Abstract
OBJECTIVE
The purpose of our study was to compare the effect of three different full-laxative bowel preparations on patient compliance, residual stool and fluid, reader confidence, and polyp detection at CT colonography (CTC).
SUBJECTS AND METHODS
A total of 2531 patients underwent CTC followed by colonoscopy for the American College of Radiology Imaging Network (ACRIN) National CTC Trial. Of this total, 2525 patients used one of three bowel preparations with bisacodyl tablets and stool and fluid tagging: 4 L of polyethylene glycol (PEG); 90 mL of phosphosoda; or 300 mL of magnesium citrate. Patients reported percent compliance with the bowel preparation and radiologists graded each CTC examination for the amount of residual fluid and stool on a scale from 1 (none) to 4 (nondiagnostic). Reader confidence for true-positive findings was reported on a 5-point scale: 1 (low) to 5 (high). Sensitivity and specificity for detecting polyps ≥ 6 mm and ≥ 1 cm compared with colonoscopy were calculated for each preparation.
RESULTS
The most commonly prescribed preparation was phosphosoda (n = 1403) followed by PEG (n = 1020) and magnesium citrate (n = 102). Phosphosoda had the highest patient compliance (p = 0.01), least residual stool (p < 0.001), and highest reader confidence versus PEG for examinations with polyps (p = 0.06). Magnesium citrate had significantly more residual fluid compared with PEG and phosphosoda (p = 0.006). The sensitivity and specificity for detecting colon polyps ≥ 6 mm and ≥ 1 cm did not differ significantly between preparations.
CONCLUSION
Polyp detection was comparable for all three preparations, although phosphosoda had significantly higher patient compliance and the least residual stool.
Keywords: bowel preparation, CT, CT colonography
Bowel preparations at CT colonography (CTC) have evolved from cathartic preparations only [1–3] to cathartic preparations with fluid tagging and stool tagging [4, 5] to minimal preparations with only fluid and stool tagging [6–9]. Cathartic preparations, however, remain an integral part of most preparations because they provide superior bowel cleansing compared with preparations without a cathartic component [10–12]. In addition, some practices now offer same-day colonoscopy after CTC, for which a cathartic preparation is usually required.
The optimal cathartic agent for CTC is unclear. The most commonly used cathartic bowel agents for CTC are polyethylene glycol (PEG), phosphosoda, and magnesium citrate. PEG is available in multiple ways, without or with sodium sulfate, or in low volume packages with bisacodyl. At the time of this study, the larger volume PEG solution was most commonly in use (PEG–electrolyte lavage solution). Some practices prefer PEG because it can be safely used in most patients, unlike phosphosoda or magnesium citrate, which are contraindicated in some patients [13–15]. Others prefer phosphosoda or magnesium citrate because of higher patient acceptability of phosphosoda [16] and lower required volumes. Recent studies showing the risk of nephrocalcinosis and renal failure in patients ingesting the double-dose phosphosoda preparation (90 mL), however, have decreased the popularity of phosphosoda for CTC [13]. To our knowledge, a direct comparison of all three agents in a large screening population has not been performed.
The purpose of this study was to evaluate patient compliance, residual stool and fluid, reader confidence, and polyp detection performance of three CTC bowel preparations using identical fluid and stool tagging regimens.
Subjects and Methods
Patients
A total of 2531 asymptomatic participants 50 years or older (1205 men and 1326 women; mean age, 58 years) prescheduled for routine screening colonoscopy were recruited from 15 participating sites across the United States in the American College of Radiology Imaging Network (ACRIN) National CTC Trial between February 2005 and December 2006 [4]. The prospective study was reviewed and approved by each participating institution’s institutional review board, and subjects gave their informed consent to participate and to have their private health information accessed for the purposes of the study. Only patients who took one of three types of cathartic laxatives (polyethylene glycol, sodium phosphate, magnesium citrate) for colon preparation were included in this study (n = 2525 patients).
Bowel Preparation
Patients were instructed to stay on a clear liquid diet the day before the CTC examination. The stool tagging requirement was a minimum of 15 g of liquid barium sulfate administered in three divided doses at mealtimes the day before the examination. The protocol did not specify the type of barium tagging to be used; however, the vast majority (14/15 sites) used Tagitol V (Bracco) (40% weight/volume), which was given according to the following regimen: 10 mL at breakfast, 10 mL at lunch, and 20 mL at dinner, which resulted in a total of 16 g of barium sulfate.
After finishing the stool tagging, patients began one of three widely used cathartic laxatives: 4 L of PEG (PEG-3350, GoLytely, Braintree Laboratories), 90 mL of sodium phosphate solution in two divided doses of 45 mL (phosphosoda, Phospho-soda, Fleet Laboratories), or 300 mL of magnesium citrate (Magnesium Citrate, SunMark). After completing the laxative preparation, all participants ingested bisacodyl tablets (10 mg, or according to their current institutional standard of care) to reduce the presence of any residual stool or fluid. The final step was to ingest a 6-oz (177-mL) glass of liquid containing at least 5 mL and up to 60 mL of water-soluble iodinated oral contrast material (diatrizoate meglumine and sodium diatrizoate, Gastroview, Mallinckrodt Imaging) the night before the examination to label any residual colonic fluid.
