Abstract
Multiple neurodegenerative disorders are associated with altered mitochondrial bioenergetics. Although mitochondrial O2 consumption is frequently measured in isolated mitochondria, isolated synaptic nerve terminals (synaptosomes), or cultured cells, the absence of mature brain circuitry is a remaining limitation. Here we describe the development of a method that adapts the Seahorse Extracellular Flux Analyzer (XF24) for the microplate-based measurement of hippocampal slice O2 consumption. As a first evaluation of the technique, we compared whole slice bioenergetics to previous measurements made with synaptosomes or cultured neurons. We found that mitochondrial respiratory capacity and O2 consumption coupled to ATP synthesis could be estimated in cultured or acute hippocampal slices with preserved neural architecture. Mouse organotypic hippocampal slices oxidizing glucose displayed mitochondrial O2 consumption that was well-coupled, as determined by the sensitivity to the ATP synthase inhibitor oligomycin. However stimulation of respiration by uncoupler was modest (<120% of basal respiration) compared to previous measurements in cells or synaptosomes, although enhanced slightly (to ~150% of basal respiration) by the acute addition of the mitochondrial complex I-linked substrate pyruvate. These findings suggest a high basal utilization of respiratory capacity in slices and a limitation of glucose-derived substrate for maximal respiration. The improved throughput of microplate-based hippocampal respirometry over traditional O2 electrode-based methods is conducive to neuroprotective drug screening. When coupled with cell type-specific pharmacology or genetic manipulations, the ability to efficiently measure O2 consumption from whole slices should advance our understanding of mitochondrial roles in physiology and neuropathology.
Keywords: mitochondria, bioenergetics, oxygen, respiration, pyruvate
INTRODUCTION
Mitochondrial functional impairments are associated with traumatic brain injury, stroke, Alzheimer’s disease, epilepsy, and other neurodegenerative disorders (Fiskum et al., 1999; Lin and Beal, 2006). The hippocampus, a brain region implicated in learning and memory, is particularly susceptible to degenerative damage in these disorders (Noraberg et al., 2005). The Clark electrode, developed by Leland Clark in the early 1950s (Clark, Jr. et al., 1953), was instrumental in the identification of bioenergetic defects using preparations of isolated brain mitochondria. However, a number of aspects of mitochondrial physiology and pathology cannot be adequately modeled in isolated preparations. These include mitochondrial trafficking, fission, fusion, biogenesis, and interaction with cytoplasmic signaling pathways or other organelles. Mitochondrial yield from discrete brain regions such as the hippocampus is also a limitation of such studies, as is the potential for damage or segregation of a sub-population of mitochondria during the isolation procedure. Advances in respirometry over the last decade include self-referencing O2 electrode microsensors (Land et al., 1999), flow-through cell respirometers (Jekabsons and Nicholls, 2004), and the microplate-based Seahorse Extracellular Flux (XF24) Analyzer (Gerencser et al., 2009; Wu et al., 2007). These technologies enabled the investigation of bioenergetics in intact neural cells and facilitated the identification of mitochondrial spare respiratory capacity as a parameter that influences survival during glutamate excitotoxicity (Johnson-Cadwell et al., 2007; Yadava and Nicholls, 2007). However, the absence of mature brain circuitry is a remaining limitation in the study of neural bioenergetics.
Organotypic and acute hippocampal slice preparations retain the endogenous cytoarchitecture and neural circuitry of the hippocampus (De Simoni et al., 2003; Gahwiler et al., 1997; Noraberg et al., 2005). Consequently, aspects of brain injury that cannot be readily studied in cell culture (e.g., spreading depression (Dietz et al., 2009)), can be modeled in slice preparations. O2 consumption from hippocampal slices was measured previously using O2 electrodes (Foster et al., 2005; Huchzermeyer et al., 2008; Kudin et al., 1999; Kudin et al., 2002; Kunz et al., 1999; Nishizaki and Okada, 1988; Poli et al., 1983). However, poor sensitivity and the degree of difficulty have hindered analysis of bioenergetic parameters in intact slices compared to populations of cells or synaptosomes. The Seahorse XF24 is an instrument that uses an O2-quenchable fluorophore to measure oxygen consumption in microplates (Gerencser et al., 2009; Wu et al., 2007). Because this system is in widespread use and offers improved throughput compared to traditional O2 electrode-based methods, we tested whether it could be adapted to bioenergetic studies using intact hippocampal slices.
MATERIALS AND METHODS
Materials
Basal Medium Eagle (BME), horse serum, L-glutamine and Hank’s Buffered Salt Solution (HBSS) were purchased from Invitrogen (Carlsbad, CA). Chicken plasma was obtained from Cocalico Biologicals (Philadelphia, PA). XF Islet Capture Microplates equipped with nylon mesh inserts (“Capture Screens”) were purchased from Seahorse Bioscience (Billerica, MA). All other reagents including thrombin were purchased from Sigma-Aldrich (St. Louis, MO).
Preparation of organotypic hippocampal slices
Protocols were approved by the Institutional Animal Care and Use Committee (IACUC) and were in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Organotypic hippocampal slices were prepared from 7-8 day old C57BL/6J mouse or Sprague Dawley rat pups essentially as described (Schuh et al., 2008; Vornov et al., 1995). Hippocampi were rapidly dissected following decapitation and 300-μm slices were made using a McIlwain tissue chopper under aseptic conditions. The slices were placed in Gey’s Balanced Salt Solution containing 0.5 mg/ml D-glucose and then centered onto the flat surface of nylon mesh inserts (XF Islet Capture Screens). Four inserts (with one slice per insert) were placed into individual 9.6 cm2 wells of 6-well plates (Nalge Nunc International, Rochester, NY) containing 1 ml of culture medium consisting of BME (50%), HBSS (25%), horse serum (25%), L-glutamine (2 mM), and D-glucose (25 mM). This volume of medium barely covered the slice surface, allowing ready diffusion of O2 into the tissue. Slice cultures were maintained at 37°C in a 95% air/5% CO2 humidified incubator with half medium changes every 3-4 days. Slices were used for experiments after culture for 12-18 days in vitro (DIV) when they had thinned to approximately 200 μm.
