Abstract
At the time of fertilization, an increase in the intracellular Ca2+ concentration ([Ca2+]i) underlies egg activation and initiation of development in all species studied to date. The inositol 1,4,5-trisphosphate receptor (IP3R1), which is mostly located in the endoplasmic reticulum (ER) mediates the majority of this Ca2+ release. The sensitivity of IP3R1, i.e. its Ca2+ releasing capability, is increased during oocyte maturation so that the optimum [Ca2+]i response concurs with fertilization, which in mammals occurs at metaphase of second meiosis. Multiple IP3R1 modifications affect its sensitivity, including phosphorylation, sub-cellular localization and ER Ca2+ concentration ([Ca2+]ER). Here we evaluated using mouse oocytes how each of these factors affected IP3R1 sensitivity. The capacity for IP3-induced Ca2+ release markedly increased at the germinal vesicle breakdown stage, although oocytes only acquire the ability to initiate fertilization-like oscillations at later stages of maturation. The increase in IP3R1 sensitivity was underpinned by an increase in [Ca2+]ER and receptor phosphorylation(s) but not by changes in IP3R1 cellular distribution, as inhibition of the former factors reduced Ca2+ release, whereas inhibition of the latter had no impact. Therefore, the results suggest that the regulation of [Ca2+]ER and IP3R1 phosphorylation during maturation enhance IP3R1 sensitivity rendering oocytes competent to initiate oscillations at the expected time of fertilization. The temporal discrepancy between the initiation of changes in IP3R1 sensitivity and acquisition of mature oscillatory capacity suggest that other mechanisms that regulate Ca2+ homeostasis also shape the pattern of oscillations in mammalian eggs.
Keywords: Oocyte maturation, mammalian eggs, Ca2+, IP3 receptor
Introduction
Before fertilization, vertebrate eggs are arrested at the metaphase stage of the second meiosis (MII). Sperm entry induces cortical granule exocytosis, extrusion of the second polar body (2PB), pronuclear (PN) formation and entry into first mitosis (Schultz and Kopf, 1995); these phenomena are collectively known as ‘egg activation’. In all species studied to date, egg activation requires a dramatic increase in the intracellular Ca2+ concentration ([Ca2+]i) (Stricker, 1999). In mammals, a series of increases in [Ca2+]i that last for several hours after sperm entry, generally known as [Ca2+]i oscillations, is responsible for egg activation (Miyazaki et al., 1993). The correct spatio-temporal pattern of these oscillations is not established until late during oocyte maturation, as for instance, in vitro fertilized immature germinal vesicle (GV) oocytes show fewer oscillations and each [Ca2+]i rise exhibit lesser duration and amplitude than those observed in fertilized MII eggs (Jones et al., 1995; Mehlmann and Kline, 1994). However, the mechanisms underlying the enhanced Ca2+ releasing ability of matured oocytes, here referred to as eggs, are not well understood.
In vertebrate eggs, inositol 1,4,5-trisphosphate (IP3)-mediated Ca2+ release from intracellular stores is primarily responsible for the increase in [Ca2+]i at fertilization (Miyazaki et al., 1992). Fittingly, the discovery of the sperm-specific phospholipase C ζ(plcζ) (Saunders et al., 2002), which in the presence of basal concentrations of [Ca2+]i effectively hydrolyzes phosphatidylinostitol (4,5)-bisphosphate generating IP3(Rebecchi and Pentyala, 2000), supports the involvement of this pathway in mammalian fertilization. The type 1 IP3 receptor (IP3R1), which in mammalian eggs is the predominantly expressed isoform (Fissore et al., 1999; Parrington et al., 1998) and is located in the endoplasmic reticulum (ER), the main Ca2+ reservoir in the cell (Berridge, 2002), acts as a IP3-gated Ca2+ channel. The importance of this system in mammalian fertilization is further evidenced by the findings that specific inhibition of IP3R1 prevents Ca2+ release at fertilization and blocks the initiation of development (Miyazaki et al., 1992).
Changes in IP3R1 conductivity may underpin the changes in the spatio-temporal [Ca2+]i responses that occur during oocyte maturation. In agreement with this notion, research has shown that IP3R1 sensitivity, i.e. the receptor's ability to conduct Ca2+ in response to increase in IP3, is enhanced at the MII stage (Fujiwara et al., 1993; Mehlmann and Kline, 1994; Sun et al., 2009). Nevertheless, the receptor’s modifications responsible for enhancing its function have not been clearly defined, although several possibilities exist. Studies have reported that phosphorylation of different IP3R isoforms by various kinases in somatic cells generally increases IP3-induced Ca2+ release (Bezprozvanny, 2005; Vanderheyden et al., 2009a). Most of these studies comprise kinases such as protein kinase A (PKA) and protein kinase C (PKC), whose activities are not restricted to M-Phase like stages of the cell cycle, which is when IP3R1 function in eggs is enhanced. On the other hand, since the initiation and progression of meiosis are controlled by M-phase kinases, it is logical to propose that these kinases may also regulate IP3R1 function in eggs. In agreement with this possibility, our previous studies demonstrated that IP3R1 becomes phosphorylation at an MPM-2 epitope, which is commonly phosphorylated by M-phase kinases during oocyte maturation (Ito et al., 2008; Lee et al., 2006; Vanderheyden et al., 2009b). Although it is still unclear what kinase(s) is responsible for this phosphorylation, and at what site(s) or domain(s) these modification(s) takes place.
A second mechanism that might underlie the increased IP3R1 sensitivity in oocytes at the end of maturation is the differential redistribution of IP3R1. In mice, the architecture of the ER in MII eggs displays a fine tubular network appearance and dense accumulation in the cortex (Mehlmann et al., 1995), which is thought to be an important factor for sperm-induced [Ca2+]i oscillations (Kline et al., 1999). ER reorganization during mouse oocyte maturation is underpinned by distinct components of the cytoskeleton (FitzHarris et al., 2007). An initial migration of the ER around the condensing chromosomes at germinal vesicle breakdown (GVBD) is dependent on microtubules, whereas the ER reorganization into cortical clusters at MII stage is dependent on microfilaments. Similar to the ER distribution, conspicuous cortical clusters of IP3R1 were observed in MII eggs (Fissore et al., 1999; Mehlmann et al., 1996), yet the relationship between ER and IP3R1 clustering is unclear. Furthermore, whether IP3R1 redistribution is controlled by the same mechanisms as those of the ER, and whether it plays a role in IP3R1 sensitivity remains to be clarified.
Changes in other cellular parameters may also increase IP3R1 sensitivity during oocyte maturation. For example, a marked increase in the ER Ca2+ concentration ([Ca2+]ER) is observed during this period (Jones et al., 1995). Furthermore, the Ca2+ influx and efflux pathways, which supply the Ca2+ for the ER and maintain Ca2+ homeostasis, respectively, are actively modulated in Xenopus oocytes during maturation (El-Jouni et al., 2005; Machaca and Haun, 2000; Yu et al., 2009), yet we remain largely uninformed of their identity and function in mammalian eggs. Another complicating factor in determining which IP3R1 modification affects the receptor’s function is that several of these changes occur simultaneously during maturation. Therefore, in this study, we independently evaluated the effects of IP3R1 phosphorylation, IP3R1 redistribution and [Ca2+]ER content on IP3-mediated Ca2+ release. We found that IP3R1 phosphorylation and [Ca2+]ER are the critical determinants underlying the increase in IP3R1 sensitivity during oocyte maturation required for successful fertilization.
Materials and Methods
Collection of oocytes and culture conditions
GV oocytes were collected from the ovaries of 4- to 6-week-old CD-1 female mice. Females were injected with 5 IU pregnant mare serum gonadotropin (PMSG; Sigma, St Louis, MO). Cumulus cell-enclosed oocytes were recovered 42–46 hr post-PMSG into HEPES-buffered Tyrode-Lactate solution (TL-HEPES) supplemented with 5% heat-treated fetal calf serum (Gibco, GGrand Island, NY) and 100 µM isobutyl-1-methylxanthine (IBMX). The cumulus cells were removed by repeated pipetting and denuded oocytes were matured in Chatot, Ziomek, and Bavister (CZB) medium (Chatot et al., 1989) containing 0.1% polyvinyl alcohol (PVA; Sigma, St Louis, MO) at 37 ˚C under an atmosphere of 5% CO2. Animal handing and procedures were approved by the University’s IACUC committee.
Preparation of pharmacological inhibitors
Cyclopiazonic acid (CPA), Roscovitine, RO-3306, H-89 and Latrunculin A (Lat-A) were purchased from Calbiochem (San Diego, CA). The stock solutions were prepared in dimethyl sulfoxide (DMSO; Sigma, St Louis, MO) and further diluted in CZB-PVA medium to their final concentration.
Antibodies
Two polyclonal antibodies raised against the same peptide sequence on the C-terminal end of the molecule were used to detect IP3R1. Rbt03 (Parys et al., 1995) was used for western blot analysis, whereas CT1 (Wojcikiewicz et al., 1994), which was affinity purified, was used for immunofluorecent staining. The MPM-2 monoclonal antibody (Upstate biotechnology, Lake Placid, NY) was used to ascertain IP3R1 phosphorylation as previously reported (Jellerette et al., 2004). Phospho-PKA (Ser/Thr) substrate antibody (Cell Signaling Technology, Beverly, MA) was used to detect substrates of PKA. Two polyclonal antibodies raised in rabbits against phosphopeptide sequences (MLKIGTS*PVKEDKEA and DPQEQVT*PVKYARL) within mouse IP3R1 that contain the S421 and T799 phosphorylation residues, respectively, were used for western blotting (Malathi et al., 2005). These antibodies were a kind gift of Dr. T. Jayaraman (St. Luke’s Roosevelt Hospital Center, New York, NY). Species-specific HRP-conjugated secondary antibodies were used for western blotting and immunofluorescent staining.
Western blotting
Cell lysates were prepared by adding 2X LSB buffer to mouse oocytes/eggs after which the samples were boiled for 5 min. Samples were loaded onto NuPAGE Novex 3–8% Tris-acetate gel (Invitrogen, Carlsbad, CA). After electrophoresis, proteins were transferred onto nitrocellulose membranes and probed with MPM-2 (1/500), phospho-PKA substrate (1/1000), phospho-S421 (1/500) or phospho-T799(1/500) antibodies. Species-specific secondary antibodies were used for detection with chemiluminescence (NEN Life Science Products, Boston, MA) according to the manufacturer's instructions, and blots were digitally captured using a Kodak Imaging Station (440 CF, Rochester, NY). To detect total IP3R1, membranes were stripped and re-probed with Rbt03 antibody (1/1000) and captured as above.
Immunofluorescent staining
Immunofluorescent staining of IP3R1 was performed as previously described (Ito et al., 2008). In brief, zona pellucida-free oocytes/eggs (removed by brief treatment with acid Tyrode solution, pH 2.7), were washed several times in 0.1% BSA-supplemented Dulbecco's PBS (PBS-BSA) and then fixed in PBS-BSA containing 3.7% paraformaldehyde and permeabilized with 0.1 % Triton X-100. Samples were then transferred into PBS-PVA supplemented with 5% goat serum for 2 hr at 4°C followed by incubation with CT1 antibody overnight at 4°C. Oocytes/eggs were then washed with PBS-BSA, and incubated with Alexa fluor 555-conjugated goat anti rabbit IgG (Molecular Probes, Eugene, OR) for 1 hr at room temperature. Samples were mounted on slide glasses and examined using a laser-scanning confocal microscope (LSM 510 META, Carl Zeiss Microimaging Inc., Germany) outfitted with a 63×1.4 NA oil immersion objective lens.
