Abstract
Chronic immune activation is a hallmark of HIV infection, yet the underlying triggers of immune activation remain unclear. Persistent antigenic stimulation during HIV infection may also lead to immune exhaustion, a phenomenon in which effector T cells become dysfunctional and lose effector functions and proliferative capacity. Several markers of immune exhaustion, such as PD-1, LAG-3, Tim-3, and CTLA-4, which are also negative regulators of immune activation, are preferentially upregulated on T cells during HIV infection. It is not yet clear whether accumulation of T cells expressing activation inhibitory molecules is a consequence of general immune or chronic HIV-specific immune activation. Importantly, however, in vitro blockade of PD-1 and Tim-3 restores HIV-specific T-cell responses, indicating potential for immunotherapies. In this review we discuss the evolution of our understanding of immune exhaustion during HIV infection, highlighting novel markers and potential therapeutic targets.
Keywords: T-cell exhaustion, Immune activation, PD-1, Tim-3, HIV, LAG-3, SIV
Introduction
Major advances in antiretroviral therapy have controlled HIV viremia in most infected individuals under treatment and have resulted in a great reduction in mortality in Western countries. However, despite these advances in our understanding of HIV pathogenesis, specifically how it causes chronic immune activation and why the immune system is unable to control the virus in most people, remains unclear. Indeed, chronic immune activation is one of the hallmarks of HIV infection, characterized by expression of activation markers on lymphocytes, polyclonal B-cell expansion, increased T-cell turnover, and elevated serum levels of proinflammatory cytokines and chemokines [1–4]. Over a decade ago, Giorgi et al. [5, 6] described the prognostic value of a T-cell activation marker, CD38, expressed on cytotoxic lymphocytes during HIV infection. It is now well established that an activated phenotype of CD8+ and CD4+ T cells defined by expression of CD38 is one of the best biomarkers for disease progression, surpassing the prognostic value of clinical markers CD4+ T-cell counts and HIV viral load [6]. The importance of immune activation in HIV pathogenesis is further demonstrated in non-human primate models of SIV infection. African green monkeys (AGMs) infected with natural SIV neither develop chronic immune activation nor progress to AIDS, despite viral loads similar to those detected in humans infected with HIV-1 or rhesus macaques (RMs) infected with pathogenic SIV [7]. Thus it is clear that immune activation is a crucial element of HIV pathogenesis, yet the underlying triggers or causes of this persistent immune activation remain elusive.
Perturbations in the homeostasis of different T-cell subsets during HIV infection have been linked to immune activation. As such, several separate populations of immune cells have been correlated with immune activation, including regulatory T cells (Tregs), dendritic cells, and gut-infiltrating lymphocytes. Tregs play an important role in the development of peripheral tolerance and counter immune activation with suppressive activity. Some studies demonstrated that removal of Tregs increases HIV-specific T-cell responses [8–11], while others show HIV-positive individuals with low Tregs have higher immune activation [12, 13]. Plasmacytoid dendritic cells (pDC) producing type I IFN have been related to increased activation of CD8+ T cells and the progressive depletion of CD4+ T cells [14]. A recently identified subset of T cells called Th17 cells also may also be compromised by immune activation during HIV infection [15]. While systemic perturbations in the immune system are evident, the most drastic depletion of T cells occurs in the gut mucosa. Brenchley et al. [16] recently reported that compromises to the mucosal barrier allow microbial translocation of bacterial products into systemic circulation causing immune activation. They demonstrated that plasma lipopolysaccharide (LPS) and soluble CD14 correlated with systemic immune activation, and sooty mangabeys with nonpathogenic SIV infection did not have evidence of microbial translocation [16]. Thus, several mechanisms of immune activation have been proposed, yet many questions remain unanswered. If HIV primes and activates immune cells, why do these cells fail to mount an effective response to HIV? Are these activated cells dysfunctional or does HIV evade the immune response? Could the phenomenon of immune exhaustion provide a partial explanation to these questions?
What is Immune Exhaustion?