Preparation of acute hippocampal slices
Acute slices were prepared from postnatal day 24-31 Sprague Dawley rats. Hippocampi were rapidly dissected following decapitation and 200-μm slices were made using a McIlwain tissue chopper. Slices were centered onto nylon inserts containing 20 μl of chicken plasma (1 slice/insert) and 20 μl of thrombin was added to fix slices within O2 permeable clots. The slices were transferred to artificial cerebrospinal fluid (aCSF) at ~23 °C consisting of (in mM) 120 NaCl, 3.5 KCl, 1.3 CaCl2, 0.4 KH2PO4, 1 MgCl2, 5 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), pH 7.4 and supplemented with glucose (25 mM) and pyruvate (0.23 mM). Inserts were then placed into the XF Islet Capture Microplates for O2 consumption measurements (see below)
XF24 microplate-based respirometry
Respirometry of hippocampal slices was performed using an XF24 Extracellular Flux Analyzer (Seahorse Bioscience, Billerica, MA). Slices on nylon inserts were individually inserted face down into 20 wells of 24-well XF Islet Capture Microplates that contained 675 μl of aCSF supplemented with glucose (25 mM) and pyruvate (0.23 mM) for rat slices or glucose alone (15 mM) for mouse slices. Fatty acid free bovine serum albumin (BSA, 4 mg/ml, Sigma-Aldrich Catalogue# A6003) was also present when indicated. Four wells contained inserts but no slices to control for temperature-sensitive fluctuations in O2 fluorophore emission. Once the transfer of inserts was complete, slices in XF Islet Capture Microplates were incubated in a CO2-free incubator at 37°C for 1hr to allow temperature and pH equilibration. Slices were then loaded into the XF24 and further equilibrated for 15 min by three 3 min mix, 2 min wait cycles prior to the first measurement. XF assays consisted of 3 min mix, 3 min wait, and 2 min measurement cycles and were performed at 37°C as described (Wu et al., 2007). Using this protocol, it was possible to calculate an O2 consumption rate every 8 min. Drugs of interest prepared in aCSF assay medium (75 μl) were preloaded into reagent delivery chambers A, B, C, and D at 10X, 11X, 12X, and 13X the final working concentration, respectively, and injected sequentially at intervals of 24 to 56 min as indicated.
Propidium iodide fluorescence imaging
Organotypic mouse hippocampal slices were incubated with 10 μM propidium iodide (PI) for 10 minutes. Following two washes with aCSF, PI fluorescence was imaged using the 10x objective of a Nikon Eclipse E800 microscope (Nikon Instruments, Melville, NY). The filter sets for excitation/dichroic mirror/emission were (in nm): 540(10)/565/620(30).
Statistical Analysis
The basal O2 consumption rates (OCR) of organotypic and acute rat hippocampal slices were compared by the Student’s t-test. The power analysis of organotypic mouse hippocampal OCR data was based on two-way analysis of variance (with treatment and subjects as two factors) and implemented using PASS software (NCSS, Kaysville, UT). Data are expressed as means ± SD unless otherwise indicated. P<0.05 was considered significant.
RESULTS
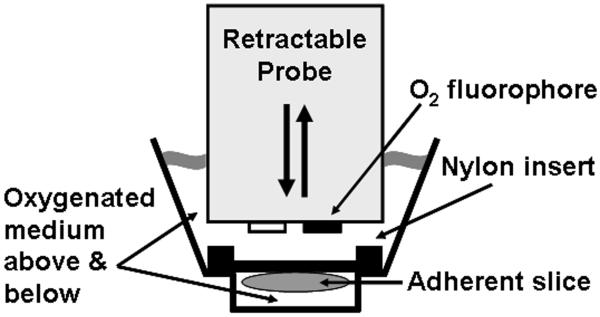
Organotypic hippocampal slice cultures are typically cultured on porous membrane inserts (Stoppini et al., 1991) or roller-tubes (Gahwiler, 1981; Gahwiler et al., 1997). Initially, we tested whether postnatal day 7 (P7) rat organotypic hippocampal slices cultured on 30 mm diameter porous membrane inserts (Millipore, Billerica, MA) for 14 days in vitro could be transferred into XF Islet Capture Microplates for measurements of O2 consumption. Islet microplates consist of a modified XF24 V7 plate (Seahorse Bioscience) containing a 3 mm diameter 1 mm deep microchamber at the bottom of wells. An accompanying fitted nylon mesh insert (70 μm pore size) is designed to trap pancreatic islet tissue (Fig. 1) but can be adapted for other tissue types. The XF24 lowers a retractable probe with a spotted O2 sensor into each well, sealing off a small volume of medium above the inserts in which tissue O2 consumption is assessed (Gerencser et al., 2009; Wu et al., 2007). When rat hippocampal slices were detached from porous membrane inserts and transferred to XF Islet Capture Microplates ~1 hour prior to measurements as free-floating tissue beneath capture screens, O2 consumption rates were noisy and did not respond to the ATP synthase inhibitor oligomycin or the uncoupler carbonylcyanide p-trifluoromethoxyphenylhydrazone (FCCP, data not shown).
Fig. 1.
Schematic diagram of hippocampal slices attached to nylon mesh inserts (“Capture Screens”) within XF Islet Capture Microplates. A retractable probe limits O2 diffusion to the tissue when in the measurement position, enabling estimation of O2 consumption rates based on the emission of an O2-sensitive fluorophore. Mixing between measurements allows rapid re-oxygenation of medium surrounding adherent slices.
To overcome the problems of free floating tissue moving laterally and vertically with respect to the O2 sensitive fluorophore spots and the poor drug responsiveness, P7-8 rat or mouse organotypic hippocampal slices were cultured directly on the XF nylon mesh inserts before transfer to the XF measurement plates (Fig. 1, Fig. 2A-D). Slices cultured for up to 18 DIV on the inserts were generally viable, with the majority of slices exhibiting low to moderate staining by propidium iodide (PI), a nuclear dye excluded by healthy cells. In a few slices, PI staining localized strongly to the CA1 pyramidal layer, indicating extensive cell death within this region. Images in Fig. 2B-D illustrate the range of PI staining observed in slices, with Fig. 2C representative of the majority.
Fig. 2.
