The perspective that cancer may be causally linked to stress has a long history. In 200 AD, Galen proposed that melancholic women were more susceptible to cancer than women who were sanguine. Rigorous examinations of related observations have lagged over the ensuing centuries. More recently, epidemiologic studies have shown that psychologic and social characteristics (e.g., chronic stress and negative life events, social isolation and support, socioeconomic burden, and emotional processes) might be associated with differential cancer incidence, progression, and mortality (Antoni et al., 2006; Lutgendorf et al., in press). The biologic mechanisms (e.g., signaling pathways) that may account for such observations are being discovered through the convergence of relevant molecular, cellular, and clinical data.
In this article, we review the clinical and experimental evidence regarding the effects of stress on tumor development, growth, and progression. Within this context, we define “stress” as an external event (“stressor”) or perception of such events that engender psychologic and physiologic changes (“stress responses”) designed to approach, avoid, or defend against the external event. “Stress” initiates a cascade of information-processing pathways in the central nervous system (CNS) and periphery, which activate the autonomic nervous system (ANS) or the hypothalamic-pituitary-adrenal (HPA) axis. Cognitive and emotional feedback from cortical and limbic areas of the brain modulate the activity of hypothalamic and brain stem structures directly controlling autonomic nervous system and hypothalamic-pituitary-adrenal activity. Hypothalamic-pituitary-adrenal responses are mediated by hypothalamic production of corticotrophin-releasing factor (CRF) and arginine vasopressin, which activate the secretion of pituitary hormones such as adrenocorti-cotrophic hormone (ACTH), enkephalins, and endorphins. ACTH induces downstream release of glucocorticoids such as cortisol from the adrenal cortex. Glucocorticoids control growth, metabolism, and immune function, and have a pivotal role in regulating basal function and stress reactivity across a wide variety of organ systems. Autonomic nervous system responses to stress are mediated primarily by activation of the sympathetic nervous system (SNS), and subsequent release of catecholamines (principally norepinephrine and epinephrine) from sympathetic neurons and the adrenal medulla. Individual differences in the perception and evaluation of external events (appraisal and coping processes) create variability in autonomic nervous system and hypothalamic-pituitary-adrenal activity levels. Stressors that have been associated with alterations in neuroendocrine dynamics include marital disruption, bereavement, depression, chronic sleep disruption, severe trauma, and post-traumatic stress disorder. For a review of central and periphery stress systems, see Chrousos and Gold (1992) and Charmandari et al. (2005).
Stress, Neuroendocrine Responses, and Cancer Pathogenesis
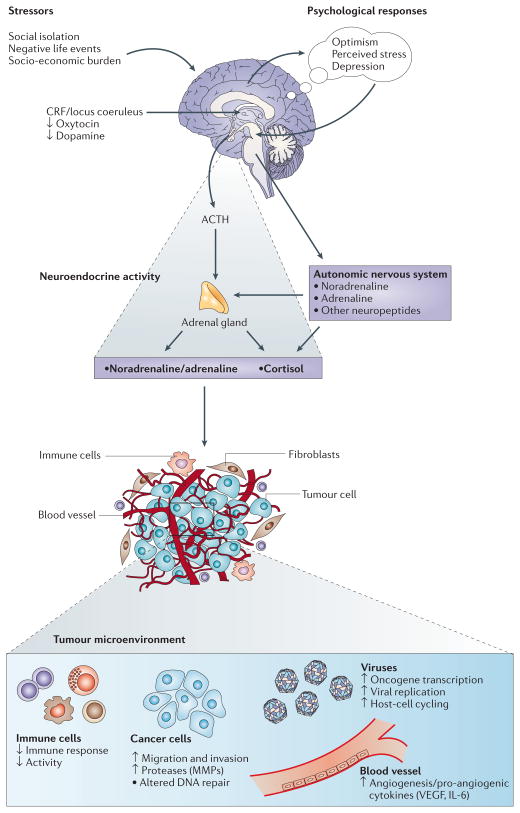
Physiologic stress responses are generally considered adaptive because they prepare an individual to survive a threat. However, most physiologic systems are negatively affected by prolonged exposure to glucocorticoids and catecholamines (referred to collectively as stress hormones). Over time, chronic changes in stress hormones can potentially modulate the activity of multiple components of the tumor microenvironment, including DNA repair, oncogene expression by viruses and somatic cells, and production of growth factors and other regulators of cell growth (Figure 1). Once tumor development has begun, neuroendocrine factors can also regulate the activity of proteases, angiogenic factors, chemokines, and adhesion molecules involved in invasion, metastasis, and other aspects of tumor progression.
