Abstract
Obesity is associated with an aggressive course in chronic viral hepatitis; however its impact in the development of clinical decompensation (CD) in patients with established cirrhosis is uncertain. We evaluated the role of obesity, in relationship to other recognized predictors, in the development of CD in patients with compensated cirrhosis. The study population, a subset of patients included in a randomized trial of beta-blockers in the prevention of varices in whom data on body mass index (BMI) was available, consisted of 161 patients with compensated cirrhosis. Laboratory tests and portal pressure (assessed by the hepatic venous pressure gradient or HVPG) were assessed on inclusion. Patients were followed-up until CD (ascites, hepatic encephalopathy or variceal hemorrhage), or until September 2002.
29% had a normal BMI, 40% were overweight and 30% were obese. In a median follow-up of 59 months, CD occurred in 48/161 (30%) patients with an increasingly higher rate according to BMI group (15% in those with normal BMI; 31% in overweight; 43% in obese patients, p=0.011). The actuarial probability of developing CD was significantly higher in the abnormal BMI groups (p=0.022). In a multivariable model that included parameters previously identified as being predictive of CD (HVPG, albumin, MELD), etiology and treatment group, BMI [HR 1.06 (1.01-1.12), p=0.02] was an independent predictor of decompensation, together with HVPG and albumin.
Conclusion
Obesity has a deleterious effect on the natural history of compensated cirrhosis of all etiologies, independent of portal pressure and liver function. Weight reduction may be a valuable therapeutic measure in this patient population.
Keywords: Portal pressure, HVPG, ascites, variceal bleeding
The natural history of chronic liver diseases is characterized by the progression of fibrosis and nodule formation leading to the development of cirrhosis. Once cirrhosis is established, patients progress from a frequently asymptomatic compensated stage to a decompensated stage, marked by the development of clinical complications of portal hypertension and liver failure. While the risk of mortality is very low in the compensated stage, it markedly increases upon decompensation (1). Therefore, prognostic markers of progression to clinical decompensation are needed in patients with compensated cirrhosis. In this population serum albumin, MELD (Model of End stage Liver Disease) score and the degree of portal hypertension, as determined by the hepatic venous pressure gradient (HVPG) are independent predictors of first clinical decompensation (2).
Obesity is a growing epidemic worldwide, involving 20-35% of the population in Western countries (3, 4). In addition to known deleterious health consequences outside the liver(5), obesity is a frequent cause of chronic liver disease that can progress to cirrhosis (6-8). Recent data from a cohort study of middle aged women in the UK suggested that an estimated 17% of liver cirrhosis is attributable to excess body weight (9). Moreover, patients with cirrhosis due to obesity-related liver disease have a lower survival than patients with viral cirrhosis (10), and there is increasing evidence of a deleterious effect of obesity on pre-existing chronic liver disease due to hepatitis C, hepatitis B or alcoholic disease. In these settings obesity has been associated with more advanced fibrosis in cross-sectional studies (11, 12) and with faster histological and/or clinical progression in longitudinal studies of patients with chronic hepatitis C (13, 14).
Taken together, these data strongly support that obesity per se is a risk factor for progression in the natural history of cirrhosis. Therefore, it can be hypothesised that increased body weight could be an additional risk factor for the transition from compensated to decompensated cirrhosis. However, this aspect has not been adequately evaluated so far, which was the objective of this study.
Patients and Methods
The current study was conducted in a subset of patients included in a multicenter randomized controlled trial (RCT) of beta-blockers in the prevention of varices (timolol study) (15). Briefly, between August 1993 and March 1999, patients aged 18-75 y/o, with compensated cirrhosis were enrolled in a prospective placebo-controlled, double-blind RCT designed to evaluate the efficacy of non-selective-blockers in preventing the development of gastroesophageal varices in patients with compensated cirrhosis and portal hypertension. Four centers participated in the study: 2 in the U.S. (New Haven/West Haven and Boston) and 2 in Europe (Barcelona, London). Patients were considered for inclusion if they had compensated cirrhosis and portal hypertension (defined by an HVPG ≥ 6 mmHg), without gastroesophageal varices. The diagnosis of cirrhosis was either biopsy proven or clinically suspected and confirmed by the presence of an HVPG ≥ 10 mmHg. Exclusion criteria included ascites, diuretic treatment, hepatocellular carcinoma, splenic or portal vein thrombosis, comorbidity with life expectancy < 1 year, use of drugs affecting splanchnic hemodynamics or portal pressure, primary biliary cirrhosis or primary sclerosing cholangitis, contraindications to beta-blockers, pregnancy and alcohol intake during the dose titration phase. The timolol study included 213 patients that were randomly assigned to receive placebo or timolol, a non-selective beta-blocker. At baseline clinical history, physical examination including body weight, blood tests, upper gastrointestinal endoscopy, abdominal ultrasonography, and HVPG measurement were performed. Patients were followed at 1 and 3 months after random assignment and then every 3 months until the primary end point of the study (development of small varices observed in 2 consecutive endoscopies, large varices, or variceal haemorrhage), the secondary end point (death or liver transplantation), or until the end of the study in September 2002. Eighty-four patients developed the primary endpoint of the trial, without any differences between timolol or placebo (15). Of these, 62 had not developed clinical decompensation (ascites, encephalopathy or variceal hemorrhage) prior to development of the primary endpoint and, in a subsequent study (2), their followup was completed regarding clinical decompensation until the end of the study (September 2002). This database, which therefore included a complete follow-up of all patients until September 2002, was used for the present study.
