Abstract
Background
Our objective was to document the clinical and imaging features of Othello's syndrome (delusional jealousy).
Methods
The study design was a retrospective case series of 105 patients with Othello's syndrome that were identified by using the Electronic Medical Record system of Mayo Clinic.
Results
The average age at onset of Othello's syndrome was 68 (25–94) years with 61.9% of patients being male. Othello's syndrome was most commonly associated with a neurological disorder (73/105) compared with psychiatric disorders (32/105). Of the patients with a neurological disorder, 76.7% had a neurodegenerative disorder. Seven of eight patients with a structural lesion associated with Othello's syndrome had right frontal lobe pathology. Voxel-based morphometry showed greater grey matter loss predominantly in the dorsolateral frontal lobes in the neurodegenerative patients with Othello's compared to matched patients with neurodegenerative disorders without Othello's syndrome. Treatment success was notable for patients with dopamine agonist induced Othello's syndrome in which all six patients had improvement in symptoms following decrease in medication.
Conclusions
This study demonstrates that Othello's syndrome occurs most frequently with neurological disorders. This delusion appears to be associated with dysfunction of the frontal lobes, especially right frontal lobe.
Keywords: Othello's Syndrome, Right frontal lobe, Delusions, Dementia
Introduction
Othello's syndrome (OS), named after the character in Shakespeare's play refers to the delusion of infidelity of a significant other, which is sometimes used interchangeably with delusional or morbid jealousy. OS has been associated with psychiatric and neurological disorders including strokes, brain trauma, brain tumors, neurodegenerative disorders, encephalitis, multiple sclerosis, normal pressure hydrocephalus, endocrine disorders, and drugs.(1–8) Many of these reports, however, have been single case reports or small case series. Several case reports have suggested that the right frontal lobe is the neuroanatomical correlate for OS(9–12) although, others have reported thalamic and left frontal lobe lesions.(13, 14) There are no quantitative imaging studies on OS. In one study assessing the prevalence of delusional jealousy in psychiatric patients, delusional jealousy was most frequently associated with an organic cause.(6)
While cases reports and small cases series have associated OS with neurological and psychiatric diseases, there are no comprehensive studies on a large series of patients with OS.. The aim of the study was to investigate clinical and imaging characteristics of OS in patients with different neurological and psychiatric conditions.
Methods
Subject selection
Using a text word search for “Othello” or “Delusions and Infidelity” or “Delusions of Jealousy or Infidelity”, the Mayo Clinic Medical Records Linkage system was used to identify patients with possible OS who were evaluated at our institution in any department between January 1st, 1998 and October 31st, 2009. Given this approach, any case where the delusion was mentioned as present or absent by the examining physician was identified (n=185). These medical records were reviewed to determine whether the patient met inclusion criteria for OS. Specifically, the delusion had to be one of infidelity or jealousy, clearly stated in the medical records and described. Cases not meeting criteria (n=80) were excluded. These were cases in which either OS was incorrectly used or the delusion did not involve infidelity or jealousy. Clinical data were abstracted in all 105 cases that met our inclusion criteria, including age at onset of delusion of infidelity, age at onset of first neurological sign/symptom, gender, description of the infidelity delusion, first and final clinical diagnosis, treatment and response to treatment of the infidelity delusion, physical findings on examination, behavioral or personality changes, hallucinations and other delusions. This study was approved by the Mayo Clinic Institutional Review Board in Rochester, Minnesota.
All 105 subjects were initially divided into the following groups for comparison based on the context of the occurrence of the delusion of infidelity (i.e. diagnosis): psychiatric, drug-related, neurodegenerative (Alzheimer disease (AD), Lewy body disease (LBD) and behavioral variant of frontotemporal dementia (bvFTD)), vascular dementia and focal lesions. The group of patients with diseases, where Lewy bodies are the predominant pathology included the following subtypes: dementia with Lewy bodies (DLBD), Parkinson's disease (PD) and Parkinson's disease with dementia (PDD).(15, 16) There are approximately thirty two inpatient adult neurology beds and fifty five inpatient psychiatric beds available.
MRI analysis
Given that neurodegenerative disorders are associated with cerebral atrophy, we used Voxel-based morphometry (VBM)(17) to assess patterns of grey matter loss in the OS patients that had a neurodegenerative disorder and a T1-weighted volumetric MRI performed after the onset of OS as previously described.(18) These neurodegenerative patients were matched by age and gender to patients that had the identical clinical diagnoses but no evidence of delusions on the Neuropsychiatric Inventory (NPI),(19) and to healthy controls. In order to identify regions of grey matter loss that may be contributing to the OS two comparisons were performed. First, the OS patients were compared to the matched neurodegenerative patients. Age, gender and Short Test of Mental Status(20) score were included in the analysis as covariates. Second, a conjunction analysis was performed to identify regions of grey matter loss that were common to the DLBD, AD and bvFTD patient groups with OS compared to controls. Age and gender were included in the analysis as covariates. Due to the exploratory nature of the analyses, results were assessed uncorrected for multiple comparisons at p<0.001.