Microinjections
Oocytes were microinjected as previously described (Kurokawa et al., 2005). Reagents were loaded into glass micropipettes and delivered by pneumatic pressure (PLI-100 picoinjector, Harvard Apparatus, Cambridge, MA). Each oocyte received 7–12 pl (∼1–3% of the total volume of the egg). The full-length coding sequence of mouse plcζ was a kind gift from Dr K. Fukami (Tokyo University of Pharmacy and Life Science, Japan) and that of human YFP-STIM1 was generously contributed by Dr. T. Meyer (Stanford University, Stanford, CA). These sequences were subcloned into pcDNA6/myc-His (Invitrogen, San Diego, CA), which contains a T7 promoter suitable for in vitro transcription. Plasmids were linearized and cDNA was in vitro transcribed using the T7 mMESSAGE mMACHINE Kit (Ambion, Austin, TX), as reported by us (Kurokawa et al., 2004).
[Ca2+]i imaging
[Ca2+]i was measured using the Ca2+ sensitive dyes Fura-2AM or Fluo-4AM (Molecular Probes). Recording of [Ca2+]i oscillations was performed as previously described (Kurokawa et al., 2005). Oocytes were loaded with 1.25 µM Fura-2AM supplemented with 0.02% pluronic acid (Molecular Probes) for 20 min at room temperature. Oocytes were then placed in drops of TL-HEPES under mineral oil and fluorescence ratios of 340/380 nm were obtained every 20 sec. To estimate [Ca2+]ER, oocytes were monitored in Ca2+-free TL-Hepes supplemented with 1 mM EGTA, after which they were treated with 2 µM ionomycin. To analyze IP3-induced Ca2+ release, 0.25 mM caged-IP3 (cIP3) (Molecular Probes) was microinjected into oocytes. Oocytes were loaded with 1.25 µM Fluo-4AM for 20 min and photolysis was performed 1–2 hr after microinjection of cIP3 in Ca2+-free TL-HEPES + EGTA. cIP3 photolysis was accomplished with a 360 nm wavelength UV light provided by a 75 W Xenon arc lamp and modulated by neutral density filters, which also produced the 480 nm wavelength required to excite Fluo-4. The emitted light above 510 nm was collected by a cooled Photometrics SenSys CCD camera (Roper Scientific, Tucson, AZ). [Ca2+]i changes and filter wheel changes were controlled by using the software SimplePCI (C-Imaging System, Cranberry Township, PA).
Statistical analysis
Values from three or more experiments performed on different batches of oocytes/eggs were analyzed by the Student's t-test or one-way ANOVA followed by Fishers’protected least significant difference test using the STATVIEW program (Abacus Concepts, Inc.). Differences were considered significant at p < 0.05. Values are given as means±SEM. Significance among groups/treatments is denoted in bar graphs by different superscripts or by the presence of asterisks.
Results
[Ca2+]i oscillatory activity and IP3R1 sensitivity are enhanced during oocyte maturation
Multiple compounds have in various studies been used to probe the enhanced Ca2+ releasing ability of mouse oocytes during maturation, including sperm (Deng and Shen, 2000; Jones et al., 1995; Mehlmann and Kline, 1994), sperm extracts (Carroll et al., 1994), IP3 (Carroll and Swann, 1992; Mehlmann and Kline, 1994), thapsigargin (Jones et al., 1995) and ionomycin (Jones et al., 1995; Mehlmann and Kline, 1994), strontium chloride (Cui et al., 2005) and thimerosal (Carroll and Swann, 1992; Mehlmann and Kline, 1994). While all studies concur that IP3R1 sensitivity increases during maturation, the precise stage at which this occurs and how it relates to the acquisition of MII-like oscillatory ability cannot be determined from those studies. We thus examined in mouse oocytes the temporal association between the acquisition of the [Ca2+]i oscillatory activity and the changes in IP3R1 sensitivity. The observations were performed at 0, 4, 8 and 12 hr of in vitro maturation, which corresponded with GV, GVBD, MI and MII stages of meiotic progression, respectively.
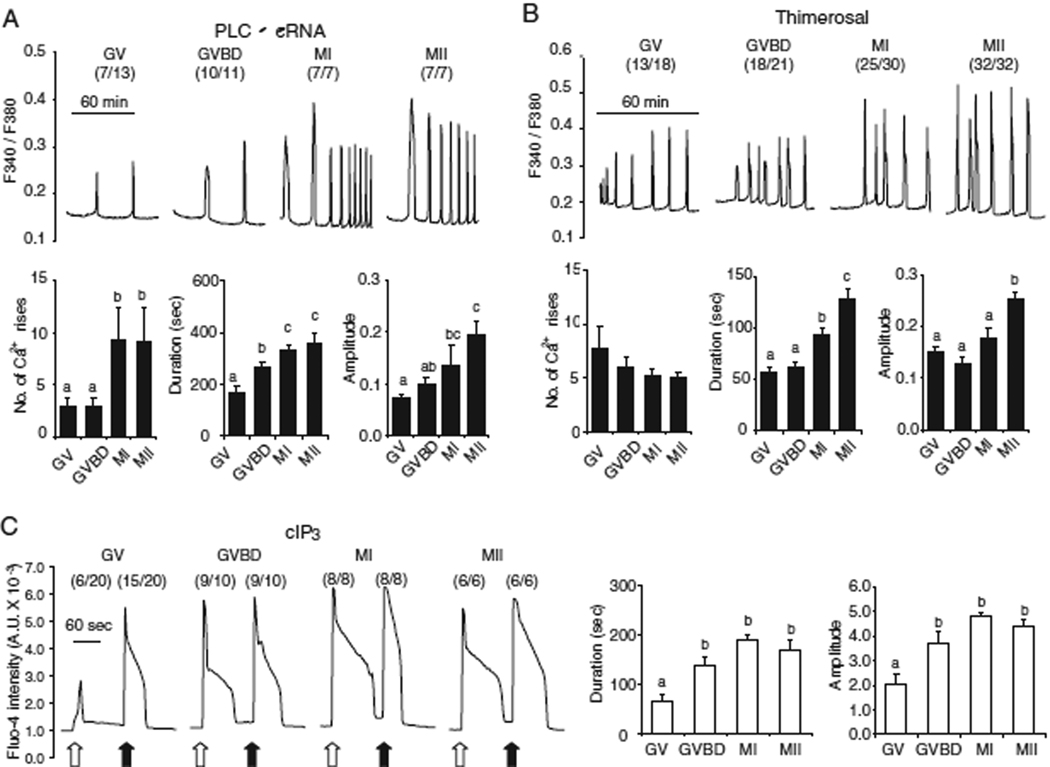
Oscillations were first induced by injection of 0.05 µg/µl plcζ mRNA. In MII eggs, as expected, injection of plcζ mRNA initiated [Ca2+]i oscillations in all cells (5/5), and the first [Ca2+]i rise displayed longer duration and greater amplitude than subsequent [Ca2+]i rises (Fig. 1A). In GV oocytes, however, only 7/13 injected cells showed [Ca2+]i, rises, which were of low amplitude and frequency (Fig. 1A; left trace). For example, the duration of the first rise in GV oocytes was less than half of that in MII oocytes (165±25 sec vs. 360±38 sec) and the amplitude was ∼ one-third (0.07±0.01 vs. 0.20±0.25). In addition, the first [Ca2+]i peak in GV oocytes was generally similar in magnitude to the subsequent [Ca2+]i rises. As maturation progressed, the pattern of oscillations initiated by plcζ mRNA injection gradually changed (Fig. 1A) and by the MI stage (Fig. 1A; center right trace) nearly all individual parameters became indistinguishable of those at the MII stage. It is important to note the lower responses induced by plcζ mRNA injection at the GV stage are unlikely to be due to lower protein expression as this stage, as GV stage oocytes exhibit maximal translational activity, as determined by expression of Venus mRNA (data not shown).
Fig. 1.
[Ca2+]i oscillatory activity and IP3R1 sensitivity change at different times during oocyte maturation. (A and B) [Ca2+]i responses were induced by 0.05 µg/µl plcζ cRNA (A) and 25 µM thimerosal (B). The number of oocytes showing more than two [Ca2+]i rises is shown in parentheses. The number of [Ca2+]i rises and the duration and amplitude of the first [Ca2+]i rise were compared between the different stages (GV, GVBD, MI and MII) of maturation. (C) IP3-induced Ca2+ release obtained after photolysis of cIP3 (0.25mM) by a flash of UV light. The flashes were of 0.001 sec (open arrow) and of 0.1 sec (closed arrow) duration. A representative trace is shown for each stage, and the number of oocytes responding to cIP3 is shown in parentheses. The duration and amplitude of Ca2+ release caused by the 0.001 sec UV pulse were compared between the different stages. Error bars represent SEM. Bars with different superscripts are significantly different (P < 0.05).
We next initiated [Ca2+]i oscillations with 25 µM thimerosal, a selective sulphydryl reagent that is known to promote [Ca2+]i oscillations through IP3R1 without inducing IP3 production (Cheek et al., 1993; Swann, 1992). Treatment with thimerosal induced [Ca2+]i oscillations at all stages of maturation (Fig. 1B), although as it was the case with injection of plcζ mRNA, the pattern of [Ca2+]i oscillations gradually changed attaining a near mature configuration by the MI stage. The duration and amplitude of first [Ca2+]i rise peaked at the MII stage. In contrast to plcζ mRNA, there was no significant difference in the number of [Ca2+]i rises between the different stages.
The previous results showed a marked change in individual [Ca2+]i rise parameters and oscillatory ability between the GVBD and MI stages. Nevertheless, whether these changes coincided with increases in IP3R1 sensitivity was not known. We therefore directly examined IP3R1 function during maturation using cIP3, which was released into the ooplasm by a flash of UV light. To exclude the participation of Ca2+ influx, these experiments were performed in Ca2+-free medium. UV flashes of different durations (0.001 and 0.1 sec) were applied to oocytes of all stages and each pulse generated a single [Ca2+]i rise. The 0.001 sec UV pulse induced Ca2+ release in 6/20 GV oocytes but in all or nearly all oocytes at the GVBD, MI and MII stage. While the few GV oocytes that responded to the short pulse did so with [Ca2+]i rises of reduced duration and amplitude (Fig. 1C), the magnitude of the [Ca2+]i responses appeared comparable among all other stages (Fig. 1C, bar graph). In contrast, the longer UV flash induced [Ca2+]i responses in most GV oocytes (15/20) as well as in all or nearly oocytes of more advances stages of maturation (Fig. 1C), and the parameters of the induced [Ca2+]i rises were not different among the stages. Collectively, our results show that IP3R1 sensitivity increases between the GV and GVBD stages, although the acquisition of mature oscillatory ability is delayed until the MI to MII transition.