CTC Examinations
Enrolled participants completed a clinical CTC examination using a low-dose technique without IV contrast administration as previously described [4]. Specific CT parameters included 16-MDCT or 64-MDCT scanner; collimation, 0.5–1.0 mm; reconstructed slice thicknesses, 1–1.25 mm; reconstruction interval, 0.8 mm; pitch, 0.98–1.5; matrix, 512 × 512; field-of-view to fit the patient; 50 effective mAs; and peak voltage of 120 kV. Data were obtained in the supine and prone positions; 1 mg of glucagon was administered subcutaneously 7–15 minutes before the CT examination unless contraindicated or refused by the patient. Colonic distention was achieved with an automated carbon dioxide insufflator (PROTOCO2L, E-Z-EM).
Outcome Parameters
Patient compliance
To assess compliance with the bowel preparation, participants were asked before their CTC if the prescribed cathartic laxative, barium tagging, and oral iodinated contrast were taken as directed, not taken as directed but fully consumed, or not taken as directed and partially consumed. Responses were given separately for each material (cathartic laxative, barium, iodinated contrast material) and recorded by the study coordinator or nurse.
Residual fluid and stool assessment
The interpreting radiologists completed a questionnaire grading the amount of residual stool and residual fluid present in six segments of the colon: rectum, sigmoid, descending colon, transverse colon, ascending colon, and cecum. The amount of fluid was graded on a 4-point scale with 1, no fluid; 2, less than 25% of the lumen filled with fluid; 3, 25–50% of the lumen filled with fluid; and 4, greater than 50% of the lumen filled with fluid (Figs. 1–4). The amount of stool was also graded on a similar 4-point scale: 1, none; 2, less than 25% of the lumen filled with stool; 3, 25–50% of the lumen filled with stool; and 4, greater than 50% of the lumen filled with stool (Figs. 1–4). Residual stool and fluid were considered “good” with a grade of 1 or 2 and “poor” with a grade of 3 or 4.
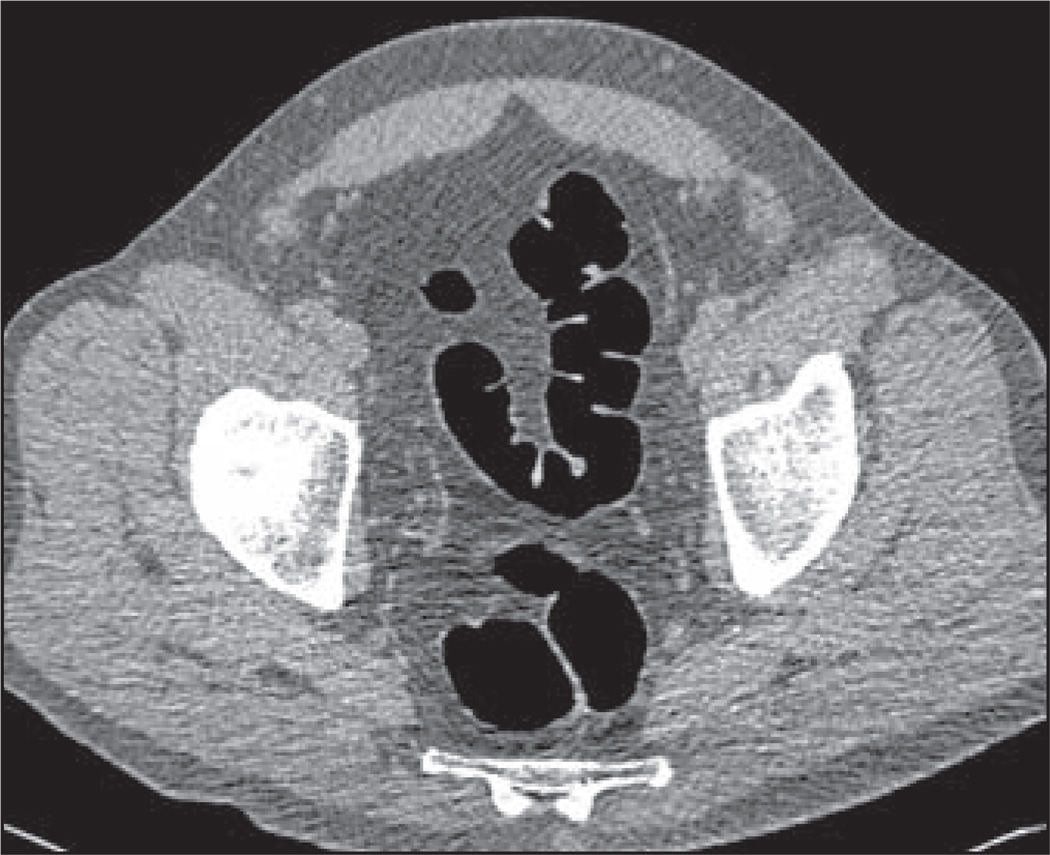
Fig. 1.
CT colonography image in 60-year-old man shows score of 1, no residual fluid or stool.
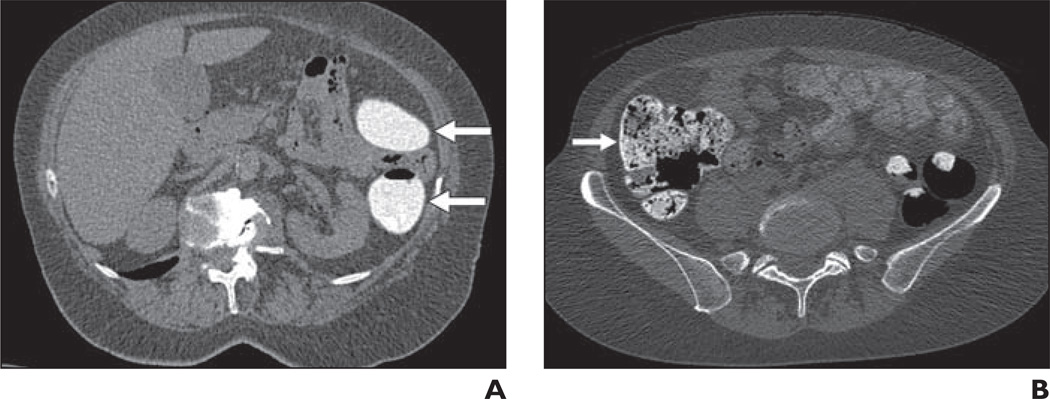
Fig. 4.
