Abstract
Targeted contrast-enhanced ultrasound (molecular ultrasound) is an emerging imaging strategy that combines ultrasound technology with novel molecularly-targeted ultrasound contrast agents for assessing biological processes at the molecular level. Molecular ultrasound contrast agents are nano- or micro-sized particles that are targeted to specific molecular markers by adding high-affinity binding ligands onto the surface of the particles. Following intravenous administration, these targeted ultrasound contrast agents accumulate at tissue sites overexpressing specific molecular markers, thereby enhancing the ultrasound imaging signal. High spatial and temporal resolution, real-time imaging, non-invasiveness, relatively low costs, lack of ionizing irradiation and wide availability of ultrasound systems are advantages compared to other molecular imaging modalities. In this article we review current concepts and future directions of molecular ultrasound imaging, including different classes of molecular ultrasound contrast agents, ongoing technical developments of preclinical and clinical ultrasound systems , the potential of molecular ultrasound for imaging different diseases at the molecular level, and the translation of molecular ultrasound into the clinic.
INTRODUCTION
In recent years, targeted contrast-enhanced ultrasound imaging (molecular ultrasound) has emerged as a promising new non-invasive imaging strategy for imaging biological processes at the molecular level. It combines the advantages of ultrasound, including high spatial and temporal resolution, relatively low costs, portability, lack of ionizing irradiation, and the widespread availability in imaging departments throughout the world, with the use of molecularly-targeted ultrasound contrast agents. These ultrasound contrast agents are decorated with binding ligands such as antibodies or small peptides that recognize receptor proteins involved in various disease processes. Combined with dedicated ultrasound imaging sequences and the latest transducer technology, molecular ultrasound allows quantitative assessment of molecular target expression with a high sensitivity, which opens exciting new applications for ultrasound including early detection of diseases such as cancer or atherosclerosis as well as monitoring treatment effects of novel drugs such as anti-angiogenic agents at the molecular level. Through further advances in ultrasound contrast agent design, molecular ultrasound may soon achieve a clinical translation into human studies.
In this article we review the concepts of molecular ultrasound imaging, including the various types of molecular ultrasound contrast agents, ultrasound imaging techniques, and possible applications of molecular ultrasound imaging in medicine.
Ultrasound Contrast Agents
Ultrasound contrast agents can be divided into two main classes: 1) microbubble based contrast agents and 2) non microbubble based contrast agents (Figure 1) 1. Microbubbles are gas-liquid emulsions consisting of a gaseous core surrounded by a shell and are usually 1 to 4 microns in size. Following insonification with ultrasound, the gaseous core of the microbubbles causes a very high echogenic response that results in a high contrast to tissue background ratio on ultrasound images. The microbubble shell prevents both gas leakage and aggregation of the microbubbles. Different types of contrast microbubbles have been synthesized by combining different shell compositions such as albumin, galactose, lipids, or polymers, with different gaseous cores such as air, or high molecular weight gases (perfluorocarbon, sulfur hexafluoride or nitrogen) 2. Heavy gases are less water-soluble and, thus, dissolve into the surrounding solution at a much slower rate, than for example, air. This prolongs the effective lifetime of microbubbles 3. Introduction of a grafted arm of polyethylene glycol (PEG) polymer onto the microbubble shell provides additional stearic protection and prevents microbubble aggregation4,5. Following intravenous administration, microbubbles are rapidly cleared by the reticuloendothelial system (RES) with a serum half life of only a few minutes 6. Due to their size of a few microns, microbubbles stay within the vascular compartment, and do not leak out into the extra-vascular space. This limits their use for imaging of disease processes that are reflected on vascular endothelial cells such angiogenesis, inflammation, or thrombus formation. Preparation protocols for microbubbles are reviewed elsewhere 7.
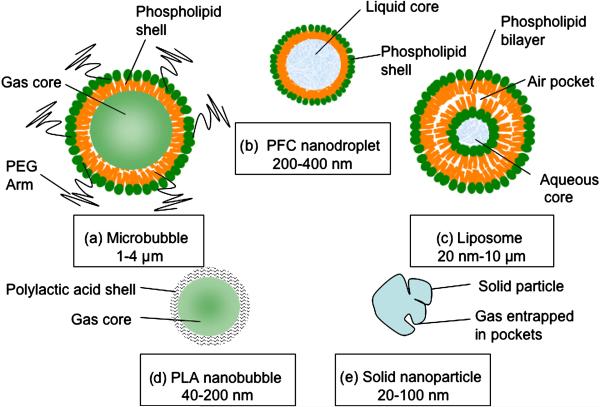
Figure 1.
Different types of ultrasound contrast agents. a) Microbubbles are gas-liquid emulsions with a polyethylene gycol (PEG) polymer on the surface to prevent aggregation. Microbubbles are highly echogenic and the most commonly used contrast agent for molecular ultrasound imaging. b) Perfluorocarbon emulsion (PFC) nanodroplets are liquid-liquid emulsions that can be vaporized into echogenic gas-bubbles following administration of acoustic energy. c) Liposomes are phospholipid bilayers that can enclose air pockets for ultrasound imaging. d) Nanobubbles are gas-liquid emulsions that can fuse into echogenic microbubbles at the target site. e) Solid nanoparticles are solid amorphous substances with gas entrapped in their pores or fissures increasing echogenicity.
Non microbubble based contrast agents consist of either submicron or nano sized particles. These particles consist of either liquid or solid colloids that range in size between 10 and 1000 nanometers 1. Non microbubble based contrast agents are advantageous over microbubbles in terms of their ability to enter the extravascular space providing the opportunity to image targets beyond the vascular compartment. However, most of the non microbubble based contrast agents cannot be detected individually by ultrasound imaging due to their poor inherent acoustic reflectivity. The relative incompressible liquid/solid core or the small size of these particles makes visualization by ultrasound difficult. Hence, other mechanisms for visualization of these particles with ultrasound have been proposed (see below).
Based on their composition and size, different types of sub micron or nano sized particles have been synthesized for ultrasound imaging: Echogenic liposome, perfluorocarbon emulsion (PFC) nanodroplets, nanobubbles and solid nanoparticles. Echogenic liposomes consist of a lipid bilayer with an aqueous core. Air pockets within the lipid bilayer can generate acoustic reflectivity 8. An example for the use of targeted echogenic liposome was shown in an atheroma model in miniswines 9. Echogenic liposomes were targeted against various markers of inflammation such as anti-intercellular adhesion molecule 1 (ICAM1), anti-vascular cell adhesion molecule 1 (VCAM1), anti-fibrin, anti-fibrinogen, and anti-tissue factor (TF). These targeted liposomes accumulated at the site of atheroma and increased the imaging signal 5 min after intravenous administration 9.
PFC nanodroplets are liquid-liquid emulsions consisting of a liquid perfluorocarbon core encapsulated by a phospholipid monolayer, with typical diameters ranging between 200 and 400 nm. The liquid composition makes them resistant to pressure and mechanical stress compared with the gas core of microbubbles. When a large number of PFC nanodroplets accumulate on the surface of a biological target these PFC nanodroplets can become acoustically detectable with ultrasound. Lanza et al 10 successfully increased the detection of blood clots by using fibrin-targeted PFC nanodroplets and intravascular ultrasound in the carotid arteries of dogs. Targeted PFC nanodroplets were administered either in situ or systemically and aggregated at the surface of blood clots, thereby increasing the echogenicity of the clots. In a related study, stretch induced tissue factor targeted PFC nanodroplets which were used to enhance the detection of injured pig carotid arteries in vivo using intravascular ultrasound 11. A segment of the injured carotid artery was isolated and infused with targeted PFC nanodroplets. High echogenicity was observed at the site of arterial injury due to the accumulation of PFC nanodroplets in the tunica media and internal elastic lamina 11. To overcome the general limitation of low acoustic reflectivity a novel technology has been introduced where the liquid core of PFC nanodroplets can be vaporized into gas using acoustic energy, thereby substantially increasing the echogenicity of PFC nanodroplets (Figure 2). However, whilst low-boiling point perfluorocarbons such as dodecafluoropentane can spontaneously vaporize at physiological body temperature, high-boiling point liquid perfluorocarbons such as perflurohexyl bromide are difficult to vaporize and need relatively high energy to transfer them into a gaseous phase 12. To overcome this trade-off, Amirriazi et al showed in vitro that nanodroplets filled with high-boiling point perfluorcarbons can be modified by adding iron oxide nanoparticles as nucleation sites, thereby substantially lowering the boiling-point of nanodroplets 13. Future studies are warranted to assess biodistribution and feasibility for in vivo molecular ultrasound imaging using targeted perfluorocarbon nanodroplets and this vaporization technique.