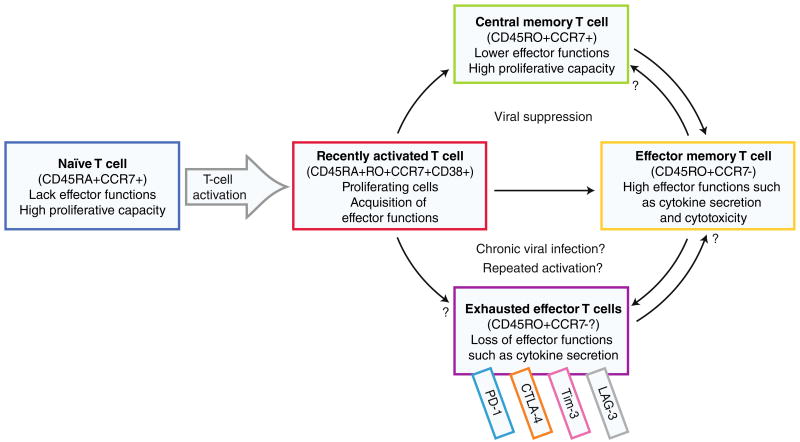
The term “immune exhaustion” is defined by loss of effector functions and proliferative capacity in memory T cells. During an adaptive immune response, human naïve T cells are activated by antigen-presenting cells, such as dendritic cells, through MHC-peptide and costimulatory molecules. This activation leads to robust proliferation and acquisition of effector functions, accompanied by cell surface phenotype changes that reflect both the activation (recent vs. late) and differentiation state of the T cells (Fig. 1). Most of the activated T cells are differentiated into relatively short-lived effector T cells, while a small portion become long-lived central memory T cells (Fig. 1). It is thought that chronic activation by viral antigens or other mechanisms causes some effector or activated T cells to persist but become functionally unresponsive to further antigen stimulation (Fig. 1), which is generally termed immune exhaustion. However, the mechanisms that lead to exhausted cells and whether these differ from terminally differentiated or senescent cells remain unclear.
Fig. 1.
Progression from naïve to exhausted T cells. Upon activation by an antigen, a naïve T cell becomes activated, begins to proliferate, and acquires effector functions. The activated cell differentiates to either effector memory T cells with cytotoxic potential and effector functions or long-lived central memory T cells with low effector functions but the ability to rapidly proliferate upon repeated stimulation. Persistent antigenic exposure and repeated activation may lead to exhausted effector T cells with loss of effector functions and proliferative capacity
During the course of HIV infection, T-cell functions such as cytokine secretion, proliferation, and cytotoxic potential appear to diminish gradually and define stages of immune exhaustion [17]. While dysfunctional T cells in HIV were described early in the HIV epidemic, recently this notion of exhaustion has gained renewed interest with the identification of a “molecular signature” of exhaustion [18••]. Most importantly, some of these negative regulators in HIV infection were found to be reversible, with potential for immunotherapies.
Immune exhaustion in the context of persistent viral infections was first described in the mouse model of lymphocytic choriomeningitis (LCMV) infection. In a landmark study, Zajac et al. [19] demonstrated that LCMV-specific CD8+ T cells persisted during chronic infection but lacked cytotoxic potential. This study illustrated a mechanism for viral immune evasion in chronic persistent viral infections [19]. These dysfunctional cytotoxic lymphocytes were termed “exhausted.” Subsequently T-cell exhaustion was described in humans with chronic viral infections such as HIV, hepatitis B virus, and hepatitis C virus infections [20–22, 23••, 24••]. Emerging data suggest immune exhaustion is a crucial factor for viral persistence in these chronic infections [25]. Below we discuss the evolution of our understanding of immune exhaustion, highlighting novel markers to better define it and potential therapeutic targets.
What are Markers of Immune Exhaustion?
Programmed-Death 1
Programmed-death 1 (PD-1) was among the first inhibitory receptors associated with immune exhaustion. PD-1 is a member of the B7:28 family of inhibitory molecules initially found in a T-cell hybridoma undergoing apoptosis, hence labeled programmed-death 1 [26]. As a negative regulator, PD-1 is critical for the balance between T-cell activation and tolerance [27]. Deletion of PD-1 in mice results in disease resembling systemic lupus erythematosis and polymorphisms in humans are associated with autoimmune disorders, highlighting its importance in self-tolerance [28, 29]. PD-1 is expressed on CD4+ and CD8+ T cells, B cells, natural killer cells, and monocytes [27]. It binds to ligands PD-L1 (B7-H1;CD274), broadly expressed on antigen-presenting cells, T cells, and non-hematopoietic cells, and PD-L2 (B7-DC;CD273), expressed only on dendritic cells and macrophages [27]. The role of PD-1 in chronic viral infections was discovered by Barber et al. [30••] via an RNA microarray of dysfunctional virus-specific CD8+ T cells in the LCMV mouse model showing marked upregulation of messenger RNA encoding PD-1. Antibody staining confirmed the expression of PD-1 in LCMV-specific CD8+ T cells, thereby establishing it as a marker for dysfunctional T cells [30••]. Remarkably, in vivo blockade of PD-1 resulted in reduction of virus and recovery of virus-specific T-cell responses and proliferation [30••], suggesting that PD-1 signals do not cause irreversible differentiation into an exhausted T-cell phenotype.