Score of 4, > 50% of lumen filled with fluid or stool.
A, CT colonography image in 74-year-old woman shows residual fluid score of 4 in descending colon (arrows).
B, CT colonography image in 53-year-old woman shows residual stool score of 4 in cecum (arrow).
The worst assessment score for all segments was recorded as the overall residual fluid and stool grades. Therefore, if one segment was graded as having a moderate amount of residual fluid but the remaining segments had none, the overall score would be moderate for residual fluid. Each examination was graded by one of 15 radiologists who were blinded to the type of preparation used. A subset analysis was performed for those patients who did not ingest any iodinated oral contrast material.
Reader confidence
Reader confidence was reported for test positives of ≥ 6 mm and ≥ 1 cm using a 5-point scale: 1, low confidence; 2, possible; 3, indeterminate; 4, probable; and 5, high confidence. The confidence scores were summarized for all preparations. In addition, confidence scores were compared with scores for residual fluid and stool to determine the association of bowel cleansing and reader confidence.
Polyp detection
Each reader evaluated CTC images using one of five self-chosen software platforms and a randomly assigned primary image review method (primary 2D or primary 3D). In the original study, readers were instructed to record the size and location of all findings 5 mm or larger. Lesion size was determined from the pathology report unless the lesion was not wholly resected; for these lesions, colonoscopy-derived size estimates based on visual estimation or forceps were used.
Same-day colonoscopy was performed immediately after CTC to serve as the reference standard in 2512 of 2525 patients (99%). The remainder of the studies were completed within 90 days. Perpatient sensitivity and specificity analyses were conducted using a 5-mm threshold for determining a positive result in the CTC interpretation. The reference standard was identical to the primary manuscript [4]; patients with at least one cancer or adenoma sized 10 mm or larger at pathology or colonoscopy were considered positive. Additionally we considered cancers or adenomas sized 6 mm or above. Polyp detection results were compared for each preparation.
Statistical Methods
The primary aim of this analysis was to determine whether any cathartic preparation outperforms remaining preparations in areas of patient compliance, residual fluid, residual stool, reader confidence, sensitivity, and specificity. Patient compliance results were compared by each preparation and patient age group using chi-square exact tests. To determine whether reader confidence is associated with examination quality, we used rank tests. To estimate the effect of preparation type on residual fluid and stool and reader confidence scores, we used proportional odds models.
To summarize diagnostic accuracy while accounting for reader correlation, estimates of sensitivity and specificity are averaged over all readers using resampling methods. Fifteen reader estimates were sampled with replacement and then averaged. Additionally, differences in the averaged estimates for each preparation were calculated. These bootstrap procedures were repeated 10,000 times for sensitivity and specificity of all three preparations and their differences; 95% CIs are reported as 250th and 9750th estimate from the ordered estimates. As a safeguard against multiple tests of significance, the Bonferroni correction was used; all p values ≤ 0.01 from significance tests remain significant after adjusting for multiple comparisons.
Results
Of the 2531 participants who completed both CTC and optical colonoscopy, 2525 patients took one of the three designated preparations and were used for analysis. Six patients who took only bisacodyl tablets or could not recall the preparation were excluded from analysis.
Of the 2525 patients, the most commonly prescribed preparation was phosphosoda (n = 1403) followed by PEG (n = 1020) and magnesium citrate (n = 102). Table 1 shows patient compliance and quality scores for the three colon preparations. PEG examinations were read by 13 readers, phosphosoda examinations were read by 15 readers, and magnesium citrate examinations were read by four readers, with a single reader evaluating the majority of magnesium citrate examinations (98/102, 96%).
TABLE 1.
Preparation Compliance and Quality Scores for Three Colon Preparations
| Parameter | PEG (n = 1020) | PS (n = 1403) | MC (n = 102) |
|---|---|---|---|
| Patient compliance “taken as directed”a | 948 (93) | 1393 (99) | 100 (98) |
| Residual fluid | |||
| Good (grades 1–2) | 533 (52) | 783 (56) | 37 (36) |
| Poor (grades 3–4) | 487 (48) | 620 (44) | 65 (64) |
| Residual stool | |||
| Good (grades 1–2) | 855 (84) | 1255 (89) | 85 (83) |
| Poor (grades 3–4) | 165 (16) | 148 (11) | 17 (17) |
| Odds ratio less residual fluid (95% CI)b | 1.67 (1.16–2.40) | 1.90 (1.32–2.72) | Reference |
| Odds ratio more residual stool (95% CI)c | 2.2 (1.8–2.6) | Reference | 3.04 (2.03–4.55) |
Note—Data are number with percentage in parentheses. PEG = polyethylene glycol, PS = phosphosoda, MC = magnesium citrate.
Phosphosoda was significantly more likely to be taken as directed compared with polyethylene glycol and magnesium citrate (p = 0.01).