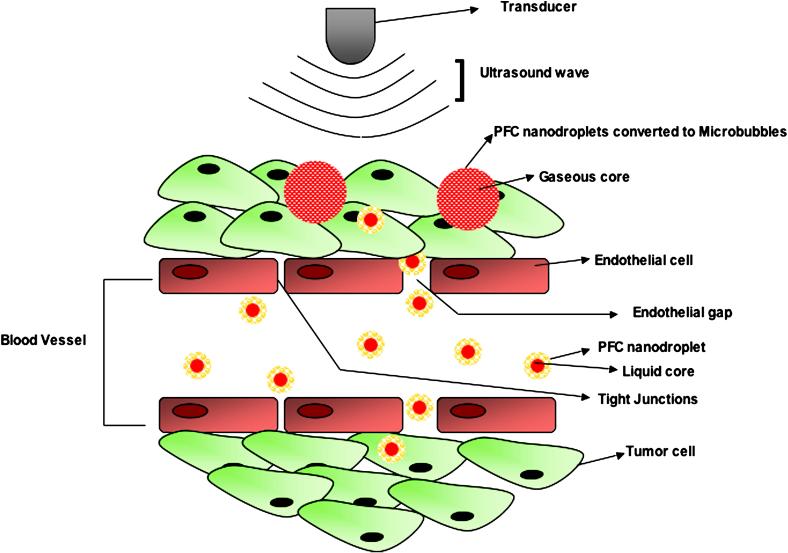
Figure 2.
Schematic representation of the use of PFC nanodroplets as ultrasound contrast agents. Due to their small size, PFC nanodroplets extravasate into the tumor interstitium. Following administration of acoustic energy, the liquid perfluorocarbon core can be vaporized and transferred into gaseous phase, thereby increasing echogenicity at the site of PFC nanodroplet accumulation.
Nanobubbles are gas-liquid emulsions enclosed by a biodegradable polymer such as polylactic acid and range between 40 and 200 nm in size. A recent study assessed the application of nanobubbles for both ultrasound imaging and ultrasound mediated delivery of the drug doxorubicin in subcutaneous human breast cancer xenograft tumors in mice 14. In that study, doxorubicin encapsulated, non-targeted nanobubbles accumulated and were retained in the tumor tissue via the enhanced permeability and retention (EPR) effect, thus accomplishing passive drug targeting to tumors 14. The EPR effect refers to the phenomenon of small particles entering the extravascular space of tumors through leaky and defective tumor vessels and accumulating due to the defective lymphatic drainage of the tumor tissue 15. As the nanobubbles extravasate and accumulate in the tumor tissue, they coalesce into larger, better detectable gas bubbles, thus providing strong signal on ultrasound imaging (Figure 3). Furthermore, when subjected to tumor directed therapeutic ultrasound, these gas bubbles oscillate and collapse, thereby releasing the therapeutic drug at the site of the tumor.
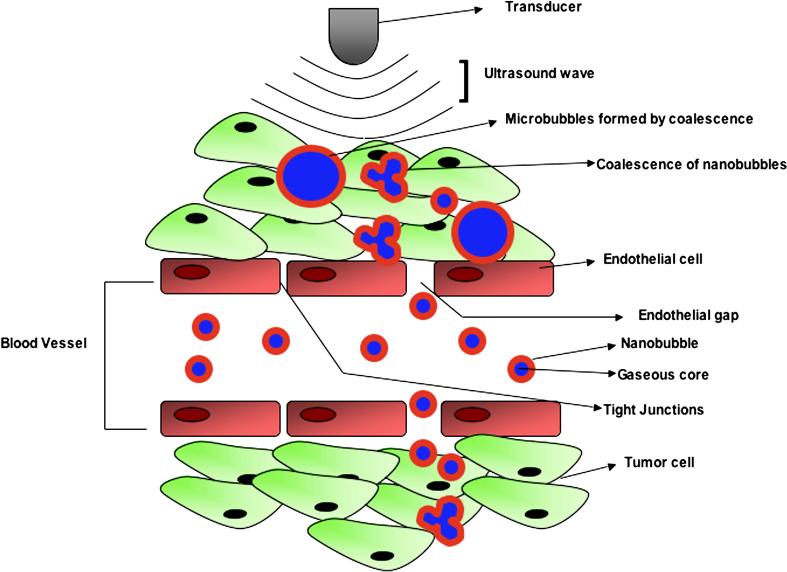
Figure 3.
Schematic representation of the use of nanobubbles as ultrasound contrast agents. Tumors are characterized by defective vasculature with large gaps between the endothelial cells, allowing extravasation of non-targeted nanobubbles and passive accumulation in tumor interstitium. In the tumor interstitium, these nanobubbles can coalesce to form echogenic microbubbles, detectable by ultrasound imaging (modified from Rapport et al, J Natl Cancer Inst 200714).
Solid nanoparticles such as amorphous solid particles including silica or iron oxide particles contain gas pockets in their pores and fissures and have been shown to generate detectable backscatter for ultrasound imaging. Liu et al 16 imaged the liver of mice after intravenous administration of 100 nm silica nanoparticles and showed that the mean acoustic intensity increased by 30% compared to background. Iron oxide based nanoparticles (SPIO) were used to delineate malignant gliomas orthotopically implanted into the brain of rats in another study 17. Ex vivo ultrasound imaging of the brains showed that malignant gliomas could be well delineated from the surrounding brain tissue due to the presence of echogenic SPIO that accumulated in the macrophages situated at the tumor margin. In another study, SPIO were used for imaging autoimmune encephalomyelitis (EAE, characterized by demyelination, axonal damage and inflammation with the most prevalent cell type being macrophages) 18. These iron oxide nanoparticles accumulated in macrophages creating a front of small echogenic scatterers in the EAE regions of the brain that could be detected by ultrasound imaging.
Microbubble-based contrast agents are the most commonly used contrast media for contrast-enhanced ultrasound imaging and the following part of this review focuses on the use of contrast microbubbles for molecular ultrasound imaging.
Cellular and Molecular Targeting of Contrast Microbubbles
Targeting of contrast microbubbles to a cellular or molecular target can be either passive or active. Passive targeting refers to a nonspecific accumulation of microbubbles at the target site. Lindner et al 19 have shown that activated leukocytes engulf and absorb microbubbles that are surrounded by an albumin or a lipid shell. In this study, passive targeting of leukocytes was achieved by the incorporation of negatively charged phosphatidylserine into the shell of microbubbles, which promotes microbubble attachment to activated leukocytes. These microbubbles were used to image inflamed venules of mouse cremaster muscles 19. Ultrasound images after microbubble infusion demonstrated increased contrast enhancement from phagocytosed and adherent microbubbles retained at the site of inflammation.
Active targeting refers to the modification of the contrast agent shell to allow selective binding to cellular epitopes or other receptors of interest. Avidin or streptavidin is commonly used for non-covalent attachment of biotinylated ligands onto the shell of ultrasound contrast microbubbles due to its wide availability, very high effective dissociation constant Kd of 10−15 molar, and flexible use for pre-clinical experiments 20. The use of (strept)avidin is advantageous in preclinical research because it allows binding of virtually any biotinylated ligand onto the microbubble surface without the need to design new microbubbles for each vascular molecular target under investigation. A drawback of this approach, however, is that streptavidin can cause immunogenic and allergic reactions in patients 21. Another possible side effect of streptavidin is the potential binding of streptavidin to physiologically available biotin within the human body, which is needed for fatty acid synthesis and gluconeogenesis 22. Therefore, (strept)avidin containing microbubbles are unlikely to be clinically translated.
Several alternate strategies that allow covalent binding of ligands to the microbubble shell have been explored 7. One approach involves the binding of activated carboxylic groups in the microbubble shell with amino-groups from lysine residues of binding ligands 20. However, coupling yields of ligands to microbubbles are limited with this reaction as large excess amounts of binding ligands have to be added to yield reasonable ligand/microbubble coupling ratios (corresponding to at least 105 attached ligands per microbubble) 20,23. In another approach, maleimide is introduced into the microbubble shell and reacted with an activated thiol group on ligands to form stable thioether bonds 24. Since single thiol residues can be introduced into the non-functional parts of the ligand (for example by site-directed mutagenesis or by direct synthesis), site-specific attachment of the binding ligand to the microbubble shell can be achieved 20. In a third approach, binding ligands are coupled to components of the microbubble shell (e.g. to phospholipids), which is followed by the formation of microbubbles using these functionalized components in a second procedure 20. This approach has been used to design targeted contrast microbubbles for clinical use 25,26.
Imaging and quantification of molecular ultrasound signal
The goal of different molecular ultrasound imaging and quantification strategies is to isolate imaging signals derived from accumulating microbubbles from the surrounding tissue signal in vivo. On many early ultrasound systems non-targeted microbubbles were imaged by destroying them with high powered ultrasound pulses. This technique is often referred to as Loss of Correlation (LOC). A microbubble undergoing disruption generates a different acoustic response between successive imaging pulses, resulting in a decorrelation detectable with Power Doppler signal processing 27. While this approach can be easily implemented on existing Power Doppler imaging systems, the detection of contrast agent relative to tissue is limited and it is not appropriate for molecular ultrasound imaging because the microbubble signal of interest is immediately destroyed while imaging. Therefore, other imaging techniques harnessing particular properties of the microbubbles have been developed.
Due to their high compressibility and shell elasticity properties, microbubbles oscillate nonlinearly in an ultrasound field 28. This causes nonlinear scattering of ultrasound waves, which have harmonics at twice the center frequency (second harmonic) and above (third harmonic, fourth harmonic, etc.) 29,30, or at half of the center frequency (subharmonic) 31. Therefore, most ultrasound imaging techniques try to preferentially detect this nonlinear energy from microbubbles, thereby segmenting the microbubble-generated imaging signal from the tissue signal 32. With increasing acoustic pressure, tissue also responds nonlinearly to ultrasound 33, which always causes some residual nonlinear background tissue signal. This background tissue signal can be substantially reduced by using low acoustic pressures (below ~ 500 kPa), thereby improving the contrast-to-tissue-ratio (CTR) of the ultrasound image.