Statistical analysis
Statistical analyses were performed using JMP statistical software (JMP, version 7.0.0; SAS Institute Inc. Cary, North Carolina) with statistical significance set at P<0.05. Sex ratios and binary variables collected were compared across groups and subgroups using the Chi squared test; Fisher's Exact test if there were cells with small numbers (<5). Age at onset of OS was compared across groups using analysis of variance. If significance was found, the Tukey-Kramer post hoc test was then used to compare all pairs of groups.
Results
We identified 105 patients who met our inclusion criteria for having OS. Examples of the type of delusions that the patients experienced are shown in Table 1. The mean age at onset of the delusion for all 105 patients was 68 years (25–94). Sixty five (61.9%) of all patients were male.
Table 1.
Examples of Othello's Syndrome (description by Physician)
| DLBD | He reports that he sees his wife having sex in a theatre, and hears his wife's voice moaning as though she is having an orgasm. |
| AD | He says the reason for his hunger strike is that his wife is having an affair with several other patients in the nursing home and that she entertains them in the lounge periodically. |
| FTD | She repeatedly accuses him of infidelity specifically with one woman, although he has not seen this person for well over 10 years. |
| Vascular dementia | She has delusions that he is having sexual relations with several women. The husband reports that they have daily arguments about her delusions. He said what precipitated the presentation to the Emergency Department today is that they were at home arguing about his infidelity when the wife picked up the phone, dialed 911, and when the person answered, she hung up on them. The husband has had erectile dysfunction for over 20 years |
| Methamphetamine use | She states, “When we are making love, he turns away and goes out to the living room and dances for her and then comes back.” When challenged whether the patient has actually seen the woman or talked to her she states, “No.” |
| Pramipexole induced in PD | The patient states that she has become so “scrutinizing” of his activities and her suspicions of his infidelity that she has begun to investigate financial records and tracking things on the Internet to prove his infidelity. |
| Right frontal trauma | He ruminates about his wife's infidelity. He states that his wife is self-centered and evil and that he doesn't deserve to be “in a throw away relationship.” He states that even though she assures him, he is skeptical because “she knows what I want to hear”. His wife reports was he pleasant at baseline which changed after his head trauma. |
Diagnoses
Seventy-three patients had OS associated with a neurological disorder (Table 2). Of the 56 patients with a neurodegenerative disorder, the majority (n=29) met criteria for one of the Lewy body diseases including DLBD in 20,(16, 21) PD in six and PDD in three patients. The onset of the delusion was associated with a dopamine agonist in six patients with PD. Of the 27 patients with a neurodegenerative diagnosis, 22 met criteria for AD(16) and 5 met criteria for bvFTD.(22)
Table 2.
Demographic and psychiatric features
| Psych | Drugs | Neurological | P values | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All NEUROL | Neurodegeneration | Lesion | Vascular | Psych vs. Drugs vs. All NEUROL | NDEG vs. lesion vs. vascular | LBD vs. AD vs. bvFTD | ||||||
| LBD | AD | bvFTD | All NDEG | |||||||||
| N | 27 | 5 | 73 | 29 | 22 | 5 | 56 | 8 | 8 | NA | NA | NA |
| Age at onset of Othello's (yrs.) | 52.1 (15.0) | 41.4 (16.3) | 71.2 (12.5) | 70.7 (10.6) | 75.6 (11.3) | 58.6 (14.9) | 71.5 (12.1) | 63.8 (17.0) | 77.5 (5.8) | <0.001 | 0.08 | 0.01 |
| Frequency of female gender | 10 (37%) | 2 (40%) | 28 (38%) | 7 (24%) | 12 (55%) | 3 (60%) | 22 (39%) | 2 (25%) | 3 (38%) | 0.92 | 0.41 | 0.09 |
| Frequency of hallucinations | 6 (22%) | 1 (20%) | 37 (50%) | 22 (76%) | 6 (27%) | 2 (40%) | 30 (54%) | 1 (13%) | 5 (63%) | 0.02 | 0.07 | 0.002 |
| Frequency of other delusions | 10 (37%) | 2 (40%) | 36 (49%) | 12 (41%) | 12 (55%) | 2 (40%) | 26 (46%) | 4 (50%) | 5 (63%) | 0.53 | 0.69 | 0.62 |
Data are shown as number (%) for categorical data, and mean (standard deviation) for age at onset of Othello's syndrome. AD = Alzheimer's disease, bvFTD = behavioral variant frontotemporal dementia; LBD = Lewy body disease; NEUROL = Neurological; NDEG = Neurodegenerative; Psych = Psychiatric
PD patients are included in the LBD including those with dopamine agonist associated Othello's syndrome
Of the eight patients with lesions, two had a right frontal meningioma, one had a left frontal stroke, one had bifrontal hemorrhagic contusions, one had right frontal encephalomalacia from trauma, two had right frontal strokes and one had a right frontal subdural hematoma. Head MRI were completed in six of these patients (Figure 1), with the remaining two patients having CT scans.