Since height was not among the variables included in the original dataset, we retrieved this information from the clinical records in order to calculate the body mass index (BMI). Data on height was available in 161 of the 213 patients. These 161 patients constitute the object of the present study.
BMI was calculated as weight in kilograms/height in meters squared.
According to the World Health Organization and the US Department of Health and Human Services (16, 17), the following scale of BMI was used to classify the patients: underweight= BMI < 18.5; normal weight = BMI 18.5-24.9 Kg/m2; overweight = BMI 25-29.9 Kg/m2; obese = BMI >30 Kg/m2.
Statistical analysis
Statistical analysis was performed using SPSS 16.0 statistical package (SPSS Inc., Chicago, IL, USA). All results are expressed as frequencies, median and range or as mean ± standard deviation (SD). Comparisons between BMI classes were done by Chi-square test or Kruskal-Wallis test when appropriate for frequencies; ANOVA and Student's t-test were used for continuous variables; Kruskall-Wallis H test was used to assess differences in numerical variables among ordinal categories (normal BMI, overweight and obese). Linear regression was used to evaluate the presence of a linear association between continuous variables. Correlations were performed using Pearson's test. Multivariate analysis was conducted using backward stepwise Cox proportional hazards regression analysis for the probability of developing clinical decompensation including variables that were previously identified as predictors of decompensation, etiology and treatment group, and BMI. Survival was analysed by Kaplan-Meier curves. Comparisons among the different BMI groups were performed using the log-rank test. Since this study included a subset of patients from the original RCT (15) in whom height was available, and in order to rule out inclusion bias, baseline characteristics and incidence of decompensation were compared between the 161 patients with BMI and the 52 patients excluded because of lack of BMI. Additionally, multiple imputation analysis (18) was performed to exclude potential bias derived from missing data. The variables used to impute BMI were age, gender, weight and study site.
A p value of < 0.05 was considered statistically significant.
Results
Body mass index in the studied population
Table 1 shows the characteristics of the population included in this study.
Table 1.
Main baseline clinical, biochemical and hemodynamic characteristics of the patients included in the study. Data are shown as mean ± SD or as median values (interquartile range) * p <0.05 vs. normal and overweight.
| Overall (n=161) |
Normal BMI (n=47) |
Overweight (n=65) |
Obese (n=49) |
|
|---|---|---|---|---|
| Age (yrs), Mean ± SD | 54±10 | 54±11 | 55±10 | 52±10 |
| Median (range) | 54 (23-75) | 54 (27-75) | 58 (23-70) | 49 (34-70) |
|
| ||||
| Male gender (n) (%) | 95 (59%) | 24 (51%) | 39 (60%) | 32 (65%) |
|
| ||||
| BMI (kg/m2), Mean ± SD | 27.9 ± 4.8 | 22.8±1.7 | 27.2 ± 1.5 | 33.8 ± 3.3* |
| Median | 27.3 | 23.4 | 27.3 | 32.4 |
| (range) | (18.5-44.7) | (18.5-24.9) | (25.0-29.9) | (30.1-44.7) |
|
| ||||
| Etiology of cirrhosis: virus/alcohol/crypto/other (% of each) | ||||
| 109/35/8/9 | 37/9/0/1 | 45/14/2/4 | 27/12/6/4 | |
| (68/22/5/5) | (79/19/0/2) | (69/22/3/6) | (55/24/12/8)* | |
|
| ||||
| Blood Pressure(mmHg) | ||||
| Systolic BP | 130±18 (130) | 130±19 (125) | 130±19 (130) | 131± 17 (130) |
| Diastolic BP | 75±10 (75) | 75±10 (75) | 75±9 (73) | 76 ± 10 (77) |