Increased ER Ca2+ store content enhances IP3R1-mediated Ca2+ release and is required for complete [Ca2+]i responses to Plcζ mRNA
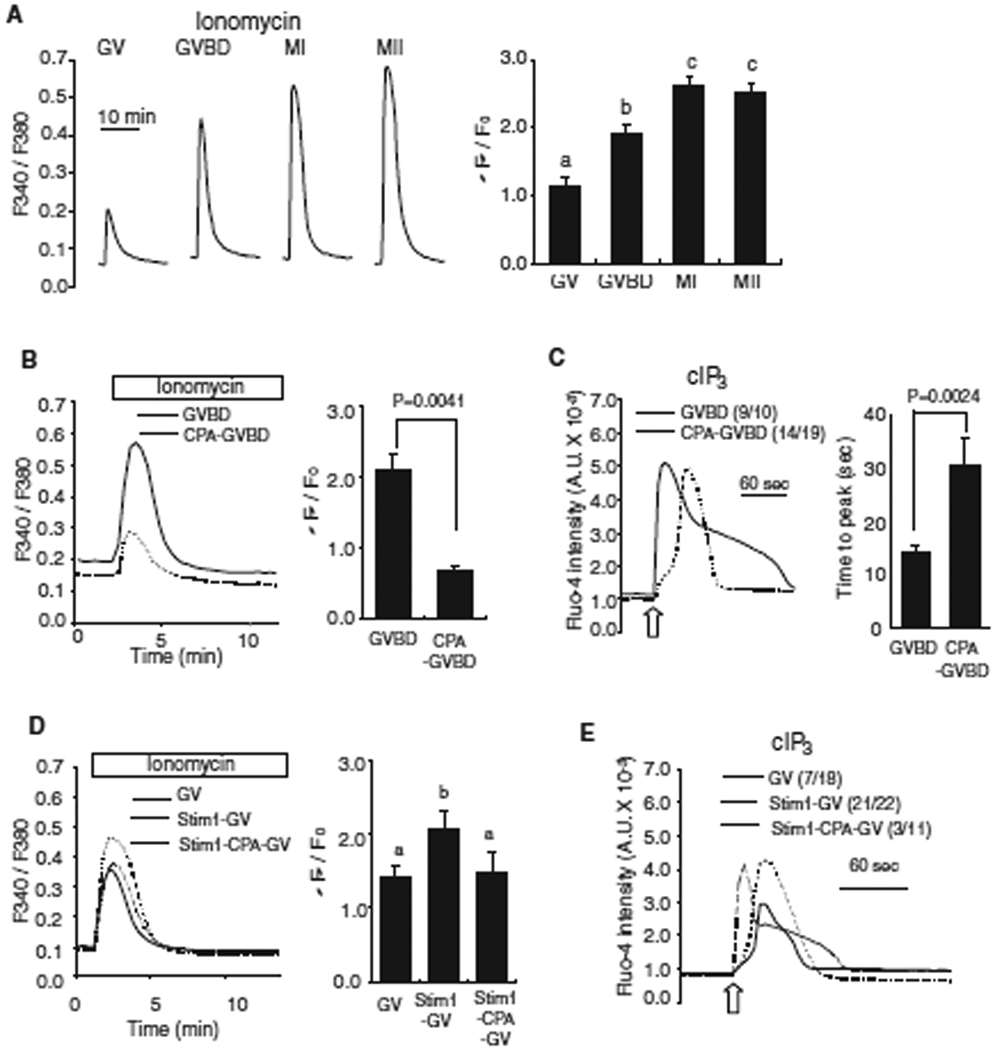
Previous reports have documented the increase in the [Ca2+]ER during mouse oocyte maturation (Jones et al., 1995). Therefore, we asked whether this was one of the factors underlying the increase in IP3R1 sensitivity between the GV and GVBD stages in oocytes. We first confirmed that [Ca2+]ER increased throughout maturation by exposing oocytes of all stages of maturation to 2 µM ionomycin. Ionomycin-induced Ca2+ release sharply increased between the GV and GVBD stages and less so thereafter until the MII stage (Fig. 2A). A similar result was obtained when the oocytes were treated with 10 µM thapsigargin (data not shown), which is a inhibitor of the sarco-endoplasmic reticulum Ca2+ ATPase pump (SERCA) (Thastrup et al., 1990). To ascertain how [Ca2+]ER contributes to IP3R1-mediated Ca2+ release, we treated oocytes with 50 µM CPA, a reversible SERCA inhibitor (Seidler et al., 1989), with the expectation to decrease [Ca2+]ER content. CPA-matured GV oocytes advanced to the GVBD stage without delay or gross abnormalities, although, as expected, they failed to increase their [Ca2+]ER, as demonstrated by the reduced response to ionomycin (Fig. 2B). We used this experimental paradigm to examine whether lower [Ca2+]ER impacted IP3R1-mediated Ca2+ release. To accomplish this, Ca2+ release in control and in CPA-treated oocytes was induced using cIP3, as previously described. Treatment with CPA modestly decreased the number of oocytes responding to IP3, and it significantly delayed (P < 0.01) [Ca2+]i responses (Fig. 2C).
Fig. 2.
The increase in [Ca2+]ER during oocyte maturation enhances IP3R1-mediated Ca2+ release. (A) [Ca2+]ER was estimated from the [Ca2+]i responses induced by the addition of 2 µM ionomycin while oocytes were in Ca2+ free medium. The mean fluorescence peak was compared between the different stages of oocytes and shown on a bar graph. Error bar, SEM (n=10). Bars with different superscripts are significantly different (P < 0.05). (B) [Ca2+]ER was compared in GVBD oocytes matured in the absence (solid line; n=4) or presence (dashed line; n=3) of CPA (50µM). The comparison of fluorescent [Ca2+]i peak is shown in bar graph to the right of the traces. (C) IP3-induced Ca2+ release using cIP3 was measured in control (solid line) and CPA-treaded (dashed line) oocytes. A 0.001 sec UV pulse was applied to oocytes (open arrow) and a representative trace is shown with the number of oocytes responding to cIP3 presented in parentheses. The time needed to reach peak [Ca2+]i fluorescence was measured in each oocyte and compared between the two groups (right graph). (D) [Ca2+]ER was measured in GV-stage control oocytes (solid line; n=10), oocytes injected with Stim1 cRNA (dashed line; n=10) and Stim1-injected oocytes treated with CPA for 2 hrs (dotted line; n=5) after addition of ionomycin; comparisons are shown in a bar graph. (E) A representative [Ca2+]i trace caused by cIP3 release in control (solid line), Stim1 over-expressed oocytes (dashed line) and Stim1-injected oocytes treated with CPA for 2 hrs (dotted line) is shown. The number of oocytes responding to cIP3 is shown in parentheses.
We also tested whether increased [Ca2+]ER content could enhance IP3R1 sensitivity in GV stage oocytes. To accomplish this, we over-expressed Stim1, a protein recently suggested to be required for store-operated Ca2+ entry (Liou et al., 2005; Roos et al., 2005), acting as a sensor of decreased [Ca2+]ER levels and leading to activation of CRAC channels in the plasma membrane (Luik et al., 2006). We found that over-expression of STIM1 mRNA increased [Ca2+]ER levels in GV oocytes (Fig. 2D) and used this approach to ascertain IP3R1 sensitivity in the presence of elevated [Ca2+]ER. As shown in Fig. 2E, IP3R1 sensitivity in GV oocytes was enhanced by higher [Ca2+]ER conditions (Fig. 2E). To ascertain whether the effects of STIM1 over expression were mostly due to the enhanced levels of [Ca2+]ER, STIM1 over-expressing oocytes were treated with CPA for 2 hrs, which negated the effect of STIM1 over-expression on [Ca2+]ER levels (Fig. 2D), followed by monitoring of cIP3-induced Ca2+ release. cIP3 pulse induced an enhanced Ca2+ release in STIM1 over-expressing oocytes (21/22), whereas CPA abrogated this gain and delayed responses in STIM1 over expressing oocytes (3/11). Therefore, we interpret these results to mean that STIM1 over-expression enhances IP3-induced Ca2+ release because it augments [Ca2+]ER levels. The results also suggest that higher [Ca2+]ER levels increase both the amount of Ca2+ available for release and IP3R1 sensitivity.
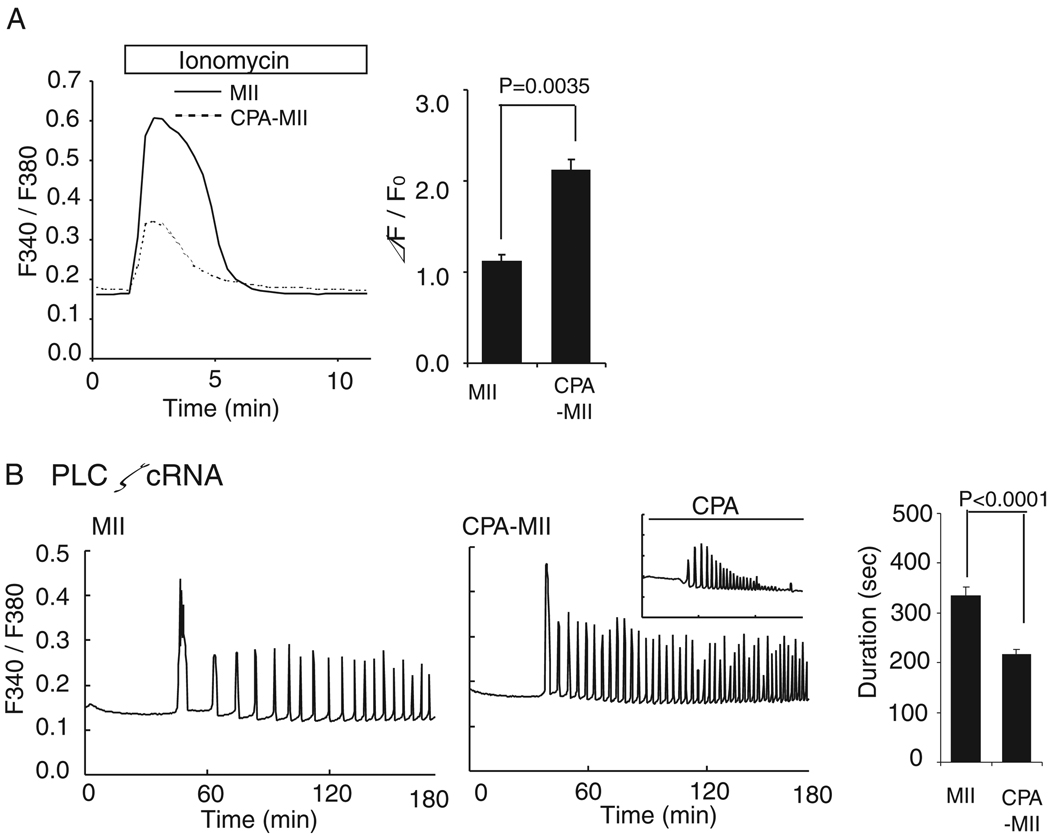
To investigate the overall role of increased [Ca2+]ER on fertilization, oocytes matured for 12hr in CPA were injected with PlcζmRNA and CPA washed away just prior to the initiation of [Ca2+]i measurements. CPA-matured oocytes progressed to the MII stage without delay or gross abnormalities (data not shown), but they failed to increase their [Ca2+]ER, as estimated from the [Ca2+]i responses induced by ionomycin at the MII stage (Fig. 3A). PlcζmRNA-injected CPA-matured oocytes showed a reduced first [Ca2+]i rise (P<0.0001), although subsequent [Ca2+]i rises appeared unchanged and even occurred with higher frequency than controls (Fig. 3B). As expected, in the continuous presence of CPA, Plcζ mRNA-induced [Ca2+]i oscillations displayed reduced amplitude and terminated prematurely (Fig. 3B; inset). Thus, our results suggest that to obtain a complete initial [Ca2+]i rise and maintain undiminished [Ca2+]i oscillations in response to Plcζ mRNA and most likely sperm, [Ca2+]ER must increase during maturation and must be refilled during oscillations.
Fig. 3.