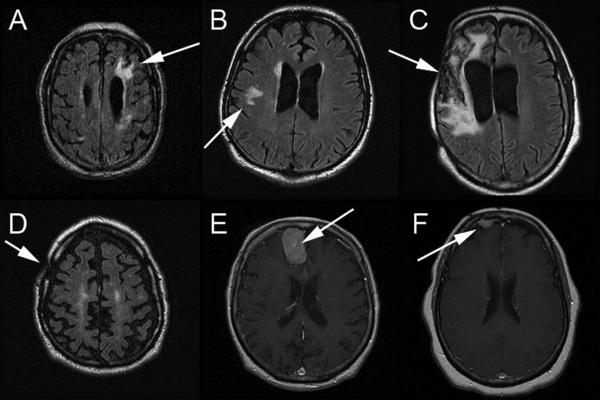
FIGURE 1.
MRI scans highlighting major abnormalities in six patients with OS resulting from lesions are summarized below. Fluid attenuated inversion recovery images are shown in A–D, and gadolinium enhanced T1 images are shown in E and F:
(A) Chronic infarct is in the left frontal lobe. Small cortical infarcts are present in the left frontal lobe. Thalamic chronic lacunar infarcts present.
(B) There is a white matter hyperintensity within both frontal lobes. There is a right internal capsule infarct.
(C) Encephalomalacia in the right frontal and temporal lobes, beneath the right craniectomy at the site of the prior subdural and intraparenchymal hemorrhages. There is associated ex vacuo dilatation of the right lateral ventricle with slight midline shift.
(D) Post-surgical changes in the right calvaria compatible with previous subdural hematoma evacuation.
(E) Meningioma within the right side of the interhemispheric fissure. Old lacunar infarcts. Old right occipital lobe infarct.
(F) Right frontal meningioma.
Thirty-two patients (Table 2) had non-neurological causes associated with OS. Fourteen patients were diagnosed with a delusional disorder not otherwise specified (NOS), six with major depressive disorder and two with a mood disorder NOS. There was one case each of anxiety disorder, major depression with psychosis, psychotic disorder, schizoaffective disorder and bipolar disorder type 2. Of those with drug induced Othello's, two patients used methamphetamine, one was started on valproic acid, and the other two were thought to be related to a combination of multiple drugs.
Other Delusions and Hallucinations
Forty-four patients in the series had hallucinations (Table 2). Four patients with DLBD had hallucinations specifically related to their delusions of infidelity e.g. visual hallucination of the patient's spouse engaging in an act of infidelity and to the patients this served as proof of the infidelity (Table 1). Forty-eight patients also had delusions other than OS (Table 2). One patient with AD had a delusion of reference.
Behavioral and Personality Changes
In the majority of patients, there were substantial changes in their behavior and personality (Table 3).
Table 3.
Examples of Behavioral and Personality Changes (description by physician)
| DLBD | The Patient became unpredictable with an increase in risk taking. He kicked his wife out of the house 3 weeks ago after he accused her of having an affair. He is consumed by sexual thoughts and has purchased a “penis pump” and several lubricants through television advertisements. |
| AD | The patient took her grandchild on a high speed chase with the police. Her driving privileges were revoked by the court, and she was ordered to move into a nursing facility. She had frequent physical altercations with other residents in the nursing home. She then bought a car against court order, and began making false accusations to the police that her brother was vandalizing her car. |
| FTD | The patient bought a gun and started toting it around with her. |
| Vascular dementia | She was also described as demanding, stubborn, refusing to eat, shower, or even use the bathroom. She had been urinating in a Tupperware bowl she kept under the bed. |
| Methamphetamine use | The patient was pounding on the wall in his room. He escalated to where he tore light fixtures and other built-ins off the walls and also punched a hole in the ceiling. |
| Pramipexole induced in PD | The patient has demanded sex many times a day. He has done this in the middle of the night, such as awakening at 3 in the morning and demanding sex. He also has developed compulsive shopping. |
| Right Frontal Trauma | He has become increasingly aware of his gray hair and started using her makeup to cover it up and will not look in the mirror because he hates his white hair. He has started talking more about sex and suddenly very concerned that he “cannot satisfy” his wife. His wife says that he never said sexually inappropriate comments until now. |
Comparisons of demographics and psychiatric features across groups
The 105 cases of OS were divided into neurological, psychiatric and medication induced delusional jealousy groups (Table 2).
