Abstract
The incidence of human urinary bladder cancer increases markedly with age, suggesting a mechanistic connection between aging and bladder carcinogenesis and a potential use of anti-aging agents in bladder cancer chemoprevention. Rhodiola rosea, growing in high-altitude or cold regions of the world, has been reported to have anti-aging effects in Drosophila. We demonstrated that a R. rosea extract and one of its bioactive components, salidroside, inhibited the growth of bladder cancer cell lines with a minimal effect on non-malignant bladder epithelial cells TEU-2. Interestingly, the R. rosea extract and salidroside component exhibited a selective ability to inhibit the growth of p53 knockout primary mouse embryo fibroblasts (p53−/− MEFs) compared to their wild type counterparts. The growth inhibitory effects of the R. rosea extract and salidroside were, however, attenuated in TSC2 and p53 double knock MEFs (TSC2−/−, p53−/− MEFs), suggesting that TSC2 protein is, at least in part, required for the growth inhibitory effects of the R. rosea extract and salidroside. The R. rosea extract and salidroside treatment of UMUC3 cells resulted in an increase of AMP-activated protein kinase (AMPK)-α phosphorylation and a decrease of 4EBP1 phosphorylation, leading to increased binding of 4EBP1 to m7GTP. These results indicate that the R. rosea extract and salidroside inhibit translation initiation. Furthermore, both the R. rosea extract and salidroside treatment of UMUC3 cells caused a significant percentage of cells undergoing autophagy. Therefore, the R. rosea extract and salidroside deserve further study as novel agents for chemoprevention of bladder carcinogenesis.
Keywords: Rhodiola rosea extracts, Salidroside, TSC2, Autophagy, Translation initiation, p53 deficiency, bladder cancer
Introduction
Bladder cancer arises most commonly in the elderly with over 71% of first diagnoses and 85.5% of deaths occurring in patients older than 65 years old [1, 2]. Although cigarette smoking and occupational exposure to aromatic amines are known risk factors for bladder cancer [1, 2], many bladder cancer cases occur with no apparent exposure to these carcinogens [3], suggesting that aging or other factors also play an important role. Therefore, anti-aging may represent a unique approach to delay or prevent the onset of bladder cancer in the elderly.
So far, long-term calorie restriction (CR) without malnutrition and inhibition of the activity of nutrient-sensing pathways (insulin/IGF-1/mTOR signaling pathways) by mutations or chemical inhibitors consistently and robustly delay aging and extends life span in diverse species [4-8]. In addition, both CR and decreased nutrient-sensing pathway activity can delay the onset of age-related diseases, including diabetes, cancer, cardiovascular disease and brain atrophy [4-8]. In a 20-year longitudinal study of adult-onset CR in rhesus monkeys, Colman et al. [4] demonstrated that moderate CR reduced the incidence of cancer by 50%. However, given severe CR can lead to several harmful health effects such as amenorrhea, infertility, sarcopenia, osteoporosis, and immune deficiencies, CR is not likely a practical option for cancer prevention in an extended period. Alternatively, agents that target nutrient-sensing pathways, such as the mTOR pathway, could potentially mimic the benefit of CR for cancer prevention [9]. CR is critically involved in the mTOR pathway. Since the mTOR pathway is one of the most commonly deregulated pathways in human urinary bladder cancer through Ras oncogene activation or via gene mutations in PIK3CA, TSC1/2, and PTEN [10-14], agents that have anti-aging properties by targeting the mTOR pathway could be ideal candidates for bladder cancer chemoprevention.
Rhodiola rosea L, also known as “golden root”, is a perennial herbaceous plant of the Crassulaceae family, widely distributed at high altitudes (up to 2280 m) in the arctic and mountainous regions throughout Europe and Asia [15]. R. rosea extracts are commercially available as nutrient supplements and primarily used to reduce the effect of fatigue on physical and mental performance [15]. The R. rosea extract SHR-5 is the only one that has passed extensive toxicological studies and has been certified safe for both animals and humans [15, 16]. Rhodiola has a history of centuries of folk use and has been the subject of many clinical studies. No side effects or drug interactions have been reported [15, 17]. In a screen for anti-aging compounds using Drosophila melanogaster model systems, a R. rosea extract was found to significantly increase both mean and maximum life span of the fly [18]. A further study using SHR-5 with increased contents of its bioactive compounds, salidroside and/or rosavin, resulted in an increased efficacy of the R. rosea extract in extending mean and maximum life span of the fly [19]. Consistent with the above report, a R. rosea extract was also shown to significantly increase mean and maximum life span of C. elegans [20]. These results indicated the anti-aging property of R. rosea extracts and its potential usefulness in maintaining and promoting general health of the elderly.
There are a few studies demonstrating the anticancer activities of R. rosea extracts against liver tumors, breast cancer cells, and others [21, 22]. Anecdotal evidence from a study in Russia involving 12 patients with superficial bladder cancer showed that a R. rosea extract decreased the average frequency of cancer relapse by half [23]. However, mechanisms of R. rosea extracts’ anticancer action remain little known, and none of these studies has linked the anticancer effects of R. rosea extracts to its anti-aging property.
In this study, we examined the effect of the R. rosea extract, known as SHR-5, and one of its chemical component salidroside on the growth of cancer cell lines derived from different stages of human urinary bladder cancer and on the mTOR pathway, a conserved longevity pathway. We found that SHR-5 and salidroside preferentially inhibited the growth of bladder cancer cell lines and p53 deficient cells with minimal effects on non-malignant cells. The growth inhibitory effect of SHR-5 and salidroside on p53 deficient fibroblasts requires, at least in part, the existence of TSC2 expression. In addition, we showed that SHR-5 and salidroside activated AMPK and decreased the phosphorylation levels of S6 and 4E-BP1, leading to inhibition of translational initiation and induction of autophagy. These results suggested a novel mechanistic link between the anti-aging and anti-cancer effects of SHR-5 and calls for further studies for the potential usefulness of SHR-5 or bioactive components of SHR-5 in bladder cancer prevention.