| Mean ± SD (median) | 94±11 (93) | 94±12 (93) | 93±11 (93) | 94±11 (97) |
|
| ||||
| Heart rate (bpm) Mean ± SD (median) | 74±10 (73) | 74±12 (72) | 73±9 (74) | 74±10 (74) |
|
| ||||
| MELD score, Mean ± SD | 8.7±2.1 | 8.2±1.6 | 8.6±2.1 | 9.2±2.4 |
| (Median) | (8.2) | (7.7) | (8.1) | (8.8) |
|
| ||||
| Bilirubin (mg/dl) Mean ± SD (median) | 1.1±0.6 | 0.9±0.6 | 1.20±0.67 | 1.17±0.67 |
| (1.0) | (0.8) | (1.00) | (1.00) | |
|
| ||||
| Albumin (g/dL) Mean ± SD (median) | 3.9±0.5 (4.0) | 4.0±0.5 (4.0) | 3.9±0.4 (4.0) | 3.9±0.5 (4.0) |
|
| ||||
| INR, Mean ± SD (median) | 1.12±0.14 | 1.10±0.10 | 1.10±0.13 | 1.15±0.17 |
| (1.10) | (1.10) | (1.04) | (1.11) | |
|
| ||||
| AST (U/l), Mean ± SD (median) | 92±63 (77) | 94±71 (82) | 99±67 (78) | 81±47 (62) |
|
| ||||
| ALT (U/l), Mean ± SD | 111±99 (79) | 114±104 (82) | 124±112 (85) | 88±69 (71) |
|
| ||||
| Creatinine (mg/dl), Mean ± SD (median) | 0.9± 0.2 (0.9) | 0.90±0.2 | 0.90±0.22 | 0.95±0.22 |
| (0.80) | (0.90) | (0.93) | ||
|
| ||||
| Glucose (mg/dl), Mean ± SD (median) | 118±46 (103) | 108±31 (98) | 122±55 (105) | 120±44(110) |
|
| ||||
| Platelets (n3/mmc) Mean ± SD (median) | 118±48 (110) | 114±49 (104) | 112±49 (105) | 125±53 (119) |
|
| ||||
| HVPG (mmHg), Mean ± SD | 12.0±4.3 | 11.7± 4.5 | 12.4 ± 4.6 | 12.1 ± 4.0 |
| Median (range) | 11.0 (6-25) | 10.8(6.0-24.5) | 11.5 (6.0-25.0) | 11.0(6.0-21.5) |
According to BMI, the majority of the patients (114/161 or 71%) were overweight or obese with only 29% of the patients having a normal BMI. There were no underweight patients. The proportion of obese patients was significantly greater in patients enrolled in the U.S.A (53.7%) compared to those enrolled in Europe (18.7%, p<0.0001). Conversely, a higher proportion of European patientswere in the normal weight category (32.7 vs. 22.2%, p=0.05) and in the overweight category (48.6 vs. 24.1%, p=0.001), compared to American patients.
As shown in Table 1, the only variable that differed significantly among groups was the etiology of cirrhosis, with “cryptogenic” cirrhosis being more frequent among obese patients (12.2% vs. 1.8% in overweight and normal weight patients, p=0.005). There was a tendency for obese patients to have a higher MELD score (p=0.06 by ANOVA) at baseline, mainly because of significantly higher serum creatinine levels (p=0.04). All the remaining variables, including other components of MELD score and HVPG, were not different among the three BMI groups.
Body mass index and risk of clinical decompensation
Decompensation occurred in 48/161 patients (30%) in a median follow-up of 59 months (range 1-109), and was due to ascites in 33 cases (69%), to hepatic encephalopathy in 15 (31%) and to variceal hemorrhage in 5 (10%). Five patients presented with more than one complication: ascites and variceal hemorrhage in 2, ascites and encephalopathy in 2 and variceal hemorrhage and encephalopathy in 1. Notably, the rate of decompensation was not different from the 29% (62 of 213) observed in the whole study population (2).
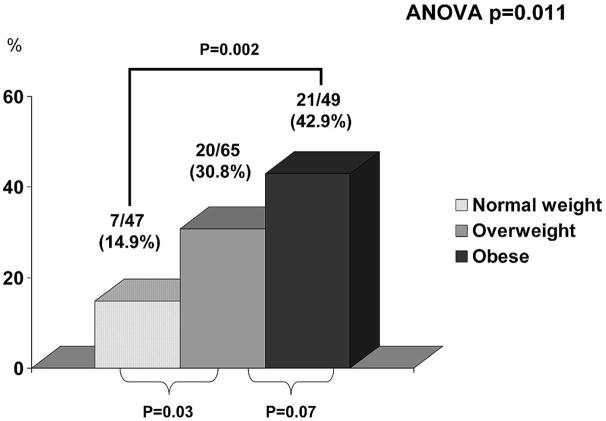
The proportion of patients with clinical decompensation increased with higher baseline BMI: it developed in 7/47 (14.9%) of patients with normal weight, in 20/65 (30.8%) overweight patients and in 21/49 (42.9%) obese patients (p=0.011) (Figure 1, panel A). Patients who were obese and overweight at baseline developed decompensation at a significantly higher rate than patients with a normal weight (p=0.002 and p=0.03 respectively). Obese patients had a tendency to develop decompensation at a higher rate compared to overweight subjects (p=0.07).