The subjects with a neurological diagnosis were then further subdivided into those with a neurodegenerative diagnosis, those with vascular dementia and those with a lesion-specific diagnosis (Table 2). We also looked for differences across the different types of neurodegenerative disorders (Table 2). We also subdivided the 29 LBD patients into those with DLBD (n=20), those with PDD (n=3) and those with PD (n=6). There was a significant difference in age at onset of the delusional jealousy across these three LBD groups (p<0.0001), with the PD group being younger (54.3 ± 7.3) than the DLBD (74.6 ± 6.5) and PDD (77.3 ± 8.0) groups. There was no gender difference across these three groups. There was a significant difference in the frequency of hallucinations (p=0.01), which occurred most frequently in the DLBD group (DLBD=90% vs PDD=67% & PD=33%), but not in the frequency of other delusions across these three groups.
Treatment and responses
Table 4 shows the medication responses and dose ranges. In the medication subgroup, one patient had improvement after decreasing the dose of valproic acid that was recently started, and one subject had resolution of symptoms after stopping adderall (Amphetamine and Dextroamphetamine) and darvocet (Acetaminophen and Dextropropoxyphene). In the PD subgroup, 5/6 subjects developed OS secondary to pramipexole therapy. The average daily dose of pramipexole was 4.4mg daily when the delusions started in four of the patients. The delusions resolved when pramipexole was discontinued in each of these four patients. In the fifth patient, the patient's total daily dose of pramipexole was reduced to 6mg daily from an unspecified higher dose. The sixth patient was taking ropinirole and carbidopa/levodopa. Detailed information regarding these cases has been previously reported.(23)
Table 4.
Treatments for Othello's Syndrome
| Diagnoses | Medications associated with improvement in delusion range of daily dose in mg | Medications without improvement in delusion range of daily dose in mg | Medications started without follow-up available to document response |
|---|---|---|---|
| DLBD/PDD | Quetiapine (5) 25–137.5 | Quetiapine (6) 25–300 | Quetiapine (1) |
| Olanzapine (2) 2.5 | Mirtazapine (1) 15 | ||
| Clozapine (1) 12.5 | |||
| Risperidone (2) 1 | |||
| Olanzapine (1) 7.5 | |||
|
| |||
| AD | Olanzapine (2) 6–32.5 | Quetiapine (7) 50–800 | Quetiapine (3) |
| Quetiapine (2) 50–150 | Valproic Acid (1) 125 | ||
|
| |||
| VaD | None | Quetiapine (2) 12.5–25 | Olanzapine (1) |
| Olanzapine (1) 17.5 | Risperidone (1) | ||
| Quetiapine (1) | |||
| Citalopram (1) | |||
|
| |||
| Lesions | None | Quetiapine (3) 50–200 | Quetiapine (1) |
| Ziprasidone (1) 160 | Risperidone 1.5 | ||
|
| |||
| FTD | Risperidone (1) 1 | Quetiapine (1) 50 | |
| Olanzapine(1) 20 | |||
|
| |||
| Delusional Disorder | Risperidone (3) 1–4 | Risperidone (2) 0.5–1.5 | Aripiprazole (1) |
| Olanzapine (1) 5 | Quetiapine (2) 50 | Quetiapine (1) | |
| Quetiapine (1) 200 | |||
|
| |||
| Mood Disorder | Risperidone (1) 2 | Citalopram (1) 20 | Risperidone (1) |
| Buproprion (1) 300 | Mirtazipine (1) 1 | Depakote (1) | |
| Quetiapine (1) 50 | Aripiprazole (1) 20 | Tegretol (1) | |
| Lithium (1) 300 | Ziprasidone (1) dose unknown | ||
| Citalopram (1) 20 | |||
Number in parenthesis indicate the number of patients with response or not response to medication
MRI analysis
A total of 14 patients with a neurodegenerative disorder had a volumetric T1-weighted MRI (DLBD n=5, AD, n=6, bvFTD n=3). These patients were therefore matched by age and sex to 14 patients that had identical clinical diagnoses (DLBD n=5, AD, n=6, bvFTD n=3) but no delusions, and 14 healthy controls. The mean (standard deviation) age at MRI was 73.3 years (10.6) in the Othello's group, 73.1 years (10.8) in the matched neurodegenerative group, and 73.1 years (11.2) in controls, with 50% female in each group. Time from disease onset to scan was 5.1 years (4.0) in the Othello's group and 4.0 years (2.1) in the matched neurodegenerative group (p=0.35) while Short Test of Mental Status scores,(24) a tests of cognitive severity, was 22.9/38 (5.2) and 24.9/38 (7.4), respectively, (p=0.42). The average time from the onset of the Othello's delusion to the MRI was 1.2 (1.3) years (range 0–5 years).