The role of increase in [Ca2+]ER during oocyte maturation in [Ca2+]i oscillatory activity. (A) [Ca2+]ER was compared in GVBD oocytes matured in the absence (solid line; n=5) or presence (dashed line; n=8) of CPA (50µM) after addition of ionomycin; comparisons are shown in a bar graph. (B) [Ca2+]i responses were induced by 0.05 µg/µl plcζ cRNA. A representative [Ca2+]i trace in MII-stage control (left, n=15) and oocytes matured in the presence of CPA (right, n=22) is shown. [Ca2+]i oscillations performed in the continuous presence of CPA is shown in a small panel (n=6). The duration of the first [Ca2+]i rise was compared between control and CPA-matured oocytes and shown on a bar graph.
Mechanisms independent of ER Ca2+ store content also enhance IP3R1-mediated Ca2+ release during maturation
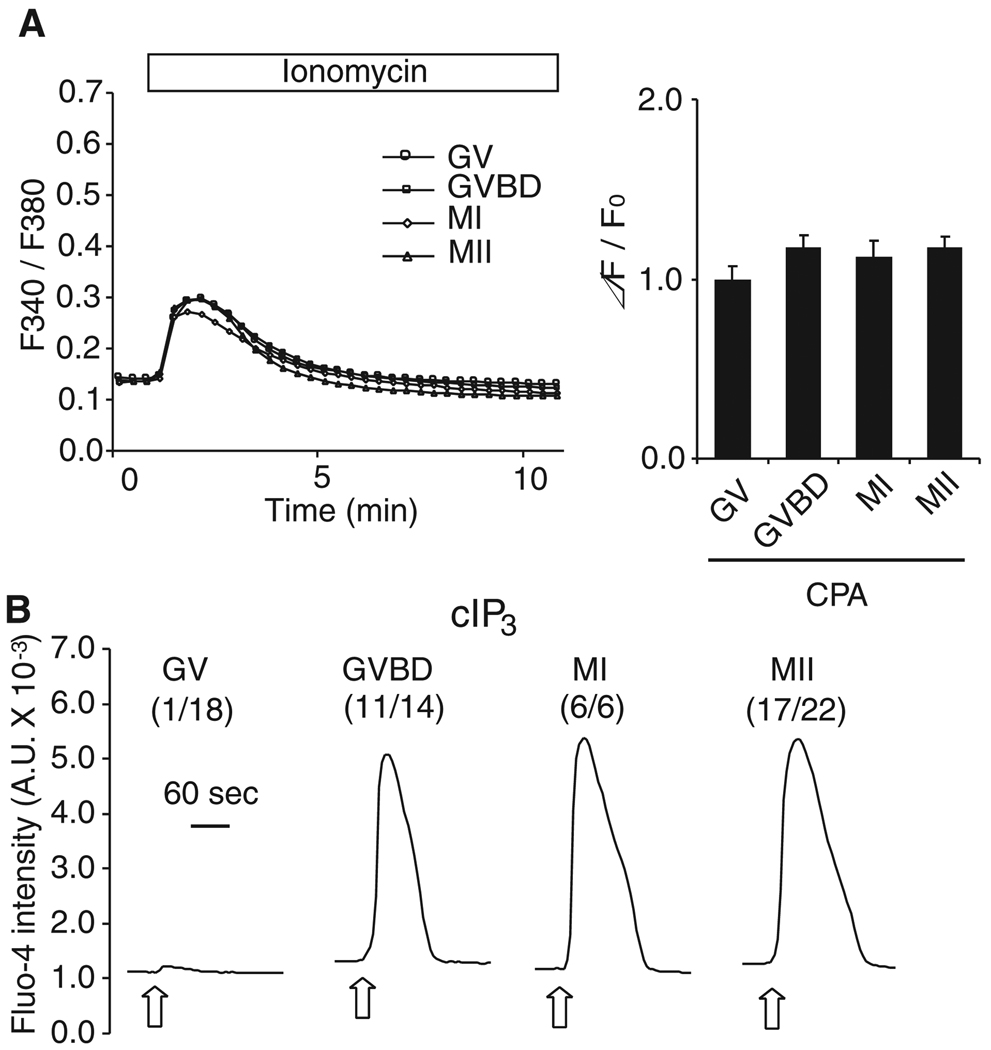
While IP3R1-mediated Ca2+ release is impaired in GVBD oocytes matured in the presence of CPA, IP3-induced Ca2+ release was far greater in these oocytes than in GV oocytes, implying that mechanisms other than [Ca2+]ER content also modulate IP3R1 sensitivity. To investigate this possibility in more detail, IP3R1-mediated Ca2+ release had to be performed in oocytes at different stages of maturation but with similar [Ca2+]ER content. To prevent the maturation-associated increase in [Ca2+]ER, we harnessed the ability of CPA to inhibit SERCA without altering meiotic progression. Importantly, to expose all oocytes to CPA for the same time but taking into account that all stages of maturation needed to be evaluated simultaneously, oocytes were cultured in the presence of CPA for 12 hr but for variable time in IBMX (GV, GVBD and MI oocytes were cultured with IBMX for 12, 8 and 4 hr, respectively). Ionomycin was used to examine whether this treatment equalized [Ca2+]ER despite meiotic progression. We found that maturation in the presence of CPA successfully prevented the increase in [Ca2+]ER, as ionomycin promoted similar [Ca2+]i increases in oocytes at all stages of maturation (Fig. 4A). We then proceeded to examine the effect on IP3R1-mediated Ca2+ release using cIP3. We found that even under conditions where the increase in [Ca2+]ER was prevented, IP3-induced Ca2+ release was greater at the GVBD stage and stages thereafter, whereas only 1/18 GV stage oocytes showed small responses to cIP3 with the short UV pulse (Fig. 4B). Thus, we interpret these results to mean that IP3R1 sensitivity in oocytes is controlled by other factors besides the increase in [Ca2+]ER.
Fig. 4.
IP3R1-mediated Ca2+ release is enhanced independently of [Ca2+]ER during oocyte maturation. (A) Oocytes were cultured in the presence of CPA (50µM) for 12hr but for variable times in IBMX (GV, GVBD and MI oocytes were cultured in IBMX for 12, 8 and 4 hr, respectively). [Ca2+]ER content was estimated following addition of 2 µM ionomycin in Ca2+ free medium and shown to be comparable in all stages of maturation (bar graph on right). Error bar, SEM (n=9–10). (B) IP3-induced Ca2+ release was caused by release of cIP3 in CPA-treated oocytes. UV flashes of 0.001 sec (open arrow) were applied to oocytes in all stages of maturation. A representative trace is shown for each stage, and the number in parentheses represents oocytes responding to cIP3 in each stage.
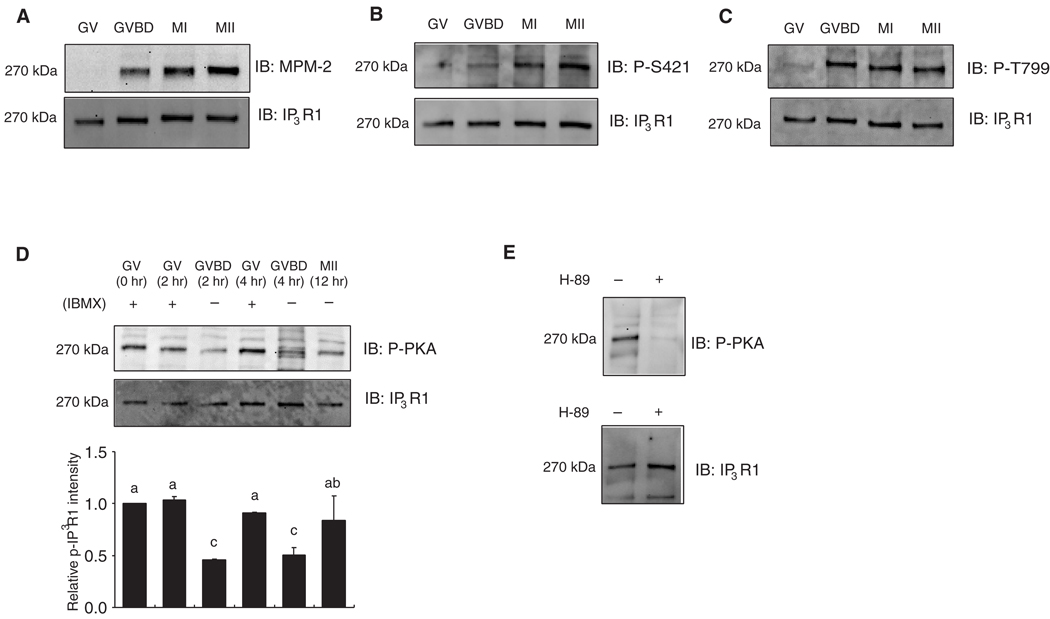
IP3R1 undergoes multiple phosphorylations during oocyte maturation
Phosphorylation is known to modify IP3R1 sensitivity in somatic cells, although the probable impact on IP3R1 sensitivity and possible sites of these phosphorylations in mammalian oocytes has not been determined. Our own studies have shown that IP3R1 becomes phosphorylated during maturation at a site(s) recognized by an antibody raised against the MPM-2 epitope and at a time that coincides with GVBD (Lee et al., 2006), which we confirmed in the present study (Fig. 5A). A number of M-phase kinases including cyclin-dependent kinase 1 (Cdk1), mitogen-activated protein kinase (MAPK) and polo-like kinase 1 (Plk1) become activated around the GVBD stage and reportedly phosphorylate MPM-2 epitopes (Lee et al., 2006; Vanderheyden et al., 2009b), which has hindered the identification of the responsible kinase(s) and the site(s) at which it takes place. IP3R1 displays two highly conserved Cdk1 consensus sites, S/T-P-X-K/R (Nigg, 2001), which are centered on amino acids S421 and T799 and that have been shown to be phosphorylated in somatic cells (Malathi et al., 2003; Malathi et al., 2005). We used these site- and phospho-specific IP3R1 antibodies developed in the latter studies to probe the phosphorylation status of the receptor in mouse oocytes. Our results show that while both S421 and T799 are dephosphorylated at the GV stage, they gain immunoreactivity by the GVBD stage and remain so until the MII stage (Fig. 5B and C).
Fig. 5.
Multiple phosphorylations of IP3R1 during oocyte maturation. (A–C) Immunoblots (IB) of oocytes lysates at the different stages of maturation were probed with the MPM-2 (A), phospho-S421 (B) and phospho-T799 (C) antibodies (upper panel), and after stripping of the blot, re-probed with IP3R1 antibody (lower panel). A representative result of 2–3 independent experiments with similar results is shown. Thirty oocytes for MPM-2 and 100 oocytes for phospho-S421 and phospho-T799 analysis were used per lane, both here and elsewhere in this study. (D) IP3R1 PKA (upper panel) and IP3R1 reactivity (lower panel) in oocytes at the different stages (GV, GVBD and MII). One of two independent experiments with similar results is shown. Fifty oocytes were used per lane. The intensity of the PKA reactive band from GV oocytes was arbitrarily given the value of 1 and values in the other lanes were expressed relative to this band. Data are presented as mean±SEM (n=2). Bars with different superscripts are significantly different (P < 0.05). (B) Oocytes were cultured in the absence or presence of H-89 (25 µM) for 2hr and in the presence of IBMX. Lysates from 50 GV oocytes were probed with the phospho-PKA substrate (upper panel), and after stripping of the blot, re-probed with IP3R1 antibody (lower panel).