Materials and methods
Cell lines, compounds and reagents
The RT4, T24, UMUC3, 5637, and J82 cell lines were obtained from American Type Culture Collection (Manassas, VA). RT4 and T24 cells were maintained in McCoy’s 5A medium containing 10% fetal bovine serum (FBS). UMUC3 cells were cultured in EMEM medium with 10% FBS. 5637 and J82 cells were cultured in RPMI 1640 medium with 10%FBS. Normal bladder epithelial cells immortalized by viral proteins E6/E7 (TEU-2) were obtained from Dr. David J. Klumpp (Northwestern University Medical School, Chicago, Illinosis). p53 +/+, p53−/−, p53−/−TSC2−/−, p53−/−TSC2+/+, TSC1−/− and TSC1+/+ mouse embryonic fibroblasts (MEFs) were generous gifts from David Kwiatkowski (Brigham Women’s hospital) and MEFs were maintained in DMEM supplemented with 10% FBS. The R. rosea extract (SHR-5) was obtained from the Swedish Herbal Institute (Göteborg, Sweden) and dissolved in DMSO, aliquoted, and stored at −80°C. The DMSO in culture medium never exceeded 0.1% (v/v), a concentration known not to affect cell proliferation. Pure salidroside, rosin, rosavin, and rosarin (99%) were obtained from Chromadex (Irvine, CA). The pEGFP-LC3, PcDNA3-TSC1, and PcDNA3-TSC2 constructs were purchased from Addgene (Cambridge, MA). Antibodies for phospho-AMPK α, AMPK α, 4E-BP1, phospho-4E-BP1, TSC1, TSC2, eIF4G, eIF4E, phosphorylated p70S6K (Thr389), p70S6K, phosphorylated rpS6 (Ser240/244), rpS6, p62, ACC and phospho-ACC were from Cell Signaling Technology, Inc. (Beverly, MA). Beta-actin antibody, protein A/G-plus agarose and protein A-plus agarose beads were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). m7βGTP Sepharose and ECL detection system were from Amersham Biosciences (Arlington Heights, IL). Thymidine, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) was obtained from Sigma (Saint Louis, MO). RNAazol B was purchased from Tel-Test (Friendswood, TX.). The Reverse Transcription System kit was from Promega (Mandison, WI).
MTT assay [24]
TEU-2, RT4, T24, UMUC3, 5637, J82, and p53 KO and wild-type HCT116 cells, as well as p53 +/+, p53−/−, p53−/−TSC2−/−, p53−/−TSC2+/+, TSC1−/− and TSC1+/+ MEFs were plated at a density of 2 × 105 per well in 24-well culture plates in medium containing 10% FBS. After 24 hours, the medium was refreshed with fresh medium and left untreated or was treated as indicated in the figures or table legends. After treatment, MTT was added to the wells at a final concentration of 1 mg/mL and incubated at 37°C for 3 hours. The absorbance was determined at 570 nm.
Colony Formation Assay [25]
UMUC-3 cells were seeded in top agar containing 0.35% agar with EMEM and 10% FBS. Bottom agar is consisted of 0.8% agar, EMEM and 10% FBS. Cultures were maintained under standard culture conditions. Media with 0.1% DMSO or indicated doses of SHR-5 or salidroside was added and replaced every 3 days. After 3 weeks, the number of colonies was determined with an inverted phase-contrast microscope at ×100 magnification where a group of >10 cells was counted as a colony. Crystal violet (0.1%) in methanol was used to stain the 6-well plates. After washing in PBS, colonies were photographed.
Western blot analysis
Cells were treated with 0.1% DMSO, SHR-5, or salidroside under each experimental condition. After treatment, clarified protein lysates (20-80 μg) were denatured at 95 °C for 5 min and resolved by 8-16% SDS-PAGE. Proteins were transferred to nitrocellulose membranes, and probed with antibodies and visualized by an enhanced chemiluminescence detection system.
7-Methyl-Guanosine Cap Binding Assay
Total of 1 mg of cellular proteins in lysis buffer (20mM Tris-HCl, pH 7.5, 150mM NaCl, 1mM EDTA, 1mM EGTA, 1% Triton, 2.5mM sodium pyrophosphate, 1mM β-glycerophosphate, 1mM Na3VO4, 1μg/ml leupeptin) was mixed with 50μl of 7-methyl-GTP-Sepharose-4B bead suspension (Amersham Biosciences) and incubated overnight. After washing the pellet, the affinity complex was recovered and boiled with SDS sample buffer. The supernatant was recovered after centrifugation and subjected to SDS-PAGE. The amount of 4E-BP1 or eIF4E in the bound fraction was detected by Western blotting using specific antibodies.
Stable transfection [25]
UMUC-3 or J82 cells were plated at 1 × 106 per 100-mm dish. At 60% confluency, cultures were transfected with PCDNA3.1, pEGFP-LC3, or TSC1 using FuGENE 6 (Roche, Indianapolis, IN). Transfected cells were selected with G418 (800 μg/mL) starting at 48 hours after transfection, and all of the stable transfectants were pooled to avoid cloning artifacts. Pooled stable clones of UMUC-3 or J82 cells expressing pEGFP-LC3, TSC1 or PCDNA3.1 were maintained in RPMI containing 10% FBS and 500 μg/mL G418.
Fluorescence microscopy
UMUC-3/ pEGFP-LC3 cells were cultured in chamber slides (Lab-Tek). After treatment with different concentrations of SHR-5, salidorside and chloroquine, cells were fixed in 4% paraformaldehyde solution for 20 min and methanol for 2 min. Slides were mounted in Vectashield (Vector Laboratories Inc.). Immunostaining was analyzed with a Nikon Eclipse TE2000-S fluorescent microscope (magnification, 200×) using the 488-excitation wavelength. For quantification of autophagic cells, cells with >10 GFP-LC3 punctuate dots were considered positive. Data obtained from counting triplicates of 100 cells was average (mean ± SD).
Electron microscopy
After treatments, cell pellets were collected, fixed with 1.6% glutaraldehyde, post-fixed in 1% OsO4, dehydrated in alcohol series, and embedded in epoxy resin. Thin sections were contrasted with uranyl acetate and lead citrate. Preparations were observed either with a Philips CM12 electron microscope operating at 80 kV (FEI) or with a Jeol 1400 mounted with CCD cameras (Morada, Olympus SIS). Percentage of cell with autophagosomes obtained from counting triplicates of 30 cells was average (mean ± SD).
Statistics
Microsoft Excel software was used to calculate the mean and the standard error of the mean. Comparisons of cell viabilities, number of colony formation in soft agar and number of positive cells with LC3-GFP puncta between treatment and control were conducted using analysis of variance (ANOVA) or Student’s t test. All statistical tests were two sided. P values <0.05 were considered significant.