Harmonic Imaging 34,35 was developed for imaging microbubbles nonlinearly, to overcome intrinsic limitations in CTR with LOC approaches. By implementing frequency-based filters in the analog electronics of the ultrasound system, particular nonlinear frequency components (harmonics) were retained. These implementations improved detection when compared with LOC, however, with the tradeoff of limited signal bandwidth, a restriction imposed by the design of analog harmonic filters. This is due to the fact that frequency-based filtering will not distinguish between linear and nonlinear signal components that overlap in frequency. By restricting the frequency bandwidth of the imaging, the overlap of the signals is minimized (however, at the expense of reduced axial imaging resolution).
Current contrast-enhanced ultrasound imaging techniques
Most contrast ultrasound imaging modes on current clinical ultrasound systems employ multi-pulse techniques for nonlinear contrast agent detection (Table 1) 36,37. Multi-pulse techniques rely on detecting nonlinear imaging signals by modulating either the phase or amplitude between successive ultrasound pulses. Since microbubbles oscillate nonlinearly, scaling their receive echoes and cancelling them results in a residual signal difference that can be exploited to detect microbubbles and cancel signal from the surrounding tissue. Since the signal filtering is not frequency-based, linear and nonlinear signal components overlapping in frequency can still be extracted, avoiding the bandwidth restrictions imposed by Harmonic Imaging as described above. Also, broadband imaging pulses can be used which improves the axial imaging resolution. This is particularly important if one tries to detect nonlinear signals from microbubbles imaged at the fundamental frequency (the frequency of insonation) 38, which cannot be detected with Harmonic Imaging since linear and nonlinear harmonic components overlap at the fundamental frequency. Many clinical ultrasound systems actually utilize this nonlinear fundamental component because it is most easily detected at the sensitivity peak of the transducer. Multi-pulse implementations also have the added benefit of signal averaging (which reduces noise in the image) since two or more pulses are used to form a given image line.
Table 1.
Current state of the art clinical ultrasound systems and their respective contrast imaging technologies
| Manufacturer | System | Technology |
|---|---|---|
| Philips | iU22 | Pulse Inversion (PI) Power Modulation (PM) PM/PI Coded Contrast Harmonics (CCH) |
| General Electric | Logiq 9 | Coded Harmonic |
| Siemens | Sequoia | Cadence Contrast Pulse Sequencing (CPS) |
| Hitachi | HI Vision 900 | Dynamic Contrast Harmonic Imaging (dCHI) using wideband Pulse Inversion (PI) |
| Toshiba | Aplio XG | Pulse Subtraction |
| Aloka | Prosound Alpha 7 | Contrast Harmonic Echo using ePureHD (Extended Pure Harmonic Detection) |
| Esaote | MyLab GOLD | CnTI (Contrast Tuned Imaging) |
The two most common two-pulse sequences are Pulse Inversion (PI) and Amplitude Modulation (AM) (often referred to as Power Modulation) and the majority of multi-pulse techniques are based on the fundamentals of either PI, AM, or their combination (PIAM). The basic implementation involves a combination of two pulses, however, this can be extended to repetitions of multiple pulse pairs 39,40. In general, adding more pulses to a sequence helps suppress tissue motion (which improves sensitivity), however, with the tradeoff of reduced frame rate and, thus, reduced temporal resolution 41,42.
High-frequency ultrasound imaging systems
Ultrasound imaging performed at high frequency (also known as micro-ultrasound) increases image resolution, however at the expense of imaging penetration depth. It is currently widely used in pre-clinical imaging of small animals 43 where high spatial resolution is needed. Typical frequencies range from 20-70 MHz (resulting in axial spatial resolution of 20-80 μm) compared with frequencies commonly used for clinical purposes in the range of 1-15 MHz (with an axial spatial resolution of 100-1500 μm). High-frequency imaging is well suited to molecular imaging since the resolution approaches a much smaller scale (closer to the cellular level) and in turn increases the specificity of the detection. However, the design of high frequency array transducers is technically challenging because the size of transducer elements is on the order of 50-100 μm, with spacing between elements requiring cuts (kerfs) on the order of 10 μm. Dicing equipment capable of creating kerfs of this size was, until recently, not possible. First generation small animal high-frequency ultrasound systems, therefore, used mechanically scanned transducers with a single active element which reduced the fabrication complexity since kerfs between elements were not required 44. Although there were efforts in measuring nonlinear signals from microbubbles using sub- and second-harmonic imaging approaches 45,46, non-linear imaging with first generation high-frequency transducers was of limited success due to the fixed focus zone of the transducers, which limited the depth-of-field, and thus, the sensitivity to contrast agent outside of the focal zone.
As a result, molecular imaging with first generation micro-ultrasound systems used a frame subtraction technique for microbubble detection. Using fundamental B-mode imaging, targeted microbubbles were separated from freely circulating microbubbles using a destruction/replenishment approach 47-49. By using a sum of absolute differences algorithm50, the best match between pre- and post-destruction image frames was identified and subtracted from each other. The subtracted signal was then displayed as a colored overlay on the B-mode images and was considered to be the imaging signal from microbubbles attached to the molecular target (Figure 4). The challenge with this quantification approach, however, is that often microbubbles and background tissues cannot be easily differentiated from each other due to high echogenicity of the tissue. This ultimately limits the sensitivity of this approach to detecting all microbubbles present in the imaging field of view.
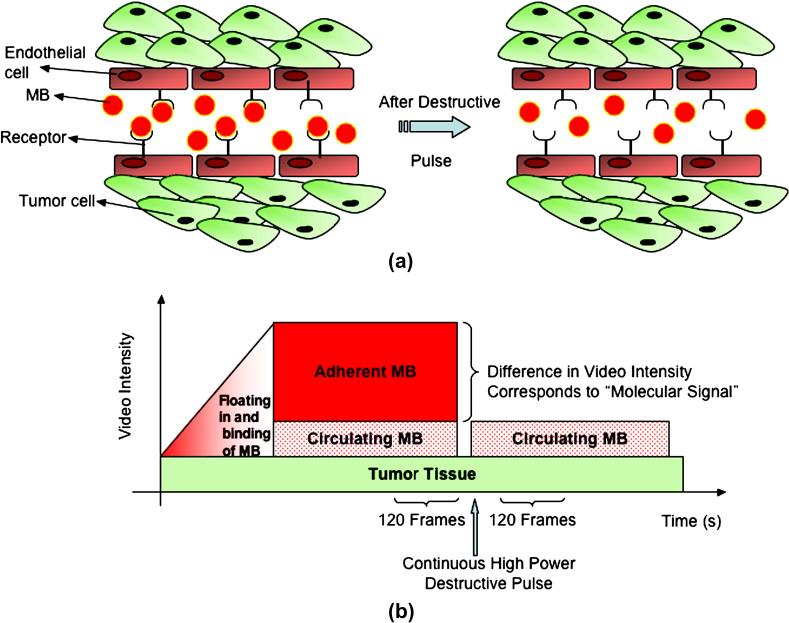
Figure 4.
a) Schematic representation of targeted microbubbles (MB) attached to receptors expressed on tumor endothelial cells after intravenous administration. Microbubbles remain predominantly in the vasculature due to their size (several micrometers) and thus adhere only to the tumor endothelial cells and not to tumor cells. Some microbubbles do not attach to receptors and freely float. After high-power destructive pulse, adherent microbubbles are destroyed and freely circulating microbubbles replenish from outside imaging plane after several seconds. b) Graphical summary of the approach for quantification of imaging signal from attached microbubbles. Adapted from Willmann et al, Radiology Vol 246, number 2, 2008 49.
In the meantime, high-frequency linear array technologies continued to develop 51,52,53, improving depth of field and eliminating mechanical motions, which improved the implementation of multi-pulse sequencing for high-frequency ultrasound imaging. Recently, a linear array based high-frequency ultrasound imaging system has been developed 54 that allows nonlinear contrast imaging at frequencies ranging between 18 and 24 MHz 55.
Quantification of molecular ultrasound imaging signal
The imaging signal in contrast-enhanced ultrasound imaging is proportional to the concentration/number of the administered contrast microbubbles 56. Provided that the uncompressed linear data on the US system can be stored, or a calibrated linearization scheme exists with sufficient dynamic range to undo the image compression 57, it is possible to perform an absolute or relative approach for quantifying attached microbubbles in molecular ultrasound imaging. For absolute quantification, the goal is to quantify the absolute number of accumulating microbubbles at the target site. This requires the ultrasound system to be calibrated for detected ultrasound signal power corresponding to a given number of microbubbles (e.g., performed by in vitro characterization) for a particular combination of system settings (e.g. gain, dynamic range, mechanical index, etc.). The detected ultrasound signal will also be influenced by attenuation through tissue (the loss of energy primarily through absorption; the conversion of ultrasound energy into other forms such as heat) as well as the geometry of the imaging beam (which is not perfectly uniform with depth). These effects must be accounted for, and are not always known with certainty in vivo. Also, the interaction between the microbubble and vascular endothelial cells must be better understood so that the relationship between the number of microbubbles per cell surface marker is known, in order to infer the amount of cellular expression. Finally, the effect of non-specific attachment of targeted microbubbles to endothelial cells needs to be characterized with certainty. Preset system parameters could be stored on an ultrasound system, along with standard values of attenuation and ultrasound beam geometry (which would be measured separately) and used for calculation of absolute values. These types of corrections have been proposed for blood perfusion imaging with microbubbles 58 and could potentially be applied to molecular imaging with ultrasound. The combination of all this, however, requires careful measurement procedures and experimental technique. Thus, absolute measures of molecular expression with ultrasound so far are rare, especially with current commercial ultrasound systems, and have not found widespread use.