Figure 1.
Panel A. Proportion of patients developing first clinical decompensation according to BMI group. As shown, obesity was associated with a significantly higher proportion of clinical decompensation in a median follow-up of 59 months.
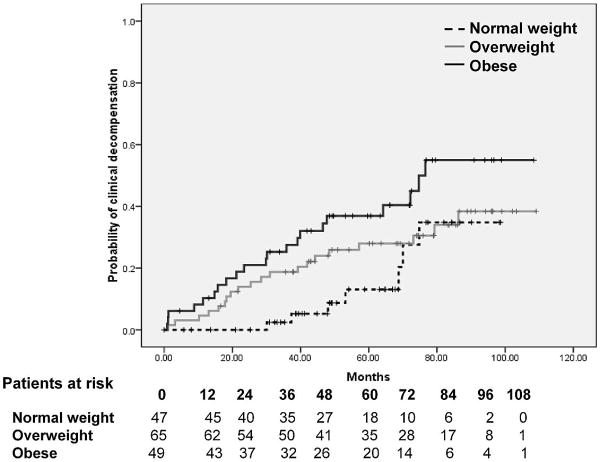
Panel B. Probability of first clinical decompensation of cirrhosis according to BMI group. As shown, obese patients had the highest probability of decompensation and patients with a normal BMI had the lowest probability of decompensation, with overweight patients having an intermediate probability. Differences among groups were statistically significant (Log-Rank 7.60, p=0.022).
The actuarial probability of developing clinical decompensation was significantly different among the three BMI groups (log-rank 7.60, p=0.022), being highest in obese patients, intermediate in overweight patients and lowest in those with a normal BMI (Figure 1 panel B). The cumulative probability of clinical decompensation at 2 and 5 years for each BMI group was: normal weight 0% (95% CI 0% to 0%) and 13% (95% CI 3% to 23%), respectively; overweight patients 14% (95% CI 6% to 22%) and 28 % (95% CI 17% to 39%), respectively; obese patients 21% (95% CI 10% to 32%) and 37% (95% CI 23% to 50%), respectively.
In a sensitivity analysis, the increased risk of decompensation of obese patients was documented both in American patients (5-year probability: 35%; 95% CI 18% to 53%) and in European patients (5-year probability: 39%; 95% CI 18% to 61%).
To evaluate whether BMI is an independent predictor of decompensation, we performed a Cox regression analysis including previously defined predictors of decompensation (HVPG, albumin and MELD) and variables that could potentially act as confounders on the association (etiology and treatment). Therefore, variables introduced into the analysis were: etiology (alcoholic vs. non-alcoholic); MELD score, albumin, HVPG; BMI; and treatment group (timolol or placebo).
Table 2 shows the results of the uni- and multivariate Cox analysis. As shown, HVPG [per 1 mmHg increase hazard ratio, HR: 1.14 (95%CI 1.07-1.20), p<0.0001], albumin [per 1 g/dL decrease HR 4.54 (2.44-8.33), p<0.0001] and BMI [per 1 unit increase HR 1.06 (1.01-1.12), p=0.02] remained independently associated to clinical decompensation in the final model, while MELD score was excluded. Therapeutic group (timolol or placebo) was unrelated to outcome (Table 2). The results were similar when the analysis was restricted to the subgroup of patients with HCV-related cirrhosis (n=103), with HVPG, albumin and BMI being the only variables independently associated with clinical decompensation: HVPG [per 1 mmHg increase hazard ratio, HR: 1.19 (95%CI 1.09-1.30), p<0.0001], albumin [per 1 g/dL decrease HR 2.78 (1.06-7.14), p=0.04] and BMI [per 1 unit increase HR 1.09 (1.01-1.19), p=0.03].
Table 2.
