Abstract
Aging men and women display both increased incidence of cardiovascular disease and complications of myocardial infarction and heart failure. We hypothesized that altered glucose metabolism, in particular, flux of glucose via the polyol pathway (PP) may be responsible, in part, for the enhanced vulnerability of aging myocardium to ischemic injury, even in the absence of superimposed disease processes linked to PP flux, such as diabetes. To test our hypothesis, we determined the expression and products of PP enzymes aldose reductase (AR) and sorbitol dehydrogenase (SDH) in hearts from Fischer 344 aged (26 month) and young (4 month) rats subjected to global ischemia followed by reperfusion in the presence or absence of blockers of PP and the measures of ischemic injury and functional recovery were determined. Expression and activities of AR and SDH were significantly higher in aged vs. young hearts, and induction of ischemia further increased AR and SDH activity in the aged hearts. Myocardial ischemic injury was significantly greater in aged vs. young hearts, and blockade of AR reduced ischemic injury and improved cardiac functional recovery on reperfusion in aged hearts. These data indicate that innate increases in activity of the PP enzymes augment myocardial vulnerability to I/R injury in aging, and that blockers of PP protect the vulnerable aging hearts.
Keywords: ldose reductase, polyol pathway, myocardial ischemic injury, aging hearts, cardiac function
1. INTRODUCTION
Lakatta and Levy summarized the complexities of understanding aging and the risk of cardiovascular disease, and concluded that the major factor underlying the propensity of cardiovascular diseases in human subjects is advancing age itself, even after taking into account aging-associated diseases, such as diabetes, sedentary life style, and genetic predisposition (Lakatta et al., 2003a; Lakatta et al., 2003b; Lakatta et al, 2003c) Innate dysfunction of the aging cardiovascular system in the vascular arterial structures and myocardium, in part, primes aging subjects for increased vulnerability to superimposed stresses. In this work, we focused on one such stress, ischemia/reperfusion (I/R) injury (Wei et al., 1992). Studies in aging animals have demonstrated increased cell death and poor survival after cardiac I/R injury compared to young animals (Azhar et al, 1999a; Liu et al., 2002; Isoyama et al., 2002; Azhar et al., 1999b). Although multiple pathways contribute to the above findings, we have focused on the role of glucose metabolizing pathways in aging and ischemic stress.
A number of mechanisms with diverse etiologies have been postulated to contribute to myocardial damage due to ischemia in young animals. Although reperfusion, especially early after the onset of occlusion, has provided immense benefit during acute myocardial ischemia, recovery of function after ischemia is predicated not only on restoration of nutritive perfusion but also on the ability of the myocyte to maintain sufficient substrate metabolism during ischemia. Numerous studies (Camici et al., 1989; Stanley et al., 1997; Taegtmeyer et al., 1998; Young et al.,1997; Ramasamy et al., 2001; Vanoverschelde et al., 1994; Lopaschuk et al., 1997; Lopaschuk et al., 1988; Neely et al., 1974; Schaefer et al., 1995; Steenbergen et al., 1990; Steenbergen et al., 1993), have shown that the recovery of function after ischemia is enhanced by interventions that (a) maintain tissue ATP availability, and (b) limit derangements in ionic homeostasis. The presence of inherent metabolic alterations seen in aging animals and humans (Hall et al., 1994; Cartee, 1993; McMillin et al., 1993; Lesnefsky et al., 1994) restricts the availability of therapeutic interventions to protect ischemic myocardium in aging subjects. Impaired metabolism and energy homeostasis have been associated with poor metabolic and functional recovery after I/R in hearts (Kates et al., 2003; Misare et al., 1992; Headrick et al., 1998). In this context, the relationship between altered glucose metabolism and response to I/R is poorly understood in hearts from aging subjects. Studies by us and others have demonstrated that increased substrate flux via aldose reductase impairs and impedes recovery of myocardium after ischemic insult in young animals (Ramasamy et al., 1997; Ramasamy et al., 1998; Hwang et al., 2002; Hwang et al., 2004; Tracey et al., 2000). In this study, we investigated if aging impacts flux via the aldose reductase pathway and as a consequence increases vulnerability of hearts to I/R injury.
2. MATERIALS AND METHODS
All animal studies were performed with the approval of the Institutional Animal Care and Use Committee at Columbia University, New York and New York University School of Medicine. This investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication No. 85-23, 1996). Fischer 344 young (4 months) and aged (24 months) rats were purchased from NIA colony (Bethesda, MD). Transgenic mice expressing human aldose reductase driven by the major histocompatibility type 1 promoter and nontransgenic littermates in the C57BL/6 background, young (4 months) and old (24 months), were obtained from our established breeding colony. (Hwang et al., 2004).