IP3R1 also display consensus sites for other more general kinases, such as PKA and PKC, and these sites have been shown to be phosphorylated in somatic cells (Bezprozvanny, 2005; Vanderheyden et al., 2009a). Using phospho-specific antibodies, we detected PKA IP3R1 phosphorylation in oocytes; this phosphorylation was maximal at the GV stage and declined by the GVBD stage, although it remained detectable throughout maturation (Fig. 5D). PKA IP3R1 immunoreactivity remained stably elevated in oocytes kept in IBMX (Fig. 5D), although it was abrogated by treatment with 25 µM H-89, a PKA inhibitor (Fig. 5E). We were unable to detect IP3R1 phosphorylation by PKC in mouse oocytes at any stage of maturation even when using upwards of 200 oocytes per lane for the immunoblotting procedure (data not shown). Therefore, these results suggest that M-phase and PKA-associated IP3R1 phosphorylations occur during mouse oocyte maturation, although it is unlikely that the enhanced IP3R1 function during maturation is underpinned by PKA-mediated modifications.
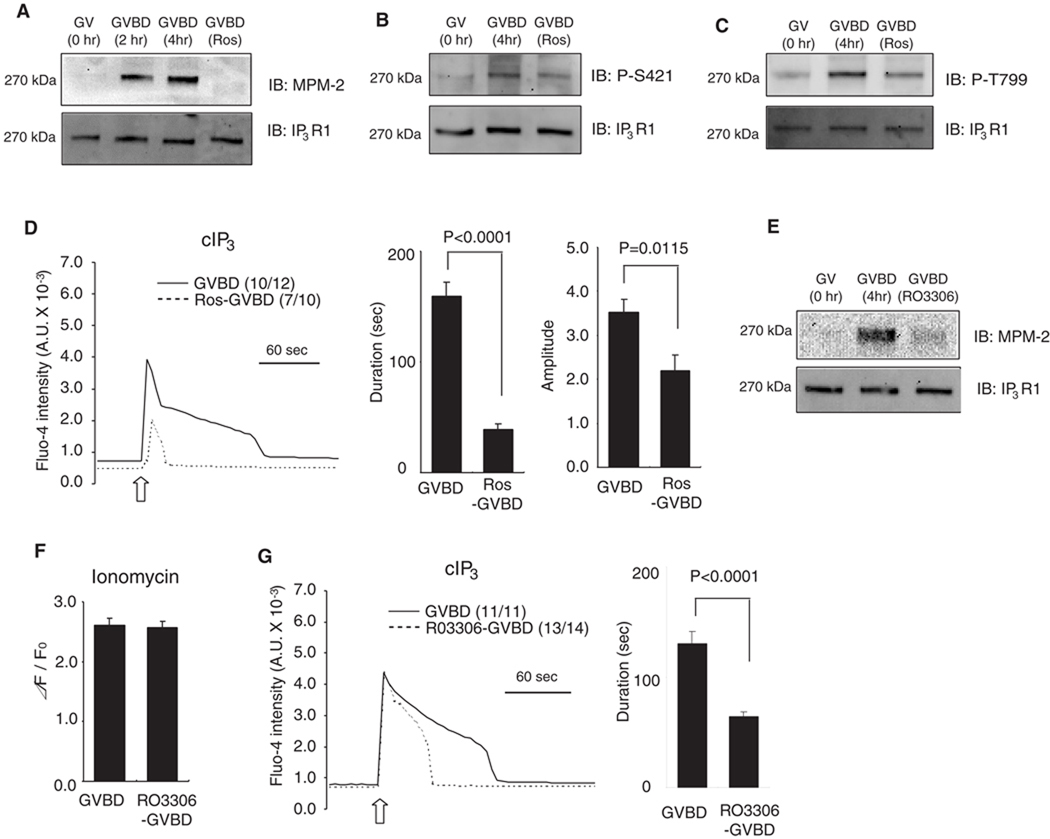
Inhibition of cdk1 activity reduces IP3R1 phosphorylation and decreases IP3R1 sensitivity
To examine the impact of phosphorylation of the MPM-2 epitope and the Cdk1-dependent sites on IP3R1 sensitivity, it was necessary to prevent or erase them prior to inducing Ca2+ release. Roscovitine, a competitive and reversible Cdk inhibitor (Meijer et al., 1997), is widely used to reverse Cdk1 phosphorylations in mammalian oocytes (Phillips et al., 2002). Nevertheless, given that roscovitine prevents meiosis resumption and GVBD, GV oocytes were first allowed to undergo GVBD and 50 µM roscovitine was added 2 hr after start of maturation. Within 2 hr of addition, we found that cdk1 activity was significantly reduced (data not shown). During this interval, roscovitine abrogated IP3R1 MPM-2 immunoreactivity (Fig. 6A) and reduced the reactivity (relative p-IP3R1/IP3R1 intensity) corresponding to T799 and S421 phosphorylation sites between 73.6±8.2 and 69.8±7.6 % (mean±SEM), respectively. Whether the absence of these phosphorylations was associated with reduced IP3-induced Ca2+ release was examined next. As shown in Fig. 6D, roscovitine-treated GVBD oocytes showed [Ca2+]i responses with greatly reduced durations and amplitude ( P < 0.0001, P < 0.05, respectively). While these results point to a specific role of phosphorylation on IP3R1-mediated Ca2+ release, roscovitine reportedly reduces [Ca2+]ER (Deng and Shen, 2000). To avoid this confounding factor, we tested an alternative cdk1 inhibitor, RO-3306, which specifically inhibits cdk1. As expected, treatment of GVBD oocytes with 10 µM RO-3306 reduced IP3R1 MPM-2 phosphorylation (Fig. 6E). Importantly, [Ca2+]ER was not significantly altered in RO3306-treated oocytes (Fig. 6F), although the duration of cIP3-induced response was shortened (P < 0.0001) compared to control GVBD oocytes (Fig 6G). Thus, we interpret these results as an indication that phosphorylation by M-phase kinases, and especially by Cdk1, is one of the factors that enhance IP3R1 sensitivity during oocyte maturation. Whether these kinases modulate IP3R1 function as a result of direct IP3R1 phosphorylation or due to phosphorylation changes on IP3R1-associated proteins remains to be demonstrated.
Fig. 6.
Phosphorylation-dependent sensitization of IP3R1 during oocyte maturation. (A–D) Inhibition of cdk1 activity was achieved by the addition of roscovitine (Ros). GV oocytes were allowed to undergo GVBD and 50 µM Ros was added 2 hr after the start of maturation. Lysates from GV, GVBD and Ros-treated GVBD oocytes were probed with MPM-2 (A), phospho-S421 (B) and phospho-T779 (C) antibodies (upper panel), and re-probed with IP3R1 antibody (lower panel), as described above. A representative result of 2–3 independent experiments with similar results is shown. (D) IP3-induced Ca2+ release using cIP3 was measured in control (solid line) and Ros-treaded (dashed line) oocytes. A 0.001 sec UV pulse was applied to oocytes (open arrow), number of oocytes responding to cIP3 is shown in parentheses. The bar graphs show comparison of the duration and amplitude of cIP3-caused Ca2+ release. (E–G) RO3306, another cdk1 inhibitor was used to block MPF activity. (E) IP3R1 MPM-2 (upper panel) and IP3R1 reactivity (lower panel) in GV, GVBD and RO3306-treated GVBD oocytes. (F) [Ca2+]ER was examined in control (n=9) and RO3306-treated GVBD oocytes (n=10) by the addition of 2 µM ionomycin in Ca2+ free medium. (G) IP3-induced Ca2+ release using cIP3 was measured in control (solid line) and Ros-treaded (dashed line) GVBD oocytes and induced by the application of a 0.001 sec UV pulse (open arrow). The number of oocytes responding to cIP3 is shown in parentheses. The bar graph shows the comparisons of the duration of the cIP3-induced Ca2+ release.
IP3R1 redistribution during oocyte maturation does not affect receptor sensitivity
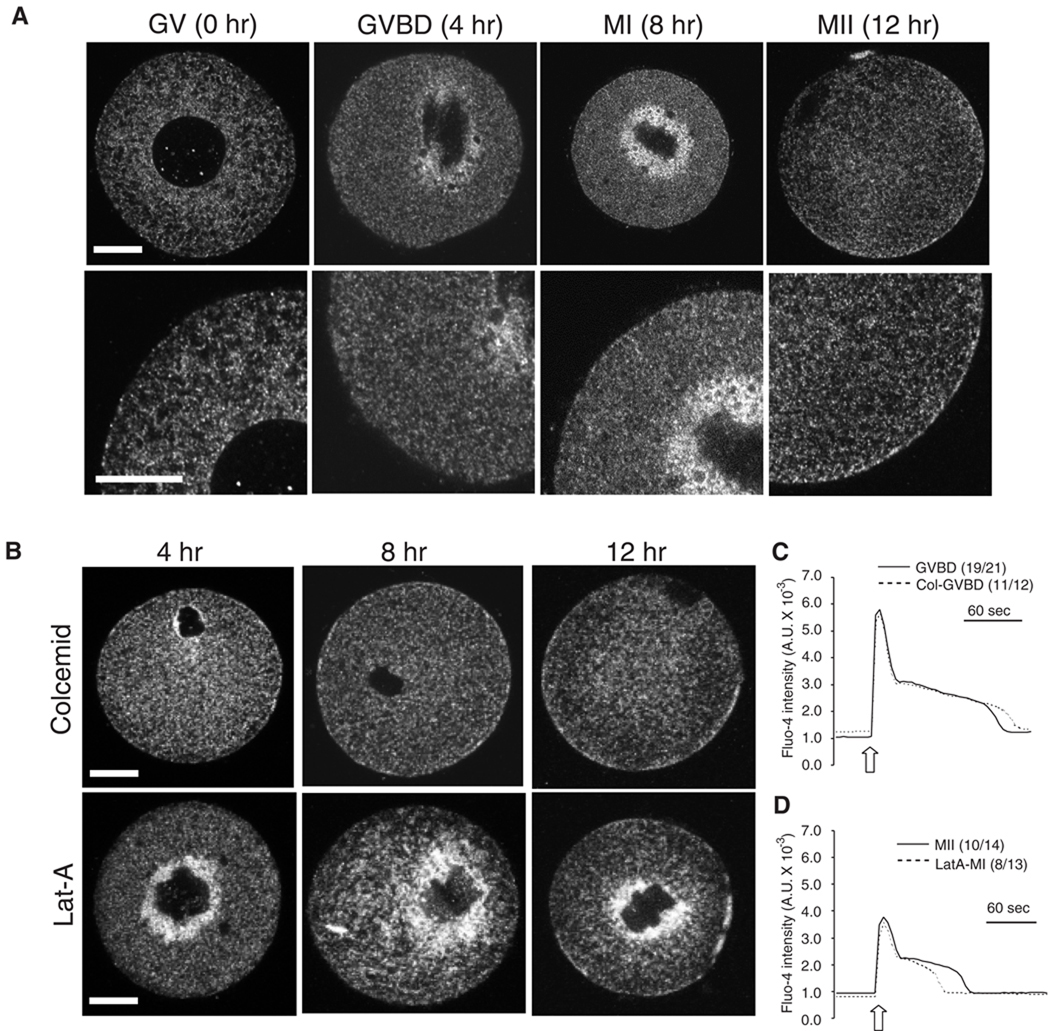
During oocyte maturation the distribution of the ER undergoes dramatic cellular reorganization that is controlled in a step-wise fashion first by microtubules and then by microfilaments (FitzHarris et al., 2007). Importantly, the role of this reorganization on IP3R1 mediated Ca2+ release has not been evaluated in mammalian oocytes. Furthermore, while the location of IP3R1 changes between GV and MII, it is unclear whether its distribution between intermediates stages and the underlying regulatory mechanisms are similar to those that control ER reorganization. Using IF and confocal microscopy we found that at the GV stage IP3R1 is mostly diffusely distributed in the ooplasm (Fig. 7A, GV panels), although during the transition to the GVBD stage IP3R1s begin to accumulate around the chromosomes (Fig. 7A, GVBD panels). At MI, IP3R1s further accumulate in the spindle region (Fig. 7A, MI panels), and this distribution changes during the transition to the MII stage, as by this time IP3R1 disperses away from the spindle region into the cortical and sub-cortical areas (Fig. 7A; MII panels).