T-cell Immunoglobulin and Mucin Domain-Containing Molecule-3
One negative regulator that has been recently studied during HIV infection is T-cell immunoglobulin and mucin domain-containing molecule-3 (Tim-3). Tim-3 was first identified as a cell surface marker on mouse IFNγ secreting Th1 cells but not Th2 cells [31]. Zhu et al. [32] identified the ligand for Tim-3 as galectin-9 and demonstrated that in mice interactions between Tim-3 and galectin-9 result in the death of Th1 CD4+ T cells by calcium flux. Blockade of Tim-3 in mice resulted in hyperproliferation of Th1 cells, illustrating the importance of Tim-3 in peripheral tolerance [33]. In humans Tim-3 is expressed preferentially on Th1 cells and constitutively on macrophages and dendritic cells [34]. Tim-3 was found to also regulate a novel effector T-cell subset called Th17 cells [35••], which have inflammatory properties and play a pathologic role in autoimmune disorders [36]. The relation between Tim-3 and Th17 cells was discerned from the autoimmune disorder, multiple sclerosis, initially thought to be mediated by Th1 cells and later found to also involve Th17 cells [36]. These patients exhibit defective Tim-3 expression and high IL-17 levels [37, 38]. This discovery prompted studies by Hastings et al. [35••] in which blockade of Tim-3 resulted in enhanced IFNγ and IL-17 production by CD4+ T cells, suggesting Tim-3 negatively regulates both Th1 and Th17 cytokines in human T cells.
Lymphocyte Activation Gene-3
In mice studies of chronic LCMV infection, PD-1 was most expressed on virus-specific CD8+ T cells followed by lymphocyte activation gene-3 (LAG-3) [39••]. LAG-3, a member of the immunoglobulin superfamily, is an activation-induced cell-surface molecule with a preferential expression on Th1 cells [40•]. IL-12, a Th1-polarizing cytokine, upregulates LAG-3 expression [41]. In humans, LAG-3 blockade resulted in T-cell proliferation and production of Th1 cytokines. During LCMV infection, LAG-3 was highly expressed on virus-specific T cells, but inhibition of LAG-3 failed to rescue T-cell function or diminish plasma viremia, like PD-1 [39••, 40•]. However, simultaneous blockade of LAG-3 with PD-1 resulted in synergistic enhancement of virus-specific CD8+ T cells in the spleen and in the peripheral blood and improved viral control [39••]. Interestingly, LAG-3 was found to be co-expressed on antiviral CD8+ T cells with PD-1 and Tim-3 during LCMV infection in mice, but the functions of these triple-negative regulators are unexplored [40•].
Immune Exhaustion and HIV Infection
PD-1 as a Marker of Immune Exhaustion in HIV or SIV Infection
The findings of reversible dysfunction mediated by PD-1 in LCMV infection rapidly led to the study of PD-1 in humans with HIV infection. Almost simultaneously, Day and Trautmann reported upregulated PD-1 expression in HIV-specific CD8+ T cells [23••, 24••]. Both groups demonstrated that PD-1 expression in HIV-specific CD8+ T cells was higher than in CMV-specific T cells. In HIV-infected individuals, PD-1 expression on CD4+ and CD8+ T cells correlated with plasma viral load and CD4+ T-cell counts [23••, 24••], both markers of disease progression. Moreover, HIV-specific CD4+ and CD8+ T-cell responses were rescued with blockade of PD-1 [23••, 24••]. In two small longitudinal analyses, PD-1 expression in HIV-infected subjects lowered after the initiation of antiretroviral therapy [23••, 24••]. Finally, Zhang et al. [42] reported long-term nonprogressors (LTNPs), HIV-infected individuals who independently maintain CD4+ T-cell counts, had lower PD-1 expression on HIV-specific CD8+ T cells, compared with individuals with progressive HIV infection.
PD-1 expression was evaluated in rhesus macaques with pathogenic SIV infection in two independent studies [43•, 44•]. In both reports, the majority of SIV-specific CD8+ T cells expressed PD-1, and blockade of PD-1 enhanced SIV-specific cytotoxic T lymphocyte (CTL) proliferation, similar to HIV infection in humans [43•, 44•]. Velu et al. [44•] found the highest expression of PD-1 occurred in gut and lymphoid tissue rather than blood, corresponding with sites of highest viral replication. Petrovas et al. [43•] demonstrated that repeated TCR stimulation is a crucial factor in PD-1 expression, as SIV epitopes with mutational escapes led to decreased PD-1 expression.
Velu et al. [45••] followed their first study with an in vivo trial of the safety and immune restoration potential of PD-1 blockade in macaques with chronic SIV infection. A human anti–PD-1 antibody was used to block the interaction of PD-1 with its ligand PD-L1, administered to macaques in early (10 weeks) or late (∼90 weeks) SIV infection. PD-1 blockade in both early and late infection resulted in rapid expansion of HIV-specific CD8+ T cells and improved polyfunctionality with IFNγ, IL-2, and TNFα secretion. These findings were apparent in the blood and in the gut. Macaques tolerated the anti–PD-1 antibody well, thus establishing both safety and efficacy of PD-1 blockade in the SIV/macaque model [45••].