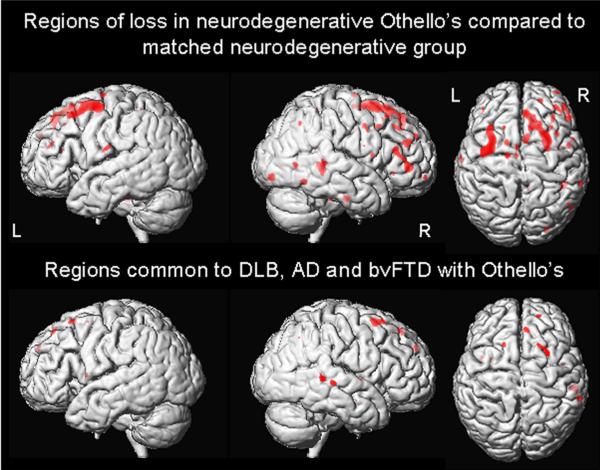
The neurodegenerative patients with OS showed greater grey matter loss predominantly in the dorsolateral frontal lobes, particularly in superior frontal gyri, and right posterior lateral temporal lobe compared to the matched neurodegenerative patients without delusions (Figure 2). No regions of grey matter loss showed greater involvement in the matched neurodegenerative patients compared to the neurodegenerative patients with OS. The only regions of loss that were found to be common to each of the different neurodegenerative groups (DLBD, AD, bvFTD) with OS were also found in the superior frontal lobe, particularly on the right, and the posterior temporal lobe (Figure 2).
FIGURE 2.
Three dimensional renderings showing VBM results. The top panel shows regions of greater grey matter loss in neurodegenerative patients with OS than matched neurodegenerative patients without delusions. The bottom panel shows the regions of grey matter loss that were common to each of the different neurodegenerative disorders (AD, bvFTD and DLBD) with OS when compared to healthy controls.
Discussion
The main finding in this study is that OS is found in a wide array of disorders commonly related to frontal lobe dysfunction with seven of the eight focal lesion patients showing right frontal lobe damage. In addition, VBM showed that the neurodegenerative cases with OS had greater grey matter loss in the dorsolateral frontal lobes, compared to neurodegenerative patients without Othello's.
Previous smaller studies have linked OS to right hemisphere events such as strokes.(9, 11, 12) In addition OS has been associated with right frontal lobe hypometabolism on single photon emission computer tomography in one study,(9) and has also occurred after right orbitofrontal resection for a meningioma.(10) Our study also found an association between the development of OS and frontal lobe, in particular right frontal lobe, dysfunction. Specifically frontal lobe lesions were a common theme in our lesion associated cases, and were independently identified in the neurodegenerative patients on VBM. One hypothesis linking right frontal lesions to delusions suggests that the loss of frontal lobe function impairs the ability to monitor interactions with oneself and with others leading to false beliefs that persist despite being incorrect. Impairments in monitoring reality, memory and familiarity due to a damaged right hemisphere may lead to the unopposed left hemisphere categorizing without emotional familiarity resulting in false explanations.(25) There were also other reports that implicated the right hemisphere and/or right frontal lobe dysfunction to be associated with delusions in general. For example, in 1984, Levine and Grek described nine patients with right sided strokes who developed frequent delusions. These patients had strokes with underlying generalized atrophy.(26) Using functional imaging, Staff et al. also demonstrated right frontal lobe hypoperfusion in Alzheimer's patients with delusions compared to Alzheimer's patients without delsusions.(27)
Although there is strong evidence that links OS and delusions in general, to the right frontal lobe, delusional jealousy has also been associated with lesions in other regions of the brain. Indeed, in our study one patient developed OS after a left frontal stroke. Similarly, Silva and Leong describe delusional jealousy in a patient after left frontal stroke.(13) A case of OS has also been attributed to a right-sided thalamic infarct, although this patient had 3 prior right hemispheric infarcts leaving him with a left hemiparesis.(14)
Another important finding in this paper is the fact that six PD subjects without dementia developed OS after starting or increasing the dose of dopamine agonist. In support of the dopamine agonist as the cause of their delusional jealousy, the delusions stopped or significantly improved, when the medication was discontinued or the dose was decreased. OS has been described in a handful of cases after other dopamine therapy. Cannas et al. describe six young male PD patients without dementia who developed OS from dopamine therapy.(28) Two of these patients were on pramipexole, while the others were on pergolide, ropinirole, levodopa and amantadine, levodopa and pergolide respectively.