Fig. 7.
Redistribution of IP3R1 during oocyte maturation. (A and B) Oocytes were labeled with IP3R1 and imaged by confocal microcopy. The observations were performed at 0, 4, 8 and 12 hr of maturation, which corresponded with GV, GVBD, MI and MII stages. Oocytes were matured in the absence (A) or in the presence of 5 µM colcemid (B; upper panel) or 2.5 µM Lat-A (B; lower panel). A typical equatorial section is shown. Scale bar, 30 µm. (C) IP3-induced Ca2+ release using cIP3 was examined in control (solid line) and colcemid-treaded (dashed line) GVBD oocytes. The 0.001 sec UV pulse was applied to oocytes (open arrow). The number of oocytes responding to cIP3 is shown in parentheses. (D) Oocytes were matured for 8 hr in the absence of LatA, cIP3 was injected at this time, after which maturation continued for 4 hr in the absence or presence of LatA. IP3-induced Ca2+ release using cIP3 was examined in control MII (solid line) and LatA-treaded MI arrested (dashed line) oocytes. The 0.001 sec UV pulse was applied to oocytes (open arrow). The number of oocytes responding to cIP3 is shown in parentheses.
Using two common pharmacological disruptors of the cytoskeleton, colcemid, a depolymerizing microtubule agent (Winston et al., 1995), and Lat-A, an actin polymerization inhibitor (Spector et al., 1983), we found that the centripetal IP3R1 movement between the GVBD and MI stages is entirely dependent on microtubule-mediated mechanisms, whereas the dispersal of the receptor between the MI and MII stages is accomplished by microfilament-based mechanisms (Fig. 7B). We next examined whether IP3R1 redistribution influenced the magnitude of IP3-induced [Ca2+]i responses. We focused on the stages where the inhibitors had maximal impact on IP3R1 distribution, namely, the GVBD stage for colcemid and the MII stage for LatA. Oocytes were matured for 4 hr in the presence of the 5 µM colcemid and injection of cIP3 was performed 2–3 hr after the initiation of maturation. For LatA, because this treatment hinders plasma membrane penetration and because cIP3 losses effectiveness after 4 hr, oocytes were matured for 8 hr in the absence of 2.5 µM LatA, cIP3 was injected at this time, after which maturation continued for 4 hr in the presence of LatA. LatA effectively prevented extrusion of the first polar body and IP3R1 cortical dispersal (data not shown). cIP3-induced Ca2+ release was unaffected by colcemid (Fig. 7C) at the GVBD stage. Similarly, the lag time and amplitude of the responses were not compromised by treatment with LatA, although these oocytes showed modestly shorted [Ca2+]i rises (Fig. 6D). Under these conditions, [Ca2+]ER were not influenced by LatA (data not shown). We interpret these results to mean that the reorganization that IP3R1 undergoes during maturation is not a major factor in enhancing IP3R1 sensitivity.
Discussion
The [Ca2+]i responses associated with fertilization and responsible for egg activation in vertebrate eggs are primarily mediated by IP3R1. The function of IP3R1 is optimized during oocyte maturation so that maximal Ca2+ conductivity concurs with the time of sperm entry. Among the factors affecting IP3R1 function during maturation are biochemical modifications, cellular reorganization and overall changes in cellular Ca2+ homeostasis, although the relative impact of these factors in enhancing IP3R1 sensitivity is not well known. Herein, we demonstrated that while mouse oocytes acquire the ability to initiate fertilization-like oscillations late during maturation, IP3R1 sensitivity increases earlier ∼at the GVBD stage. We determined that the increase in IP3R1 sensitivity is underpinned, at least in part, by increases in [Ca2+]ER and receptor phosphorylation(s) but not by changes in IP3R1 distribution. These results set the stage for investigating the mechanisms that regulate [Ca2+]ER and IP3R1 phosphorylation, the identification of which may be used as markers of maturation and whose understanding may increase the production of developmentally competent oocytes.
IP3R1 sensitivity and MII-like oscillations
To investigate the factors that control IP3R1 sensitivity in oocytes and to determine how it relates to the ability to mount MII-like oscillations, we induced [Ca2+]i responses throughout maturation with a variety of agonists. Several groups have examined the parameters of sperm-initiated [Ca2+]i responses during maturation, although their results and end points differed, and it was therefore difficult to draw conclusions. One study observed an increase in the magnitude of the first [Ca2+]i rise by 5 hr, which is around the GVBD stage (Mehlmann and Kline, 1994), while another demonstrated normal pattern of oscillations after 10–12 hr, coinciding with the transition from MI to the MII stage (Jones et al., 1995). In our study, oscillations were induced by plcζ and thimerosal, as the initiation of oscillations could be more closely controlled. Both compounds induced oscillations at all stages of maturation, although the MII-like oscillatory pattern was only attained near the MI stage. It is noteworthy that the amplitude and duration of the 1st rise induced by both compounds progressively increased throughout maturation, while the parameters of subsequent rises did not seem to change after the GVBD stage (data not shown). As observed in other studies (Carroll and Swann, 1992; Jones et al., 1995), spontaneous oscillations in GV stage oocytes were also observed here, although the agonists-induced responses were of lower frequency and higher amplitude than the spontaneous [Ca2+]i oscillations.
Unlike the responses induced by plcζ and thimerosal, IP3-induced Ca2+ release experienced its maximal change in responsiveness earlier during maturation, as nearly all Ca2+ parameters in GVBD stage oocytes were indistinguishable from oocytes in more advanced stages of maturation. The increase in IP3R1 sensitivity during maturation is widespread among species, as it has also been observed in starfish (Chiba et al., 1990), hamster (Fujiwara et al., 1993) and Xenopus oocytes (Sun et al., 2009). In the latter species, as it is the case in the mouse, transition to the GVBD stage is accompanied by marked changes in IP3R1 phosphorylation (Ito et al., 2008; Vanderheyden et al., 2009b), ER reorganization (FitzHarris et al., 2007) and [Ca2+]ER content (Jones et al., 1995), although which of these modifications underlies the increase in IP3R1 sensitivity is unknown. Collectively, our results support the view that while the distinct species-specific pattern of [Ca2+]i responses in response to fertilization is attained late during maturation near the time of ovulation/fertilization, IP3R1 sensitivity is enhanced earlier during this process
[Ca2+]ER and phosphorylation regulate IP3R1 function during oocyte maturation
To discriminate how each of the aforementioned cellular changes contributes to regulate IP3R1 function, we isolated them and examined [Ca2+]i responses vs. untreated controls oocytes. To assess the impact of [Ca2+]ER content, Ca2+ release was induced in GVBD stage oocytes where the increase in [Ca2+]ER was prevented by CPA. Further, [Ca2+]ER in GV stage oocytes was increased by over-expression of STIM1. Under both conditions, higher [Ca2+]ER increased IP3R1 sensitivity because while the amplitude of the rises was similar to those observed in control oocytes, the time to peak was significantly delayed in lower [Ca2+]ER conditions. These results support earlier findings that higher [Ca2+]ER not only increases the amount of Ca2+ available for release, but also increases the receptor’s sensitivity (Missiaen et al., 1991). It is unknown how [Ca2+]ER affects IP3R1 sensitivity, although it could be the result of direct interaction with the receptor protein or via luminal Ca2+ binding proteins that interact with IP3R1 (Horne and Meyer, 1995; Missiaen et al., 1992; Sienaert et al., 1997). Additionally, it can not be ruled out that the effect of [Ca2+]ER is related to changes in cytosolic [Ca2+]i in the IP3R1 microdomain, as it is well known that cytosolic Ca2+ is a major regulator of IP3R1 activity. Furthermore, little is known about how [Ca2+]ER is controlled in oocytes, although the presence of SERCA has been surmised in these cells by the alteration in [Ca2+]i levels caused by exposure to thapsigargin (Kline and Kline, 1992; Lawrence and Cuthbertson, 1995; Machaty et al., 2002) and here to CPA. Importantly, our results with CPA and plcζ show that without a full [Ca2+]ER the first [Ca2+]i rise is compromised, and that under persistent inhibition of SERCA and inability to refill [Ca2+]ER, the amplitude of the rises is diminished and oscillations cease prematurely. Nonetheless, the molecular presence, cellular distribution and possible regulation of SERCA function during maturation and fertilization have not yet been examined in mammalian oocytes.
Evidence that factors besides [Ca2+]ER also regulate IP3R1 sensitivity stemmed from our studies showing that oocytes in more advanced stages of maturation displayed greater [Ca2+]i rises in response to cIP3 than GV-stage oocytes, even though the maturation-associated increase in [Ca2+]ER was precluded. Among the possible factors, we first examined the role of phosphorylation, as we have demonstrated that beginning at the GVBD stage IP3R1 becomes phosphorylated at an MPM-2 epitope, which is thought to be a target for M-phase kinases (Ito et al., 2008; Lee et al., 2006; Vanderheyden et al., 2009b). Here, we extended those results using phospho-specific antibodies and found phosphorylation at S421 and T799 residues, which lie within conserved Cdk1 consensus sites in IP3R1 (Malathi et al., 2003; Malathi et al., 2005). This is in line with previous findings in mouse oocytes where Plk1 kinase was identified as involved in MPM-2 IP3R1 phosphorylation (Ito et al., 2008), addition of the Plk1 inhibitor BI 2556 (Lenart et al., 2007) eliminated only ∼half of the MPM-2 IP3R1 reactivity (Vanderheyden et al., 2009b), which implied the participation of another M-phase kinase in this phosphorylation. Our present results do not permit to resolve the kinase(s) responsible for the MPM-2 phosphorylation, although the evidence that the Cdk1 inhibitor roscovitine collectively reduced the MPM-2 phosphorylation suggests a role for Cdk1. Cdk1 and Plk1 however participate in the same signaling pathway and form feedback loops during meiosis (Okano-Uchida et al., 2003), and therefore additional studies are needed to ascribe a specific kinase to these M-phase kinase associated phosphorylations. A phospho-proteomic study in Xenopus oocytes revealed that Cdk1 and MAPK consensus sites in IP3R1 are phosphorylated during maturation and subsequent independent activation of MPF and MAPK during maturation increased IP3R-mediated Ca2+ release in these oocytes (Sun et al., 2009). These results are consistent with present findings where addition of roscovitine greatly diminished IP3-induced release in mouse oocytes. Despite studies showing that MAPK affects MPM-2 IP3R1 phosphorylation levels in mouse eggs and in vitro and in vivo evidence that it phosphorylates IP3R1 (Lee et al., 2006), IP3R1 sensitivity at the GVBD stage was not affected by inhibition of this pathway with U0126 (data not shown), which is consistent with our previous evidence that MAPK affects IP3R1 MPM-2 phosphorylation only after the MI stage (Ito et al., 2008).