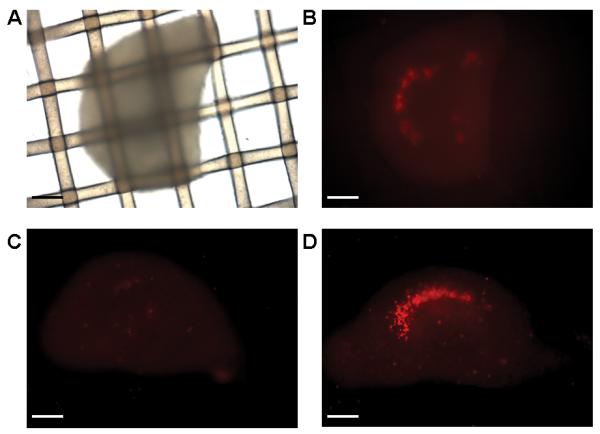
Representative brightfield (A) and propidium iodide (PI)-stained fluorescent images (B-D) of organotypic mouse hippocampal slices cultured on nylon mesh inserts for 12-18 DIV. The same slice is depicted in A and B. Scale bars, 50 μm.
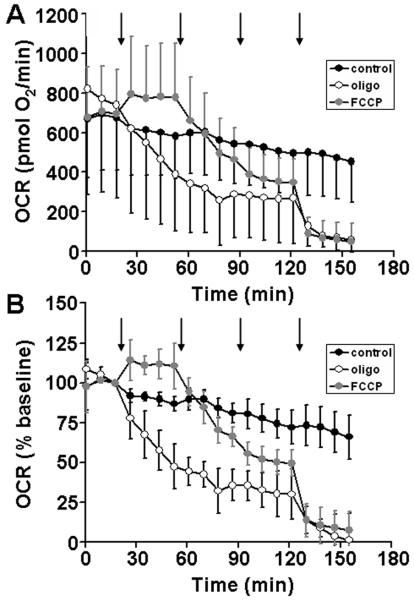
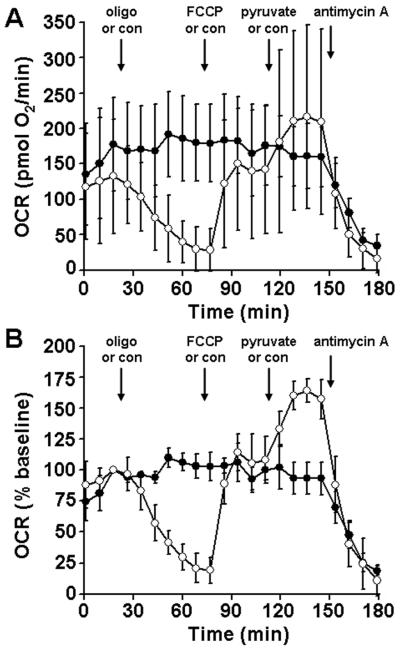
Next, we tested whether stable O2 consumption rates (OCR) and drug responses could be measured from rat organotypic slices cultured directly on the nylon inserts and then inverted into XF Islet Capture Microplates. For these pilot experiments we used an assay medium consisting of a relatively standard artificial cerebrospinal fluid solution (see Materials and Methods) that was supplemented with glucose (25 mM) and pyruvate (0.23 mM). We observed a large variability in OCR from 14 DIV slices of a single rat brain, with a mean ± SD of 703 ± 307 pmol O2/min (n=12 slices). In the absence of drug additions, respiration exhibited a slow, progressive decline but remained at 72% of the initial baseline after two hours (Fig. 3A, B, filled black circles). Addition of the uncoupler FCCP (1 μM) caused an initial stimulation of OCR (Fig. 3A, B, filled gray circles). However, further additions lead to a progressive decrease in OCR, most likely due to toxicity, with OCR reaching ~50% of the baseline rate at ~1.5 hours after the first addition. Titration with the ATP synthase inhibitor oligomycin led to a slow drop in OCR to ~30% of the initial rate, with a peak response at ~1 hour subsequent to the first addition (Fig. 3A, B, open circles). The majority (~90%) of hippocampal O2 consumption was attenuated by the mitochondrial complex III inhibitor antimycin A (10 μM), indicating that it was of mitochondrial origin.
Fig. 3.
Oligomycin and FCCP titrations for organotypic rat hippocampal slices. Absolute (A) and baseline-normalized (B) O2 consumption rates (OCR) of 14 DIV organotypic rat hippocampal slices exposed to successive additions of respiratory modulators (arrows) are shown. Slices received three additions of oligomycin (1 μg/ml each, open circles), FCCP (1 μM each, filled gray circles), or control (75 μl of aCSF each, filled black circles) followed by a final addition of antimycin A (10 μM, open and filled gray circles) or control (75 μl of aCSF, filled black circles). Rates in B are normalized to the third baseline measurement point. Data are mean ± SD, n = 4 slices per trace.
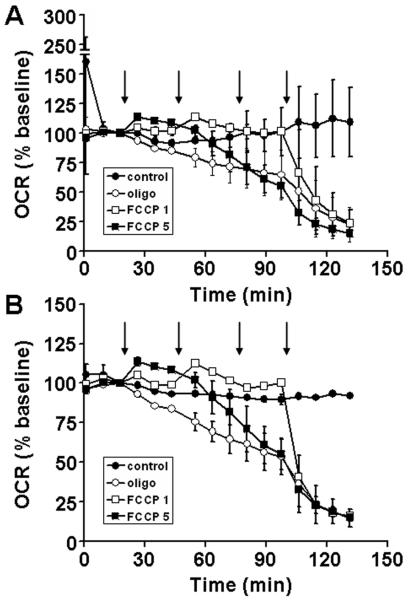
Because organotypic slice cultures are typically restricted to brain tissue from immature animals while neurodegenerative disorders are most prevalent in the aging population, we tested whether the same technique could be applied to acute rat hippocampal slices. Acute slices were attached to the nylon inserts within two hours of slicing by centering them on a small volume of chicken plasma and initiating the formation of an O2 permeable clot by thrombin addition (see Materials and Methods). The basal OCR of acute rat slices of 798 ± 377 pmol O2/min (n=10 slices) did not differ significantly from that of organotypic rat hippocampal slices cultured for 14 DIV. As in organotypic slices, FCCP caused a slight (~13%) stimulation of OCR while oligomycin decreased OCR to ~53% of baseline after 1 hour (Fig. 4A). Of 10 slices, one slice displayed an unstable baseline (170% drop in OCR from the first to the third measurement) and residual OCR that was antimycin A-insensitive. Antimycin A-insensitive (non-mitochondrial) O2 consumption was greater than 30% of the total OCR for two additional slices. These slices had a reduced ratio of mitochondrial to non-mitochondrial O2 consumption compared to the majority of the slices, likely due to an increased number of cells lacking functional mitochondria. Therefore they were considered damaged and were excluded from analysis here (Fig. 4B) and in subsequent experiments.