Sooty mangabeys (SMs) are natural primate hosts for SIV and do not develop pathogenic infection. During acute SIV infection SMs develop immune activation, but this resolves rapidly, unlike macaques with continued activation during chronic infection [7]. Thus, studies of nonpathogenic SIV infection in SMs offer unique insight to HIV immunopathogenesis. Interestingly, resolution of immune activation in SMs was associated with early induction of PD-1, suggesting that the inhibitory effects of PD-1 may mediate early control of hyperimmune activation during acute SIV infection [46].
Alternative Mechanism for PD-1–mediated Immune Exhaustion During HIV Infection
While most studies of PD-1 during HIV infection focus on cytotoxic T lymphocytes, PD-1 expression on CD4+ T cells was shown to mark dysfunctional HIV-specific CD4+ T cells and correlate with HIV disease progression [27]. PD-1 is also expressed on B cells, natural killer cells, and monocytes [27]. A recent report suggests that PD-1 expression on monocytes may induce CD4+ T-cell dysfunction [47•]. In this study, Said et al. [47•] demonstrated that PD-L1 triggering of PD-1 expressed on monocytes induced IL-10 production and reversible CD4+ T-cell dysfunction. They also showed that TLR ligands LPS, lipoteichoic acid (LTA), and CpG DNA induced a dose-dependent increase in PD-1 expression on monocytes, whereas HIV ssRNA, CCR5-tropic or CXCR4-tropic, HIV failed to induce PD-1 [47•].
It was recently suggested that chronic immune activation during HIV infection could be due to circulating bacterial elements such as LPS translocated from the gut due to HIV disruption of the intestinal mucosal barrier [16]. Since PD-1 expression depended on TLR ligands including LPS, Said et al. [47•] investigated whether PD-1 levels correlated with microbial translocation products in HIV-infected individuals. They found that sera from viremic HIV-infected subjects induced PD-1 expression and IL-10 production from monocytes. HIV-infected individuals had high PD-1 expression on monocytes, which correlated with plasma IL-10 levels, but there was no direct correlation between PD-1 or IL-10 and LPS levels [47•].
Tim-3 as a Novel T-cell Exhaustion Marker in HIV Infection
Despite the high expression of PD-1 in exhausted T cells during HIV infection, not all dysfunctional cells display PD-1, suggesting a role of other inhibitory molecules. Moreover, blockade of PD-1 only partially restores T-cell function, suggesting additional mediators may be involved in T-cell exhaustion [30••]. Other negative regulators that have been tested or proposed as markers to define exhausted T cells are CTLA-4, LAG-3, and Tim-3. Tim-3 is the most recently described of these markers, and HIV-infected individuals were found to have elevated Tim-3 expression on CD4+ and CD8+ T cells, which correlated with HIV disease progression as assessed by viral loads, CD4+ T-cell counts, and immune activation marker CD38 [48•]. In this study, Jones et al. [48•] showed Tim-3 upregulation of HIV-specific CD8+ T cells in individuals with chronic progressive HIV infection. Tim-3–expressing cells were also shown to be dysfunctional with muted cytokine responses and proliferation [48•]. Indeed, in vitro blockade of Tim-3 with a soluble Tim-3 immunoglobulin resulted in enhanced proliferation of HIV-specific CD4+ and CD8+ T cells [48•]. In a longitudinal analysis of seven HIV+ subjects initiating antiretroviral therapy, four had decreases in Tim-3 expression CD4+, and CD8+ T cells, whereas three maintained high levels despite viral suppression [48•]. The consequences of this differential Tim-3 expression on the T cells of treated patients for HIV disease progression remain to be determined.
LAG-3 and HIV Infection
In the microarray analysis of dysfunctional cells in the LCMV model, PD-1 was the most notable gene expressed, yet other potential inhibitory receptors were also identified. Further analysis of the gene expression data identified several inhibitors that correlated with the PD-1 gene Pdcd1, including LAG-3, CTLA-4, PIR-B, GP49, 2B4, and CD160, of which LAG-3 was the second most prominent [18••]. LAG-3 was initially evaluated during HIV infection as a marker of the Th1 environment rather than a mediator of T-cell exhaustion. During HIV infection, LAG-3 was shown to correlate with HIV viral load irrespective of CCR5- or CXCR4-tropism in patients unresponsive to antiretroviral therapy [49]. However, another study reported no difference in LAG-3 expression on CD4+ or CD8+ T cells between HIV-negative and treated or untreated HIV-infected subjects [50]. In a recent study of HIV-infected patients initiating antiretroviral therapy, baseline PD-1 levels were elevated in patients who developed immune reconstitution syndrome. Interestingly, LAG-3 and CTLA-4 expression were elevated on the PD-1+CD4+ T cells of these subjects [51•].