Besides M-phase kinases, numerous studies in somatic cells have shown that IP3R isoforms can be phosphorylated by various, more wide-ranging kinases (Bezprozvanny, 2005; Vanderheyden et al., 2009a). The most commonly implicated kinases include PKA, PKC and CaMKII, all of which have important physiological functions in oocytes and eggs (Ducibella and Fissore, 2008). Interestingly, using a phospho-PKA substrate antibody we found that IP3R1 is maximally phosphorylated at the GV stage, although a significant proportion of this phosphorylation is lost when oocytes resume meiosis, which mirrors the changes in intra-oocyte cAMP concentrations (Norris et al., 2009). In somatic cells, IP3R1-PKA phosphorylation is associated with increased channel activity (DeSouza et al., 2002; Nakade et al., 1994) and reduced [Ca2+]ER levels (Oakes et al., 2005). It is unknown whether IP3R1 phosphorylation by PKA has any functional consequence in oocytes, although given that [Ca2+]ER content in GV oocytes is low and the function of cAMP and PKA in maintaining GV arrest (Conti et al., 2002), a role for it on the regulation of [Ca2+]ER content cannot be ruled out and deserves to be studied further.
Our present results show that IP3R1 undergoes marked reorganization throughout oocyte maturation, extending data from previous studies that examined IP3R1 distribution mostly at the GV and MII stages (Mehlmann et al., 1996; Shiraishi et al., 1995). We also demonstrate that IP3R1 redistribution until the MI stage is controlled by microtubules-associated pathways, whereas the cortical dispersal that occurs between the MI and MII transition is controlled by actin-associated mechanism; similar regulatory pathways are thought to control ER re-organization (FitzHarris et al., 2007). Using appropriate and well-characterized cytoskeletal inhibitors that prevent IP3R1/ER redistribution we show that IP3R1/ER reorganization during maturation affects neither IP3R1 sensitivity nor the ability of eggs to initiate oscillations. These results are consistent with the evidence that during mouse fertilization the ER-, and also possibly IP3R1, cortical clusters disappear ahead of the termination of the oscillations (Kline et al., 1999), suggesting that they are dispensable for the maintenance of oscillations. Nonetheless, it is possible that the ER/IP3R1 reorganization is important for the completion of cortical events, such as cortical granule exocytosis or plasma membrane reorganization, or for propagation of the Ca2+ wave, aspects which were not examined in this manuscript.
IP3R1 sensitivity, Ca2+ homeostasis and MII [Ca2+]i oscillations
Our results suggest that IP3R1 attains near maximal sensitivity prior to the ability of oocytes to mount MII-like [Ca2+]i oscillations, which occurs approximately at the time of MI to MII transition. These results agree in part with those obtained in hamster oocytes, where IP3R1 sensitivity was ascertained using different iontophoretic pulses to inject IP3 (Fujiwara et al., 1993). It was observed that while gradual and greater responses were observed between 1–5hr of maturation, which is similar to results observed in our study, maximal sensitivity, as assessed using near threshold pulses, was only attained after 10 hr of maturation. It was proposed that this was due to the late acquisition of Ca2+ sensitized IP3-induced Ca2+ release, although what molecular mechanisms regulated this sensitization was not investigated. Our results demonstrating the temporal discordance between acquisition of IP3R1 sensitivity, which is reportedly biphasic, and the ability to initiate fertilization-like oscillations, suggests that several mechanisms that control IP3R1 function are actively modulated during maturation and may ultimately shape the responses at fertilization. For example, in contrast to the direct injection of IP3 used here to test IP3R1 sensitivity, sperm-induced IP3 production may involve the organization of positive-feedback loops (Swann and Yu, 2008), the assembly of which may not fully develop until the late stages of maturation. Furthermore, although [Ca2+]i responses at fertilization are underpinned by changes in IP3R1 function, the maintenance of basal [Ca2+]i and [Ca2+]ER levels needed for the persistence of oscillations require the participation of Ca2+ influx or efflux mechanisms as well as of other organelles, such as the mitochondria. Remarkably, we remain largely uninformed of the mechanism(s) that regulate in mammalians oocytes and eggs the function of SERCA, which recycles and refills the ER Ca2+ stores, and of the molecules that underpin Ca2+ influx and efflux. Therefore, identification and elucidation of the regulatory mechanisms responsible for Ca2+ release and Ca2+ homeostasis in these cells will deepen our understanding of fertilization, information which could be then used in the clinic for the diagnosis of infertility, and to enhance developmental competence of embryos generated by a variety of Assisted Reproductive Technology procedure.
Acknowledgments
We thank Ms. Changli He for technical assistance. V.V. was recipient of a postdoctoral fellowship of the Research Foundation-Flanders (FWO). This work was supported in part by a grant HD051872 from NIH to R.A.F.
References
- Berridge MJ. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium. 2002;32(5–6):235–249. doi: 10.1016/s0143416002001823. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I. The inositol 1,4,5-trisphosphate receptors. Cell Calcium. 2005;38(3–4):261–272. doi: 10.1016/j.ceca.2005.06.030. [DOI] [PubMed] [Google Scholar]
- Carroll J, Swann K. Spontaneous cytosolic calcium oscillations driven by inositol trisphosphate occur during in vitro maturation of mouse oocytes. J Biol Chem. 1992;267(16):11196–11201. [PubMed] [Google Scholar]
- Carroll J, Swann K, Whittingham D, Whitaker M. Spatiotemporal dynamics of intracellular [Ca2+]i oscillations during the growth and meiotic maturation of mouse oocytes. Development. 1994;120(12):3507–3517. doi: 10.1242/dev.120.12.3507. [DOI] [PubMed] [Google Scholar]
- Chatot CL, Ziomek CA, Bavister BD, Lewis JL, Torres I. An improved culture medium supports development of random-bred 1-cell mouse embryos in vitro. J Reprod Fertil. 1989;86(2):679–688. doi: 10.1530/jrf.0.0860679. [DOI] [PubMed] [Google Scholar]
- Cheek TR, McGuinness OM, Vincent C, Moreton RB, Berridge MJ, Johnson MH. Fertilisation and thimerosal stimulate similar calcium spiking patterns in mouse oocytes but by separate mechanisms. Development. 1993;119(1):179–189. doi: 10.1242/dev.119.1.179. [DOI] [PubMed] [Google Scholar]
- Chiba K, Kado RT, Jaffe LA. Development of calcium release mechanisms during starfish oocyte maturation. Dev Biol. 1990;140(2):300–306. doi: 10.1016/0012-1606(90)90080-3. [DOI] [PubMed] [Google Scholar]
- Conti M, Andersen CB, Richard F, Mehats C, Chun SY, Horner K, Jin C, Tsafriri A. Role of cyclic nucleotide signaling in oocyte maturation. Mol Cell Endocrinol. 2002;187(1–2):153–159. doi: 10.1016/s0303-7207(01)00686-4. [DOI] [PubMed] [Google Scholar]
- Cui LB, Huang XY, Sun FZ. Nucleocytoplasmic ratio of fully grown germinal vesicle oocytes is essential for mouse meiotic chromosome segregation and alignment, spindle shape and early embryonic development. Hum Reprod. 2005;20(10):2946–2953. doi: 10.1093/humrep/dei143. [DOI] [PubMed] [Google Scholar]
- Deng MQ, Shen SS. A specific inhibitor of p34(cdc2)/cyclin B suppresses fertilization-induced calcium oscillations in mouse eggs. Biol Reprod. 2000;62(4):873–878. doi: 10.1095/biolreprod62.4.873. [DOI] [PubMed] [Google Scholar]
- DeSouza N, Reiken S, Ondrias K, Yang YM, Matkovich S, Marks AR. Protein kinase A and two phosphatases are components of the inositol 1,4,5-trisphosphate receptor macromolecular signaling complex. J Biol Chem. 2002;277(42):39397–39400. doi: 10.1074/jbc.M207059200. [DOI] [PubMed] [Google Scholar]
- Ducibella T, Fissore R. The roles of Ca2+, downstream protein kinases, and oscillatory signaling in regulating fertilization and the activation of development. Dev Biol. 2008;315(2):257–279. doi: 10.1016/j.ydbio.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Jouni W, Jang B, Haun S, Machaca K. Calcium signaling differentiation during Xenopus oocyte maturation. Dev Biol. 2005;288(2):514–525. doi: 10.1016/j.ydbio.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Fissore RA, Longo FJ, Anderson E, Parys JB, Ducibella T. Differential distribution of inositol trisphosphate receptor isoforms in mouse oocytes. Biol Reprod. 1999;60(1):49–57. doi: 10.1095/biolreprod60.1.49. [DOI] [PubMed] [Google Scholar]
- FitzHarris G, Marangos P, Carroll J. Changes in endoplasmic reticulum structure during mouse oocyte maturation are controlled by the cytoskeleton and cytoplasmic dynein. Dev Biol. 2007;305(1):133–144. doi: 10.1016/j.ydbio.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Nakada K, Shirakawa H, Miyazaki S. Development of inositol trisphosphate-induced calcium release mechanism during maturation of hamster oocytes. Dev Biol. 1993;156(1):69–79. doi: 10.1006/dbio.1993.1059. [DOI] [PubMed] [Google Scholar]
- Horne JH, Meyer T. Luminal calcium regulates the inositol trisphosphate receptor of rat basophilic leukemia cells at a cytosolic site. Biochemistry. 1995;34(39):12738–12746. doi: 10.1021/bi00039a033. [DOI] [PubMed] [Google Scholar]
- Ito J, Yoon SY, Lee B, Vanderheyden V, Vermassen E, Wojcikiewicz R, Alfandari D, De Smedt H, Parys JB, Fissore RA. Inositol 1,4,5-trisphosphate receptor 1, a widespread Ca2+ channel, is a novel substrate of polo-like kinase 1 in eggs. Dev Biol. 2008;320(2):402–413. doi: 10.1016/j.ydbio.2008.05.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellerette T, Kurokawa M, Lee B, Malcuit C, Yoon SY, Smyth J, Vermassen E, De Smedt H, Parys JB, Fissore RA. Cell cycle-coupled [Ca(2+)](i) oscillations in mouse zygotes and function of the inositol 1,4,5-trisphosphate receptor-1. Dev Biol. 2004;274(1):94–109. doi: 10.1016/j.ydbio.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Jones KT, Carroll J, Whittingham DG. Ionomycin, thapsigargin, ryanodine, and sperm induced Ca2+ release increase during meiotic maturation of mouse oocytes. J Biol Chem. 1995;270(12):6671–6677. doi: 10.1074/jbc.270.12.6671. [DOI] [PubMed] [Google Scholar]
- Kline D, Kline JT. Thapsigargin activates a calcium influx pathway in the unfertilized mouse egg and suppresses repetitive calcium transients in the fertilized egg. J Biol Chem. 1992;267(25):17624–17630. [PubMed] [Google Scholar]
- Kline D, Mehlmann L, Fox C, Terasaki M. The cortical endoplasmic reticulum (ER) of the mouse egg: localization of ER clusters in relation to the generation of repetitive calcium waves. Dev Biol. 1999;215(2):431–442. doi: 10.1006/dbio.1999.9445. [DOI] [PubMed] [Google Scholar]