Factors associated with clinical decompensation in univariate and multivariate analysis in the study cohort.
| Univariate Cox Regression | |||
|---|---|---|---|
| Variable | Hazard Ratio | 95% CI | P Value |
| Gender (male) | 1.60 | 0.87 – 2.95 | 0.130 |
| MELD score | 1.27 | 1.12 – 1.44 | < 0.0001 |
| Serum albumin (per 1 g/dL decrease) | 4.55 | 2.50 – 7.69 | < 0.0001 |
| Serum Creatinine (per 1 mg/dL increase) | 1.03 | 0.28 – 3.81 | 0.962 |
| Serum bilirubin (per 1 mg/dL increase) | 2.06 | 1.40 – 3.02 | <0.0001 |
| INR | 10.92 | 2.02 – 58.99 | 0.005 |
| HVPG (per 1 mmHg increase) | 1.12 | 1.06 – 1.18 | <0.0001 |
| BMI (per 1 Kg/m2 increase) | 1.06 | 1.00 – 1.11 | 0.036 |
| Treatment (timolol) | 1.45 | 0.81 – 2.59 | 0.207 |
| Etiology (alcohol) | 1.26 | 0.69 – 2.31 | 0.445 |
| Etiology (viral) | 0.66 | 0.37-1.18 | 0.161 |
| Multivariate Cox Regression | |||
|---|---|---|---|
| Variable | Hazard Ratio | 95% CI | P Value |
| Serum albumin (per 1 g/dL decrease) | 4.54 | 2.44-8.33 | <0.0001 |
| HVPG (per 1 mmHg increase) | 1.14 | 1.07-1.20 | <0.0001 |
| BMI (per 1 Kg/m2 increase) | 1.06 | 1.01-1.12 | 0.02 |
Body mass index and HVPG change after 1 year of follow-up
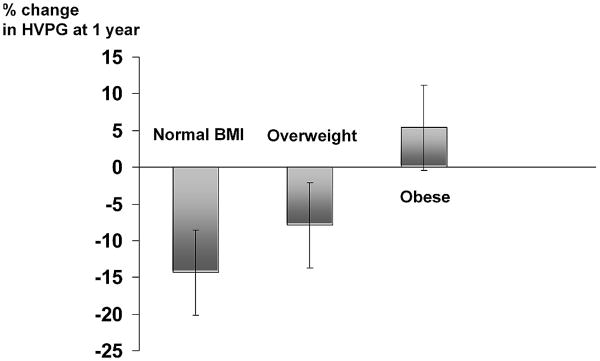
One hundred eighteen patients (30 normal BMI, 47 overweight and 41 obese) underwent a second HVPG measurement after 1 year of follow-up. The 1-year change in HVPG was linearly correlated to baseline BMI (R=0.348, p<0.01) and to 12-month BMI (r=0.306, p<0.01). While patients with a normal BMI had a significant reduction in HVPG (mean reduction of 14.3 ± 26.8%; 95%CI 4.3% to 23.7%; median reduction 15.2%, p=0.007 vs. baseline), as did overweight patients (mean reduction 7.9 ± 16.4%; 95% CI 2.3% to 14.7%; median reduction 11.5%; p=0.14 vs. normal BMI, p=0.002 vs. baseline), obese patients had a slight, non significant increase in HVPG (mean increase of 5.4 ± 32.4%; 95%CI -5.1% to 15.1%); median 0%; p=0.004 vs. normal BMI; p=0.015 vs. overweight) (Figure 2).
Figure 2.
Change in HVPG after 1 year of follow-up according to BMI group (normal BMI n= 30, overweight n=47, obese n=41). Change is expressed as percentage, and bars represent mean change; vertical lines over bars indicate standard errors of the mean. As shown, patients with normal BMI and overweight patients had a decrease of HVPG at 1 year, while in obese patients the HVPG did not decrease (p=0.004 vs. normal BMI; p=0.015 vs. overweight).
Consequently, the proportion of patients showing a reduction of HVPG ≥ 10% from baseline (“responders”) was significantly lower in obese patients (24.5% vs.40.4 % normal weight and 36.9% overweight, p=0.019). Changes in HVPG at 1 year were not related to treatment (timolol or placebo) group.
Comparison between patients included in the present study and patients with missing BMI included in the original RCT(15)
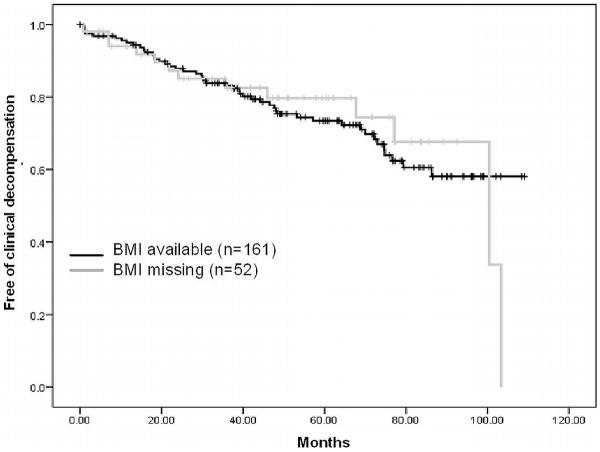
Table 3 shows the comparison between the 161 patients included in the present study in whom height (and consequently BMI) was available, and the 52 originally included in the RCT (15) but in whom BMI could not be calculated since height was unavailable (missing information). As shown, there were no significant differences in baseline characteristics between both groups. Importantly, the incidence of clinical decompensation in the follow-up (Figure 3) did not differ in the two groups, suggesting that no inclusion bias exists and that the population explored in the present study can be considered a random sample from the original cohort. This is further supported by the findings of a multiple imputation analysis of missing values. This analysis showed that obesity was indeed an independent predictor of first clinical decompensation in the entire RCT cohort.