Polyethylene glycol and phosphosoda were nearly twice as likely to have less residual fluid than magnesium citrate (p < 0.01).
Polyethylene glycol and magnesium citrate were more than twice as likely to have more residual stool than phosphosoda (p < 0.01).
The majority of patients (92%, 2322/2525) received glucagon. Two-hundred three patients did not receive glucagon because of brittle diabetes (n = 78); patient refusal (n = 69); glucagon was not available (n = 47); or pheochromocytoma, radiologist not present, or borderline glucose level (n = 9).
Patient Compliance
PEG was significantly (p = 0.01) less likely to be taken as directed (93%) compared with phosphosoda (99%) and magnesium citrate (98%). The barium sulfate was consumed as directed by 98% (2476/2525) of patients and all patients ingested at least a partial dose. Iodinated oral contrast material was taken as directed by 94% (2384/2525) of participants. Of the remaining 141 patients who did not take the iodinated oral contrast material as directed, 4% (110/2525) consumed a partial or complete dose and the remaining 1.2% (31/2525) did not ingest any.
Of the 31 patients who did not ingest any oral iodinated contrast material, 19 had phosphosoda, 10 PEG, and two magnesium citrate. For both residual fluid and stool scoring, 23 of 31 patients (74%) had “good” scores, indicating none to minimal fluid and stool, whereas eight of 31 (26%) had “poor” scores, indicating moderate or more stool. Compared with scores in patients who did ingest oral iodinated contrast material, the “good” residual fluid scores were higher (74% vs 36–56%) and “good” residual stool scores were slightly lower (74% vs 83–89%). Therefore, patients who did not ingest the oral iodinated contrast were more likely to have less residual fluid and slightly more residual stool.
Residual Fluid
A fluid score of 1 or 2, indicating no or minimal fluid, was seen in 82.7% (5061/6120) of segments with PEG, 83.0% (6980/8411) of segments with phosphosoda, and 70.9% (434/612) with magnesium citrate. A fluid score of 3 or 4, indicating moderate or more, fluid was seen in 17.3% (1059/6120) of segments with PEG, 17.0% (1431/8411) of segments with phosphosoda, and 29.1% (178/612) with magnesium citrate.
More magnesium citrate examinations were graded as poor (i.e., having moderate or residual fluid) compared with PEG and phosphosoda (64% vs 48 and 44%, respectively). When compared with magnesium citrate, a patient examination using PEG or phosphosoda was almost twice as likely to have lower scores for residual fluid (odds ratio [OR], [95% CI], 1.7 [1.2–2.4]; 1.9, [1.3–2.7]; p < 0.01). There was no significant difference when comparing residual fluid using PEG and phosphosoda.
Residual Stool
A stool score of 1 or 2, indicating none or minimal stool, was seen in 92.9% (5688/6120) of segments with PEG, 96.6% (8125/8411) of segments with phosphosoda, and 94.0% (575/612) with magnesium citrate. A stool score of 3 or 4, indicating moderate or more stool, was seen in 7.1% (432/6120) of segments with PEG, 3.4% (286/8411) of segments with phosphosoda, and 6.0% (37/612) with magnesium citrate.
Fewer phosphosoda examinations were graded as poor (i.e., having a moderate or greater amount of stool) compared with PEG and magnesium citrate (11% vs 16 and 17%, respectively). When compared with phosphosoda, a patient examination using PEG or magnesium citrate was more than twice as likely to have higher scores for residual stool (OR, [95% CI], 2.2, [1.8–2.6]; 3.0, [2.0–4.5]; p < 0.01). There was no significant difference when comparing residual stool between PEG and magnesium citrate.
Polyp Detection
Colonoscopy-proven cases with polyps ≥ 1 cm were found in the following number of cases per preparation: 43 (PEG), 52 (phosphosoda), and 14 (magnesium citrate). Colonoscopy-proven cases with polyps ≥ 6 mm were found in the following number of cases per preparation: 83 (PEG), 102 (phosphosoda), and 25 (magnesium citrate).
The reader-averaged sensitivity and specificity for correctly identifying cases with colon polyps ≥ 1 cm did not differ significantly between preparations, and the results per preparation are shown in Table 2. For colon polyps ≥ 1 cm, the sensitivity was 90% or higher and specificity was between 87% and 89%. Two readers reviewing too few positive cases (n = 14) with magnesium citrate prohibited direct comparison of magnesium citrate with phosphosoda and PEG.
TABLE 2.
Polyp Detection Sensitivity and Specificity by Preparation
| Parameter | PEG (n = 1020 patients) | PS (n = 1403 patients) | MCa (n = 102 patients) |
|---|---|---|---|
| No. of readers (reviewed case with polyp ≥ 1 cm) | 13 (10) | 15 (9) | 4 (2) |
| No. of cases with colonoscopy-proven polyps ≥ 1 cm | 43 | 52 | 14 |
| No. of cases with colonoscopy-proven polyps ≥ 6 mm | 83 | 102 | 25 |
| Average sensitivity for polyps ≥ 1 cm (95% BCI) (%) | 90 (84–95) | 91 (84–97) | 100a |
| Average specificity for polyps ≥ 1 cm (95% BCI) (%) | 88 (83–93) | 89 (83–93) | 87 (76–95) |
| Average sensitivity for polyps ≥ 6 mm (95% BCI) (%) | 80 (72–89) | 79 (70–88) | 96a |
| Average specificity for polyps ≥ 6 mm (95% BCI) (%) | 90 (84–95) | 90 (86–94) | 89 (66–100) |
Note—Bootstrap methods were used to calculate sensitivity and specificity. There were no statistically significant differences in sensitivity and specificity comparing the three preparations. PEG = polyethylene glycol, PS = phosphosoda, MC = magnesium citrate, BCI = Bootstrap CI.