In comparison, the relative quantification approach is more practical and allows trends of microbubble attachment to be quantitatively correlated with trends in expression levels of molecular markers. Comparing detected molecular signal (M) between regions within an image can give a relative value of molecular expression. For example, the regions may be sections of normal and pathological tissues within an organ. Thus, the signal from two regions, M1 and M2, (where M is the Root-Mean-Square (RMS) of the signal within the region) can be expressed as: Molecular Expression = M1 / M2. (equation 1). If the regions of interest encompass a similar depth range in the image, then the effects due to attenuation and ultrasound beam geometry can be considered negligible as they will influence the values of M1 and M2 equally and are effectively cancelled in equation 1. Rather than calculating an absolute quantity of cellular expression, equation 1 simply relates the amount of molecular signal expressed in region 1 relative to the amount in region 2 as a linear ratio, which could be presented as a percentage increase or decrease over time. This approach to quantification is very straightforward, and thus, most current research with microbubbles for molecular imaging uses relative measures of linear ultrasound signal level to infer the amount of molecular expression.
Applications of molecular ultrasound imaging
Since microbubbles remain in the intravascular space due to their size of several microns, disease processes within the vascular compartment such as angiogenesis, inflammation or thrombus formation are ideal targets for molecular ultrasound imaging.
Molecular ultrasound imaging of tumor angiogenesis
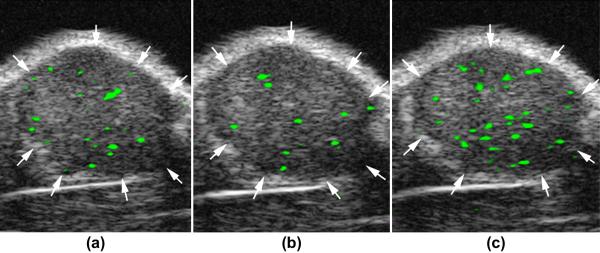
Tumor angiogenesis is the formation and recruitment of new blood vessels from the host surrounding tissue. In this process various molecular markers are over-expressed on tumor vascular endothelial cells 59,60. Microbubbles targeted to these molecular markers of angiogenesis such as vascular endothelial growth factor receptor type 2 (VEGFR2), αvβ3 integrin, or endoglin, have been used to image tumor angiogenesis with molecular ultrasound imaging. In the study of Lee et al 61, a positive correlation between the in vivo ultrasound imaging signal and actual VEGFR2 expression on tumor endothelial cells of subcutaneous breast cancer tumors could be demonstrated. Since angiogenesis marker expression can vary in different tumor types and at different stages of tumor development, another group 62 explored the potential of a dual-targeted microbubble (targeted to VEGFR2 and αvβ3 integrin using the same microbubble) to increase in vivo imaging signal in subcutaneous human ovarian cancer xenografts in mice. In vivo imaging signal was substantially higher using dual-targeted microbubbles compared to either singly-targeted microbubble or a combination of the two singly-targeted microbubbles, suggesting that multi-targeting of contrast microbubbles may improve detection of tumor angiogenesis (Figure 5). This may be of significance to molecular ultrasound imaging for early tumor detection of cancer with different markers expressed at different time points during cancer development.
Figure 5.
Molecular ultrasound images of a subcutaneous human ovarian adenocarcinoma xenograft tumor (arrows) in a nude mouse after intravenous administration of singly-targeted microbubbles, targeted at vascular endothelial growth factor receptor type 2 (a) or αvβ3 integrin (b), and dual-targeted microbubbles (c). Molecular ultrasound imaging signal is shown as green overlay on B-mode images. Note that in vivo imaging signal is substantially higher after administration of dual-targeted microbubbles compared to either of the singly-targeted microbubbles. Reprinted with permission from Willmann et al, Radiology Vol 248, number 3, 2008 62.
In addition, three studies have shown that molecular ultrasound allows longitudinal monitoring of anti-angiogenic therapy in human cancer xenograft tumors in mice. Subcutaneous and orthotopically implanted pancreatic cancers were treated with anti-VEGF monoclonal antibodies and/or gemcitabine and longitudinal molecular imaging was performed with microbubbles targeted to VEGFR2, the VEGF-VEGFR complex, or endoglin 63. Imaging signal from targeted microbubbles decreased in tumors undergoing anti-angiogenic or cytotoxic therapy, and correlated with the level of expression of the target and with microvessel density. Similarly, an upregulation of VEGFR2 and αvβ3 integrin was shown in subcutaneous human squamous cell xenografts in mice without treatment, and a downregulation of both markers following therapy with matrix metalloproteinase inhibitor treatment 64. Pysz et al 26 have compared molecular ultrasound imaging signal in human colon cancers in mice with and without anti-angiogenic treatment using ultrasound and a novel clinically-translatable human VEGFR2-targeted microbubble. As early as 24 hours after initiation of anti-angiogenic treatment, molecular ultrasound showed a significant decrease in imaging signal in treated versus non-treated mice, while there was no difference in terms of tumor size at early time points after anti-angiogenic treatment. This study suggested that molecular ultrasound allows early assessment of successful anti-angiogenic treatment before overt morphological-anatomical changes become visible in tumors.
Molecular ultrasound imaging of inflammatory processes
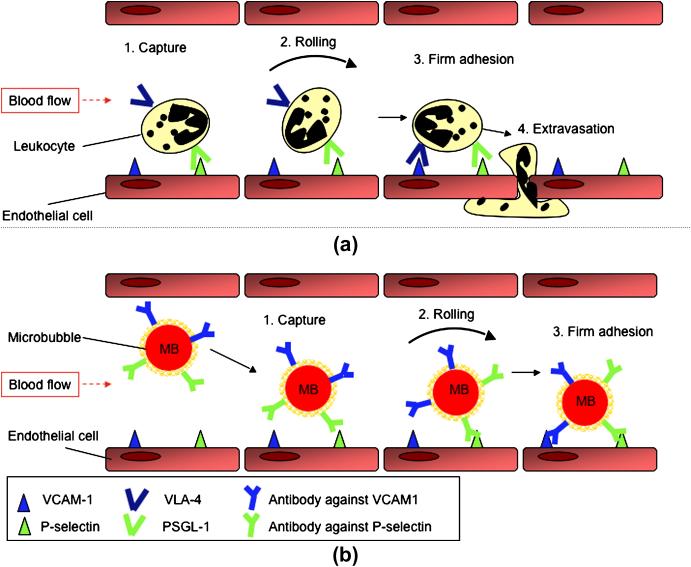
Inflammation is a common pathophysiological process in a number of disease states. Therefore, a noninvasive imaging method to assess inflammation in vivo at the molecular level can be beneficial for early diagnosis and treatment monitoring. A common feature of inflammation is the activation of leukocytes in the blood pool and their transmigration to the extravascular compartment. The recruitment and transmigration of leukocytes is mediated by the interaction between adhesion molecules on the leukocytes and various receptors expressed on the endothelial cell surface 65,66. Molecules such as E- and P-selectin mediate the initial capturing and then promote rolling of leukocytes on the inner vessel wall. Subsequently, a firm arrest of the rolling leukocytes, a necessary precondition for migration of leukocytes into the tissue, is promoted by adhesion molecules such as ICAM1 and VCAM1 (Figure 6a). Recently, a contrast agent system was modeled based on the behavior of leukocytes during inflammation 67. The aim of this study was to improve adherence of microbubbles under conditions of sheer stress that is present due to blood flow for detection of inflammation in atherosclerosis. Dual-targeted microbubbles (simultaneously targeted to both P-selectin and VCAM1) were shown to adhere with increased efficiency compared to single targeted microbubbles in cell culture experiments. It was hypothesized that similar to rolling and attachment of leukocytes, targeting the P-selectin marker aided in capturing the microbubbles to the endothelial cell wall with subsequent rolling of the microbubbles on the endothelial cells, while targeting to VCAM1 aided in firm attachment of the microbubbles to the blood vessel wall (Figure 6b).
Figure 6.
Dual-targeted microbubble can be designed that mimic the behavior of leukocytes in vivo. a) Circulating leukocytes in the blood invade the site of inflammation through a process of several steps: 1) Capturing and 2) rolling of leukocytes along the vasculature mediated through transient interactions between selectin proteins (e.g., P-selectin) and their ligands (e.g., P-selectin glycoprotein ligand-1 - PSGL-1); 3) Firm adhesion of leukocytes to endothelial cells by high affinity interaction between cell adhesion molecules (e.g., VCAM1) and integrins (e.g., Very Late Antigen-4- VLA-4); and 4) Extravasation (modified from Kunkel and Butcher 2003, Nature Reviews Immunology 3, 822-829 65). b) Dual-targeted microbubble targeted against both P-selectin and VCAM1, simulating vascular attachment of a leukocyte at sites of inflammation by first interacting with P-selectin and then firmly attaching via VCAM1.