Results
SHR-5 inhibits anchorage-dependent and –independent growth of bladder cancer cell lines with minimal effect on the growth of non- malignant bladder epithelial cells
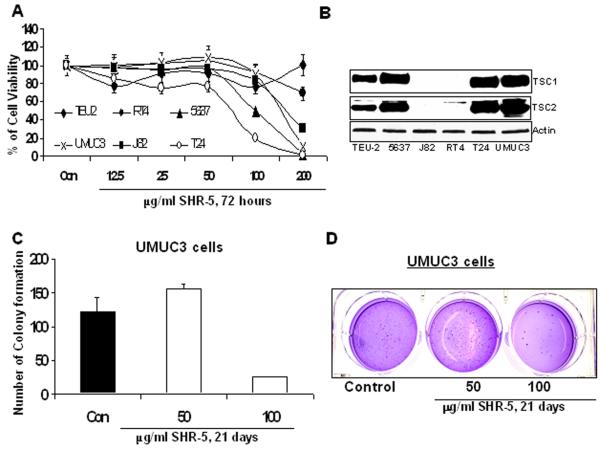
SHR-5 is a standardized Rhodiola R. extract made by the Swedish Herbal Institute (the SHI, Gothenburg, Sweden) according to Good Manufacturing Practice [15]. The bioactive components of SHR-5 have been thought to be the rosavins (rosin, rosavin and rosarin) and salidroside. SHR-5 is reported to contain a 1.7% of salidroside and a 4.5% of total rosavins [19]. To examine the potential anti-bladder cancer effects of SHR-5, human papillary (RT4) and muscle-invasive (T24, UMUC3, J82 and 5637) bladder cancer cell lines, as well as a non-malignant human bladder epithelial TEU-2 cell line were treated with different doses of SHR-5. Cell viabilities were measured by a MTT assay. Percentage of cell viabilities relative to their vehicle controls for different treatments was calculated. Figure 1A shows that SHR-5 differentially inhibits the growth of tested cell lines. IC50s in RT4, J82, T24, UMUC3, and 5637 cell lines are estimated to be 264,165, 71, 100 and 151μg/ml, respectively, whereas the same doses of SHR-5 treatment doesn’t significantly inhibits the growth of TEU-2 cells. In addition, J82 and RT4 cells are less sensitive to SHR-5 treatment compared to other bladder cancer cell lines (T24, UMUC3, and 5637) (Figure 1A). Figure 1B shows that J82 and RT4 cells have no, or minimal, expression of TSC1 and TSC2 proteins. In addition, RT4 cells have wild-type p53 [25]. T24 and UMUC-3 cells harbor TP53 mutations in the NH2-terminal transactivation domain [25]. 5637 and J82 cells contain TP53 mutations in the core domain [25]. Taken together, it appears that the growth inhibitory effect of SHR-5 on bladder cancer cell lines may be related to both p53 status and TSC1/2 expression (Table1).
Figure 1.
The effect of SHR-5 on anchorage-dependent growth of malignant and non-malignant bladder epithelial cell lines and anchorage-independent growth of UMUC-3 cells. A: TEU-2, RT4, T24, UMUC3, 5637 and J82 cells were treated with vehicle control (0.1% DMSO), different doses of SHR-5 as indicated. After 72 hours of treatments, cell viabilities were measured by a MTT assay. The growth inhibitory effects of these treatments are expressed as percentage of cell viabilities relative to their vehicle controls. Points, mean of four independent plates; bars, SE. B: Western blotting analysis of TSC1 and TSC2 protein expression. A representative of three independent experiments. C&D: A soft agar colony formation assay with indicted treatments of UMUC-3 cells was performed using 6-well plates. The data are presented by bar figures and mean ±SE of four independent wells at an optimum time of 21 days post cell seeding; Pictures are qualitative analysis of colony formation.
Table1.
The IC50s of SHR-5 and tumor suppressor gene status in bladder cancer cell lines
| Cell lines | RT4 | UMUC- 3 |
T24 | 5637 | J82 |
|---|---|---|---|---|---|
| Estimated IC50 (μg/ml) | 264 | 100 | 71 | 151 | 165 |
| P53 | WT | MT/ Exon4 |
MT/ Exon5 |
MT/ Exon8 |
MT/ Exon8 |
| TSC1 | HD | WT | WT | WT | HD |
| TSC2 expression | weak | strong | strong | strong | No detectable |
P53: WT= wild-type; MT = mutant allele. Tumor suppressor TSC1: WT = wild-type allele; HD = homozygous deletion.
Figure 1C &D shows that compared to control, treatment of UMUC-3 cells with 100 μg/mL SHR-5 resulted in 79% inhibition of colony formation in soft agar (P <0.01, Student’s t test). Since anchorage-independent growth predicts in vivo tumor growth of cancer cell lines, this result may suggest a potential in vivo anti-tumor effect of SHR-5.
Salidroside, an bioactive component of SHR-5, similarly inhibits anchorage-dependent and –independent growth of bladder cancer cell lines with minimal effect on the growth of non- malignant bladder epithelial cells
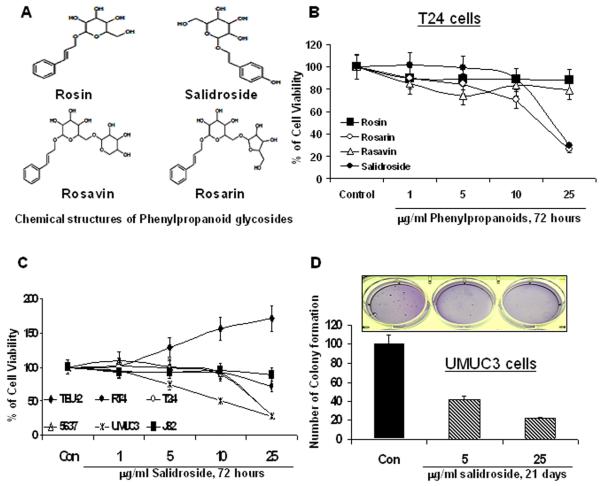
The bioactive components (rosin, rosavin, rosarin and salidroside) of SHR-5 belong to the family of phenylpropanoid glycosides [15] (Figure 2A). We next examined the effect of rosin, rosavin, rosarin and salidroside on the growth of bladder cancer T24 cells. Figure 2B shows that salidroside and rosarin are the most effective agents among tested compounds in inhibition of T24 cell growth. Given salidroside is a major compound for standardizing SHR-5 [15], we chose salidroside for our further study in comparison with SHR-5. Figure 2C shows that salidroside inhibits the growth of bladder cancer cell lines in a similar pattern to that of SHR-5. RT4 and J82 cells that express no or minimal TSC1/2 are relatively resistant to the growth inhibitory effect of salidroside compared to other bladder cancer cell lines (T24, UMUC3, and 5637) expressing high levels of TSC1/2 proteins. Salidroside also exhibits no inhibitory effect on the growth of TEU-2 cells. In addition, 5 and 25 μg/ml Salidroside inhibits the anchorage-independent growth of UMUC-3 cells by 58 and 78%, respectively (ANOVA, P<0.01, Figure 2D and insert).