- Kurokawa M, Sato K, Smyth J, Wu H, Fukami K, Takenawa T, Fissore RA. Evidence that activation of Src family kinase is not required for fertilization-associated [Ca2+]i oscillations in mouse eggs. Reproduction. 2004;127(4):441–454. doi: 10.1530/rep.1.00128. [DOI] [PubMed] [Google Scholar]
- Kurokawa M, Sato K, Wu H, He C, Malcuit C, Black SJ, Fukami K, Fissore RA. Functional, biochemical, and chromatographic characterization of the complete [Ca2+]i oscillation-inducing activity of porcine sperm. Dev Biol. 2005;285(2):376–392. doi: 10.1016/j.ydbio.2005.06.029. [DOI] [PubMed] [Google Scholar]
- Lawrence YM, Cuthbertson KS. Thapsigargin induces cytoplasmic free Ca2+ oscillations in mouse oocytes. Cell Calcium. 1995;17(2):154–164. doi: 10.1016/0143-4160(95)90084-5. [DOI] [PubMed] [Google Scholar]
- Lee B, Vermassen E, Yoon SY, Vanderheyden V, Ito J, Alfandari D, De Smedt H, Parys JB, Fissore RA. Phosphorylation of IP3R1 and the regulation of [Ca2+]i responses at fertilization: a role for the MAP kinase pathway. Development. 2006;133(21):4355–4365. doi: 10.1242/dev.02624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenart P, Petronczki M, Steegmaier M, Di Fiore B, Lipp JJ, Hoffmann M, Rettig WJ, Kraut N, Peters JM. The small-molecule inhibitor BI 2536 reveals novel insights into mitotic roles of polo-like kinase 1. Curr Biol. 2007;17(4):304–315. doi: 10.1016/j.cub.2006.12.046. [DOI] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15(13):1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol. 2006;174(6):815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machaca K, Haun S. Store-operated calcium entry inactivates at the germinal vesicle breakdown stage of Xenopus meiosis. J Biol Chem. 2000;275(49):38710–38715. doi: 10.1074/jbc.M007887200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machaty Z, Ramsoondar JJ, Bonk AJ, Bondioli KR, Prather RS. Capacitative calcium entry mechanism in porcine oocytes. Biol Reprod. 2002;66(3):667–674. doi: 10.1095/biolreprod66.3.667. [DOI] [PubMed] [Google Scholar]
- Malathi K, Kohyama S, Ho M, Soghoian D, Li X, Silane M, Berenstein A, Jayaraman T. Inositol 1,4,5-trisphosphate receptor (type 1) phosphorylation and modulation by Cdc2. J Cell Biochem. 2003;90(6):1186–1196. doi: 10.1002/jcb.10720. [DOI] [PubMed] [Google Scholar]
- Malathi K, Li X, Krizanova O, Ondrias K, Sperber K, Ablamunits V, Jayaraman T. Cdc2/cyclin B1 interacts with and modulates inositol 1,4,5-trisphosphate receptor (type 1) functions. J Immunol. 2005;175(9):6205–6210. doi: 10.4049/jimmunol.175.9.6205. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Kline D. Regulation of intracellular calcium in the mouse egg: calcium release in response to sperm or inositol trisphosphate is enhanced after meiotic maturation. Biol Reprod. 1994;51(6):1088–1098. doi: 10.1095/biolreprod51.6.1088. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Mikoshiba K, Kline D. Redistribution and increase in cortical inositol 1,4,5-trisphosphate receptors after meiotic maturation of the mouse oocyte. Dev Biol. 1996;180(2):489–498. doi: 10.1006/dbio.1996.0322. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Terasaki M, Jaffe LA, Kline D. Reorganization of the endoplasmic reticulum during meiotic maturation of the mouse oocyte. Dev Biol. 1995;170(2):607–615. doi: 10.1006/dbio.1995.1240. [DOI] [PubMed] [Google Scholar]
- Meijer L, Borgne A, Mulner O, Chong JP, Blow JJ, Inagaki N, Inagaki M, Delcros JG, Moulinoux JP. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem. 1997;243(1–2):527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- Missiaen L, Taylor CW, Berridge MJ. Spontaneous calcium release from inositol trisphosphate-sensitive calcium stores. Nature. 1991;352(6332):241–244. doi: 10.1038/352241a0. [DOI] [PubMed] [Google Scholar]
- Missiaen L, Taylor CW, Berridge MJ. Luminal Ca2+ promoting spontaneous Ca2+ release from inositol trisphosphate-sensitive stores in rat hepatocytes. J Physiol. 1992;455:623–640. doi: 10.1113/jphysiol.1992.sp019319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S, Shirakawa H, Nakada K, Honda Y. Essential role of the inositol 1,4,5-trisphosphate receptor/Ca2+ release channel in Ca2+ waves and Ca2+ oscillations at fertilization of mammalian eggs. Dev Biol. 1993;158(1):62–78. doi: 10.1006/dbio.1993.1168. [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Yuzaki M, Nakada K, Shirakawa H, Nakanishi S, Nakade S, Mikoshiba K. Block of Ca2+ wave and Ca2+ oscillation by antibody to the inositol 1,4,5-trisphosphate receptor in fertilized hamster eggs. Science. 1992;257(5067):251–255. doi: 10.1126/science.1321497. [DOI] [PubMed] [Google Scholar]
- Nakade S, Rhee SK, Hamanaka H, Mikoshiba K. Cyclic AMP-dependent phosphorylation of an immunoaffinity-purified homotetrameric inositol 1,4,5-trisphosphate receptor (type I) increases Ca2+ flux in reconstituted lipid vesicles. J Biol Chem. 1994;269(9):6735–6742. [PubMed] [Google Scholar]
- Nigg EA. Mitotic kinases as regulators of cell division and its checkpoints. Nat Rev Mol Cell Biol. 2001;2(1):21–32. doi: 10.1038/35048096. [DOI] [PubMed] [Google Scholar]
- Norris RP, Ratzan WJ, Freudzon M, Mehlmann LM, Krall J, Movsesian MA, Wang H, Ke H, Nikolaev VO, Jaffe LA. Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development. 2009;136(11):1869–1878. doi: 10.1242/dev.035238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes SA, Scorrano L, Opferman JT, Bassik MC, Nishino M, Pozzan T, Korsmeyer SJ. Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2005;102(1):105–110. doi: 10.1073/pnas.0408352102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano-Uchida T, Okumura E, Iwashita M, Yoshida H, Tachibana K, Kishimoto T. Distinct regulators for Plk1 activation in starfish meiotic and early embryonic cycles. Embo J. 2003;22(20):5633–5642. doi: 10.1093/emboj/cdg535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrington J, Brind S, De Smedt H, Gangeswaran R, Lai FA, Wojcikiewicz R, Carroll J. Expression of inositol 1,4,5-trisphosphate receptors in mouse oocytes and early embryos: the type I isoform is upregulated in oocytes and downregulated after fertilization. Dev Biol. 1998;203(2):451–461. doi: 10.1006/dbio.1998.9071. [DOI] [PubMed] [Google Scholar]
- Parys JB, de Smedt H, Missiaen L, Bootman MD, Sienaert I, Casteels R. Rat basophilic leukemia cells as model system for inositol 1,4,5-trisphosphate receptor IV, a receptor of the type II family: functional comparison and immunological detection. Cell Calcium. 1995;17(4):239–249. doi: 10.1016/0143-4160(95)90070-5. [DOI] [PubMed] [Google Scholar]
- Phillips KP, Petrunewich MA, Collins JL, Booth RA, Liu XJ, Baltz JM. Inhibition of MEK or cdc2 kinase parthenogenetically activates mouse eggs and yields the same phenotypes as Mos(−/−) parthenogenotes. Dev Biol. 2002;247(1):210–223. doi: 10.1006/dbio.2002.0680. [DOI] [PubMed] [Google Scholar]
- Rebecchi MJ, Pentyala SN. Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol Rev. 2000;80(4):1291–1335. doi: 10.1152/physrev.2000.80.4.1291. [DOI] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169(3):435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, Swann K, Lai FA. PLC zeta: a sperm-specific trigger of Ca(2+) oscillations in eggs and embryo development. Development. 2002;129(15):3533–3544. doi: 10.1242/dev.129.15.3533. [DOI] [PubMed] [Google Scholar]
- Schultz RM, Kopf GS. Molecular basis of mammalian egg activation. Curr Top Dev Biol. 1995;30:21–62. doi: 10.1016/s0070-2153(08)60563-3. [DOI] [PubMed] [Google Scholar]
- Seidler NW, Jona I, Vegh M, Martonosi A. Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasmic reticulum. J Biol Chem. 1989;264(30):17816–17823. [PubMed] [Google Scholar]
- Shiraishi K, Okada A, Shirakawa H, Nakanishi S, Mikoshiba K, Miyazaki S. Developmental changes in the distribution of the endoplasmic reticulum and inositol 1,4,5-trisphosphate receptors and the spatial pattern of Ca2+ release during maturation of hamster oocytes. Dev Biol. 1995;170(2):594–606. doi: 10.1006/dbio.1995.1239. [DOI] [PubMed] [Google Scholar]
- Sienaert I, Missiaen L, De Smedt H, Parys JB, Sipma H, Casteels R. Molecular and functional evidence for multiple Ca2+-binding domains in the type 1 inositol 1,4,5-trisphosphate receptor. J Biol Chem. 1997;272(41):25899–25906. doi: 10.1074/jbc.272.41.25899. [DOI] [PubMed] [Google Scholar]
- Spector I, Shochet NR, Kashman Y, Groweiss A. Latrunculins: novel marine toxins that disrupt microfilament organization in cultured cells. Science. 1983;219(4584):493–495. doi: 10.1126/science.6681676. [DOI] [PubMed] [Google Scholar]
- Stricker SA. Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev Biol. 1999;211(2):157–176. doi: 10.1006/dbio.1999.9340. [DOI] [PubMed] [Google Scholar]
- Sun L, Haun S, Jones RC, Edmondson RD, Machaca K. Kinase-dependent regulation of inositol 1,4,5-trisphosphate-dependent Ca2+ release during oocyte maturation. J Biol Chem. 2009;284(30):20184–20196. doi: 10.1074/jbc.M109.004515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann K. Different triggers for calcium oscillations in mouse eggs involve a ryanodine-sensitive calcium store. Biochem J. 1992;287(Pt 1):79–84. doi: 10.1042/bj2870079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann K, Yu Y. The dynamics of calcium oscillations that activate mammalian eggs. Int J Dev Biol. 2008;52(5–6):585–594. doi: 10.1387/ijdb.072530ks. [DOI] [PubMed] [Google Scholar]
- Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderheyden V, Devogelaere B, Missiaen L, De Smedt H, Bultynck G, Parys JB. Regulation of inositol 1,4,5-trisphosphate-induced Ca2+ release by reversible phosphorylation and dephosphorylation. Biochim Biophys Acta. 2009a;1793(6):959–970. doi: 10.1016/j.bbamcr.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderheyden V, Wakai T, Bultynck G, De Smedt H, Parys JB, Fissore RA. Regulation of inositol 1,4,5-trisphosphate receptor type 1 function during oocyte maturation by MPM-2 phosphorylation. Cell Calcium. 2009b;46(1):56–64. doi: 10.1016/j.ceca.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston NJ, McGuinness O, Johnson MH, Maro B. The exit of mouse oocytes from meiotic M-phase requires an intact spindle during intracellular calcium release. J Cell Sci. 1995;108(Pt 1):143–151. doi: 10.1242/jcs.108.1.143. [DOI] [PubMed] [Google Scholar]
- Wojcikiewicz RJ, Tobin AB, Nahorski SR. Muscarinic receptor-mediated inositol 1,4,5-trisphosphate formation in SH-SY5Y neuroblastoma cells is regulated acutely by cytosolic Ca2+ and by rapid desensitization. J Neurochem. 1994;63(1):177–185. doi: 10.1046/j.1471-4159.1994.63010177.x. [DOI] [PubMed] [Google Scholar]
- Yu F, Sun L, Machaca K. Orai1 internalization and STIM1 clustering inhibition modulate SOCE inactivation during meiosis. Proc Natl Acad Sci U S A. 2009;106(41):17401–17406. doi: 10.1073/pnas.0904651106. [DOI] [PMC free article] [PubMed] [Google Scholar]