Figure 1.
The response to stress involves central nervous system (CNS) perceptions of threat and subsequent activation of the autonomic nervous system (ANS) and the hypothalamic-pituitary-adrenal (HPA) axis. Catecholamines, glucocorticoids, and other stress hormones can modulate the activity of multiple components of the tumor microenvironment, creating a permissive environment for tumor initiation, growth, and progression. [Reprinted from Antoni MH, Lutgendorf SK, Cole SW, et al. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nature Reviews Cancer 6:240–248, 2006. Copyright, Antoni et al.]
Studies with animal models provide the most compelling evidence about the effects of behavioral stress on tumorigenesis and the biologic mechanisms involved. Increased growth of tumors has been associated with immobilization stress, social isolation, and restraint stress, and increased metastasis has been related to social confrontation and surgical stress (Antoni et al., 2006). Studies of human tumor biology have also helped to explain the mechanisms that mediate the effects of stress on tumorigenesis. Hormones associated with sympathetic nervous system activation may enhance angiogenesis in human tumors. For example, norepinephrine has been shown to up-regulate vascular endothelial growth factor (VEGF), a proangiogenic factor, in two ovarian cancer cell lines and to increase migration of colon cancer cells, while both epinephrine and norepinephrine have promoted invasion of ovarian cancer cells. Furthermore, β-adrenergic receptors (βARs), which mediate most effects of catecholamines, have been identified on breast and ovarian cancer cells.
Glucocorticoids regulate a wide variety of cellular processes through glucocorticoid receptor-mediated activation or repression of target genes. For example, cortisol may act synergistically with catecholamines to facilitate cancer growth; thus, it is plausible that stressful situations characterized by elevated levels of catecholamines and cortisol (e.g., uncontrollable stress) may significantly impact cancer-related processes. Stress also affects the expression and activity of other hormones associated with cancer growth. Studies have shown that prolactin, a hormone known to increase in response to stress, promotes the growth and survival of tumor cells. Conversely, stress-associated decreases in oxytocin have been associated with inhibition of epithelial cell tumor growth and stimulation of trophoblastic and endothelial tumors (Antoni et al., 2006).
Collectively, these studies demonstrate the growing evidence that mediators of sympathetic nervous system and the hypothalamic-pituitary-adrenal axis activate cellular pathways within tumors that contribute to growth and progression. Although the clinical relevance of stress-associated mechanisms remains to be demonstrated in studies involving humans, clinical studies have begun to corroborate the findings in animal studies. For example, higher levels of social support have been linked to lower serum levels of VEGF and another proangiogenic factor, interleukin-6 (IL-6), in patients with ovarian cancer (Lutgendorf et al., in press).
Stress, Circadian Regulation, and Cancer
Evidence indicates that stress can disrupt circadian rhythms, and studies in humans and animals have shown that circadian dysregulation enhances tumorigenesis and progression. For example, night-time shift work, an occupational characteristic known to disrupt endocrine rhythms, has been shown to increase the risk of breast and colorectal cancer. Gene mutations that result in circadian disruption are associated with tumor development and early death in mice. Tumors in animals and humans are associated with disrupted endocrine, metabolic, and immunologic cycles; and the disruption is greater when the tumor is advanced or fast growing. Elimination of circadian rhythms in experimental studies dramatically accelerates tumor progression and mortality. The status of circadian cycles, such as the cortisol cycle or the 24-hour rest-activity cycle, is associated with long-term survival in metastatic breast and colorectal cancer (Antoni et al., 2006).