Figure 2.
The growth inhibitory effect of phenylpropanoids on malignant and non-malignant bladder epithelial cell lines. A: Chemical structures of phenylpropanoids in the R. rosea extract SHR-5. B: T24 cells were treated with vehicle control (0.1% DMSO), different doses of salidroside, rosavin, rosarin and rosin as indicated. After 72 hours of treatments, cell viabilities were measured by a MTT assay. Points, mean of four independent plates; bars, SE. C: TEU-2, RT4, T24, UMUC3, 5637 and J82 cells were treated with vehicle control (0.1% DMSO), different doses of salidroside as indicated. After 72 hours of treatments, cell viabilities were measured by a MTT assay. Points, mean of four independent plates; bars, SE. D: A soft agar colony formation assay with indicted treatments of UMUC-3 cells was performed using 6-well plates. The data are presented by bar figures and mean ±SE of four independent wells at an optimum time of 21 days post cell seeding; Pictures are qualitative analysis of colony formation.
SHR-5 selectively inhibits the growth of MEF p53−/− cells and this inhibitory effect of SHR-5, at least in part, requires the existence of TSC2
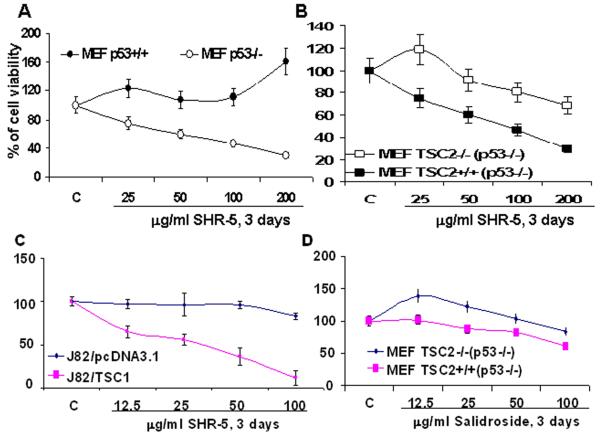
P53 alterations in bladder cancer plays a major role in disease progression [26] and the mTOR pathway is deregulated in cells with loss of p53 function [27]. We therefore examined whether SHR-5 can selectively inhibit the growth of p53 defective cells by targeting the mTOR pathway. Figure 3A shows that treatment of MEF p53−/− cells with SHR-5 caused a dose–dependent cell growth inhibition, whereas there is no inhibition on the growth of MEF p53+/+ cells. However, by employing MEF p53 −/−/TSC2+/+ and p53−/−/TSC2−/− cell lines, we further shows that loss of TSC2 in p53−/− MEFs significantly attenuates the growth inhibitory effect of SHR-5 on p53 deficient MEFs by about 30% (Figure 3B). These results suggest that the growth inhibitory effect of SHR-5 on p53 deficient MEFs, at least in part, requires the existence of TSC2, which also suggests a novel role of TSC2 in the growth of p53 null or deleted cells. In addition, gene transfer and overexpression of TSC1 in J82 cells which normally lack TSC1/2 expression increases the sensitivity of J82 cells to the growth inhibitory effect of SHR-5 (Figure 3C). Figure 3D also shows that p53 −/−/TSC2+/+ MEFs are relatively more resistant to the growth inhibitory effect of salidroside compared to p53 −/−/TSC2−/− MEFs. This pattern of SHR5 and salidroside’s growth inhibitory effect on MEFs with genetic knockout of p53 and/or TSC2 (Figure 3) is consistent with their effect on the native bladder cancer cell lines (Figure 1 and 2). Therefore, our results suggested that SHR-5 and salidroside selectively inhibit the growth of p53 defective cells via a TSC2-dependent mechanism. Since the major function of TSC1/2 is to inhibit the mTOR pathway, we examined whether the mechanism of SHR-5 or salidroside’s action is associated with the mTOR pathway.
Figure 3.
The growth inhibitory effect of SHR-5 and salidroside is associated with p53 and TSC2 expression. MEFs were treated as indicated for 72 hours. J82 cells were stably transfected with TSC1 or PCDNA3.1 (vector control). Cell viabilities were measured by a MTT assay. Points, mean of four independent plates; bars, SE. A. The effect of SHR-5 on the growth of MEFs p53+/+ and p53−/− cells. B. The effect of SHR-5 on the growth of MEFs TSC2−/− (p53−/−) and TSC2 +/+ (p53−/−). C. The effect of SHR-5 on the growth of J82/PcDNA3.1 and J82/TSC1 cells. D. The effect of salidroside on the growth of MEFs TSC2−/− (p53−/−) and TSC2 +/+ (p53−/−).