Role of PD-1 and Tim-3 Coexpression During HIV Infection
Parallels between PD-1 and Tim-3 expression and function during HIV infection beg the question whether they are mutually exclusive populations. Jones et al. [48•] examined coexpression of PD-1 with Tim-3 in ten individuals with chronic progressive HIV infection, and demonstrated a small Tim-3+PD-1+ population that exhibited lower HIV-specific responses than either Tim-3 or PD-1 single positive populations. A more recent study evaluated PD-1 and Tim-3 coexpression in mouse LCMV infection [52•]. They first evaluated Tim-3 expression in acute and chronic LCMV infection and found that Tim-3 was transiently upregulated in viral-specific CD8+ T cells during acute infection, whereas it is more highly and persistently expressed during chronic infection [52•]. In contrast, only a small portion of Tim-3+PD-1+ cells was apparent in acute infection but a large population rose during chronic infection that persisted even at 90 days post-infection [52•]. Thus, CD8+ T cells with chronic viral infection can be divided into Tim-3−PD-1+ and Tim-3+PD-1+ populations. When stimulated with LCMV peptide pools, Tim-3+PD-1+ T cells lacked apparent proliferation and produced minimal TNFα and IL-2 cytokines compared with Tim-3 PD-1+ cells [52•]. This result suggests the double positive population may represent exhausted CD8+ T cells with more severe proliferative and functional impairment [52•]. The most interesting finding from this study, however, came out of the blockade experiments. Jin et al. [52•] evaluated the effects of Tim-3 and PD-1 inhibition, individually and combined, on virus-specific CD8+ T-cell responses. Dual blockade resulted in synergistic rescue of T-cell responses and reduction of viral loads in all tissues, compared with PD-1 blockade alone [52•]. In humans with HIV/HCV coinfection, Tim-3+PD-1+ frequencies were higher in HCV-specific CD8+ T cells compared with HIV-specific T cells [53]. However, patients with HIV/HCV coinfection had higher levels of Tim-3+PD-1+ CD8+ T cells as opposed to HCV monoinfection [53]. Finally, coexpression of Tim-3 and PD-1 correlated with progression of liver disease in these patients [53]. Recently investigators have begun to evaluate the coordination between PD-1 and other inhibitory molecules in search of synergistic therapies.
Immune Activation Versus Exhaustion Markers in HIV Infection
Immune activation marked by CD38 expression remains the strongest predictor of disease progression, yet immune exhaustion has also been shown to correlate with HIV disease progression [48•, 54]. Since the “molecular signature” of immune exhaustion is still being decoded, it is not yet clear whether PD-1 may be relevant to clinical applications during HIV infection. Two recent studies suggest an association between PD-1 and CD38 expression [54, 55]. One study reported a positive correlation between CD38 and PD-1 expression on CD8+ T cells in HIV-infected subjects [54]. Another demonstrated that CD38/PD-1 coexpression was higher in HIV-specific CD8+ T cells in HIV progressors, compared with HIV controllers; moreover, coexpression decreased after initiation of anti-retroviral therapy and viral suppression [55]. Whether these two molecules act in concert or antagonistically remains to be determined. Future studies assessing this question will be interesting since CD38 and PD-1 appear to share opposite functions but coincide with identical clinical markers of HIV disease progression.
Conclusions
Immune exhaustion has emerged as a new potential contributor to HIV pathogenesis or insufficient control of the infection. We have outlined several key mediators of T-cell exhaustion during HIV infection, with an emphasis on cell surface negative T-cell regulators, PD-1 and Tim-3. The recent report of a PD-1 antibody with demonstrated safety and immune restoration in SIV-infected macaques brings optimism for immunotherapies in human HIV infection or potential uses as a vaccine adjunct [45••]. Blockade of Tim-3 also revealed reversible dysfunction of HIV-specific T cells. Both markers correlate with clinical parameters of HIV disease progression. Finally, Tim-3+PD-1+ cells may represent a subset of T cells with more severe exhaustion. Initial reports of synergistic effects of Tim-3 and PD-1 dual blockade reveal a direction for future investigation of potential therapeutic interventions. It will be important to determine in future studies the relative contribution of these molecules and others both as a mechanism contributing to an exhausted phenotype and as biomarkers to better discriminate exhausted or dysfunctional cells from recently activated or senescent populations.
Acknowledgments
This work was supported by NIH grant R21AI087973-01 to DU and the NYU Physician Scientist Training Program award to AK, supported in part by grant 1 UL1 RR029893 from the National Center for Research Resources, National Institutes of Health.
Footnotes
Disclosure No potential conflicts of interest relevant to this article were reported.
Contributor Information
Alka Khaitan, Email: Alka.Khaitan@nyumc.org, Department of Pediatrics, New York University School of Medicine, New York, NY 10016, USA.