Sensitivity of magnesium citrate is limited to point estimate because only two readers contributed to this finding.
The reader-averaged sensitivity and specificity for correctly identifying cases with colon polyps ≥ 6 mm also did not differ significantly between preparations. For colon polyps ≥ 6 mm, the sensitivity was between 80% and 96% and specificity between 89% and 90%. Two readers reviewing too few positive cases (n = 25) with magnesium citrate prohibited direct comparison of magnesium citrate with phosphosoda and PEG.
Reader Confidence
High reader confidence for a polyp ≥ 1 cm was scored in 74% (63/85) of examinations with none to minimal residual stool. High reader confidence was scored in only 20% (2/10) of examinations with moderate or greater residual stool. Thus, reader confidence scores were significantly higher when residual stool was lower (p = 0.0005).
On the other hand, reader confidence for a polyp ≥ 1 cm improved slightly when there was more residual fluid. Specifically, if residual fluid was graded as poor, there was high reader confidence in 74% (31/42) of examinations. When residual fluid was graded as good (i.e., less fluid), high reader confidence decreased to 64% (34/53) of examinations. This difference, however, was not statistically significant.
Confidence scores compared by different preparations were not significantly different overall, i.e., when evaluating both positive and negative examinations (Table 3). Compared with PEG, however, phosphosoda was more likely to have higher reader confidence scores in positive examinations with ≥ 1 cm polyps (OR, [95% CI], 2.5 [1.0–6.3], p = 0.06) and with ≥ 6 mm polyps (OR, [95% CI], 2.4, [1.2–4.7]; p = 0.01).
TABLE 3.
Reader Confidence
| Parameter | PEG (n = 1020 patients) |
PS (n = 1403 patients) |
MC (n = 102 patients) |
|---|---|---|---|
| Odds ratio higher reader confidence for detected polyps ≥ 1 cm (95% CI)a | 0.73 (0.21–2.54) | 1.83 (0.50–6.63) | Reference |
| Odds ratio higher reader confidence for detected polyps ≥ 6 mm (95% CI)a | 0.70 (0.28–1.73) | 1.68 (0.67–4.22) | Reference |
| CT colonography–positive cases with polyps ≥ 1 cm graded with high reader confidence (%) | 54 (20/37) | 77 (34/44) | 64 (9/14) |
| CT colonography–positive cases with polyps ≥ 6 mm graded with high reader confidence (%) | 44 (28/64) | 68 (50/74) | 52 (12/23) |
Note—Six cases had other colon preparations. PEG = polyethylene glycol, PS = phosphosoda, MC = magnesium citrate.
Reader confidence was not significantly different between preparations: for polyps ≥ 1 cm, p = 0.09; for polyps ≥ 6 mm, p = 0.06.
Discussion
The mechanism of action and delivery of the three most commonly used cathartic agents for bowel cleansing differ, explaining their relative advantages and disadvantages in various patient populations. PEG is an osmotically balanced electrolyte lavage solution, which results in minimal water and electrolyte absorption and secretion [14]. Because PEG does not result in internal fluid shifts, it has the advantage of being safe in the vast majority of patients and provides a gentle colonic lavage [17]. Phosphosoda often has better patient tolerance than PEG, primarily because of the lower ingested volume [16]. Phosphosoda, however, is a hyperosmotic solution, drawing fluid into the bowel lumen and can result in electrolyte imbalances [14]. In addition, phosphosoda preparations have been shown to induce nephrocalcinosis with hypercalcemia, leading to acute renal failure even in patients with previously normal calcium levels [13]. Therefore, the use of phosphosoda has been limited, especially in patients at risk of dehydration, age greater than 55 years, or with an underlying risk of renal dysfunction [13, 18–20], and warnings are now required by the Food and Drug Administration for prescription phosphate products using the 90 mL-dose [21].
A saline laxative sometimes used for colon cleansing is magnesium citrate. This hyperosmotic saline laxative increases intraluminal volume, thereby increasing motility [15]. It is also thought to stimulate cholecystokinin, promoting fluid secretion and bowel peristalsis. Because magnesium citrate is eliminated entirely by the kidneys, it must be used carefully in patients with renal disease [15]. The use of these different agents for CTC bowel preparation varies between practices and no head-to-head comparisons have previously been done in a large population.
Patient compliance is often the most challenging aspect for successful CTC bowel preparations. In this study, all three types of bowel preparations had > 90% compliance and 99–100% of patients ingested some or all of the barium tagging and iodinated contrast material. Phosphosoda and magnesium citrate, however, were significantly more likely to be taken as directed compared with PEG. The relatively worse compliance with PEG is likely attributed to the higher volume of fluid required (4000 mL) compared with phosphosoda (90 mL) and magnesium citrate (300 mL). Previous studies have shown similar results, finding that as required ingested volumes increases, both patient acceptability [11, 22] and compliance decrease [16]. More patient discomfort with PEG compared with magnesium citrate has been reported both in the colonoscopy and CTC literature [23, 24]. Although PEG had the worst compliance in this study, detection of polyps ≥ 6 mm and ≥ 1 cm was essentially identical to phosphosoda. Therefore, even if the full 4 L of PEG was not consumed, it still appeared adequate for a diagnostic CTC. In fact, a reduced PEG preparation consisting of 2 L instead of 4 L is now widely accepted accepted for colonoscopy and has been shown to be more acceptable for patients, with similar bowel cleansing efficacy [25, 26]. However, this 2-L preparation was not commonly used at the onset of this study. Had this reduced PEG preparation been used, it is likely that patient compliance with PEG may have been higher.