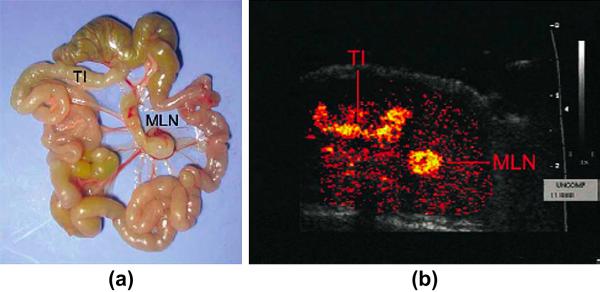
Inflammatory markers such as E- and P-selectin, ICAM1 and VCAM1 have been targeted with microbubbles to quantify inflammation in various organs such as the kidneys, the heart and the colon 68-71. Using P-selectin targeted microbubbles in a myocardial ischemia reperfusion model in rats allowed identifying the presence of recently ischemic myocardium in the absence of necrosis 69. A similar study used the P-selectin ligand sialyl Lewis conjugated to microbubbles in myocardial ischemia reperfusion model in rats that adhered to inflammatory endothelial cells of recently ischemic myocardium 72. Both studies highlight the potential of molecular ultrasound imaging as a rapid and straight forward bedside test for imaging “ischemic memory” in patients with atypical chest pain. Furthermore, the potential clinical uses for P-selectin-targeted ultrasound imaging may also be extended to other myocardial inflammatory processes such as transplant rejection and myocarditis. Another promising clinical application of molecular ultrasound is the quantification of inflammation in inflammatory bowel disease. In a recent proof of principle study, inflammation in the terminal ileum of transgenic mice could be visualized non-invasively at the molecular level by using the mucosal addressin cellular adhesion molecule 1 (MAdCAM1)-targeted microbubbles 71 (Figure 7).
Figure 7.
Inflammation imaging in inflammatory bowel disease using contrast enhanced transabdominal ultrasound and microbubbles targeted at MAdCAM-1 (Mucosal Addressin Cellular Adhesion Molecule). A) anatomical image of excised shows terminal ileum (TI) and enlarged draining mesenteric lymph node (MLN). B) Corresponding in vivo color-coded molecular ultrasound image shows inflammation in TI and MLN. Reprinted with permission from Bachmann et al, Gastroenterology, Vol 130: 8-16, 2006 71.
Molecular ultrasound imaging of intravascular thrombus formation
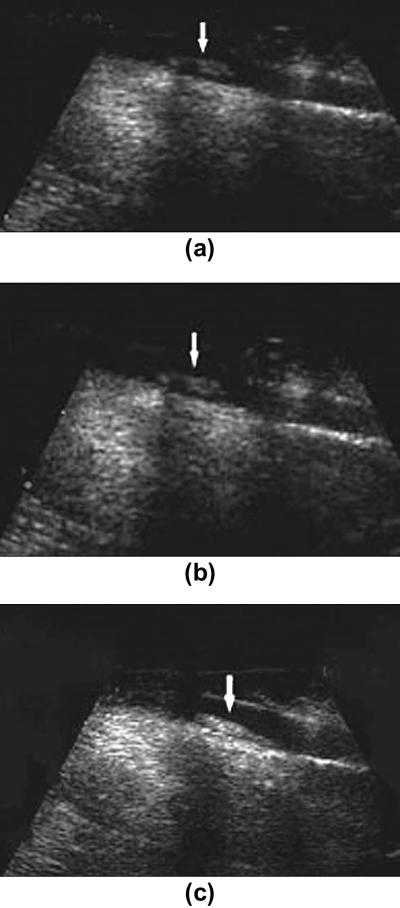
Imaging thrombus formation by molecular ultrasound has tremendous potential not only as a diagnostic tool but for making treatment decisions in patients with stroke or in patients at high risk for cerebral embolic disease. Furthermore, the use of microbubbles along with ultrasound energy can be utilized for declotting thrombosis. Although research in molecular imaging of thrombus is still at its infancy, several studies have shown feasibility of molecular ultrasound for thrombus imaging by targeting contrast agents to components of blood coagulation in animals. Studies by Schumann et al. showed binding of targeted microbubbles to arterial and venous blood clots in the cremaster muscle in mouse 73. These targeted agents were synthesized by adding Platelet glycoprotein (GP) IIb/IIIa receptor-targeted peptides onto the microbubble shell. A similar study used microbubbles with GP IIb/IIIa targeted peptides for imaging thrombi in femoral veins of dogs 74. (Figure 8).
Figure 8.
Molecular ultrasound images of thrombi in femoral vein in the hind limb of dogs. A) at baseline without any contrast agent b) after the administration of non targeted microbubbles (SonoVue) c) after the administration of microbubbles targeted against GP (Platelet Glycoprotein) IIb/IIa receptor. Note better visualization of intravascular thrombus with targeted microbubbles. Reprinted with permission from Wang et al, Acad Radiol, Vol 13: 428-433, 2006 74.
Future directions of molecular ultrasound imaging
Radial modulation imaging
In this approach, two ultrasound pulses with 2 different frequencies are applied with one frequency (usually the lower one) exciting the microbubbles and the second one being used for signal detection 75,76,77. Another more general formalism of the technique has been called Second Order UltRasound Field (SURF) Imaging 78. SURF Imaging has been demonstrated in clinical applications of prostate and abdominal imaging79,80. The major benefit of using Radial Modulation, particularly for high frequencies, is that the microbubble detection is not dependent on its resonance frequency. This is advantageous since many of the microbubbles are typically resonant at much lower frequencies than the imaging frequency used for high-resolution imaging 81. This represents a significant opportunity for pre-clinical molecular imaging, as a means for improving sensitivity at significantly higher frequencies (> 30 MHz), which in turn will improve specificity.
Improved microbubble attachment with acoustic radiation force
In addition to improving microbubble detection through improved transducer and software design, advances in molecular ultrasound imaging can be made by improving microbubble attachment to the molecular target. While microbubble attachment can be increased by increasing affinity of the binding ligand on the microbubble surface or improved avidity of the microbubble/ligand complex82, microbubbles can also be mechanically pushed to the vascular endothelial cell wall with a series of low frequency ultrasound pulses, thereby increasing vessel wall contact and, thus, the rate of microbubbles actually binding to the molecular targets expressed on the vascular endothelial cells. Radiation force is created by ultrasound waves and becomes more pronounced as frequency decreases. Therefore, similar to Radial Modulation imaging, dual-frequency transducers can be designed to aid in the generation of radiation force at lower frequencies (e.g. 1-2 MHz) to push microbubbles to the endothelial surface before imaging them at higher frequencies. Recent studies have demonstrated increased microbubble attachment using radiation force 83-85, without substantially increasing non-specific binding of targeted microbubbles to vascular endothelial cells 86.
Improved methods for differentiating bound from non-bound/freely circulating microbubbles
Recently, a new approach has been proposed using high frame rate ultrafast imaging (500 Hz) with a system capable of transmitting plane waves and capturing parallel receive beams 87. This study demonstrated that following microbubble disruption the gas inside bound and freely flowing microbubbles dissolves at different rates over time, producing different time-varying responses to ultrasound. By using ultrafast imaging, these differences in dissolution time (on the order of 20-30 ms) can be tracked, and used to differentiate regions of targeted versus freely circulating microbubbles. Since targeted bubbles dissolve very quickly after disruption (on the order of 10 ms), any signal present beyond this time after the disruption pulse can be segmented as a targeted signal and displayed on the contrast image. Other recent studies have demonstrated signal processing algorithms to differentiate attached from flowing microbubbles 83,88. These algorithms rely on a nonlinear detection of microbubbles to segment them from tissue, and then use Doppler processing to filter out signals from moving versus attached microbubbles (Figure 9). Using such an approach not only reduces the amount of time required for imaging targeted bubbles (since a real-time differentiation between freely circulating and attached microbubbles is possible), but also has the advantages that binding of targeted microbubbles can be visualized over an extended time period since the microbubbles are not destroyed with this approach.
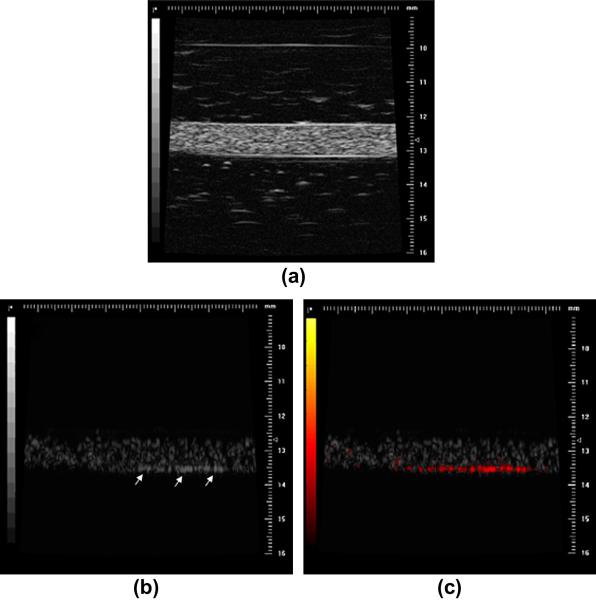
Figure 9.
(a) High-frequency (30 MHz) B-Mode image of a biotinylated gelatin vessel phantom which is perfused with avidinated microbubbles. (b) Nonlinear subharmonic (15 MHz) image of the avidinated microbubbles inside the phantom, without interframe filtering signal processing (microbubbles bound to the vessel wall through the avidin-biotin complex are highlighted with white arrows). (c) Subharmonic image of avidinated microbubbles inside the phantom, using interframe filtering to isolate signal from bound microbubbles in the presence of flowing ones (bound microbubbles highlighted with red overlay) Reprinted with permission from Needles et al, Ultrasound Med Biol, 35(9): 1564-1573, 2009.