Derya Unutmaz, Email: derya@mac.com, Department of Microbiology, New York University School of Medicine, New York, NY 10016, USA; Department of Pathology, New York University School of Medicine, New York, NY 10016, USA; Department of Medicine, New York University School of Medicine, New York, NY 10016, USA; New York University Langone Medical Center, 522 First Avenue, Smilow Research Center, Room 1011, New York, NY 10016, USA.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Lane HC, Masur H, Edgar LC, et al. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1983;309(8):453–8. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- 2.Hellerstein M, Hanley MB, Cesar D, et al. Directly measured kinetics of circulating T lymphocytes in normal and HIV-1-infected humans. Nat Med. 1999;5(1):83–9. doi: 10.1038/4772. [DOI] [PubMed] [Google Scholar]
- 3.Hazenberg MD, Stuart JW, Otto SA, et al. T-cell division in human immunodeficiency virus (HIV)-1 infection is mainly due to immune activation: a longitudinal analysis in patients before and during highly active antiretroviral therapy (HAART) Blood. 2000;95(1):249–55. [PubMed] [Google Scholar]
- 4.Valdez H, Lederman MM. Cytokines and cytokine therapies in HIV infection. AIDS Clin Rev. 1997:187–228. [PubMed] [Google Scholar]
- 5.Giorgi JV, Liu Z, Hultin LE, et al. Elevated levels of CD38+ CD8 + T cells in HIV infection add to the prognostic value of low CD4 + T cell levels: results of 6 years of follow-up. The Los Angeles Center, Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 1993;6(8):904–12. [PubMed] [Google Scholar]
- 6.Giorgi JV, Hultin LE, McKeating JA, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179(4):859–70. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 7.Silvestri G, Sodora DL, Koup RA, et al. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity. 2003;18(3):441–52. doi: 10.1016/s1074-7613(03)00060-8. [DOI] [PubMed] [Google Scholar]
- 8.Legrand FA, Nixon DF, Loo CP, et al. Strong HIV-1-specific T cell responses in HIV-1-exposed uninfected infants and neonates revealed after regulatory T cell removal. PLoS One. 2006;1:e102. doi: 10.1371/journal.pone.0000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aandahl EM, Michaelsson J, Moretto WJ, et al. Human CD4+ CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. J Virol. 2004;78(5):2454–9. doi: 10.1128/JVI.78.5.2454-2459.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinter AL, Hennessey M, Bell A, et al. CD25(+)CD4(+) regulatory T cells from the peripheral blood of asymptomatic HIV-infected individuals regulate CD4(+) and CD8(+) HIV-specific T cell immune responses in vitro and are associated with favorable clinical markers of disease status. J Exp Med. 2004;200(3):331–43. doi: 10.1084/jem.20032069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiss L, Donkova-Petrini V, Caccavelli L, et al. Human immunodeficiency virus-driven expansion of CD4+CD25+ regulatory T cells, which suppress HIV-specific CD4 T-cell responses in HIV-infected patients. Blood. 2004;104(10):3249–56. doi: 10.1182/blood-2004-01-0365. [DOI] [PubMed] [Google Scholar]
- 12.Oswald-Richter K, Grill SM, Shariat N, et al. HIV infection of naturally occurring and genetically reprogrammed human regulatory T-cells. PLoS Biol. 2004;2(7):E198. doi: 10.1371/journal.pbio.0020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eggena MP, Barugahare B, Jones N, et al. Depletion of regulatory T cells in HIV infection is associated with immune activation. J Immunol. 2005;174(7):4407–14. doi: 10.4049/jimmunol.174.7.4407. [DOI] [PubMed] [Google Scholar]
- 14.Swiecki M, Colonna M. Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunol Rev. 2010;234(1):142–62. doi: 10.1111/j.0105-2896.2009.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El Hed A, Khaitan A, Kozhaya L, et al. Susceptibility of human Th17 cells to human immunodeficiency virus and their perturbation during infection. J Infect Dis. 2010;201(6):843–54. doi: 10.1086/651021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12(12):1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 17.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77(8):4911–27. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18••.Wherry EJ, Ha SJ, Kaech SM, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27(4):670–84. doi: 10.1016/j.immuni.2007.09.006. This is the study that identified several markers of immune exhaustion by a microarray analysis of dysfunctional CD8+ T cells during LCMV infection PD-1 was the most prominent gene identified. [DOI] [PubMed] [Google Scholar]
- 19.Zajac AJ, Blattman JN, Murali-Krishna K, et al. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188(12):2205–13. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Urbani S, Amadei B, Tola D, et al. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80(22):11398–403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radziewicz H, Ibegbu CC, Fernandez ML, et al. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J Virol. 2007;81(6):2545–53. doi: 10.1128/JVI.02021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boni C, Fisicaro P, Valdatta C, et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol. 2007;81(8):4215–25. doi: 10.1128/JVI.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23••.Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443(7109):350–4. doi: 10.1038/nature05115. This study showed that PD-1 was expressed on HIV-specific CD8+ and CD4+ T cells and correlated with markers of HIV disease progression HIV-specific CD4+ and CD8+ T-cell responses rescued with blockade of PD-1. [DOI] [PubMed] [Google Scholar]