SHR-5 and salidroside activate AMPK α., inhibits mTOR, and dephosphorylates 4E-BP1, leading to increased binding of 4E-BP1 to m7 GTP in UMUC-3
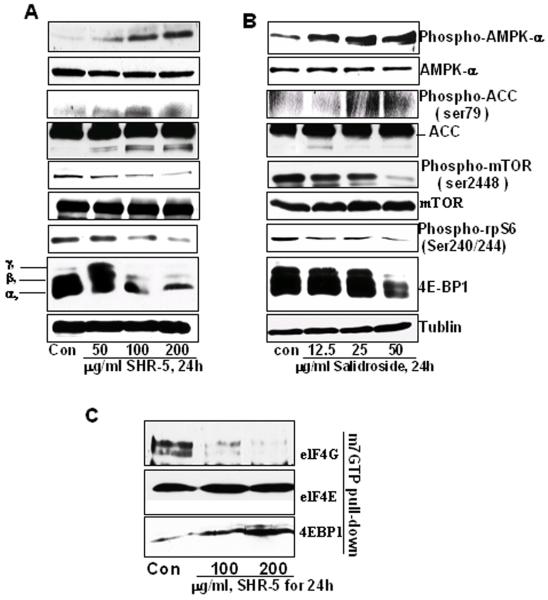
We first have examined the effect of SHR-5 and salidroside on the up-stream and down-stream events of TSC1/2 along the mTOR pathway. Figure 4 A and B show that both SHR-5 and salidroside increase the expression of phospho-AMPKα and ACC without a change in their total protein levels. A protein degradation band of ACC appears to be associated with SHR-5 and salidroside treatments. Consistent with the result described above, SHR-5 and salidroside decrease the phosporylation levels of mTOR at serine 2448 in a dose-dependent manner in UMUC-3 cells. Phosphorylations of rpS6 and 4E-BP1 represent major effectors of the mTOR pathway, which directly regulate translation initiation. SHR-5 and salidroside treatment caused a significant decrease in the phosphorylation of rpS6. In addition, three differentially phosphorylated forms of 4E-BP1 were detected in control, SHR-5 and salidroside treatment. 100 and 200 μg/ml SHR-5 and 50 μg/ml salidroside treatment caused a marked decrease in the levels of slower migrating bands γ and β corresponding to the hyperphosphorylated forms of 4E-BP1. This result indicates that SHR-5 and salidroside inhibit 4E-BP1 phosphorylation. Consequently, SHR-5 treatment resulted in a decreased binding of translational initiation factor eIF4G and an increased binding of 4EBP1 (a rate-limiting translation initiation blocker) to m7 GTP (Figure 4C). This result suggests that SHR-5 may inhibit cap-dependent translation initiation.
Figure 4.
The effects of SHR-5 and salidroside on AMPKα, mTOR and protein translation initiation in UMUC-3 cells. UMUC-3 cells were treated with vehicle control or indicated doses of SHR-5 or salidroside for 24 hours. At the end of each treatment time, cell lysates were prepared as mentioned in Materials and Methods. A & B: Western blotting analysis of phospho-AMPKα, AMPKα, phosphor-ACC, ACC, mTOR, phospho-mTOR, Phospho-rpS6, and 4E-BP1, and membranes were stripped for reprobing with anti-tubulin antibody for protein loading correction. A representative blot was shown from three independent experiments. C: eIF4E was purified from cell extracts by m7GTP affinity chromatography and probed with antibodies against eIF4E, eIF4G and 4E-BP1. A representative blot was shown from three independent experiments.
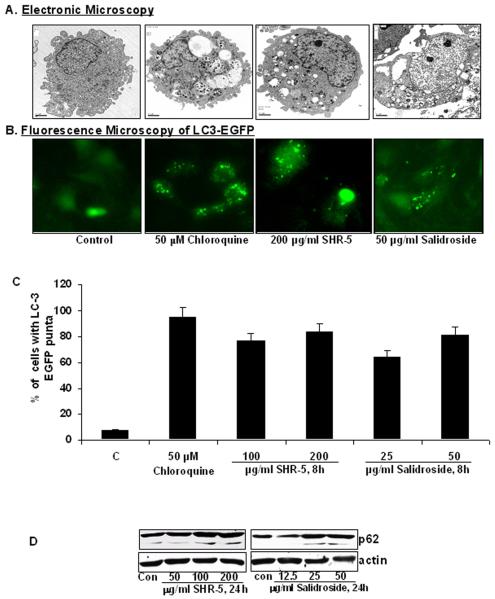
SHR-5 and salidroside induce autophagy in UMUC-3 cells
The mTOR pathway critically regulates autophagy as a cell survival mechanism under stress conditions induced by nutrient deprivation, hypoxia and drug treatments [28]. We examined the cellular consequences of SHR-5 and salidroside inhibiting the mTOR pathway in UMUC-3 cells. Electron microscopy photographs show that UMUC-3 cells treated with chloroquine (which is known to increase the numbers of autophagosomes in cells by blocking the fusion of autophagosome with lysosome), SHR-5 or salidroside exhibits a significant increase in the percentage of UMUC-3 cells with typical double-membrane autophagic vesicles compared to control (Figure 5A). In addition, the redistribution of LC3 protein from the cytoplasm to autophagic vesicles is a hallmark of autophagy [28]. We therefore have established stable UMUC-3 cell line expressing pEGFP-LC3 for studying cellular distribution of LC3. After treatment with chloroquine, SHR-5 or salidroside, LC3 in UMUC-3/ LC3-EGFP cells redistributed from a diffuse cytoplasmic staining to punctate structures (Figure 5B). Quantitative analysis of the percentage of UMUC-3 cells with LC3-EGFP puncta staining reveals that 100 μg/ml of SHR-5 and treatment for 8 hours increased the presence of LC3-GFP puncta in UMUC-3 cells by about 76% and 64%, respectively, whereas control treatment only have about 8% cells with LC3-GFP puncta (Figure 5C, ANOVA, Ps<0.01). Furthermore, Western blotting analysis revealed that SHR-5 and salidroside treatments increased the cleavage of LC-3II (data not shown) and p62 degradation (Figure 5D).
Figure 5.
SHR-5 and salidorside induce autophagy in bladder cancer UMUC-3 cells. A: UMUC-3 Cells were treated with vehicle control, 50 μM Chloroquine, 200 μg/ml SHR-5, or 50 μg/ml salidroside for 8 hours. After treatments, cells are fixed and examined by an electronic microscopy for the presences of autophagosomes. B: UMUC-3 Cells stably expressing pEGFP-LC3 were treated with vehicle control, 50 μM Chloroquine, 100 and 200 μg/ml SHR-5, or 25 and 50 μg/ml salidroside for 8 hours. After treatments, cells were examined by a fluorescence microscopy. C: Quantitative analysis of percentage of UMUC-3 cells with LC3-puncta. Bar: mean ±SE. D: Western blotting analysis of p62.