Fig. 4.
Oligomycin and FCCP titrations for acute rat hippocampal slices. Baseline-normalized O2 consumption rates (OCR) of acute rat hippocampal slices exposed to successive additions of respiratory modulators (arrows) are shown without (A) and with (B) exclusion criteria (see text). Rates are normalized to the third baseline measurement point. Slices received three additions of oligomycin (1 μg/ml each, open circles), FCCP (1 μM each, open squares or 5 μM each, filled squares), or control (75 μl aCSF each, filled circles) followed by a final addition of antimycin A (10 μM, open circles and open and filled squares) or control (75 μl aCSF, filled circles). Data in A are mean ± SD, n = 3, 3, 2, and 2 slices per trace for filled circles, open circles, filled squares, and open squares, respectively. In B, one slice per trace was excluded with the exception of filled squares.
One of the technical challenges of adapting XF24 technology for use with hippocampal slices is preventing tissue hypoxia. Organotypic hippocampal slice cultures are adapted to culture at atmospheric (20-21%) O2 while acute hippocampal slices are typically maintained in aCSF continuously bubbled with 95% O2. Because O2 diffusion is limited within tissue, hyperoxic conditions of 20% or 95% O2 (with respect to normal tissue pO2) are intended to prevent hypoxia in the slice core and are particularly important with thicker sections. While O2 tension at the surface of organotypic cultures 180-210 μm thick was previously measured at ~148 mm Hg in air (near the expected atmospheric value), O2 tension in the slice core was measured at ~51 mm Hg (Huchzermeyer et al., 2008). This pO2 approaches measured pO2 values of rat cortex, which vary with energy demand and cortical layer between ~15-40 mm Hg (~2-5% O2) (Erecinska and Silver, 2001; Grote et al., 1996; Liu et al., 1995). Although pO2 in the slice core is tolerable with respect to physiological normoxia, O2 levels in the XF24 drop during the measurement period when the microchamber above the slices is sealed off from the atmosphere. Thus, pO2 in the slice core may transiently decline below physiologically normoxic levels if tissue O2 consumption rates are sufficiently high.
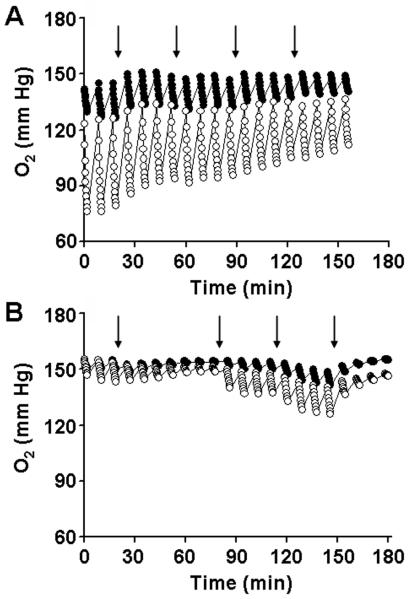
While it was not possible to get a measure of pO2 in the slice core using the XF24, the measurement of O2 levels in the aCSF are expected to approximate pO2 in the surface layer of cells. We found that even under basal conditions (i.e. in the absence of uncoupler to stimulate respiration), the pO2 in aCSF dropped below 80 mm Hg (Fig. 5A, open circles) in a slice respiring with an initial rate of 1029 pmol O2/min. The pO2 remained above 125 mm Hg (Fig. 5A, filled circles) in a sister slice respiring at 329 pmol O2/min. However, the basal OCR of this slice was more than a standard deviation below the average OCR (703 ± 307 pmol O2/min). These data suggest that the majority of rat slices likely experience transient hypoxia in the slice core by the end of each two minute measurement period, even in the absence of respiratory stimulation.
Fig. 5.
Medium O2 levels (mm Hg) during measurements of O2 consumption by rat (A) and mouse (B) organotypic slices. In A, representative fast and slow respiring rat slices are depicted (open circles and filled circles, respectively, see text). Arrows represent vehicle additions (75 μl of assay medium equilibrated with air). In B, representative fast and slow respiring mouse slices are depicted (open circles and filled circles, respectively, see text). Arrows represent the successive additions of oligomycin (10 μg/ml), FCCP (5 μM), pyruvate (10 mM) and antimycin A (10 μM).
Because transient hypoxia during measurements may influence the physiology of the slice, we tested organotypic mouse hippocampal slices which contain fewer cells per slice compared to rat slices of the same thickness. Under basal conditions, O2 remained above 140 mm Hg (Fig. 5B) for representative fast and slow O2 consuming slices that had a basal OCR of 250 pmol O2/min (open circles) and 118 pmol O2/min (filled circles), respectively. Furthermore, O2 remained above 125 mm Hg for the fast respiring slice even after OCR was stimulated to 405 pmol O2/min by the uncoupler FCCP and excess exogenous substrate (10 mM pyruvate). Therefore, mouse organotypic hippocampal slices were used in all subsequent experiments to further optimize hippocampal respirometry.
In respirometry experiments with neuronal cells and isolated synaptic nerve terminals (synaptosomes), bovine serum albumin (BSA) was used as a surrogate for extracellular protein (Choi et al., 2009; Jekabsons and Nicholls, 2006; Yadava and Nicholls, 2007). Treatment of mouse organotypic hippocampal slices with 1 μM FCCP in the absence of BSA led to little initial OCR stimulation. However, there was a delayed peak response that was similar but less variable in the presence of BSA (4 mg/ml, Fig. 6). In the absence of BSA, increasing the FCCP concentration to 5 μM abolished detection of respiratory stimulation (Fig. 6) and led to progressive inhibition (data not shown), likely due to toxicity (see Fig. 3 and 4). In contrast, with BSA present, peak respiratory stimulation was observed at the initial measurement after 5 μM FCCP addition (Fig. 6). Glucose (15 mM) was used as the energy substrate in these experiments and pyruvate (10 mM) was co-injected with FCCP to exclude the possibility that substrate supply was rate-limiting for uncoupled respiration. Additional injections of FCCP up to a cumulative total of 15 μM failed to further stimulate OCR in either the absence or presence of BSA (data not shown). Because BSA facilitated the determination of maximal OCR, it was included in subsequent experiments.
Fig. 6.