Table 3.
Comparison of main baseline clinical, biochemical and hemodynamic characteristics of the patients included in the study. Data are shown median values (interquartile range). P values refer to the comparison between the two groups.
| Patients with complete data, included in this study (n=161) |
Patients with height missing, not included (n=52) |
p | |
|---|---|---|---|
| Age (yrs) | 54 (23-75) | 56 (30-75) | 0.740 |
|
| |||
| Male gender (n) (%) | 95 (59%) | 31 (60%) | 1.000 |
|
| |||
| BMI (kg/m2) | 27.3 (18.5-44.7) | NA | NA |
|
| |||
| Etiology of cirrhosis: virus/alcohol/crypto/other (% of each) | 109/35/8/9 | 33/16/2/1 | 0.325 |
| (68/22/5/5) | (63/31/4/2) | ||
|
| |||
| Blood Pressure(mmHg) | |||
| Systolic BP | 129 (96-180) | 130 (101-167) | 0.620 |
| Diastolic BP | 75 (50-109) | 78 (57-110) | 0.759 |
| Mean BP | 93 (67-131) | 93 (74-129) | 0.703 |
|
| |||
| Heart rate (bpm) | 73 (53-107) | 76 (56-118) | 0.196 |
|
| |||
| MELD score | 8.2 (6.4-16.3) | 8.4 (6.4-15.4) | 0.435 |
|
| |||
| Bilirubin (mg/dl) | 1.0 (0.2-3.6) | 0.85 (0.3-5.9) | 0.313 |
|
| |||
| Albumin (g/dL) | 4.0 (2.1-5.4) | 4.0 (2.5-5.0) | 0.832 |
|
| |||
| INR | 1.10 (1.00-2.00) | 1.10 (1.00-2.00) | 0.781 |
|
| |||
| AST (U/l) | 77 (16-510) | 71 (16-230) | 0.379 |
|
| |||
| ALT (U/l) | 79 (10-615) | 62 (16-382) | 0.120 |
|
| |||
| Creatinine (mg/dl) | 0.90 (0.50-1.90) | 0.94 (0.20-1.50) | 0.224 |
|
| |||
| Glucose (mg/dl) | 103 (52-401) | 99 (63-221) | 0.358 |
|
| |||
| Platelets (n3/mmc) | 110 (15-286) | 110 (38-559) | 0.764 |
|
| |||
| HVPG (mmHg) | 11.0 (6.0-25.0) | 11.3 (6.0-18.0) | 0.080 |
|
| |||
| Incidence of decompensation | 48/161 (30%) | 13/52 (25%) | 0.446 |
|
| |||
| Months until first decompensation | 53 (0-109) | 49 (1-104) | 0.170 |
Figure 3.
Probability of remaining free of first clinical decompensation of cirrhosis in the 161 patients included in this study and in the 52 patients of the original RCT that were excluded from the study because of missing BMI. Differences between groups were not significant (Log-Rank 0.54, p=0.816), suggesting that no inclusion bias exists in the present analysis.
Discussion
A good nutritional status has been traditionally regarded as a positive prognostic indicator in cirrhosis (19), since malnutrition is a feature of late, decompensated stages of the disease.
In the present study we demonstrate for the first time that increased body adiposity, as indicated by an increased body mass index, is a strong predictor of decompensation in patients with compensated cirrhosis of various etiologies, independent of other previously described predictors such as albumin and HVPG, and independent of receiving placebo or active treatment with beta-blockers. The robustness of the findings was further confirmed by multiple imputation analysis (18) of missing values of BMI in the whole cohort of the original RCT.
Cryptogenic cirrhosis, possibly due to earlier NAFLD/NASH, was more prevalent in our obese patients, so etiology might potentially confound the effect of BMI on outcome; nonetheless, the proportion of patients with this etiology was small (12% in the obese patients group), and, as confirmed by multivariate analysis, did not explain the poorer outcome in obese patients. Importantly, the analysis restricted to patients with HCV, the most common cause of cirrhosis, showed identical results compared to the whole study cohort with BMI being an independent predictor of decompensation.