Three-dimensional imaging
Three-dimensional molecular ultrasound imaging approaches are of paramount importance for molecular ultrasound imaging to obtain representative measures of molecular signatures within the entire region under consideration (e.g., within a primary tumor or a metastasis). Since molecular expression in e.g. tumors can be inhomogeneous due to focal areas of necrosis or hypoxia, a 2D imaging approach allowing only a limited view may be prone to substantial sampling error 49. Current pre-clinical high-resolution ultrasound systems use linear stepper motors to translate the transducer through the 3D field of view and integrating the imaging signal over the volume obtained from these consecutive 2D images. The development of clinical ultrasound transducer technology to two-dimensional arrays has advanced the field of 3D imaging to the point where real-time imaging is now possible (referred to as 4D) at lower frequencies 89. Future studies are warranted to test 3D approaches for molecular ultrasound imaging in vivo.
Clinically translatable molecular ultrasound contrast microbubbles
Non-targeted ultrasound contrast agents have been in clinical use for many years. Promising indications include detection and characterization of focal liver lesions and lesions in other solid abdominal organs, assessment of intracranial, renal, and portal venous vasculature, improved delineation of the endocardial border, and myocardial perfusion studies. Overall, microbubbles are very safe contrast agents with a very low minor adverse event rate of 0.13% (29 per 23,188 examinations; minor adverse events included dizziness, erythematous rash, itching, nausea and vomiting)90. In two patients, 2 major adverse reactions were reported (corresponding to an incidence of 0.0086% for major adverse events). Major adverse events in that study included dyspnea, bronchospasm, slight hypotension and bradycardia in one patient and clouding of consciousness, dorsolumbar pain, severe hypotension and cutaneous rash in the other patient. Both patients recovered within 30 min of contrast agent injection by the administration of corticosteroids, antihistamines and vasoactive drugs. There are no reported nephrotoxic effects and it is not necessary to perform renal function test before administration of contrast microbubbles, which is of significant advantage compared to iodinated CT contrast agents or gadolinium-based MR contrast agents 91.
The FDA ordered a black box warning on perflutren-containing microbubbles in 2007 following reports of 11 deaths in patients following intravenous administration of non-targeted contrast microbubbles 92. Four of those deaths were caused by cardiac arrest that occurred either during infusion or within 30 minutes after infusion of the microbubbles. The black box warning contraindicated the use of microbubbles in patients with acute coronary syndromes, acute myocardial infarction, and worsening or clinically unstable heart failure, severe emphysema and pumonary emboli or other conditions that cause pulmonary hypertension. This warning was revised in 2008 following a publication of a retrospective study in the Journal of the American College of Cardiology93. The review, involving 18,671 consecutive studies, found no increased mortality risk for patients who received microbubbles compared with patients who were imaged without the agent. Currently, the revised warning continues to highlight the risk of serious cardiopulmonary reactions during or within 30 minutes following the administration of microbubbles and recommends that patients are closely monitored during and for at least 30 minutes post administration of microbubbles.
Regarding the use of targeted microbubbles, the field is currently still restricted to preclinical research. Humanizing antibodies required for generation of targeted contrast agents can be very expensive and antibodies can cause immunogenic reactions 94. Novel peptides that recognize a variety of molecular markers may provide an alternative and may circumvent the problem of allergic reactions in humans82. Novel microbubble constructs designed for clinical use are currently under development to expand molecular ultrasound imaging to the clinical arena 26 .
CONCLUSIONS
Molecular ultrasound is an emerging molecular imaging approach that has rapidly found its niche among other molecular imaging modalities within the last several years. High spatial and temporal resolution, real-time imaging, non-invasiveness, relatively low costs, lack of ionizing irradiation and wide availability among the imaging community throughout the world are important advantages that will further define the role of this novel imaging technique both in preclinical research and clinical applications. In addition, ongoing improvements in ultrasound technology and sophisticated contrast agent design with novel high-affinity targeting ligands using clinically translatable binding chemistry, and further improvements in biodistribution of ultrasound contrast agents beyond the vasculature will further expand the clinical role of molecular ultrasound for imaging diseases at the molecular level in medicine.
Acknowledgments
This work has been supported by the RSNA Seed grant RSD0809 and the NIH R21 CA139279
REFRENCES
- 1.Lanza GM, Wickline SA. Targeted ultrasonic contrast agents for molecular imaging and therapy. Curr Probl Cardiol. 2003;28(12):625. doi: 10.1016/j.cpcardiol.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Klibanov AL. Molecular imaging with targeted ultrasound contrast microbubbles. Ernst Schering Res Found Workshop. 2005;(49):171. doi: 10.1007/3-540-26809-x_10. [DOI] [PubMed] [Google Scholar]
- 3.McCulloch M, Gresser C, Moos S, et al. Ultrasound contrast physics: A series on contrast echocardiography, article 3. J Am Soc Echocardiogr. 2000;13(10):959. doi: 10.1067/mje.2000.107004. [DOI] [PubMed] [Google Scholar]
- 4.Klibanov A, Gu H, Wojdyla JK, et al. Attachemnet of ligands to gas filled microbubbles via PEG spacer and lipid residues anchored at the interface. Boston: 1999. p. 124. [Google Scholar]
- 5.Walday P, Tolleshaug H, Gjoen T, et al. Biodistributions of air-filled albumin microspheres in rats and pigs. Biochem J. 1994;299(Pt 2):437. doi: 10.1042/bj2990437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willmann JK, Cheng Z, Davis C, et al. Targeted microbubbles for imaging tumor angiogenesis: assessment of whole-body biodistribution with dynamic micro-PET in mice. Radiology. 2008;249(1):212. doi: 10.1148/radiol.2491072050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klibanov AL. Preparation of targeted microbubbles: ultrasound contrast agents for molecular imaging. Med Biol Eng Comput. 2009 doi: 10.1007/s11517-009-0498-0. [DOI] [PubMed] [Google Scholar]
- 8.Buchanan KD, Huang S, Kim H, et al. Echogenic liposome compositions for increased retention of ultrasound reflectivity at physiologic temperature. Journal of Pharmaceutical Sciences. 2007;97(6):2242. doi: 10.1002/jps.21173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamilton AJ, Huang SL, Warnick D, et al. Intravascular ultrasound molecular imaging of atheroma components in vivo. J Am Coll Cardiol. 2004;43(3):453. doi: 10.1016/j.jacc.2003.07.048. [DOI] [PubMed] [Google Scholar]
- 10.Lanza GM, Wallace KD, Scott MJ, et al. A novel site-targeted ultrasonic contrast agent with broad biomedical application. Circulation. 1996;94(12):3334. doi: 10.1161/01.cir.94.12.3334. [DOI] [PubMed] [Google Scholar]
- 11.Lanza GM, Abendschein DR, Hall CS, et al. In vivo molecular imaging of stretch-induced tissue factor in carotid arteries with ligand-targeted nanoparticles. J Am Soc Echocardiogr. 2000;13(6):608. doi: 10.1067/mje.2000.105840. [DOI] [PubMed] [Google Scholar]
- 12.Kripfgans OD, Fabiilli ML, Cardon PL, et al. On the Acoustic Vaporization of Micrometer-Sized Droplets. J Acoust Soc Am. 2004;116(1):272. doi: 10.1121/1.1755236. [DOI] [PubMed] [Google Scholar]
- 13.Amirriazi S, Eghtedari M, Jin S, et al. Increasing Ultrasound Sensitivity to Perfluorocarbon Emulsion Droplets. Montreal Canada: 2009. (abstract) [Google Scholar]
- 14.Rapoport N, Gao Z, Kennedy A. Multifunctional nanoparticles for combining ultrasonic tumor imaging and targeted chemotherapy. J Natl Cancer Inst. 2007;99(14):1095. doi: 10.1093/jnci/djm043. [DOI] [PubMed] [Google Scholar]