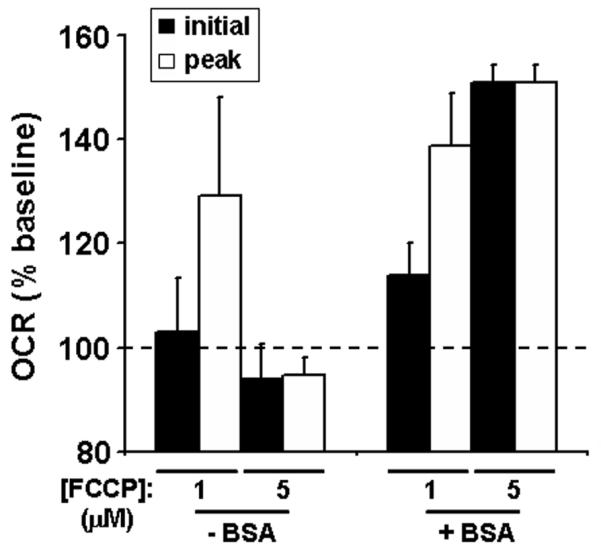
The effect of BSA on FCCP-stimulated O2 consumption rates (OCR). Baseline-normalized initial and peak OCR of organotypic mouse hippocampal slices exposed to FCCP (1 or 5 μM) in the absence (n=4) or presence (n=3) of BSA (4 mg/ml). Initial OCR (black bars) and peak OCR (white bars) were the first or the first or second measurement after FCCP addition, respectively. Data are expressed as mean ± SE and are normalized to the last OCR before FCCP addition. Two slices with baseline OCR below 50 pmol O2/min were excluded from analysis.
Next, we sequentially injected oligomycin, FCCP, pyruvate, and antimycin A to estimate coupling efficiency, maximal respiration in the presence of glucose, maximal respiration in the presence of exogenous complex I-linked substrate, and non-mitochondrial OCR, respectively (Fig. 7). As in rat hippocampal slices, antimycin A strongly inhibited organotypic mouse slice O2 consumption. Inhibition of the ATP synthase by oligomycin attenuated mitochondrial (antimycin A-sensitive) OCR by >75%, demonstrating that the majority of O2 consumption was coupled to ATP synthesis. FCCP addition restored OCR to ~basal values while the addition of pyruvate was necessary to elevate uncoupled OCR above the endogenous basal rate. Baseline OCR exhibited high variability (Fig. 7A), as was the case with rat hippocampal slices. Normalizing OCR to the third basal rate prior to drug additions (Fig. 7B) considerably reduced variability.
Fig. 7.
Estimation of coupling efficiency, maximal respiratory capacity, and the effect of exogenous pyruvate. Absolute (A) and baseline-normalized (B) O2 consumption rates (OCR) of 17 DIV organotypic mouse hippocampal slices exposed to successive additions of respiratory modulators (arrows) are shown. Slices received successive additions of oligomycin (10 μg/ml), FCCP (5 μM), pyruvate (10 mM), and antimycin A (10 μM, open circles, n=4), or three successive control (con) injections (75 μl of aCSF each) followed by antimycin A (10 μM, filled circles, n=3). Rates in B are normalized to the third baseline measurement point. Data are mean ± SD.
Finally, to provide a preliminary indication of how many replicates would be required to detect differences in bioenergetic parameters following an experimental treatment, we evaluated the variability in OCR of 10 total slices derived from three different wild type mice of the same litter that were expected to display similar responses to the sequential addition of oligomycin, FCCP plus pyruvate, and antimycin A (Fig. 8). When comparing absolute OCR, there was high variability among the mice in basal rates and drug responses (Fig. 8A). The variability in responses was greatly reduced when O2 consumption rates for each slice were normalized to the basal rate (Fig. 8B). This finding suggests that most of the variability was due to the technical variability of measurements (i.e. number of cells per slice, proximity of slices to the O2 sensor, etc., see discussion) rather than biological variability of bioenergetic parameters. We performed a power analysis to assess how many slices per animal would be required to detect a 20% difference between hypothetical control and experimental groups, assuming equal group size and groups of 5 or 10 animals (Table I). A power of 0.8 was readily achievable for comparisons of normalized data at a significance level of 0.05 but required a large number of slices for comparisons of absolute OCR. Overall, the data in Fig. 8 in combination with the power analysis illustrate that reproducible normalized O2 consumption data can be obtained from organotypic hippocampal slices while cautioning against comparisons of absolute OCR without a high number of replicates.
Fig. 8.
Assessment of inter-animal variability. Absolute (A) and baseline-normalized (B) O2 consumption rates (OCR) of 14-15 DIV organotypic mouse hippocampal slices exposed to successive additions of respiratory modulators (arrows) are shown for three different wild type mice from the same litter (mean ± SD, n=4 slices for mouse 1 and for mouse 3 and n=2 slices for mouse 2). Slices received successive additions of control (75 μl of aCSF), oligomycin (10 μg/ml), FCCP (5 μM) + pyruvate (10 mM), and antimycin A (10 μM). Two slices from mouse 2 exhibited antimycin A-insensitive respiration exceeding 30% of the total OCR and were excluded from analysis. Rates in B are normalized to the third baseline measurement point.
DISCUSSION
Henry McIlwain performed early pioneering work on optimizing brain tissue slice preparations (Collingridge, 1995; Rodnight and McIlwain, 1954), with the goal of making metabolic measurements. Over the last several decades a number of attempts have been made to measure O2 consumption from brain hippocampal slices, primarily using the Clark electrode (Foster et al., 2005; Huchzermeyer et al., 2008; Kudin et al., 1999; Kudin et al., 2002; Kunz et al., 1999; Nishizaki and Okada, 1988; Poli et al., 1983). However, to date, there are no techniques in widespread use. Therefore we adapted the microplate-based XF24 for the medium-throughput measurement of whole hippocampal slice O2 consumption, with the goal of increasing the utility of hippocampal respirometry for investigating the roles of mitochondria in brain physiology and pathology.
Pilot experiments established the necessity of attaching hippocampal slices to nylon mesh inserts to obtain reliable measurements of O2 consumption rates. While the reason for our inability to measure drug responses from free-floating tissue trapped by nylon inserts is not clear, we speculate that the increased proximity of tissue to the point of drug delivery favors drug uptake over binding to plate surfaces. Initial experiments employed rat organotypic and acute hippocampal slices. While it was possible to obtain relatively stable OCR measurements over ~2 hours and observe FCCP and oligomycin responses using rat slices, the potential for transient hypoxia during the measurements due to high OCR was a concern and may have limited our ability to achieve maximal uncoupled respiration. Therefore we shifted the focus for subsequent development to mouse organotypic hippocampal slices that exhibited lower OCR, likely due to the lower tissue mass.