A recent paper by Everhart and colleagues (13) showed that obesity-related variables (specifically insulin resistance and histological features of fatty liver), but not obesity itself, were independently associated with a worse outcome, as defined by histological and/or clinical events, in a large cohort of patients with advanced HCV liver disease. These variables were not analyzed in our study, but the strong predictive value of BMI suggests that this easily measured variable should be routinely recorded in every patient with compensated cirrhosis. In fact, in the study by Everhart et al (13), obesity was significantly associated on univariate analysis with the development of outcomes (histological and/or clinical).
The mechanism underlying the association between obesity and the development of clinical decompensation in cirrhosis is unknown. In our study, one-year changes in portal pressure (as determined by HVPG) were significantly different among BMI groups, with HVPG decreasing significantly in normal weight and overweight patients but not in obese patients (in whom it showed a slight, not statistically significant increase) (Figure 2). HVPG failed to decrease in obese patients despite the fact that half of them received a potent non-selective beta-blocker (timolol) (20). Moreover, only a minority of obese patients had a satisfactory hemodynamic response (HVPG decrease ≥10%) at 12 months. This observation suggests that the mechanism by which obesity leads to a greater incidence of clinical decompensation is, at least in part, by an increase in HVPG, as portal hypertension is responsible for the clinical complications that define this late stage of cirrhosis. In this regard it is worth noting that adipose tissue in obesity is known to acquire a pro-inflammatory, pro-fibrogenic and pro-angiogenic phenotype resulting in the production of adipokines and cytokines (leptin, IL-1 and TNF-alfa) (21-23) with deleterious vascular effects on hepatic inflammation, fibrogenesis and angiogenesis, which may mediate a further worsening of intrahepatic resistance and portal hypertension (24, 25).
From an epidemiologic point of view, it is interesting to note that over two thirds of our patients with compensated cirrhosis were overweight or obese and that this proportion is similar to that of the general population both in Europe and in the United States (4). It could therefore be predicted that, as has been occurring in the general population, obesity will increase further among patients with compensated cirrhosis. With this perspective and given the increased risk of clinical decompensation in obese patients with cirrhosis, the implementation of measures aimed at reducing obesity in patients with chronic liver diseases should be considered a priority.
From a clinical point of view our findings have important implications. Up to now the only modifiable lifestyle-associated risk factor for decompensation in cirrhosis was alcohol ingestion in patients with alcoholic cirrhosis. In a similar way, our data suggest that overweight plays an important and independent role in the progression of compensated to decompensated cirrhosis, and might become a new non-pharmacological target for preventing clinical events in this population. In fact, in patients with advanced chronic hepatitis C, weight loss has been associated with a better outcome, confirming that it can modify progression of chronic liver diseases (13).
Some limitations of the present study should be acknowledged. The source of our data is a randomized-controlled study which was not performed with the objective of evaluating the impact of obesity on clinical decompensation. However, given the prospective nature of the source data, the information we present is reliable, and not easily obtainable. Even though height was not a variable collected in the original RCT, we were able to obtain height values in 76% of the study population, and patients included in the present analysis were representative of the trial population (Table 3 and Figure 3). It should be also noted that even though BMI was a clear and strong predictor of decompensation, other factors, namely liver failure (indicated by serum albumin) and portal hypertension (as indicated by the HVPG) appeared to be more potent drivers of decompensation.
In conclusion, increased BMI is an independent predictor of clinical decompensation in patients with compensated cirrhosis of various etiologies, suggesting that obesity accelerates the progression of cirrhosis and that its correction could be a valuable non-pharmacological measure to improve prognosis in this patient population. Specific studies addressing this question are necessary.
Acknowledgments
Financial support: Supported by grants from the National Institutes of Health (RO1 DK46580, P30 DK34989 and K24 DK02727-02), and from the Instituto de Salud Carlos III (FIS 09/1261 to Jaime Bosch). CIBERehd is funded by Instituto de Salud Carlos III.
Abbreviations
- HVPG
hepatic venous pressure gradient
- MELD
Mayo end stage liver disease score
- CD
clinical decompensation
- BMI
body mass index
- CLD
chronic liver disease
- RCT
randomized controlled trial
Footnotes
No financial conflicts of interest are declared for any of the authors.