Residual fluid is another metric commonly used to measure the success of various bowel preparations. In this study, the largest amount of residual fluid was with magnesium citrate, although it required much less ingested fluid compared with PEG. Phosphosoda and PEG had nearly identical amounts of residual colonic fluid, which differs from a prior CTC study that showed that phosphosoda resulted in a drier colon [2]. This difference may be attributed to using bisacodyl after the PEG preparation in this study, which likely helps to facilitate fluid elimination. Another recent CTC study comparing single-dose phosphosoda and double-dose magnesium citrate had different results than our study because those authors found no significant differences in overall residual fluid between the two preparations [27]. The only significant difference in that study was found in the sigmoid colon, which had higher scores for residual fluid with magnesium citrate compared with phosphosoda. It is possible that our conflicting results are related to differences in the order of the colon preparation instructions. For example, in our study, the bisacodyl tablets were given after completing the laxative whereas in the other study, they were given before the laxative preparation.
Phosphosoda had the least amount of residual stool as well as the highest reader confidence for detecting polyps ≥ 1 cm. A subanalysis found that as the amount of residual stool decreases, reader confidence significantly increases. Interestingly, the same relationship was not found with residual fluid in this study in which the amount of residual fluid did not affect reader confidence. This difference may be due to the fact that residual stool, unlike fluid, has a polypoid appearance that can both simulate or obscure polyps even when tagged. A previous study found that residual stool made CTC examinations more difficult to interpret and caused polyps to be missed whereas residual fluid did not [12]. In spite of the better compliance, less stool, and higher reader confidence with phosphosoda, however, polyp detection with phosphosoda was not significantly different compared with PEG and magnesium citrate.
It is difficult to make absolute judgments concerning magnesium citrate in this study because magnesium citrate was the least commonly used preparation (4% of all examinations), and the majority of those studies were interpreted by a single reader. Although magnesium citrate studies had more overall residual stool than phosphosoda (16.7% vs 10.6%), the number of segments graded as having moderate or more stool was relatively low with magnesium citrate (6%). Prior studies evaluating the amount of residual stool with magnesium citrate have been variable. One study comparing magnesium citrate versus a 1–3 L PEG preparation found more fecal residue using the magnesium citrate preparation [20]. Another study comparing magnesium citrate and phosphosoda, however, found no difference in residual stool [24]. On the basis of the limited number of examinations using magnesium citrate in this study, we can surmise that this preparation resulted in slightly more residual stool than phosphosoda but comparable to PEG. The effect of this residual stool on performance, however, cannot be well evaluated because of the small number of examinations.
Limitations of this study include the higher volume PEG preparation used, which may have worsened patient compliance results; the use of double-dose phosphosoda, which is less commonly used today because of risks of nephrocalcinosis; and the small number of cases using magnesium citrate. This study did not evaluate minimal or no preparation options that may help to make CTC more widely acceptable to patients. Variables that were not evaluated that could impact results include colonic distention, quality of stool and fluid tagging, and impact of potentially higher residual fluid and stool scores in 6% of patients who did not ingest the iodinated oral contrast material as directed. Finally, because of differences in number of polyps detected using each technique, bootstrap methods were used to resample data.
In summary, this is the largest study comparing three different cathartic bowel preparations preparations at CTC. Our study indicates that although higher patient compliance, less residual stool, and higher reader confidence were found with phosphosoda, ultimately PEG and phosphosoda perform similarly in terms of polyp detection. Magnesium citrate was used much less commonly, and most of the magnesium citrate examinations were interpreted by a single reader, making its results more difficult to interpret and less generalizable. This study indicates that measures of compliance, residual stool, and reader confidence do not always correlate with reader performance when comparing various cathartic bowel preparations. Therefore, it is important for future studies of reduced or minimal cathartic preparations to include assessment of reader performance when evaluating these techniques.
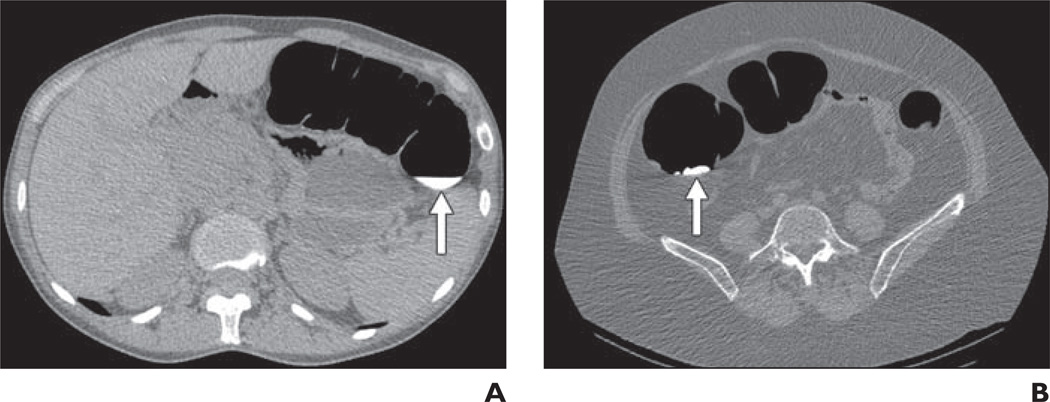
Fig. 2.