We found that reducing aCSF glucose from 25 to 15 mM or adding BSA (4 mg/ml) did not influence organotypic mouse hippocampal slice OCR (unpublished observations). However, the inclusion of BSA facilitated the determination of peak uncoupled respiration (Fig. 6). We speculate that BSA buffered the effective FCCP concentration via direct binding, broadening the peak response that could be observed prior to the onset of toxicity (i.e. the OCR decline observed during FCCP titration, see Fig. 3 and 4). The use of aCSF supplemented with 15 mM glucose and 4 mg/ml BSA allowed measurements of hippocampal slice O2 consumption under almost identical conditions previously employed for neuronal (Jekabsons and Nicholls, 2004; Johnson-Cadwell et al., 2007; Yadava and Nicholls, 2007) or synaptosomal (Choi et al., 2009) respirometry, enabling ready comparisons.
We found that the ATP synthase inhibitor oligomycin decreased the O2 consumption of rat or mouse organotypic hippocampal slices by 70-80%, a reduction similar to that reported for primary neurons (Gleichmann et al., 2009; Jekabsons and Nicholls, 2004). Oligomycin caused less reduction of the mitochondrial (antimycin A-sensitive) OCR of acute rat hippocampal slices (~50%, Fig. 4B) compared to organotypic rat hippocampal slices (~75%, Fig. 3B) although basal OCR consumption rates were similar. Because oligomycin-sensitive OCR approximates the proportion of OCR coupled to ATP synthesis, this suggests that mitochondria were not as well coupled in the acute slices. Unlike acute slices, organotypic hippocampal slices had days to recover from the initial trauma of preparation, adhered to the nylon mesh inserts without plasma-thrombin treatment, and were adapted to survival at atmospheric O2. Therefore we chose to focus on organotypic slices for optimization of slice respirometry. However, the limited data in Fig. 4 are presented as proof-of-principle that the technique can also be applied to acute slices, pending additional optimization.
The most notable difference of organotypic hippocampal slices compared to primary cultured neurons or cerebrocortical synaptosomes is a lower spare respiratory capacity. Spare respiratory capacity is defined as the difference between the basal and the maximal (uncoupled) OCR and was suggested to approximate the ability of mitochondria to upregulate OCR in response to an increased demand for ATP (Choi et al., 2009; Johnson-Cadwell et al., 2007; Yadava and Nicholls, 2007). FCCP was reported to increase OCR to ≥250% of the basal rate in cerebellar granule neurons (Jekabsons and Nicholls, 2004; Johnson-Cadwell et al., 2007; Yadava and Nicholls, 2007), cortical neurons (Gleichmann et al., 2009) or synaptosomes (Choi et al., 2009) while in slices only ~150% of the basal rate was achieved even when exogenous pyruvate was supplied (Fig. 6-8). It is possible that toxicity due to the inhibition of mitochondrial ATP synthesis by oligomycin limited our ability to detect maximal respiration in response to subsequent FCCP addition. However, the peak normalized OCR obtained when FCCP and pyruvate were added after oligomycin (Fig. 7-8) was similar to that obtained when slices were treated with the uncoupler plus substrate without prior oligomycin addition (Fig. 6). It is also possible that the uncoupled OCR was limited by O2 diffusion in the interior of the slice or the time resolution of the measured FCCP response relative to the onset of drug-induced toxicity. However, it is notable that excitatory amino acids were reported to increase guinea pig hippocampal slice O2 consumption to only 120-146% of resting levels (Nishizaki and Okada, 1988), in line with the level of stimulation we observed using uncoupler. Because neural circuitry is preserved in hippocampal slices relative to cultured neurons or isolated synaptic nerve terminals, a higher energy demand due to endogenous signaling is predicted. Consequently, cells within slices may respire at closer to maximal capacity to meet an elevated ATP requirement, therefore exhibiting less spare capacity. The increased responsiveness of slices to oligomycin (i.e. Fig. 8) relative to synaptosomes (Choi et al., 2009) is consistent with a higher energy demand (i.e. a basal rate closer to a phosphorylating or “state 3” rate). A possible difference relative to cultured neurons is less obvious and would require a direct side-by-side comparison, using a minimum of five animals with 4-5 slices per animal based on our power analysis (Table I).
A caveat in comparing hippocampal slices to cerebellar granule neurons or cerebrocortical synaptosomes, in addition to the brain region, is cell heterogeneity. While O2 consumption by mitochondria of primarily neuronal origin is measured in cell culture or synaptosomes, a mixture of neuronal and glial O2 consumption is measured in slices. The presence of glia may contribute to the apparently lower respiratory capacity measured in slices relative to neurons or synaptosomes. Cell type-specific transgenics or agonists/antagonists should prove useful when evaluating the contributions of individual cell types to measured responses in models of neurodisease. Additionally, the slice system may prove superior to neuronal-glial co-cultures for some studies of metabolic interactions within the brain.
Notably, we found that exogenous pyruvate (10 mM) was able to increase O2 consumption of organotypic mouse hippocampal slices in the presence of uncoupler, as was reported previously for neurons (Jekabsons and Nicholls, 2004), synaptosomes (Choi et al., 2009; Kauppinen and Nicholls, 1986), and acute rat hippocampal slices (Kudin et al., 1999). This finding demonstrates the utility of microplate-based hippocampal respirometry for assessing the influence of exogenous energy substrates on mitochondrial function and confirms observations made with O2 electrode-based technology. In addition, this finding suggests that the rate of glucose oxidation may limit the extent to which OCR can be upregulated in response to increased energy demand (e.g. intense excitatory stimulation). The ability of exogenous pyruvate to increase spare respiratory capacity may partly explain its neuroprotective effects (Izumi and Zorumski, 2010; Shen et al., 2010).