- 15.Maeda H, Wu J, Sawa T, et al. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65(1-2):271. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Levine AL, Mattoon JS, et al. Nanoparticles as image enhancing agents for ultrasonography. Phys Med Biol. 2006;51(9):2179. doi: 10.1088/0031-9155/51/9/004. [DOI] [PubMed] [Google Scholar]
- 17.Nolte I, Vince GH, Maurer M, et al. Iron particle enhance visualization of experimental gliomas with high resolution sonography. Am J Neuroradiol. 2005;26:1469. [PMC free article] [PubMed] [Google Scholar]
- 18.Linker RA, Kroner A, Horn T, et al. Iron particle-enhanced visualization of inflammatory central nervous system lesions by high resolution: preliminary data in an animal model. AJNR Am J Neuroradiol. 2006;27(6):1225. [PMC free article] [PubMed] [Google Scholar]
- 19.Lindner JR, Dayton PA, Coggins MP, et al. Noninvasive imaging of inflammation by ultrasound detection of phagocytosed microbubbles. Circulation. 2000;102:531. doi: 10.1161/01.cir.102.5.531. [DOI] [PubMed] [Google Scholar]
- 20.Klibanov AL. Ligand-carrying gas-filled microbubbles: ultrasound contrast agents for targeted molecular imaging. Bioconjug Chem. 2005;16(1):9. doi: 10.1021/bc049898y. [DOI] [PubMed] [Google Scholar]
- 21.Marshall D, Pedley RB, Boden JA, et al. Polyethylene glycol modification of a galactosylated streptavidin clearing agent: effects on immunogenicity and clearance of a biotinylated anti-tumour antibody. Br J Cancer. 1996;73(5):565. doi: 10.1038/bjc.1996.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaufmann BA. Ultrasound molecular imaging of atherosclerosis. Cardiovasc Res. 2009 doi: 10.1093/cvr/cvp179. [DOI] [PubMed] [Google Scholar]
- 23.Villanueva FS, Jankowski RJ, Klibanov S, et al. Microbubbles targeted to intercellular adhesion molecule-1 bind to activated coronary artery endothelial cells. Circulation. 1998;98(1):1. doi: 10.1161/01.cir.98.1.1. [DOI] [PubMed] [Google Scholar]
- 24.Alonso A, Della Martina A, Stroick M, et al. Molecular imaging of human thrombus with novel abciximab immunobubbles and ultrasound. Stroke. 2007;38(5):1508. doi: 10.1161/STROKEAHA.106.471391. [DOI] [PubMed] [Google Scholar]
- 25.Pochon S, Trady I, Bussat P, et al. PBR55: A Lipopeptide-based KDR-targeted Ultrasound Contrast Agent for Molecular Imaging of Angiogenesis. Investigative Radiology. 2010;45(2):89–95. doi: 10.1097/RLI.0b013e3181c5927c. [DOI] [PubMed] [Google Scholar]
- 26.Pysz MA, Foygel K, Rosenberg J, et al. Monitoring Anti-Angiogenic Cancer Therapy with Molecular Ultrasound and a New Clinically Translatable Contrast Agent. Radiology. 2010 doi: 10.1148/radiol.10091858. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Becher H, Burns PN. Handbook of Contrast Echocardiography. Springer; Berlin: 2000. [Google Scholar]
- 28.Leighton T. The Acoustic Bubble. Academic Press; 1997. [Google Scholar]
- 29.de Jong N, Cornet R, Lancee CT. Higher harmonics of vibrating gas-filled microspheres. Part Two: Measurement. Ultrasonics. 1994;32:455. [Google Scholar]
- 30.Bouakaz A, Frigstad S, Ten Cate FJ, et al. Super harmonic imaging: a new imaging technique for improved contrast detection. Ultrasound Med Biol. 2002;28(1):59. doi: 10.1016/s0301-5629(01)00460-4. [DOI] [PubMed] [Google Scholar]
- 31.Forsberg F, Shi WT, Goldberg BB. Subharmonic imaging of contrast agents. Ultrasonics. 2000;38:93. doi: 10.1016/s0041-624x(99)00148-1. [DOI] [PubMed] [Google Scholar]
- 32.Deng CX, Lizzi FL. A review of physical phenomena associated with ultrasonic contrast agents and illustrative clinical phenomena. Ultrasound Med Biol. 2002;28:277. doi: 10.1016/s0301-5629(02)00475-1. [DOI] [PubMed] [Google Scholar]
- 33.Hamilton M, Blackstock D. Nonlinear Acoustics: Theory and Applications. Academic Press; 1998. [Google Scholar]
- 34.Burns PN, Powers JE, Hope-Simpson D, et al. Harmonic power mode Doppler using contrast agents: An improved method for small vessel flow imaging. J Ech Med Ultra. 1994;16:132. [Google Scholar]
- 35.Chang PH, Shung KK, Levine HB. Second harmonic imaging and harmonic Doppler measurements with Albunex. IEEE Trans Ultrason Ferroelect Freq Contr. 1995;42:1020. [Google Scholar]
- 36.Hope-Simpson D, Chin CT, Burns PN. Pulse inversion Doppler: A new method for detecting non-linear echoes from microbubble contrast agents. IEEE Trans Ultrason Ferroelect Freq Contr. 1999;46:372. doi: 10.1109/58.753026. [DOI] [PubMed] [Google Scholar]
- 37.de Jong N, Frinking PJA, Buoakaz A, et al. Detection procedures of ultrasound contrast agents. Ultrasonics. 2000;38:87. doi: 10.1016/s0041-624x(99)00071-2. [DOI] [PubMed] [Google Scholar]
- 38.Haider B, Chiao RY. Higher order nonlinear ultrasonic imaging. Proc IEEE Ultrason Symp. 1999;2:1527. [Google Scholar]
- 39.Phillips PJ. Contrast pulse sequences (CPS): imaging nonlinear microbubbles. Proc IEEE Ultrason Symp. 2001;2:1739. [Google Scholar]
- 40.Eckersley RJ, Chin CT, Burns PN. Optimising phase and amplitude modulation schemes for imaging microbubble contrast agents at low acoustic power. Ultra Med Biol. 2005;31:213. doi: 10.1016/j.ultrasmedbio.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Averkiou MA. Tissue harmonic ultrasonic imaging. CR Acad Sci Ser IV-Phys Astrophys. 2001;2(1139-1151) [Google Scholar]
- 42.Hope-Simpson D, Burns PN, Averkiou MA. Techniques for Perfusion Imaging with Microbubble Contrast Agents. IEEE Trans Ultrason Ferroelect Freq Contr. 2001;48:1483. doi: 10.1109/58.971698. [DOI] [PubMed] [Google Scholar]
- 43.Foster FS, Pavlin CJ, Harasiewicz KA, et al. Advances in ultrasound biomicroscopy. Ultrasound Med Biol. 2000;26:1. doi: 10.1016/s0301-5629(99)00096-4. [DOI] [PubMed] [Google Scholar]
- 44.Foster FS, Zhang MY, Zhou YQ, et al. A new ultrasound instrument for in vivo microimaging of mice. Ultrasound Med Biol. 2002;28(9):1165. doi: 10.1016/s0301-5629(02)00567-7. [DOI] [PubMed] [Google Scholar]
- 45.Needles A, Goertz DE, Karshafian R, et al. High-frequency subharmonic pulsed-wave Doppler and color flow imaging of microbubble contrast agents. Ultrasound Med Biol. 2008;34:1139. doi: 10.1016/j.ultrasmedbio.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 46.Goertz DE, Cherin E, Needles A, et al. High frequency nonlinear B-Scan imaging of microbubble contrast agents. Ultrason Ferroelectr Freq Cont. 2005;52:65. doi: 10.1109/tuffc.2005.1397351. [DOI] [PubMed] [Google Scholar]
- 47.Rychak JJ, Graba J, Cheung AM, et al. Microultrasound molecular imaging of vascular endothelial growth factor receptor 2 in a mouse model of tumor angiogenesis. Mol Imaging. 2007;6(5):289. [PubMed] [Google Scholar]
- 48.Lyshchik A, Fleischer AC, Huamani J, et al. Molecular imaging of vascular endothelial growth factor receptor 2 expression using targeted contrast-enhanced high-frequency ultrasonography. J Ultrasound Med. 2007;26(11):1575. doi: 10.7863/jum.2007.26.11.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Willmann JK, Paulmurugan R, Chen K, et al. US imaging of tumor angiogenesis with microbubbles targeted to vascular endothelial growth factor receptor type 2 in mice. Radiology. 2008;246(2):508. doi: 10.1148/radiol.2462070536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vassiliadis S, Hakkennes EA, Wong JSSM, et al. The sum-absolute-difference motion estimation accelerator. Proc IEEE Euromicro Conference. 1998;2(559-566) [Google Scholar]
- 51.Ritter TA, Shrout TR, Tutweiler R, et al. A 30 MHz peizo-composite ultrasound array for medical imaging applications. 2002. p. 217. [DOI] [PubMed]
- 52.Lukacs M, Yin J, Pang G, et al. Performance and Characterization of New Micromachined High-Frequency Linear Arrays. IEEE Trans Ultrason Ferroelec Freq Contr. 2006;53:1719. doi: 10.1109/tuffc.2006.105. [DOI] [PubMed] [Google Scholar]
- 53.Brown JA, Foster FS, Needles A, et al. Fabrication and Performance of a 40-MHz Linear Array Based on a 1-3 Composite with Geometric Elevation Focusing. IEEE Trans Ultrason Ferroelectr Freq Cont. 2007;54(1888-1894) doi: 10.1109/tuffc.2007.473. [DOI] [PubMed] [Google Scholar]
- 54.Foster FS, Mehi J, Lukacs M, et al. A new 15-50 MHz array-based micro-ultrasound scanner for preclinical imaging. Ultrasound Med Biol. 2009;35(10):1700. doi: 10.1016/j.ultrasmedbio.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 55.Needles A, Coulthard T, Mehi J, et al. Nonlinear Molecular Imaging with a Linear Array Based Micro-Ultrasound System. Montreal, Canada: 2009. (abstract) [Google Scholar]
- 56.de Jong N, Hoff L. Ultrasound scattering properties of Albunex microspheres. Ultrasonics. 1993;31:175. doi: 10.1016/0041-624x(93)90004-j. [DOI] [PubMed] [Google Scholar]