Dr. Garcia-Tsao had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis
References
- 1.D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Ripoll C, Groszmann R, Garcia-Tsao G, Grace N, Burroughs A, Planas R, et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481–488. doi: 10.1053/j.gastro.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 3.Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132:2087–2102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization (WHO) Report of a WHO Consultation. Geneva: World Health Organization; 2000. Obesity: preventing and managing the global epidemic. [PubMed] [Google Scholar]
- 5.World Health Organization. Report of a Joint FAO/WHO Expert Consultation. Geneva: World Health Organization; 2003. Diet, Nutrition and the Prevention of Chronic Diseases. [Google Scholar]
- 6.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 7.Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356–1362. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- 8.Ratziu V, Giral P, Charlotte F, Bruckert E, Thibault V, Theodorou I, et al. Liver fibrosis in overweight patients. Gastroenterology. 2000;118:1117–1123. doi: 10.1016/s0016-5085(00)70364-7. [DOI] [PubMed] [Google Scholar]
- 9.Liu B, Balkwill A, Reeves G, Beral V. Body mass index and risk of liver cirrhosis in middle aged UK women: prospective study. BMJ. 2010;340:c912. doi: 10.1136/bmj.c912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ratziu V, Bonyhay L, Di M V, Charlotte F, Cavallaro L, Sayegh-Tainturier MH, et al. Survival, liver failure, and hepatocellular carcinoma in obesity-related cryptogenic cirrhosis. Hepatology. 2002;35:1485–1493. doi: 10.1053/jhep.2002.33324. [DOI] [PubMed] [Google Scholar]
- 11.Naveau S, Giraud V, Borotto E, Aubert A, Capron F, Chaput JC. Excess weight risk factor for alcoholic liver disease. Hepatology. 1997;25:108–111. doi: 10.1002/hep.510250120. [DOI] [PubMed] [Google Scholar]
- 12.Raynard B, Balian A, Fallik D, Capron F, Bedossa P, Chaput JC, et al. Risk factors of fibrosis in alcohol-induced liver disease. Hepatology. 2002;35:635–638. doi: 10.1053/jhep.2002.31782. [DOI] [PubMed] [Google Scholar]
- 13.Everhart JE, Lok AS, Kim HY, Morgan TR, Lindsay KL, Chung RT, et al. Weight-related effects on disease progression in the hepatitis C antiviral long-term treatment against cirrhosis trial. Gastroenterology. 2009;137:549–557. doi: 10.1053/j.gastro.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ortiz V, Berenguer M, Rayon JM, Carrasco D, Berenguer J. Contribution of obesity to hepatitis C-related fibrosis progression. Am J Gastroenterol. 2002;97:2408–2414. doi: 10.1111/j.1572-0241.2002.05995.x. [DOI] [PubMed] [Google Scholar]
- 15.Groszmann RJ, Garcia-Tsao G, Bosch J, Grace ND, Burroughs AK, Planas R, et al. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med. 2005;353:2254–2261. doi: 10.1056/NEJMoa044456. [DOI] [PubMed] [Google Scholar]
- 16.US Department of Agriculture and US Department of Health and Human Services. Nutrition and your health: dietary guidelines for Americans. 2009 [Google Scholar]
- 17.Kuczmarski RJ, Flegal KM. Criteria for definition of overweight in transition: background and recommendations for the United States. Am J Clin Nutr. 2000;72:1074–1081. doi: 10.1093/ajcn/72.5.1074. [DOI] [PubMed] [Google Scholar]
- 18.Donders AR, van der Heijden GJ, Stijnen T, Moons KG. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol. 2006;59:1087–1091. doi: 10.1016/j.jclinepi.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1–85. [PubMed] [Google Scholar]
- 20.Weiner N. Drugs that inhibit adrenergic nerves and block adrenergic receptors. In: Goodman Gilman A, Goodman LS, Gilman A., editors. Goodman and Gilman's the pharmacological basis of therapeutics. 6th. New York: Macmillan; 1980. pp. 176–210. [Google Scholar]
- 21.Marra F, Bertolani C. Adipokines in liver diseases. Hepatology. 2009;50:957–969. doi: 10.1002/hep.23046. [DOI] [PubMed] [Google Scholar]
- 22.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aleffi S, Petrai I, Bertolani C, Parola M, Colombatto S, Novo E, et al. Upregulation of proinflammatory and proangiogenic cytokines by leptin in human hepatic stellate cells. Hepatology. 2005;42:1339–1348. doi: 10.1002/hep.20965. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez M, Semela D, Bruix J, Colle I, Pinzani M, Bosch J. Angiogenesis in liver disease. J Hepatol. 2009;50:604–620. doi: 10.1016/j.jhep.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 25.Mejias M, Garcia-Pras E, Tiani C, Miquel R, Bosch J, Fernandez M. Beneficial effects of sorafenib on splanchnic, intrahepatic, and portocollateral circulations in portal hypertensive and cirrhotic rats. Hepatology. 2009;49:1245–1256. doi: 10.1002/hep.22758. [DOI] [PubMed] [Google Scholar]