Overall, we have shown that it is possible to measure stable rates of O2 consumption by hippocampal slices in microplates over a ~2-3 hour time course and assess the responsiveness to mitochondria-targeted drugs or substrates. Because it was difficult to reproducibly center slices with respect to the O2-sensing fluorophore spot, slow O2 diffusion within the temporary microchamber formed for measurements (Gerencser et al., 2009) likely contributed to the high variability in absolute OCR that was observed. Differences in viable cell number among the different slices (Fig. 2) were also a likely source of variability. Internal normalization of OCR to the baseline rate for each slice substantially reduced variability (Fig. 8) and, based on power analysis, increased the feasibility of detecting small (20%) differences in bioenergetic parameters due to genetic or experimental manipulation (Table I). Nevertheless, an increased number of animals as well as a greater number of slices per animal are required to make strong conclusions about the extent of biological variability in bioenergetics parameters (e.g. proton leak and spare respiratory capacity) among animals of the same genotype. Development of additional methods of OCR normalization that allow comparison of absolute O2 consumption rates is one of the major challenges for the future. Increasing the size of the O2 fluorophore spot with respect to the well area may decrease variability. Alternatively, an imaging-based method may be used to both correct for differences in the distance of O2 diffusion due to slice position and estimate viable cell number. We attempted to stain slices with PI and correct for differences in slice health subsequent to OCR measurements. Unfortunately the prolonged drug exposures, combined with the difficult extraction of the slice inserts that was required for imaging, led to significant toxicity (data not shown). Pre-selection of healthy slices based on PI fluorescence for respirometry may be a good alternative and is currently being evaluated. Although challenges remain, this initial description of microplate-based hippocampal respirometry should promote future investigations of the influence of excitotoxins, seizure-like activity, or neurotoxic transgenes (e.g. mutant amyloid precursor protein) on mitochondrial function. In addition, the improved throughput over previous methods should promote drug discovery.
Table I.
Power analysis of replicates required for observing a 20% difference in bioenergetic parameters
| OCR | # slices for n=5a,b | # slices for n=10a,c |
|---|---|---|
| basal | >12 | 10 |
| oligomycin | >12 | 9 |
| FCCP | 12 | 6 |
| normalized oligomycin (% basal) | 4 | 3 |
| normalized FCCP (% basal) | 5 | 3 |
Numbers in columns indicate the number of slices (replicates) per mouse necessary to detect a 20% difference between two groups at a significance level of 0.05 with a power of at least 0.8.
Assumes n=5 mice for the two groups
Assumes n=10 mice for the two groups
ACKNOWLEDGEMENTS
The authors thank Y. Logan for help with hippocampal slice preparations.
Contract grant sponsors: National Institutes of Health; Contract grant number NS064978 (to B.M.P.) Rehabilitation R & D REAP (to R.A.S.) and CDA-02 Biomedical R & D grant (to R.A.S.) from the VA Research Service
REFERENCES
- Choi SW, Gerencser AA, Nicholls DG. Bioenergetic analysis of isolated cerebrocortical nerve terminals on a microgram scale: spare respiratory capacity and stochastic mitochondrial failure. J Neurochem. 2009;109:1179–1191. doi: 10.1111/j.1471-4159.2009.06055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LC, Jr., Wolf R, Granger D, Taylor Z. Continuous recording of blood oxygen tensions by polarography. J Appl Physiol. 1953;6:189–193. doi: 10.1152/jappl.1953.6.3.189. [DOI] [PubMed] [Google Scholar]
- Collingridge GL. The brain slice preparation: a tribute to the pioneer Henry McIlwain. J Neurosci Methods. 1995;59:5–9. doi: 10.1016/0165-0270(94)00187-l. [DOI] [PubMed] [Google Scholar]
- De Simoni A, Griesinger CB, Edwards FA. Development of rat CA1 neurones in acute versus organotypic slices: role of experience in synaptic morphology and activity. J Physiol. 2003;550:135–147. doi: 10.1113/jphysiol.2003.039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz RM, Weiss JH, Shuttleworth CW. Contributions of Ca2+ and Zn2+ to spreading depression-like events and neuronal injury. J Neurochem. 2009;109(Suppl 1):145–152. doi: 10.1111/j.1471-4159.2009.05853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecinska M, Silver IA. Tissue oxygen tension and brain sensitivity to hypoxia. Respir Physiol. 2001;128:263–276. doi: 10.1016/s0034-5687(01)00306-1. [DOI] [PubMed] [Google Scholar]
- Fiskum G, Murphy AN, Beal MF. Mitochondria in neurodegeneration: acute ischemia and chronic neurodegenerative diseases. J Cereb Blood Flow Metab. 1999;19:351–369. doi: 10.1097/00004647-199904000-00001. [DOI] [PubMed] [Google Scholar]
- Foster KA, Beaver CJ, Turner DA. Interaction between tissue oxygen tension and NADH imaging during synaptic stimulation and hypoxia in rat hippocampal slices. Neurosci. 2005;132:645–657. doi: 10.1016/j.neuroscience.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Gahwiler BH. Organotypic monolayer cultures of nervous tissue. J Neurosci Methods. 1981;4:329–342. doi: 10.1016/0165-0270(81)90003-0. [DOI] [PubMed] [Google Scholar]
- Gahwiler BH, Capogna M, Debanne D, McKinney RA, Thompson SM. Organotypic slice cultures: a technique has come of age. Trends Neurosci. 1997;20:471–477. doi: 10.1016/s0166-2236(97)01122-3. [DOI] [PubMed] [Google Scholar]