- 24••.Trautmann L, Janbazian L, Chomont N, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12(10):1198–202. doi: 10.1038/nm1482. This study showed that PD-1 was upregulated on HIV-specific but not CMV-specific CD8+ T cells and correlated with markers of HIV disease progression Blocking PD-1 resulted in enhanced survival, proliferation, and cytokine secretion by HIV-specific CD8+ T cells. [DOI] [PubMed] [Google Scholar]
- 25.El-Far M, Halwani R, Said E, et al. T-cell exhaustion in HIV infection. Curr HIV/AIDS Rep. 2008;5(1):13–9. doi: 10.1007/s11904-008-0003-7. [DOI] [PubMed] [Google Scholar]
- 26.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11(11):3887–95. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaufmann DE, Walker BD. Programmed death-1 as a factor in immune exhaustion and activation in HIV infection. Curr Opin HIV AIDS. 2008;3(3):362–7. doi: 10.1097/COH.0b013e3282f9ae8b. [DOI] [PubMed] [Google Scholar]
- 28.Nishimura H, Nose M, Hiai H, et al. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11(2):141–51. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 29.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8(3):239–45. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 30••.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–7. doi: 10.1038/nature04444. This study reported that T-cell exhaustion mediated by PD-1 was reversible by in vitro blockade of PD-1 in the murine LCMV model. [DOI] [PubMed] [Google Scholar]
- 31.Monney L, Sabatos CA, Gaglia JL, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415(6871):536–41. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 32.Zhu C, Anderson AC, Schubart A, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6(12):1245–52. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 33.Sabatos CA, Chakravarti S, Cha E, et al. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat Immunol. 2003;4(11):1102–10. doi: 10.1038/ni988. [DOI] [PubMed] [Google Scholar]
- 34.Hafler DA, Kuchroo V. TIMs: central regulators of immune responses. J Exp Med. 2008;205(12):2699–701. doi: 10.1084/jem.20082429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35••.Hastings WD, Anderson DE, Kassam N, et al. TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines. Eur J Immunol. 2009;39(9):2492–501. doi: 10.1002/eji.200939274. This report was the first to demonstrate Tim-3 is a negative regulator of Th1 and Th17 cells They showed Tim-3 was expressed on activated Th17 cells in addition to Th1 cells Activated CD4+ T cells cultured with monoclonal antibodies to Tim-3 increased IL-17, IFNγ, IL-2, and IL-6 but not Il-10 secretion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev. 2008;223:87–113. doi: 10.1111/j.1600-065X.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Durelli L, Conti L, Clerico M, et al. T-helper 17 cells expand in multiple sclerosis and are inhibited by interferon-beta. Ann Neurol. 2009;65(5):499–509. doi: 10.1002/ana.21652. [DOI] [PubMed] [Google Scholar]
- 38.Koguchi K, Anderson DE, Yang L, et al. Dysregulated T cell expression of TIM3 in multiple sclerosis. J Exp Med. 2006;203(6):1413–8. doi: 10.1084/jem.20060210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39••.Blackburn SD, Shin H, Haining WN, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10(1):29–37. doi: 10.1038/ni.1679. This report demonstrates that exhausted T cells coexpress multiple negative regulators and coexpression is associated with more severe T-cell exhaustion and infection Dual blockade of PD-1 and LAG-3 synergistically improved CD8+ T-cell function and lowered viral load in the LCMV murine model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40•.Richter K, Agnellini P, Oxenius A. On the role of the inhibitory receptor LAG-3 in acute and chronic LCMV infection. Int Immunol. 2010;22(1):13–23. doi: 10.1093/intimm/dxp107. This review discusses the role of LAG-3 during LCMV infection. [DOI] [PubMed] [Google Scholar]
- 41.Romagnani S. Th1 and Th2 in human diseases. Clin Immunol Immunopathol. 1996;80(3 Pt 1):225–35. doi: 10.1006/clin.1996.0118. [DOI] [PubMed] [Google Scholar]
- 42.Zhang JY, Zhang Z, Wang X, et al. PD-1 up-regulation is correlated with HIV-specific memory CD8+ T-cell exhaustion in typical progressors but not in long-term nonprogressors. Blood. 2007;109(11):4671–8. doi: 10.1182/blood-2006-09-044826. [DOI] [PubMed] [Google Scholar]
