF, Kuhn JL, Goldstein SA, Bonadio J. Aggrecan core protein is expressed in membranous bone of the chick embryo. Molecular and biomechanical studies of normal and nanomelia embryos. J Biol Chem. 1992;267(8):5592–5598. [PubMed] [Google Scholar]
- Yamamura H, Zhang M, Markwald RR, Mjaatvedt CH. A heart segmental defect in the anterior-posterior axis of a transgenic mutant mouse. Dev Biol. 1997;186(1):58–72. doi: 10.1006/dbio.1997.8559. [DOI] [PubMed] [Google Scholar]
- Yutzey KE, Kirby ML. Wherefore heart thou? Embryonic origins of cardiogenic mesoderm. Dev Dyn. 2002;223(3):307–320. doi: 10.1002/dvdy.10068. [DOI] [PubMed] [Google Scholar]
- Zaffran S, Kelly RG, Meilhac SM, Buckingham ME, Brown NA. Right ventricular myocardium derives from the anterior heart field. Circ Res. 2004;95(3):261–268. doi: 10.1161/01.RES.0000136815.73623.BE. [DOI] [PubMed] [Google Scholar]
- Zanin MK, Bundy J, Ernst H, Wessels A, Conway SJ, Hoffman S. Distinct spatial and temporal distributions of aggrecan and versican in the embryonic chick heart. Anat Rec. 1999;256(4):366–380. doi: 10.1002/(SICI)1097-0185(19991201)256:4<366::AID-AR4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, Jiang D, von Gise A, Ikeda S, Chien KR, Pu WT. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454(7200):109–113. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]