- 57.Rognin NG, Frinking P, Costa M, et al. In-vivo perfusion quantification by contrast ultrasound: Validation of the use of linearized video data vs. raw RF data. Proc IEEE Ultrason Symp. 2008;1:1690. [Google Scholar]
- 58.Arditi M, Frinking PJ, Zhou X, et al. A new formalism for the quantification of tissue perfusion by the destruction-replenishment method in contrast ultrasound imaging. IEEE Trans Ultrason Ferroelectr Freq Control. 2006;53(6):1118. doi: 10.1109/tuffc.2006.1642510. [DOI] [PubMed] [Google Scholar]
- 59.Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 60.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 61.Lee DJ, Lyshchik A, Huamani J, et al. Relationship Between Retention of a Vascular Endothelial Growth Factor Receptor 2 (VEGFR2)-Targeted Ultrasonographic Contrast Agent and the Level of VEGFR2 Expression in an In Vivo Breast Cancer Model. J Ultrasound Med. 2008;(27):855. doi: 10.7863/jum.2008.27.6.855. [DOI] [PubMed] [Google Scholar]
- 62.Willmann JK, Lutz AM, Paulmurugan R, et al. Dual-targeted contrast agent for US assessment of tumor angiogenesis in vivo. Radiology. 2008;248(3):936. doi: 10.1148/radiol.2483072231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Korpanty G, Carbon JG, Grayburn PA, et al. Monitoring response to anticancer therapy by targeting microbubbles to tumor vasculature. Clin Cancer Res. 2007;13(1):323. doi: 10.1158/1078-0432.CCR-06-1313. [DOI] [PubMed] [Google Scholar]
- 64.Palmowski M, Huppert J, Ladewig G, et al. Molecular profiling of angiogenesis with targeted ultrasound imaging: early assessment of antiangiogenic therapy effects. Mol Cancer Ther. 2008;7(1):101. doi: 10.1158/1535-7163.MCT-07-0409. [DOI] [PubMed] [Google Scholar]
- 65.Kunkel EJ, Butcher EC. Plasma-cell homing. Nat Rev Immunol. 2003;3(10):822. doi: 10.1038/nri1203. [DOI] [PubMed] [Google Scholar]
- 66.Lindner JR, Song J, Xu F, et al. Noninvasive ultrasound imaging of inflammation using microbubbles targeted to activated leukocytes. Circulation. 2000;102(22):2745. doi: 10.1161/01.cir.102.22.2745. [DOI] [PubMed] [Google Scholar]
- 67.Ferrante EA, Pickard JE, Rychak J, et al. Dual targeting improves microbubble contrast agent adhesion to VCAM-1 and P-selectin under flow. J Control Release. 2009;140(2):100. doi: 10.1016/j.jconrel.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lindner JR, Song J, Christiansen J, et al. Ultrasound assessment of inflammation and renal tissue injury with microbubbles targeted to P-selectin. Circulation. 2001;104(17):2107. doi: 10.1161/hc4201.097061. [DOI] [PubMed] [Google Scholar]
- 69.Kaufmann BA, Lewis C, Xie A, et al. Detection of recent myocardial ischaemia by molecular imaging of P-selectin with targeted contrast echocardiography. Eur Heart J. 2007;28(16):2011. doi: 10.1093/eurheartj/ehm176. [DOI] [PubMed] [Google Scholar]
- 70.Kaufmann BA, Sanders JM, Davis C, et al. Molecular imaging of inflammation in atherosclerosis with targeted ultrasound detection of vascular cell adhesion molecule-1. Circulation. 2007;116(3):276. doi: 10.1161/CIRCULATIONAHA.106.684738. [DOI] [PubMed] [Google Scholar]
- 71.Bachmann C, Klibanov AL, Olson TS, et al. Targeting mucosal addressin cellular adhesion molecule (MAdCAM)-1 to noninvasively image experimental Crohn’s disease. Gastroenterology. 2006;130(1):8. doi: 10.1053/j.gastro.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 72.Villanueva FS, Lu E, Bowry S, et al. Myocardial ischemic memory imaging with molecular echocardiography. Circulation. 2007;115(3):345. doi: 10.1161/CIRCULATIONAHA.106.633917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schumann PA, Christiansen JP, Quigley RM, et al. Targeted-microbubble binding selectively to GPIIb IIIa receptors of platelet thrombi. Invest Radiol. 2002;37(11):587. doi: 10.1097/00004424-200211000-00001. [DOI] [PubMed] [Google Scholar]
- 74.Wang B, Zang WJ, Wang M, et al. Prolonging the ultrasound signal enhancement from thrombi using targeted microbubbles based on sulfur-hexafluoride-filled gas. Acad Radiol. 2006;13(4):428. doi: 10.1016/j.acra.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 75.Shariff HH, P.D. B, Karshafian R, et al. Radial modulation imaging: Raising the frequency for contrast imaging. Proc IEEE Ultrasonics Symp. 2006:101. [Google Scholar]
- 76.Bouakaz A, Versluis M, Borsboom J, et al. Radial modulation of microbubbles for ultrasound contrast imaging. IEEE Trans Ultrason Ferroelectr Freq Control. 2007;54(11):2283. doi: 10.1109/tuffc.2007.532. [DOI] [PubMed] [Google Scholar]
- 77.Chérin E, Brown J, Måsøy S, et al. Radial Modulation Imaging of Microbubble Contrast Agents at High Frequency. Ultrasound Med Biol. 2008;34:949. doi: 10.1016/j.ultrasmedbio.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 78.Måsøy SE, Standa O, Näsholm P, et al. SURF Imaging: In vivo demonstration of an ultrasound contrast agent detection technique. IEEE Trans Ultrason Ferroelec Freq Contr. 2008;55:1112. doi: 10.1109/TUFFC.2008.763. [DOI] [PubMed] [Google Scholar]
- 79.Måsøy SE, Hansen R, Angelsen A, et al. SURF Imaging – High-frequency ultrasound contrast agent imaging in patients with prostate cancer. Rotterdam, The Netherlands: 2008. (abstract) [Google Scholar]
- 80.Måsøy SE, Deibele JM, Tangen TA, et al. SURF imaging – A real time dual-frequency band ultrasound system. Proc IEEE Ultrason Symp. 2009 [Google Scholar]
- 81.Goertz DE, de Jong N, van der Steen AF. Attenuation and size distribution measurements of Definity and manipulated Definity populations. Ultrasound Med Biol. 2007;33:1376. doi: 10.1016/j.ultrasmedbio.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 82.Willmann JK, Kimura RH, Deshpande N, et al. Novel Integrin-Binding Ultrasound Contrast Agent for Molecular Ultrasound Imaging of Tumor Angiogenesis. J of Nuclear Medicine J Nucl Med. 2010;51(3):433. doi: 10.2967/jnumed.109.068007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao S, Kruse DE, Ferrara KW, et al. Selective imaging of adherent targeted ultrasound contrast agents. Phys Med Biol. 2007;52(8):2055. doi: 10.1088/0031-9155/52/8/002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Frinking P, Tardy I, Theraulaz M, et al. The effect of radiation force on binding efficiency and specificity of target-specific contrast-agent microbubbles. Proc IEEE Ultrason Symp. 2009 In press. [Google Scholar]
- 85.Gessner R, Lee M, Lukacs M, et al. Radiation force-enhanced targeted imaging using a dual-frequency high-resolution transducer. Proc IEEE Ultrason Symp. 2009 In press. [Google Scholar]
- 86.Tardy I, Frinking P, Theraulaz M, et al. Ultrasound radiation force application to KDR-targeted contrast agent in tumor angiogenesis model. Montreal,Canada: 2009. (abstract) [Google Scholar]
- 87.Couture O, Bannouf S, Montaldo G, et al. Ultrafast Imaging of Ultrasound Contrast Agents. Ultrasound Med Biol. 2009;35(11):1908. doi: 10.1016/j.ultrasmedbio.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 88.Needles A, Couture O, Foster FS. A Method for Differentiating Targeted Microbubbles in Real Time Using Subharmonic Micro-Ultrasound and Interframe Filtering. Ultrasound Med Biol. 2009;35:1564. doi: 10.1016/j.ultrasmedbio.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 89.Benacerraf BR, Benson CB, Abuhamad AZ, et al. Three- and 4-dimensional ultrasound in obstetrics and gynecology: proceedings of the American Institute of Ultrasound in Medicine Consensus Conference. J Ultrasound Med. 2005;24(12):1587. doi: 10.7863/jum.2005.24.12.1587. [DOI] [PubMed] [Google Scholar]
- 90.Piscaglia F, Bolondi L. The safety of Sonovue in abdominal applications: retrospective analysis of 23188 investigations. Ultrasound Med Biol. 2006;32(9):1369. doi: 10.1016/j.ultrasmedbio.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 91.Blomley M, Claudon M, Cosgrove D. WFUMB Safety Symposium on Ultrasound Contrast Agents: clinical applications and safety concerns. Ultrasound Med Biol. 2007;33(2):180. doi: 10.1016/j.ultrasmedbio.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 92.FDA Micro-bubble Contrast Agents (marketed as Definity (Perflutren Lipid Microsphere) Injectable Suspension and Optison (Perflutren Protein-Type A Microspheres for Injection) 2008.
- 93.Kusnetzky LL, Khalid A, Khumri TM, et al. Acute Mortality in Hospitalized Patients Undergoing Echocardiography With and Without an Ultrasound Contrast Agent. Journal of the American College of Cardiology. 2008;51(17):1704. doi: 10.1016/j.jacc.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 94.Mehdiratta R, Saberwal G. Bio-business in brief: Many a monoclonal. Current Science. 2007;93(6):789. [Google Scholar]