Discussion
R. Rosea has been in use for centuries to increase physical endurance, work productivity, resistance to high altitude sickness and extremely cold environments, to treat fatigue, depression and others including increasing longevity [15]. In recent clinical studies, R. Rosea extracts, particularly SHR-5, have shown their exceptional safety profile without any reported side effects or drug interactions [16, 29-31]. We demonstrated that SHR-5 and one of its major components, salidroside, selectively inhibit the growth of cancer cell lines derived from different stages of human urinary bladder cancer with minimal effect on a non-malignant bladder epithelial cell line. Since SHR-5 is conveniently ingested via in liquid form, our study suggests that SHR-5 deserves further study as a novel chemopreventive dietary supplement agent for both bladder cancer prevention and anti-aging in the elderly.
Mechanisms of R. rosea extracts’ action remain largely unknown. In this study, we provided the first evidence that R. rosea extract SHR-5 and salidroside inhibit the mTOR pathway and translational initiation via activation of AMPK α in bladder cancer UMUC-3 cells. In addition to gluconeogenesis, a major role of AMP kinase is to act as an energy (AMP/ATP ratio) sensor to inhibit energy-consuming processes, including cellular proliferation, under energy deprivation in order to maximize chance of survival [32]. Therefore, further studies are in process to determine whether the growth inhibitory effect of the R. rosea extract and salidroside on bladder cancer cells is dependent on AMP kinase–dependent pathways /AMP kinase activation. Schriner et al. [19] observed that the R. rosea extract feeding to the fruit fly lowered superoxide (O2•−) generated within isolated mitochondria. R. rosea extracts have been shown to improve endurance exercise performance [33]. Therefore, it is plausible to assume that a decreased production of O2•− by R. rosea extracts is due to its effect on oxygen consumption and thus a resulted change in ATP production and uncoupling in the mitochondria. Further studies are in progress to examine a potential link between cellular oxygen consumption and the R. rosea extract-induced AMP kinase activation.
“Superficial” and muscle-invasive bladder cancer develops along two major molecular pathways: the activation of the Ras pathway through mutations in the H-Ras, FGFR-3 and PI3K genes and loss of major tumor suppressors (p53, Rb, and PTEN), respectively [34-40]. The activation of the Ras pathway occurs in 70-90% of “superficial” bladder tumors [35-37], whereas the loss of p53 function was reported in more than 50% muscle-invasive transitional cell carcinoma [38-40]. Importantly, these pathways (Ras, p53 and PTEN) commonly cross-talk with the mTOR pathway [27, 34-35], and thus alterations of these pathways (Ras, p53 and PTEN) are expected to affect the activity of the mTOR pathway. Therefore, the mTOR pathway represents a critical target in bladder cancer prevention and treatment. Although currently there is no active clinical trial of mTOR inhibitors in bladder cancer, Seager et al. [38] recently reported that intravesical delivery of rapamycin resulted in a striking inhibitory effects on tumor progression in a bladder-specific PTEN and p53 knockout transgenic mouse model that produces carcinoma in situ lesions and develop muscle-invasive tumors. This study has provided a “proof of principle” evidence for the promising of mTOR inhibitors in bladder cancer prevention. However, concerns about the potential side effects (immunosupression) of prolonged exposure to rapamycin may preclude its widespread use for cancer prevention in human [41]. To this end, agents (e.g. R. rosea extracts) that can inhibit the mTOR pathway and have an excellent safety profile for long-term use may be desirable for bladder cancer prevention in the elderly.
Protein translation in mammalian cells is initiated by the formation of the eIF4F translation initiation complex, which binds to the cap structure (m7·GTP) at the 5′ end of mRNAs to recruit 40S ribosomal subunit. The eIF4F complex consists of the cap-binding protein eIF4E, an ATP-dependent helicase eIF4A and a scaffolding protein eIF4G that provides the docking site for the 40 S ribosomal subunit [42].The mTOR is a master regulator of translation initiation via phosphorylation of its direct downstream targets, S6K and eIF4E/4E-BP for controlling the recruitment of ribosomes to mRNA templates [43]. Hypophosphorylated 4E-BP1 inhibits cap-dependent translation initiation by competing with eIF4G for binding to eIF4E. We showed that inhibition of mTOR by a R. rosea extract and salidroside resulted in a conversion of hyperphosphorylated γ form to the hypo- or non-phosphorylated α form, which may allow 4E-BP1 to sequester eIF4G from its binding to eIF4E, and then block cap-dependent translation. It has been suggested that high levels of eIF4E activity favor the translation of a specific subset of genes with growth-promoting and oncogenic functions, such as cyclin D1 and c-Myc [42, 43]. Therefore, it is necessary to determine whether the growth inhibitory effect of R. rosea extract and salidroside in bladder cancer cells is associated with affecting the mTOR pathway mediated translation of mRNAs that encode specific oncogenic and growth promoting proteins.
We observed that the R. rosea extract and salidroside selectively inhibited the growth of MEFs with p53 deletion and that this effect of the R. rosea extract, at least in part, requires the existence of TSC2 expression. As p53 mutations occurs in more than 50% bladder cancer patients, our results suggest a potential benefits of the R. rosea extract and salidroside specifically for those bladder cancer patients with p53 deficient tumor cells. In addition, our result also suggested that TSC2 is a critical regulating point for the growth inhibitory effect of the R. rosea extract. In addition to AMPK, TSC2 is phosphorylated at multiple sites and regulated by multiple other pathways, including Wnt/GSK3β, Insulin/AKT, growth factors/ Ras/ERK or RSK, growth factors/AKT or hypoxia/Redd1 pathways [44, 45]. Therefore, in order to identify pathways by which the R. rosea extract inhibit the mTOR pathway through TSC2, further studies are in progress to determine what phosphorylation sites of TSC2 are affected by the R. rosea extract.
In summary, the concept of prevention of bladder cancer by inhibiting aging is attractive as aging is a major risk factor for human urinary bladder. We demonstrated that the R. rosea extract selectively inhibit the growth of bladder cancer cell lines and p53 defective cells with minimal effect on non-malignant bladder epithelial cells. In addition, we have shown for the first time that mechanisms of the R. rosea extract and salidroside are associated with inhibition of the mTOR pathway and translation initiation and induction of autophagy. Given R. rosea extracts also have anti-aging property and outstanding safety profiles in clinics, R. rosea extracts may represent a promising bladder cancer chemopreventive dietary supplement agent. Therefore, R. rosea extracts deserve further preclinical and clinical studies in animals and humans for determining its preventive efficacy against human urinary bladder cancer.