The influence of stress-related disruption of circadian cycles on tumor initiation and development is likely to follow genetic and/or glucocorticoid and immune pathways. Circadian genes (e.g., Per 1) regulate tumor suppression, cellular response to DNA damage, and apoptosis. Glucocorticoid rhythms have been linked to enumerative and functional immunity. For example, sleep disruption can increase cortisol release and the expression of pro-inflammatory cytokines, such as IL-6 and tumor necrosis factor (TNF)-α. These cytokines may promote tumorigenesis by several mechanisms, including the following:
DNA damage or inhibition of DNA repair
Inactivation of tumor suppressor genes
Promotion of autocrine or paracrine growth and survival of tumor cells
Stimulation of angiogenesis
Subversion of the immune response
Conversely, agents that can re-establish circadian regulation may have antitumor effects. One such agent, melatonin, reversibly inhibited cell proliferation, increased p53 expression, modulated the cell cycle, and reduced metastatic capacity in estrogen receptor-positive MCF-7 human breast cancer cells by increasing expression of cell-surface adhesion proteins (Blask et al., 2002; Sanchez-Barcelo et al., 2003). Thus, circadian regulation may play an important role in the prevention and treatment of cancer.
Stress and Virally Induced Cancers
The evidence for a viral contribution to human cancer has grown, with viral infections contributing to approximately 15% of human cancers worldwide (zur Hausen, 1991). Among the most notable cancer-related viruses are human papilloma viruses (HPVs) 16 and 33 (cervical cancer and rectal cancer, respectively), hepatitis B and C viruses (hepatocellular cancer), Epstein-Barr virus (EBV) (lymphoma and nasopharyngeal cancer), Kaposi sarcoma-associated herpesvirus (Kaposi sarcoma and primary effusion lymphoma), and human T-cell lymphotrophic viruses (adult T-cell leukemia/lymphoma). All human tumor viruses are sensitive to the intracellular signaling pathways activated by the hypothalamic-pituitary-adrenal axis and autonomic nervous system, which can reactivate latent tumor virus, stimulate oncogene expression, and inhibit host-cell antiviral responses. For example, high-risk HPVs respond to glucocorticoids by activating gene expression and interacting with cellular proto-oncogenes. Stressful life events have been found to be a risk factor for increased progression of cervical dysplasia in HPV-positive women. Furthermore, glucocorticoid antagonists can inhibit HPV activity in vitro, providing a molecular rationale for clinical interventions that target hypothalamic-pituitary-adrenal activity.
Hepatitis B and C viruses, known to cause liver cancers, originate from different viral lineages, but both respond to glucocorticoids by enhancing gene expression and replication. These dynamics are so pronounced that glucocorticoids are used clinically to activate hepatitis B or C virus for its eradication by replication-dependent antiviral drugs. Studies have shown that EBV has been reactivated in healthy people who experience prolonged psychologic stress. Hypothalamic-pituitary-adrenal activity increased in parallel, and glucocorticoids were subsequently found to enhance EBV gene expression in vitro.
Cancer-related viruses are also sensitive to catecholamines and the protein kinase A (PKA) signaling pathway. Molecular mechanisms are well defined for AIDS-associated malignancies. Catecholamines can accelerate HIV-1 replication by enhancing cellular susceptibility to infection, activating viral gene transcription, and suppressing antiviral cytokines. Catecholamines can activate the Kaposi sarcoma-associated herpesvirus and human T-cell lymphotrophic viruses through protein kinase A induction of transcription factors. Hormonal regulation of viral replication represents a major pathway by which biobehavioral factors may influence malignant processes.
Stress and Immunity
Chronic stress has been shown to suppress many facets of immune function, including antigen presentation, T-cell proliferation, and humoral and cell-mediated immunity, mainly through the release of catecholamines and/or glucocorticoids. Relevant neuroendocrine and immune system interactions include direct synapse-like connections between sympathetic nerves and lymphocytes in lymphoid organs, neural and endocrine modulation of lymphocyte trafficking, and modulation of leukocyte function through glucocorticoid and other receptors. Tumor incidence and progression based on modulation of the immune response by chronic stress has been demonstrated in many animal models. Chronic stress has been shown to significantly increase the emergence and progression of UV-induced skin cancer which is mediated by a suppression of protective T cell and Type-1 cytokine driven immunity and enhancement of suppressor T cell function. Norepinephrine and epinephrine have directly inhibited the generation of antitumor cytotoxic lymphocytes through β-adrenergic signaling mechanisms in mice with transplanted syngeneic tumors. Neuroendocrine influences on the immune response may also explain why oncogenic viruses so consistently acquire hormone-responsive replication dynamics. Viruses that coordinate their gene expression with periods of hormone-induced immunosuppression should enjoy a significant survival advantage. Similar selective pressures may also shape the evolution of nonviral malignancies, such that genomic alterations are selected based on their ability to evade immune clearance or to synergize with endocrine patterns to optimize tumor growth and metastasis.