- Gerencser AA, Neilson A, Choi SW, Edman U, Yadava N, Oh RJ, Ferrick DA, Nicholls DG, Brand MD. Quantitative microplate-based respirometry with correction for oxygen diffusion. Anal Chem. 2009;81:6868–6878. doi: 10.1021/ac900881z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleichmann M, Collis LP, Smith PJ, Mattson MP. Simultaneous single neuron recording of O2 consumption, [Ca2+]i and mitochondrial membrane potential in glutamate toxicity. J Neurochem. 2009;109:644–655. doi: 10.1111/j.1471-4159.2009.05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote J, Laue O, Eiring P, Wehler M. Evaluation of brain tissue O2 supply based on results of PO2 measurements with needle and surface microelectrodes. J. Auton. Nerv. Syst. 1996;57:168–172. doi: 10.1016/0165-1838(95)00096-8. [DOI] [PubMed] [Google Scholar]
- Huchzermeyer C, Albus K, Gabriel HJ, Otahal J, Taubenberger N, Heinemann U, Kovacs R, Kann O. Gamma oscillations and spontaneous network activity in the hippocampus are highly sensitive to decreases in pO2 and concomitant changes in mitochondrial redox state. J Neurosci. 2008;28:1153–1162. doi: 10.1523/JNEUROSCI.4105-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y, Zorumski CF. Neuroprotective effects of pyruvate following NMDA-mediated excitotoxic insults in hippocampal slices. Neurosci Lett. 2010;478:131–135. doi: 10.1016/j.neulet.2010.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jekabsons MB, Nicholls DG. In situ respiration and bioenergetic status of mitochondria in primary cerebellar granule neuronal cultures exposed continuously to glutamate. J. Biol. Chem. 2004;279:32989–33000. doi: 10.1074/jbc.M401540200. [DOI] [PubMed] [Google Scholar]
- Jekabsons MB, Nicholls DG. Bioenergetic analysis of cerebellar granule neurons undergoing apoptosis by potassium/serum deprivation. Cell Death. Differ. 2006;13:1595–1610. doi: 10.1038/sj.cdd.4401851. [DOI] [PubMed] [Google Scholar]
- Johnson-Cadwell LI, Jekabsons MB, Wang A, Polster BM, Nicholls DG. ‘Mild Uncoupling’ does not decrease mitochondrial superoxide levels in cultured cerebellar granule neurons but decreases spare respiratory capacity and increases toxicity to glutamate and oxidative stress. J. Neurochem. 2007;101:1619–1631. doi: 10.1111/j.1471-4159.2007.04516.x. [DOI] [PubMed] [Google Scholar]
- Kauppinen RA, Nicholls DG. Synaptosomal bioenergetics. The role of glycolysis, pyruvate oxidation and responses to hypoglycaemia. Eur. J. Biochem. 1986;158:159–165. doi: 10.1111/j.1432-1033.1986.tb09733.x. [DOI] [PubMed] [Google Scholar]
- Kudin A, Vielhaber S, Beck H, Elger CE, Kunz WS. Quantitative investigation of mitochondrial function in single rat hippocampal slices: a novel application of high-resolution respirometry and laser-excited fluorescence spectroscopy. Brain Res Brain Res Protoc. 1999;4:329–334. doi: 10.1016/s1385-299x(99)00037-9. [DOI] [PubMed] [Google Scholar]
- Kudin AP, Kudina TA, Seyfried J, Vielhaber S, Beck H, Elger CE, Kunz WS. Seizure-dependent modulation of mitochondrial oxidative phosphorylation in rat hippocampus. Eur J Neurosci. 2002;15:1105–1114. doi: 10.1046/j.1460-9568.2002.01947.x. [DOI] [PubMed] [Google Scholar]
- Kunz WS, Goussakov IV, Beck H, Elger CE. Altered mitochondrial oxidative phosphorylation in hippocampal slices of kainate-treated rats. Brain Res. 1999;826:236–242. doi: 10.1016/s0006-8993(99)01279-2. [DOI] [PubMed] [Google Scholar]
- Land SC, Porterfield DM, Sanger RH, Smith PJ. The self-referencing oxygen-selective microelectrode: detection of transmembrane oxygen flux from single cells. J Exp Biol. 1999;202:211–218. doi: 10.1242/jeb.202.2.211. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Liu KJ, Bacic G, Hoopes PJ, Jiang J, Du H, Ou LC, Dunn JF, Swartz HM. Assessment of cerebral pO2 by EPR oximetry in rodents: effects of anesthesia, ischemia, and breathing gas. Brain Res. 1995;685:91–98. doi: 10.1016/0006-8993(95)00413-k. [DOI] [PubMed] [Google Scholar]
- Nishizaki T, Okada Y. Effects of excitatory amino acids on the oxygen consumption of hippocampal slices from the guinea pig. Brain Res. 1988;452:11–20. doi: 10.1016/0006-8993(88)90003-0. [DOI] [PubMed] [Google Scholar]
- Noraberg J, Poulsen FR, Blaabjerg M, Kristensen BW, Bonde C, Montero M, Meyer M, Gramsbergen JB, Zimmer J. Organotypic hippocampal slice cultures for studies of brain damage, neuroprotection and neurorepair. Curr Drug Targets CNS Neurol Disord. 2005;4:435–452. doi: 10.2174/1568007054546108. [DOI] [PubMed] [Google Scholar]
- Poli A, Migani P, Contestabile A, Barnabei O. Study of differential effects of kainic acid on metabolic rates, utilizing exogenous or endogenous substrates, in rat brain slices. J Neurochem. 1983;41:989–993. doi: 10.1111/j.1471-4159.1983.tb09042.x. [DOI] [PubMed] [Google Scholar]
- Rodnight R, McIlwain H. Techniques in tissue metabolism. III. Study of tissue fragments with little or no added aqueous phase, and in oils. Biochem J. 1954;57:649–661. doi: 10.1042/bj0570649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh RA, Matthews CC, Fishman PS. Interaction of mitochondrial respiratory inhibitors and excitotoxins potentiates cell death in hippocampal slice cultures. J. Neurosci Res. 2008;86:3306–3313. doi: 10.1002/jnr.21772. [DOI] [PubMed] [Google Scholar]
- Shen H, Hu X, Liu C, Wang S, Zhang W, Gao H, Stetler RA, Gao Y, Chen J. Ethyl pyruvate protects against hypoxic-ischemic brain injury via anti-cell death and anti-inflammatory mechanisms. Neurobiol Dis. 2010;37:711–722. doi: 10.1016/j.nbd.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Vornov JJ, Tasker RC, Park J. Neurotoxicity of acute glutamate transport blockade depends on coactivation of both NMDA and AMPA/Kainate receptors in organotypic hippocampal cultures. Exp Neurol. 1995;133:7–17. doi: 10.1006/exnr.1995.1002. [DOI] [PubMed] [Google Scholar]
- Wu M, Neilson A, Swift AL, Moran R, Tamagnine J, Parslow D, Armistead S, Lemire K, Orrell J, Teich J, Chomicz S, Ferrick DA. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am. J. Physiol Cell Physiol. 2007;292:C125–C136. doi: 10.1152/ajpcell.00247.2006. [DOI] [PubMed] [Google Scholar]
- Yadava N, Nicholls DG. Spare respiratory capacity rather than oxidative stress regulates glutamate excitotoxicity after partial respiratory inhibition of mitochondrial complex I with rotenone. J. Neurosci. 2007;27:7310–7317. doi: 10.1523/JNEUROSCI.0212-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]