Score of 2, less than 25% lumen filled with fluid or stool.
A, CT colonography image in 50-year-old woman shows residual fluid score of 2 in transverse colon (arrow).
B, CT colonography image in 50-year-old woman shows residual stool score of 2 in ascending colon (arrow).
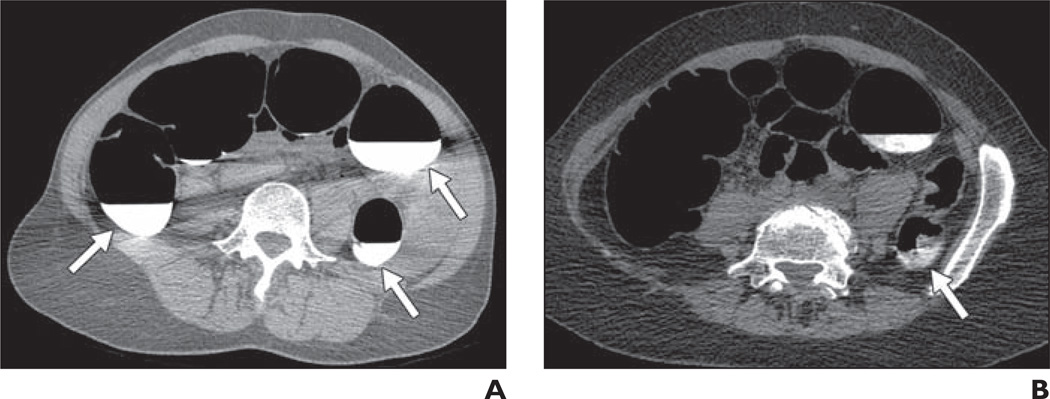
Fig. 3.
Score of 3, 25–50% of lumen filled with fluid or stool.
A, CT colonography image in 49-year-old woman shows residual fluid score of 3 in ascending and descending colons (arrows).
B, CT colonography image in 67-year-old woman shows residual stool score of 3 in descending colon (arrow).
Acknowledgments
Supported by National Institutes of Health/American College of Radiology Imaging Network grant U01 CA79778 S2.
References
- 1.Cotton PB, Durkalski VL, Pineau BC, et al. Computed tomographic colonography (virtual colonoscopy): a multicenter comparison with standard colonoscopy for detection of colorectal neoplasia. JAMA. 2004;291:1713–1719. doi: 10.1001/jama.291.14.1713. [DOI] [PubMed] [Google Scholar]
- 2.Macari M, Lavelle M, Pedrosa I, et al. Effect of different bowel preparations on residual fluid at CT colonography. Radiology. 2001;218:274–277. doi: 10.1148/radiology.218.1.r01ja31274. [DOI] [PubMed] [Google Scholar]
- 3.Rockey DC, Paulson E, Niedzwiecki D, et al. Analysis of air contrast barium enema, computed tomographic colonography, and colonoscopy: prospective comparison. Lancet. 2005;365:305–311. doi: 10.1016/S0140-6736(05)17784-8. [DOI] [PubMed] [Google Scholar]
- 4.Johnson CD, Chen MH, Toledano AY, et al. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med. 2008;359:1207–1217. doi: 10.1056/NEJMoa0800996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pickhardt PJ, Choi JH. Electronic cleansing and stool tagging in CT colonography: advantages and pitfalls with primary three-dimensional evaluation. AJR. 2003;181:799–805. doi: 10.2214/ajr.181.3.1810799. [DOI] [PubMed] [Google Scholar]
- 6.Kealey SM, Dodd JD, MacEneaney PM, Gibney RG, Malone DE. Minimal preparation computed tomography instead of barium enema/colonoscopy for suspected colon cancer in frail elderly patients: an outcome analysis study. Clin Radiol. 2004;59:44–52. doi: 10.1016/j.crad.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Iannaccone R, Laghi A, Catalano C, et al. Computed tomographic colonography without cathartic preparation for the detection of colorectal polyps. Gastroenterology. 2004;127:1300–1311. doi: 10.1053/j.gastro.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 8.Jensch S, Bipat S, Peringa J, et al. CT colonography with limited bowel preparation: prospective assessment of patient experience and preference in comparison to optical colonoscopy with cathartic bowel preparation. Eur Radiol. 2010;20:146–156. doi: 10.1007/s00330-009-1517-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensch S, de Vries AH, Peringa J, et al. CT colonography with limited bowel preparation: performance characteristics in an increased-risk population. Radiology. 2008;247:122–132. doi: 10.1148/radiol.2471070439. [DOI] [PubMed] [Google Scholar]
- 10.Nagata K, Okawa T, Honma A, Endo S, Kudo SE, Yoshida H. Full-laxative versus minimum-laxative fecal-tagging CT colonography using 64-detector row CT: prospective blinded comparison of diagnostic performance, tagging quality, and patient acceptance. Acad Radiol. 2009;16:780–789. doi: 10.1016/j.acra.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 11.Jensch S, de Vries AH, Pot D, et al. Image quality and patient acceptance of four regimens with different amounts of mild laxatives for CT colonography. AJR. 2008;191:158–167. doi: 10.2214/AJR.07.3128. [DOI] [PubMed] [Google Scholar]
- 12.Dachman AH, Dawson DO, Lefere P, et al. Comparison of routine and unprepped CT colonography augmented by low fiber diet and stool tagging: a pilot study. Abdom Imaging. 2007;32:96–104. doi: 10.1007/s00261-006-9044-9. [DOI] [PubMed] [Google Scholar]
- 13.Markowitz GS, Stokes MB, Radhakrishnan J, D’Agati VD. Acute phosphate nephropathy following oral sodium phosphate bowel purgative: an underrecognized cause of chronic renal failure. J Am Soc Nephrol. 2005;16:3389–3396. doi: 10.1681/ASN.2005050496. [DOI] [PubMed] [Google Scholar]