Acknowledgments
This work was supported by NIH award 5R01CA122558-03 and 1R21CA152804-01A1 (to X. Z.). We are grateful to the Swedish Herbal Institute for providing us the R. rosea extract (SHR-5).
References
- 1.Horner MJ, Ries LA, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975-2006. National Cancer Institute; Bethesda, MD: 2009. http://seer.cancer. Gov/csr/1975_2006. [Google Scholar]
- 2.Taylor JA, 3rd, Kuchel GA. Bladder cancer in the elderly: clinical outcomes, basic mechanisms, and future research direction. Nat Clin Pract Urol. 2009;6:135–44. doi: 10.1038/ncpuro1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Golijanin DJ, Kakiashvili D, Madeb RR, Messing EM, Lerner SP. Chemoprevention of bladder cancer. World J Urol. 2006;24:445–472. doi: 10.1007/s00345-006-0123-x. [DOI] [PubMed] [Google Scholar]
- 4.Colman RJ, Anderson RM, Johnson SC, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–4. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knutson MD, Leeuwenburgh C. Resveratrol and novel potent activators of SIRT1: effects on aging and age-related diseases. Nutr Rev. 2008;66:591–6. doi: 10.1111/j.1753-4887.2008.00109.x. [DOI] [PubMed] [Google Scholar]
- 6.Anisimov VN, Berstein LM, Egormin PA, et al. Metformin slows down aging and extends life span of female SHR mice. Cell Cycle. 2008;7:2769–73. doi: 10.4161/cc.7.17.6625. [DOI] [PubMed] [Google Scholar]
- 7.Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–5. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selman C, Tullet JM, Wieser D, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–4. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hursting SD, Smith SM, Lashinger LM, Harvey AE, Perkins SN. Calories and carcinogenesis: lessons learned from 30 years of calorie restriction research. Carcinogenesis. 2010;31:83–9. doi: 10.1093/carcin/bgp280. [DOI] [PubMed] [Google Scholar]
- 10.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–30. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 11.Mo L, Zheng X, Huang HY, et al. Hyperactivation of Ha-ras oncogene, but not Ink4a/Arf deficiency, triggers bladder tumorigenesis. J Clin Invest. 2007;117:314–325. doi: 10.1172/JCI30062. [DOI] [PMC free article] [PubMed] [Google Scholar]; Knowles MA, Habuchi T, Kennedy W, Cuthbert-Heavens D. Mutation spectrum of the 9q34 tuberous sclerosis gene TSC1 in transitional cell carcinoma of the bladder. Cancer Res. 2003;63:7652–6. [PubMed] [Google Scholar]
- 12.Platt FM, Hurst CD, Taylor CF, Gregory WM, Harnden P, Knowles MA. Spectrum of phosphatidylinositol 3-kinase pathway gene alterations in bladder cancer. Clin Cancer Res. 2009;15:6008–17. doi: 10.1158/1078-0432.CCR-09-0898. [DOI] [PubMed] [Google Scholar]
- 13.Sekulić A, Hudson CC, Homme JL, et al. A direct linkage between the phosphoinositide 3-kinase-AKT signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res. 2000;60:3504–13. [PubMed] [Google Scholar]
- 14.Chen M, Gu J, Delclos GL, et al. Genetic variations of the PI3K-AKT-mTOR pathway and clinical outcome in muscle invasive and metastatic bladder cancer patients. Carcinogenesis. 2010 doi: 10.1093/carcin/bgq110. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panossian A, Wikman G, Sarris J. Rosenroot (Rhodiola rosea): traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine. 2010;17:481–93. doi: 10.1016/j.phymed.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Panossian A, Hovhannisyan A, Abrahamyan H, Gabrielyan E, Wikman G. Pharmacokinetic and pharmacodynamic study of interaction of Rhodiola rosea SHR-5 extract with warfarin and theophylline in rats. Phytother Res. 2009;23:351–7. doi: 10.1002/ptr.2631. [DOI] [PubMed] [Google Scholar]
- 17.Gupta V, Saggu S, Tulsawani RK, Sawhney RC, Kumar R. A dose dependent adaptogenic and safety evaluation of Rhodiola imbricata Edgew, a high altitude rhizome. Food Chem Toxicol. 2008;46:1645–52. doi: 10.1016/j.fct.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 18.Jafari M, Felgner JS, Bussel II, et al. Rhodiola: a promising anti-aging Chinese herb. Rejuvenation Res. 2007;10:587–602. doi: 10.1089/rej.2007.0560. [DOI] [PubMed] [Google Scholar]
- 19.Schriner SE, Abrahamyan A, Avanessian A, et al. Decreased mitochondrial superoxide levels and enhanced protection against paraquat in Drosophila melanogaster supplemented with Rhodiola rosea. Free Radic Res. 2009;43:836–43. doi: 10.1080/10715760903089724. [DOI] [PubMed] [Google Scholar]
- 20.Wiegant FA, Surinova S, Ytsma E, Langelaar-Makkinje M, Wikman G, Post JA. Plant adaptogens increase lifespan and stress resistance in C. elegans. Biogerontology. 2009;10:27–42. doi: 10.1007/s10522-008-9151-9. [DOI] [PubMed] [Google Scholar]
- 21.Udintsev SN, Shakhov VP. The role of humoral factors of regenerating liver in the development of experimental tumors and the effect of Rhodiola rosea extract on this process. Neoplasma. 1991;38:323–31. [PubMed] [Google Scholar]
- 22.Tu Y, Roberts L, Shetty K, Schneider SS. Rhodiola crenulata induces death and inhibits growth of breast cancer cell lines. J Med Food. 2008;11:413–23. doi: 10.1089/jmf.2007.0736. [DOI] [PubMed] [Google Scholar]
- 23.Bocharova OA, Matveev BP, Baryshnikov AIu, Figurin KM, Serebriakova RV, Bodrova NB. The effect of a Rhodiola rosea extract on the incidence of recurrences of a superficial bladder cancer (experimental clinical research) Urol Nefrol (Mosk) 1995;2:46–7. Russian. [PubMed] [Google Scholar]