- 43•.Petrovas C, Price DA, Mattapallil J, et al. SIV-specific CD8+ T cells express high levels of PD1 and cytokines but have impaired proliferative capacity in acute and chronic SIVmac251 infection. Blood. 2007;110(3):928–36. doi: 10.1182/blood-2007-01-069112. This study demonstrated the in vivo relevance of PD-1 in the non-human primate model of SIV infection SIV-specific CD8+ T cells with high levels of PD-1 expression had low proliferative capacity compared with PD-1(low) T cells SIV epitopes with mutational escapes led to decreased PD-1 expression, showing that repeated TCR stimulation is a crucial factor in PD-1 expression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44•.Velu V, Kannanganat S, Ibegbu C, et al. Elevated expression levels of inhibitory receptor programmed death 1 on simian immunodeficiency virus-specific CD8 T cells during chronic infection but not after vaccination. J Virol. 2007;81(11):5819–28. doi: 10.1128/JVI.00024-07. During SIV infection in macaques, this study reports the majority of SIV-specific CD8+ T cells express PD-1 Levels of PD-1 were highest in the lymph nodes and rectal mucosa and correlated with plasma viremia They also showed that in vitro blockade of PD-1 enhanced SIV-specific CD8+ and CD4+ T-cell proliferation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45••.Velu V, Titanji K, Zhu B, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458(7235):206–10. doi: 10.1038/nature07662. This was the first in vivo trial of the safety and immune restoration potential of PD-1 blockade in macaques with chronic SIV infection PD-1 blockade was well tolerated by macaques and resulted in rapid expansion of HIV-specific CD8+ T cells with improved polyfunctionality, thus establishing both safety and efficacy of PD-1 blockade in the SIV/macaque model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Estes JD, Gordon SN, Zeng M, et al. Early resolution of acute immune activation and induction of PD-1 in SIV-infected sooty mangabeys distinguishes nonpathogenic from pathogenic infection in rhesus macaques. J Immunol. 2008;180(10):6798–807. doi: 10.4049/jimmunol.180.10.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47•.Said EA, Dupuy FP, Trautmann L, et al. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat Med. 2010;16(4):452–9. doi: 10.1038/nm.2106. In this study the authors demonstrate that PD-L1 triggering of PD-1 expressed on monocytes induced IL-10 production and reversible CD4+ T-cell dysfunction They also show that HIV-infected individuals have high PD-1 expression on monocytes, which may be triggered by products of microbial translocation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48•.Jones RB, Ndhlovu LC, Barbour JD, et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008;205(12):2763–79. doi: 10.1084/jem.20081398. This was the first study of Tim-3 in HIV-infected individuals, showing elevated levels of Tim-3 on CD8+ T cells in HIV+ individuals that correlated with CD4+ T cells and HIV viral load Blockade of Tim-3 resulted in improved HIV-specific CD8+ T-cell responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Price P, Keane N, Gray L, et al. CXCR4 or CCR5 tropism of human immunodeficiency virus type 1 isolates does not determine the immunological milieu in patients responding to antiretroviral therapy. Viral Immunol. 2006;19(4):734–40. doi: 10.1089/vim.2006.19.734. [DOI] [PubMed] [Google Scholar]
- 50.Lim AY, Price P, Beilharz MW, French MA. Cell surface markers of regulatory T cells are not associated with increased forkhead box p3 expression in blood CD4+ T cells from HIV-infected patients responding to antiretroviral therapy. Immunol Cell Biol. 2006;84(6):530–6. doi: 10.1111/j.1440-1711.2006.01467.x. [DOI] [PubMed] [Google Scholar]
- 51•.Antonelli LR, Mahnke Y, Hodge JN, et al. Elevated frequencies of highly activated CD4+ T cells in HIV+ patients developing immune reconstitution inflammatory syndrome. Blood. 2010 doi: 10.1182/blood-2010-05-285080. This report demonstrated that in HIV-infected individuals initiating antiretroviral therapy, those who developed immune reconstitution syndrome had higher baseline levels of PD-1 with CTLA-4 and LAG-3 coexpression on CD4+ T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52•.Jin HT, Anderson AC, Tan WG, et al. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci USA. 2010;107(33):14733–8. doi: 10.1073/pnas.1009731107. In this study, the authors evaluate Tim-3 and PD-1 coexpression during LCMV infection in the mouse, showing PD-1+Tim-3+ CD8+ T cells had more severe T-cell exhaustion Combined inhibition of PD-1 and Tim-3 synergistically improved CD8+ T-cell responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vali B, Jones RB, Sakhdari A, et al. HCV-specific T cells in HCV/HIV co-infection show elevated frequencies of dual Tim-3/PD-1 expression that correlate with liver disease progression. Eur J Immunol. 2010 doi: 10.1002/eji.201040340. [DOI] [PubMed] [Google Scholar]
- 54.Sachdeva M, Fischl MA, Pahwa R, et al. Immune exhaustion occurs concomitantly with immune activation and decrease in regulatory T cells in viremic chronically HIV-1-infected patients. J Acquir Immune Defic Syndr. 2010;54(5):447–54. doi: 10.1097/QAI.0b013e3181e0c7d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vollbrecht T, Brackmann H, Henrich N, et al. Impact of changes in antigen level on CD38/PD-1 co-expression on HIV-specific CD8 T cells in chronic, untreated HIV-1 infection. J Med Virol. 2010;82(3):358–70. doi: 10.1002/jmv.21723. [DOI] [PubMed] [Google Scholar]