All patients had a reduction or disappearance of jealousy after decreasing medication doses and adding an antipsychotic. Amantadine has previously been reported as a cause of OS.(4) Dopamine appears to play an important role in producing OS. Interestingly, in our study, patients on pramipexole, adderall (a racemic mixture of amphetamine and dextroamphetamine), or methamphetamine also developed OS. All of these drugs affect dopamine. Methamphetamine is an analog of amphetamine which enters nerve terminals through the dopamine reuptake transporter system and changes the pH of the terminal to release dopamine from its vesicles causing dopamine to enter the synapse.(29) Pramipexole is a dopamine agonist acting preferentially at the D3 receptor which is located in the frontal cortex, midbrain and limbic cortex.(30)–(31) In our series, and the previously reported cases, three agonists (pramipexole, ropinirole, and pergolide account for the vast majority of OS with dopamine therapy in PD. Interestingly, all three have disproportionate affinity for the D3 receptor with pramipexole not surprisingly, having the highest affinity(32–34) and hence accounting for the most cases in our series. Using PET imaging in primates, Black et al. demonstrated that pramipexole produced decreased cerebral blood flow in the bilateral orbitofrontal cortex, with greater decreases observed in the right hemisphere.(35) A subject in our cohort developed OS after increasing the dose of valproic acid which resolved after decreasing the dose. Valproic acid has been shown to increase dopamine release in rat medial prefrontal cortex.(36)
It should be noted that many individuals who take dopamine therapy with similar conditions never develop delusions and dopamine therapy has been linked to other delusions as well.
Antipsychotics were used with varying success to treat delusions in the neurodegenerative subgroups. These medications were unsuccessful in improving delusions in the vascular dementia or the lesion groups. The success of treatment was not dose dependent. Therefore, if someone does not respond to antipsychotic treatment, raising the dose and risking more side-effects is not recommended.
Our large number of patients allowed us to investigate clinical and demographic features of patients with OS. We found that 61.9% of the OS patients were male suggesting a slight male predominance associated with the syndrome. Unfortunately, the existing demographic data for OS is sparse. One previous report also found that OS occurs more frequently in males,(37) although another study found that women outnumbered men two to one in a group of psychiatric patients with delusional jealousy.(38)
We also found that OS occurred most commonly in patients with a neurological disorder (69.5%) and that the patients with neurological and psychiatric disorders were significantly older than those in the medication group. Although this latter finding could be due to a bias, since patients that are younger may be more likely to abuse drugs, the data suggests that OS is not an age dependent phenomenon. We also found that neurodegenerative disorders accounted for the majority of those with a neurological diagnosis (76.7%), in which DLBD was the most frequent. The DLBD group also had the highest frequency of hallucinations. It is perhaps not surprising then that we found visual hallucinations to be more commonly associated with OS in the context of a neurological disorder. In fact, the frequency of hallucinations in the LBD group was 76% which is significantly higher than the typically reported 40%.(15) Perhaps, the higher than anticipated number of DLBD subjects with OS and hallucinations is related to the fact that four patients in our study had hallucinations specific to spousal infidelity. This association has been reported elsewhere. Sibisi reports that two LBD patients admitted to a psychiatric ward had hallucinations of sexual infidelity of the spouse resulting in delusional jealousy.(5)
This study has a few limitations that show be mentioned. Firstly, premorbid personality may play a role in disease manifestation and the patients' families were not routinely asked about the patient's premorbid personality. Secondly, patients with sexual disorders such as impotence may be more likely to develop jealousy. Screening for these sexual disorders was not performed. Finally, neuropsychological data was not routinely performed in our patients.
Acknowledgments
Sources of Funding: KAJ is funded by the NIH grant R01 DC010367 (PI), the Dana Foundation (PI) and the Morris K. Udall PD Research Center of Excellence NIH/NINDS P50 NS40256 (Co-investigator).
Dr. Whitwell receives research support from the Dana Foundation (Co-I), R01-DC010367 (Co-I), and NIH [R01-AG11378].
Dr. Geda receives research support from the NIH grant U01 AG03949 (Co-I).