- 24.Zi X, Simoneau AR. Flavokawain A, a novel chalcone from kava extract, induces apoptosis in bladder cancer cells by involvement of Bax protein-dependent and mitochondria-dependent apoptotic pathway and suppresses tumor growth in mice. Cancer Res. 2005;65:3479–86. doi: 10.1158/0008-5472.CAN-04-3803. [DOI] [PubMed] [Google Scholar]
- 25.Tang Y, Simoneau AR, Xie J, Shahandeh B, Zi X. Effects of the kava chalcone flavokawain A differ in bladder cancer cells with wild-type versus mutant p53. Cancer Prev Res (Phila Pa) 2008;1:439–51. doi: 10.1158/1940-6207.CAPR-08-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.George B, Datar RH, Wu L, et al. p53 gene and protein status: the role of p53 alterations in predicting outcome in patients with bladder cancer. J Clin Oncol. 2007 Dec 1;25(34):5352–8. doi: 10.1200/JCO.2006.10.4125. [DOI] [PubMed] [Google Scholar]
- 27.Feng Z, Hu W, de Stanchina E, et al. The regulation of AMPK beta1, TSC2, and PTEN expression by p53: stress, cell and tissue specificity, and the role of these gene products in modulating the IGF-1-AKT-mTOR pathways. Cancer Res. 2007;67:3043–53. doi: 10.1158/0008-5472.CAN-06-4149. [DOI] [PubMed] [Google Scholar]
- 28.Jung CH, Ro SH, Cao J, Otto NM, Kim DH. mTOR regulation of autophagy. FEBS Lett. 2010;584:1287–95. doi: 10.1016/j.febslet.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Darbinyan V, Aslanyan G, Amroyan E, Gabrielyan E, Malmström C, Panossian A. Clinical trial of Rhodiola rosea L. extract SHR-5 in the treatment of mild to moderate depression. Nord J Psychiatry. 2007;61:343–8. doi: 10.1080/08039480701643290. [DOI] [PubMed] [Google Scholar]
- 30.Olsson EM, von Schéele B, Panossian AG. A randomised, double-blind, placebo-controlled, parallel-group study of the standardised extract shr-5 of the roots of Rhodiola rosea in the treatment of subjects with stress-related fatigue. Planta Med. 2009;75:105–12. doi: 10.1055/s-0028-1088346. [DOI] [PubMed] [Google Scholar]
- 31.Shevtsov VA, Zholus BI, Shervarly VI, et al. A randomized trial of two different doses of a SHR-5 Rhodiola rosea extract versus placebo and control of capacity for mental work. Phytomedicine. 2003;10:95–105. doi: 10.1078/094471103321659780. [DOI] [PubMed] [Google Scholar]
- 32.Fogarty S, Hardie DG. Development of protein kinase activators: AMPK as a target in metabolic disorders and cancer. Biochim Biophys Acta. 2010;1804:581–91. doi: 10.1016/j.bbapap.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 33.De Bock K, Eijnde BO, Ramaekers M, Hespel P. Acute Rhodiola rosea intake can improve endurance exercise performance. Int J Sport Nutr Exerc Metab. 2004;14:298–307. doi: 10.1123/ijsnem.14.3.298. [DOI] [PubMed] [Google Scholar]
- 34.Dinney CP, McConkey DJ, Millikan RE, et al. Focus on bladder cancer. Cancer Cell. 2004;6:111–6. doi: 10.1016/j.ccr.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Mo L, Zheng X, Huang HY, Shapiro E, Lepor H, Cordon-Cardo C, Sun TT, Wu XR. Hyperactivation of Ha-ras oncogene, but not Ink4a/Arf deficiency, triggers bladder tumorigenesis. J Clin Invest. 2007;117:314–325. doi: 10.1172/JCI30062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sibley K, Cuthbert-Heavens D, Knowles MA. Loss of heterozygosity at 4p16.3 and mutation of FGFR3 in transitional cell carcinoma. Oncogene. 2001;20:686–91. doi: 10.1038/sj.onc.1204110. [DOI] [PubMed] [Google Scholar]
- 37.Platt FM, Hurst CD, Taylor CF, Gregory WM, Harnden P, Knowles MA. Spectrum of phosphatidylinositol 3-kinase pathway gene alterations in bladder cancer. Clin Cancer Res. 2009;15:6008–17. doi: 10.1158/1078-0432.CCR-09-0898. [DOI] [PubMed] [Google Scholar]
- 38.Seager C, Puzio-Kuter A, Patel T, et al. Intravesical delivery of rapamycin suppresses tumorigenesis in a mouse model of progressive bladder cancer. Cancer Prev Res. 2009;2:1008–14. doi: 10.1158/1940-6207.CAPR-09-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He F, Mo L, Zheng XY, et al. Deficiency of pRb family proteins and p53 in invasive urothelial tumorigenesis. Cancer Res. 2009;69:9413–21. doi: 10.1158/0008-5472.CAN-09-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chatterjee SJ, Datar R, Youssefzadeh D, et al. Combined effects of p53, p21, and pRb expression in the progression of bladder transitional cell carcinoma. J Clin Oncol. 2004;22:1007–13. doi: 10.1200/JCO.2004.05.174. [DOI] [PubMed] [Google Scholar]
- 41.McConkey DJ, Dinney CP. The molecular, the bad, and the ugly: preventing bladder cancer via mTOR inhibition. Cancer Prev Res (Phila Pa) 2009;2:1001–2. doi: 10.1158/1940-6207.CAPR-09-0235. [DOI] [PubMed] [Google Scholar]
- 42.Livingstone M, Atas E, Meller A, Sonenberg N. Mechanisms governing the control of mRNA translation. Phys Biol. 2010;7:021001. doi: 10.1088/1478-3975/7/2/021001. [DOI] [PubMed] [Google Scholar]
- 43.Averous J, Proud CG. When translation meets transformation: the mTOR story. Oncogene. 2006;25:6423–35. doi: 10.1038/sj.onc.1209887. [DOI] [PubMed] [Google Scholar]
- 44.Rosner M, Hanneder M, Siegel N, Valli A, Hengstschläger M. The tuberous sclerosis gene products hamartin and tuberin are multifunctional proteins with a wide spectrum of interacting partners. Mutat Res. 2008;658:234–46. doi: 10.1016/j.mrrev.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 45.Yoshida T, Mett I, Bhunia AK, et al. Rtp801, a suppressor of mTOR signaling, is an essential mediator of cigarette smoke-induced pulmonary injury and emphysema. Nat Med. 2010;16:767–73. doi: 10.1038/nm.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]