Natural killer (NK) cells play an integral role in immune surveillance; low peripheral NK cell counts are prognostic for early breast cancer mortality and low NK cytotoxicity is predictive of poor clinical outcome in breast cancer. The function of NK cells has been linked to psychosocial factors. For example, social support has been associated with higher levels of NK cell cytotoxicity in patients with breast and ovarian cancer.
This relationship was found in peripheral blood and in tumor-infiltrating lymphocytes isolated from human ovarian cancers, reflecting possible psychosocial influences on the tumor microenvironment.
Implications for Treatment
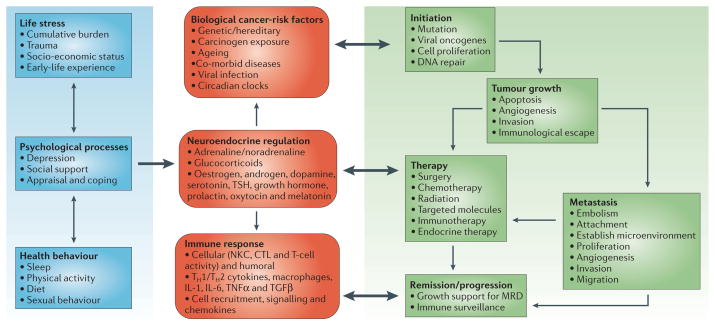
As our understanding of the biologic and clinical significance of stress-associated influences on cancer pathogenesis continues to expand, consideration of novel therapeutic paradigms that integrate a biobehavioral perspective is warranted (Figure 2). Successful management of factors such as stress and negative mood might have a salubrious effect on the neuroendocrine regulation of oncogenesis, tumor growth and metastasis, and cancer immunoediting processes. Pharmacologic interventions can potentially be used to ameliorate stress-associated influences on cancer development and progression. β-blockers have been shown to impede many of the deleterious effects of stress, and their use has been associated with a reduction in the risk for some, but not all, cancers. The use of antidepressant medications may also be promising because of their suppression of an inflammatory response that has been associated with certain types of cancer. Antiviral vaccines will have a growing role in the primary prevention of viral-mediated cancers, and biobehavioral influences on vaccine-induced immune responses will become especially relevant.
Figure 2.
This integrated model illustrates how biobehavioral factors such as life stress, psychologic processes, and health behaviors (left panel) influence tumor-related processes (right panel) through neuroendocrine regulation of hormones including epinephrine (E), norepinephrine (NE), and glucocorticoids (center panel). Central control of peripheral endocrine function also allows social, environmental, and behavioral processes to interact with biologic risk factors such as genetic background, carcinogens, and viral infections to systemically modulate malignant potential (center panel). Direct pathways of influence include effects of catecholamines and glucocorticoids on tumor cell expression of genes controlling cell proliferation, invasion, angiogenesis, metastasis, and immune evasion (right panel). Furthermore, neuroendocrine dysregulation can influence the response to conventional therapies such as surgery, chemotherapy, and immunotherapy (right panel). In addition to explaining biobehavioral risk factors for cancer, this model suggests novel targets for pharmacologic or behavioral intervention. PRL, prolactin; OT, oxytocin; DA, dopamine; 5-HT, serotonin; TSH, thyroid-stimulating hormone; GH, growth hormone; NKCC, natural killer cell cytotoxicity; CTL, cytotoxic T-lymphocyte; TH1, T-helper lymphocyte type 1 cells; TH2, T-helper lymphocyte type 2 cells; Mf, macrophage; IL-1, interleukin-1; IL-6, interleukin-6, TNF-α, tumor necrosis factor-α; TGF-β, transforming growth factor-β). [Reprinted from Antoni MH, Lutgendorf SK, Cole SW, et al. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nature Reviews Cancer 6:240–248, 2006. Copyright, Antoni et al.]