Footnotes
Conflicts of Interest: None
References
- 1.Cummings JL. Organic delusions: phenomenology, anatomical correlations, and review. Br J Psychiatry. 1985 Feb;146:184–97. doi: 10.1192/bjp.146.2.184. [DOI] [PubMed] [Google Scholar]
- 2.Hassanyeh F, Murray RB, Rodgers H. Adrenocortical suppression presenting with agitated depression, morbid jealousy, and a dementia-like state. Br J Psychiatry. 1991 Dec;159:870–2. doi: 10.1192/bjp.159.6.870. [DOI] [PubMed] [Google Scholar]
- 3.Hodgson RE, Murray D, Woods MR. Othello's syndrome and hyperthyroidism. J Nerv Ment Dis. 1992 Oct;180(10):663–4. doi: 10.1097/00005053-199210000-00010. [DOI] [PubMed] [Google Scholar]
- 4.McNamara P, Durso R. Reversible pathologic jealousy (Othello syndrome) associated with amantadine. J Geriatr Psychiatry Neurol. 1991 Jul–Sep;4(3):157–9. doi: 10.1177/089198879100400306. [DOI] [PubMed] [Google Scholar]
- 5.Sibisi CD. The phenomenology of delusional jealousy in late life. Int J Geriatr Psychiatry. 1999 May;14(5):398–9. [PubMed] [Google Scholar]
- 6.Soyka M, Naber G, Volcker A. Prevalence of delusional jealousy in different psychiatric disorders. An analysis of 93 cases. Br J Psychiatry. 1991 Apr;158:549–53. doi: 10.1192/bjp.158.4.549. [DOI] [PubMed] [Google Scholar]
- 7.Yusim A, Anbarasan D, Bernstein C, Boksay I, Dulchin M, Lindenmayer JP, et al. Normal pressure hydrocephalus presenting as Othello syndrome: case presentation and review of the literature. Am J Psychiatry. 2008 Sep;165(9):1119–25. doi: 10.1176/appi.ajp.2008.07111820. [DOI] [PubMed] [Google Scholar]
- 8.Shepard M. Morbid Jealousy: some clinical and social aspects of a psychiatric syndrome. Journal of Mental Science. 1961;107:687–753. [Google Scholar]
- 9.Luaute JP, Saladini O, Luaute J. Neuroimaging correlates of chronic delusional jealousy after right cerebral infarction. J Neuropsychiatry Clin Neurosci. 2008 Spring;20(2):245–7. doi: 10.1176/jnp.2008.20.2.245. [DOI] [PubMed] [Google Scholar]
- 10.Narumoto J, Nakamura K, Kitabayashi Y, Fukui K. Othello syndrome secondary to right orbitofrontal lobe excision. J Neuropsychiatry Clin Neurosci. 2006 Fall;18(4):560–1. doi: 10.1176/jnp.2006.18.4.560a. [DOI] [PubMed] [Google Scholar]
- 11.Richardson ED, Malloy PF, Grace J. Othello syndrome secondary to right cerebrovascular infarction. J Geriatr Psychiatry Neurol. 1991 Jul–Sep;4(3):160–5. doi: 10.1177/089198879100400307. [DOI] [PubMed] [Google Scholar]
- 12.Westlake RJ, Weeks SM. Pathological jealousy appearing after cerebrovascular infarction in a 25-year-old woman. Aust N Z J Psychiatry. 1999 Feb;33(1):105–7. doi: 10.1046/j.1440-1614.1999.00530.x. [DOI] [PubMed] [Google Scholar]
- 13.Silva JA, Leong GB. A case of organic Othello syndrome. J Clin Psychiatry. 1993 Jul;54(7):277. [PubMed] [Google Scholar]
- 14.Soyka M. Delusional jealousy and localized cerebral pathology. J Neuropsychiatry Clin Neurosci. 1998 Fall;10(4):472. doi: 10.1176/jnp.10.4.472. [DOI] [PubMed] [Google Scholar]
- 15.McKeith IG, Perry RH, Fairbairn AF, Jabeen S, Perry EK. Operational criteria for senile dementia of Lewy body type (SDLT) Psychol Med. 1992 Nov;22(4):911–22. doi: 10.1017/s0033291700038484. [DOI] [PubMed] [Google Scholar]
- 16.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984 Jul;34(7):939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 17.Ashburner J, Friston KJ. Voxel-based morphometry--the methods. NeuroImage. 2000 Jun;11(6 Pt 1):805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 18.Whitwell JL, Jack CR, Jr., Baker M, Rademakers R, Adamson J, Boeve BF, et al. Voxel-based morphometry in frontotemporal lobar degeneration with ubiquitin-positive inclusions with and without progranulin mutations. Archives of neurology. 2007 Mar;64(3):371–6. doi: 10.1001/archneur.64.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994 Dec;44(12):2308–14. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 20.Kokmen E, Naessens JM, Offord KP. A short test of mental status: description and preliminary results. Mayo Clinic proceedings. 1987;62:281–8. doi: 10.1016/s0025-6196(12)61905-3. [DOI] [PubMed] [Google Scholar]