- 14.Occhipinti KE, Di Palma JA. How to choose the best preparation for colonoscopy. Nat Rev Gastroenterol Hepatol. 2009;6:279–286. doi: 10.1038/nrgastro.2009.42. [DOI] [PubMed] [Google Scholar]
- 15.Wexner SD, Beck DE, Baron TH, et al. A consensus document on bowel preparation before colonoscopy: prepared by a task force from the American Society of Colon and Rectal Surgeons (ASCRS), the American Society for Gastrointestinal Endoscopy (ASGE), and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) Dis Colon Rectum. 2006;49:792–809. doi: 10.1007/s10350-006-0536-z. [DOI] [PubMed] [Google Scholar]
- 16.Vanner SJ, MacDonald PH, Paterson WG, Prentice RS, Da Costa LR, Beck IT. A randomized prospective trial comparing oral sodium phosphate with standard polyethylene glycol-based lavage solution (Golytely) in the preparation of patients for colonoscopy. Am J Gastroenterol. 1990;85:422–427. [PubMed] [Google Scholar]
- 17.Sharma VK, Steinberg EN, Vasudeva R, Howden CW. Randomized, controlled study of pretreatment with magnesium citrate on the quality of colonoscopy preparation with polyethylene glycol electrolyte lavage solution. Gastrointest Endosc. 1997;46:541–543. doi: 10.1016/s0016-5107(97)70011-7. [DOI] [PubMed] [Google Scholar]
- 18.Gumurdulu Y, Serin E, Ozer B, Gokcel A, Boyacioglu S. Age as a predictor of hyperphosphatemia after oral phosphosoda administration for colon preparation. J Gastroenterol Hepatol. 2004;19:68–72. doi: 10.1111/j.1440-1746.2004.03253.x. [DOI] [PubMed] [Google Scholar]
- 19.Kuehn D, May J, Bauer AJ. Phospho-soda-induced symptomatic hypocalcemia in a patient with cystic fibrosis and vitamin D malabsorption. J Pediatr Gastroenterol Nutr. 2008;47:514–516. doi: 10.1097/MPG.0b013e31811f3561. [DOI] [PubMed] [Google Scholar]
- 20.Oztas E, Bektas M, Kurt M, Onal IK, Ozden A. Oral Fleet Phospho-Soda laxative induced symptomatic hypocalcemia in an adult patient with celiac disease. Am J Gastroenterol. 2009;104:1607–1608. doi: 10.1038/ajg.2009.119. [DOI] [PubMed] [Google Scholar]
- 21.United States Food and Drug Administration Website. Oral sodium phosphate (OSP) products for bowel cleansing (marketed as Visicol and Osmo-Prep and oral sodium phosphate products available without a prescription) [Accessed January 19, 2011];FDA alert. 2008 December 11; www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ ucm103354.htm.
- 22.Liedenbaum MH, de Vries AH, Gouw CI, et al. CT colonography with minimal bowel preparation: evaluation of tagging quality, patient acceptance and diagnostic accuracy in two iodine-based preparation schemes. Eur Radiol. 2010;20:367–376. doi: 10.1007/s00330-009-1570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Regev A, Fraser G, Delpre G, et al. Comparison of two bowel preparations for colonoscopy: sodium picosulphate with magnesium citrate versus sulphate-free polyethylene glycol lavage solution. Am J Gastroenterol. 1998;93:1478–1482. doi: 10.1111/j.1572-0241.1998.00467.x. [DOI] [PubMed] [Google Scholar]
- 24.Lefere PA, Gryspeerdt SS, Dewyspelaere J, Baekelandt M, Van Holsbeeck BG. Dietary fecal tagging as a cleansing method before CT colonography: initial results polyp detection and patient acceptance. Radiology. 2002;224:393–403. doi: 10.1148/radiol.2241011222. [DOI] [PubMed] [Google Scholar]
- 25.DiPalma JA, Wolff BG, Meagher A, Cleveland M. Comparison of reduced volume versus four liters sulfate-free electrolyte lavage solutions for colonoscopy colon cleansing. Am J Gastroenterol. 2003;98:2187–2191. doi: 10.1111/j.1572-0241.2003.07690.x. [DOI] [PubMed] [Google Scholar]
- 26.Ell C, Fischbach W, Bronisch HJ, et al. Randomized trial of low-volume PEG solution versus standard PEG + electrolytes for bowel cleansing before colonoscopy. Am J Gastroenterol. 2008;103:883–893. doi: 10.1111/j.1572-0241.2007.01708.x. [DOI] [PubMed] [Google Scholar]
- 27.Borden ZS, Pickhardt PJ, Kim DH, Lubner MG, Agriantonis DJ, Hinshaw JL. Bowel preparation for CT colonography: blinded comparison of magnesium citrate and sodium phosphate for catharsis. Radiology. 2010;254:138–144. doi: 10.1148/radiol.09090398. [DOI] [PubMed] [Google Scholar]