Behavioral interventions such as relaxation and cognitive behavioral techniques that alter negative mood appear to modulate autonomic nervous system and hypothalamic-pituitary-adrenal hormonal activity. Moreover, such interventions used in conjunction with conventional therapies may maximize treatment efficacy. Interventions that dampen chronic stress-related physiologic changes may facilitate “recovery” of the immune system and thereby enhance immune surveillance during the active treatment of cancer. Cognitive behavioral stress management groups have been shown to increase indicators of immune response targeted at potentially oncogenic virus infections such as EBV. Such alterations are paralleled by decreased levels of cortisol in the serum, reduced depressive mood, increased social support, and enhanced relaxation skills. Collectively, this work indicates that stress management can modify neuroendocrine dysregulation and immunologic functions that potentially have implications for tumor development and progression. However, as seen with other treatment approaches, the effectiveness of psychosocial interventions is likely to vary with the type and stage of cancer; characteristics of the patient; and the type, dose, and delivery of the intervention.
Conclusion
Despite significant progress within the past decade, further research is needed to define the mechanisms underlying the complex circuits involving the hypothalamic-pituitary-adrenal and autonomic nervous system axes and their effects on the processes involved in cancer development and progression. The body of data outlined here supports a model in which biobehavioral factors influence multiple aspects of tumorigenesis via their impact on neuroendocrine function (Figure 2). It is important to note that stress per se does not cause cancer; however, clinical and experimental data indicate that stress and other factors such as mood, coping mechanisms, and social support can significantly influence the underlying cellular and molecular processes that facilitate malignant cell growth. The evolving discovery of stress-associated influences on cancer biology provides a unique opportunity to consider why the initiation and progression of cancer varies among individuals. Consideration of this perspective might contribute to the development of novel therapeutic interventions to preempt cancer at every opportunity.
Acknowledgments
The authors gratefully acknowledge the editorial assistance of Lori Alexander.
Contributor Information
Paige Green McDonald, Basic and Biobehavioral Research Branch, Behavioral Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892, USA
Michael H. Antoni, Departments of Psychology and Psychiatry and Behavioral Sciences, and the Sylvester Comprehensive Cancer Center, University of Miami, Coral Gables, FL 33124, USA
Susan K. Lutgendorf, Departments of Psychology and Obstetrics and Gynecology and the Holden Comprehensive Cancer Center, University of Iowa, Iowa City, IA 52242, USA
Steven W. Cole, Division of Hematology-Oncology, University of California School of Medicine, Los Angeles, CA 90095, USA
Firdaus S. Dhabhar, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, Stanford, CA 94305, USA
Sandra E. Sephton, Department of Psychological and Brain Sciences, James Graham Brown Cancer Center, University of Louisville, Louisville, KY 40202, USA
Michael Stefanek, American Cancer Society, Atlanta, GA 30329, USA
Anil K. Sood, Departments of Gynecologic Oncology and Cancer Biology, University of Texas M.D. Anderson Cancer Center, Houston, TX 77230, USA
References and Further Readings
- Antoni M, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, McDonald PG, Stefanek M, Sood AK. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nature Reviews Cancer. 2006;6:240–248. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blask DE, Sauer LA, Dauchy RT. Melatonin as a chronobiotic/anticancer agent: cellular, biochemical, and molecular mechanisms of action and their implications for circadian-based cancer therapy. Current Topics in Medicinal Chemistry. 2002;2:113–132. doi: 10.2174/1568026023394407. [DOI] [PubMed] [Google Scholar]
- Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annual Review of Physiology. 2005;67:259–284. doi: 10.1146/annurev.physiol.67.040403.120816. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA: Journal of the American Medical Association. 1992;267:1244–1252. [PubMed] [Google Scholar]
- Lutgendorf SK, Costanzo E, Siegel S. Psychosocial Influences in Oncology: An Expanded Model of Biobehavioral Mechanisms. In: Ader R, Irwin M, Glaser R, Cohen N, editors. Psychoneuroimmunology. 4. Academic Press; New York: In press. [Google Scholar]
- Sanchez-Barcelo EJ, Cos S, Fernandez R, Mediavilla MD. Melatonin and mammary cancer: a short review. Endocrine-Related Cancer. 2003;10:153–159. doi: 10.1677/erc.0.0100153. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Viruses in human cancers. Science. 1991;254:1167–1173. doi: 10.1126/science.1659743. [DOI] [PubMed] [Google Scholar]