- 21.McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005 Dec 27;65(12):1863–72. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 22.Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998 Dec;51(6):1546–54. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 23.Graff-Radford J, Ahlskog JE, Bower JH, Josephs KA. Dopamine agonists and Othello's syndrome. Parkinsonism Relat Disord. Dec;16(10):680–2. doi: 10.1016/j.parkreldis.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kokmen E, Naessens JM, Offord KP. A short test of mental status: description and preliminary results. Mayo Clinic proceedings. 1987 Apr;62(4):281–8. doi: 10.1016/s0025-6196(12)61905-3. [DOI] [PubMed] [Google Scholar]
- 25.Devinsky O. Delusional misidentifications and duplications: right brain lesions, left brain delusions. Neurology. 2009 Jan 6;72(1):80–7. doi: 10.1212/01.wnl.0000338625.47892.74. [DOI] [PubMed] [Google Scholar]
- 26.Levine DN, Grek A. The anatomic basis of delusions after right cerebral infarction. Neurology. 1984 May;34(5):577–82. doi: 10.1212/wnl.34.5.577. [DOI] [PubMed] [Google Scholar]
- 27.Staff RT, Shanks MF, Macintosh L, Pestell SJ, Gemmell HG, Venneri A. Delusions in Alzheimer's disease: spet evidence of right hemispheric dysfunction. Cortex. 1999 Sep;35(4):549–60. doi: 10.1016/s0010-9452(08)70818-9. [DOI] [PubMed] [Google Scholar]
- 28.Cannas A, Solla P, Floris G, Tacconi P, Marrosu F, Marrosu MG. Othello syndrome in Parkinson disease patients without dementia. Neurologist. 2009 Jan;15(1):34–6. doi: 10.1097/NRL.0b013e3181883dd4. [DOI] [PubMed] [Google Scholar]
- 29.Elkashef A, Vocci F, Hanson G, White J, Wickes W, Tiihonen J. Pharmacotherapy of methamphetamine addiction: an update. Subst Abus. 2008;29(3):31–49. doi: 10.1080/08897070802218554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aminoff MJ. Pharmacological Management of Parkinsonism and Other Movement Disorders. In: Katzung B, editor. Basic and Clinical Pharmacology. Ninth ed Mcgraw Hill; New York: 2004. pp. 447–61. [Google Scholar]
- 31.Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990 Sep 13;347(6289):146–51. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- 32.Perachon S, Schwartz JC, Sokoloff P. Functional potencies of new antiparkinsonian drugs at recombinant human dopamine D1, D2 and D3 receptors. Eur J Pharmacol. 1999 Feb 5;366(2–3):293–300. doi: 10.1016/s0014-2999(98)00896-6. [DOI] [PubMed] [Google Scholar]
- 33.Gerlach M, Double K, Arzberger T, Leblhuber F, Tatschner T, Riederer P. Dopamine receptor agonists in current clinical use: comparative dopamine receptor binding profiles defined in the human striatum. J Neural Transm. 2003 Oct;110(10):1119–27. doi: 10.1007/s00702-003-0027-5. [DOI] [PubMed] [Google Scholar]
- 34.Levant B. The D3 dopamine receptor: neurobiology and potential clinical relevance. Pharmacol Rev. 1997 Sep;49(3):231–52. [PubMed] [Google Scholar]
- 35.Black KJ, Hershey T, Koller JM, Videen TO, Mintun MA, Price JL, et al. A possible substrate for dopamine-related changes in mood and behavior: prefrontal and limbic effects of a D3-preferring dopamine agonist. Proc Natl Acad Sci U S A. 2002 Dec 24;99(26):17113–8. doi: 10.1073/pnas.012260599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ichikawa J, Meltzer HY. Valproate and carbamazepine increase prefrontal dopamine release by 5-HT1A receptor activation. Eur J Pharmacol. 1999 Sep 3;380(1):R1–3. doi: 10.1016/s0014-2999(99)00517-8. [DOI] [PubMed] [Google Scholar]
- 37.Munro A. Excellent response of pathologic jealousy to pimozide. Can Med Assoc J. 1984 Oct 15;131(8):852–3. [PMC free article] [PubMed] [Google Scholar]
- 38.Crowe RR, Clarkson C, Tsai M, Wilson R. Delusional disorder: jealous and nonjealous types. Eur Arch Psychiatry Neurol Sci. 1988;237(3):179–83. doi: 10.1007/BF00451287. [DOI] [PubMed] [Google Scholar]