2.1. Isolated Perfused Heart Preparation
Experiments were performed using an isovolumic isolated rat/mice heart preparation as published earlier (Ramasamy et al., 2001; Hwang et al., 2004). After deep anesthesia was achieved, hearts were rapidly excised, placed into iced saline, and retrogradely perfused at 37°C in a non-recirculating mode through the aorta. Hearts were perfused with modified Krebs-Henseleit buffer containing (in mM) NaCl 118, KCl 4.7, CaCl2 2.5, MgCl2 1.2, NaHCO3 25, glucose 5, palmitate 0.4, bovine serum albumin 0.4, and 70 mU/L insulin. The perfusate was equilibrated with a mixture of 95% O2-5% CO2, which maintained perfusate PO2 > 600 mmHg. Left ventricular developed pressure (LVDP) and left ventricular end diastolic pressure (LVEDP) were measured using a latex balloon in the left ventricle. LVDP, heart rate, and coronary perfusion pressure were monitored continuously on an ADI recorder. All rat hearts were subjected to 20 min of zero-flow ischemia and 60 min of reperfusion (I/R). All mice hearts were subjected to 30 min of zero-flow ischemia and 60 min of reperfusion.
In studies involving the use of inhibitors, hearts were perfused with modified Krebs-Henseleit buffer containing enzyme specific inhibitors of AR (ARI-gift from Pfizer, Groton, CT) 1 µM unbound zopolrestat, or SDH (SDI-gift from Pfizer, Groton, CT) 200 nM CP-407,711, 10 min prior to ischemia and was continued throughout the perfusion protocol. The specificity of ARI and SDI used in this study has been well established (Mylari et al., 1991; Mylari et al., 2003). Hearts were freeze-clamped after baseline, ischemia, and reperfusion periods for enzyme activity and expression studies. These hearts were stored frozen at −80°C until the analyses were performed.
2.2 Biochemical assays and western blot measurements
Aldose reductase and sorbitol dehydrogenase activities were measured in the heart homogenates using spectrophotometric techniques (Ramasamy et al., 1997; Ramasamy et al., 1998; Hwang et al., 2002). ATP was measured in the neutralized perchloric acid extracts of hearts using HPLC methods according to previously-published procedures (Ramasamy et al., 1997; Ramasamy et al., 1998; Hwang et al., 2002). Creatine kinase (CK) release, a marker of myocardial I/R injury, was measured as published earlier (Ramasamy et al., 1997; Ramasamy et al., 1998; Hwang et al., 2002). For western blot studies, equal amounts of proteins were separated on 4–20% SDS –PAGE and transferred on to PVDF membrane (Invitrogen), blocked with 5% nonfat dry milk for 1.5 hrs and probed with primary antibody overnight at 4°C. Polyclonal rabbit anti-AR IgG; and anti-SDH IgG at 1:500 or 1:1000 dilutions were used. After washing the blots three times in TBS-Tween (TBST), they were incubated in secondary antibody conjugated with HRP, followed by washing in TBST and signals were detected using ECL reagent (Amersham-Pharmacia). Signal intensities were quantified using software Alpha-ease (Kodak). All blots were normalized by reprobing the blots with antibody specific for β-actin or GAPDH.
2.3. Statistical methods
Data was analyzed using INSTAT (GraphPad, San Diego, CA) software operating on an IBM compatible personal computer. Differences between different groups were assessed using ANOVA for repeated measures, with subsequent Student-Newman-Keuls multiple comparisons post-tests if the p value for ANOVA was significant. All data are expressed as mean±standard deviation.
3. RESULTS & DISCUSSION
3.1. AR pathway and myocardial I/R injury in aging
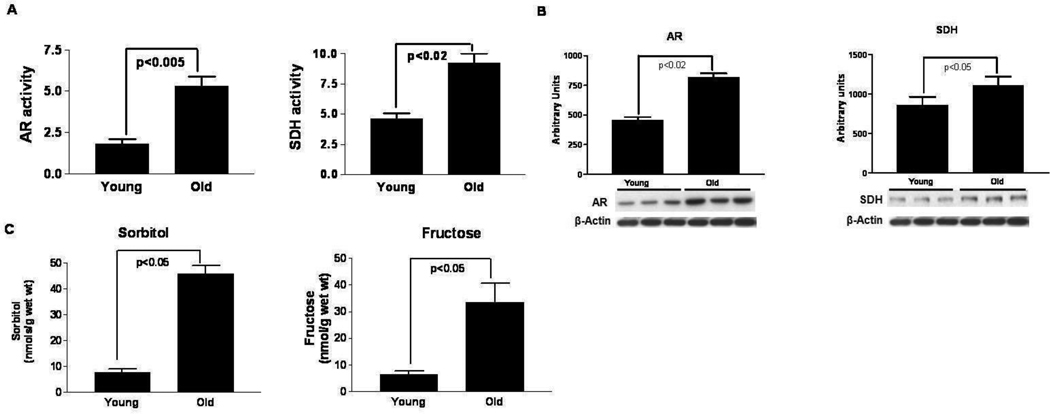
To determine if aging alters the activities of aldose reductase (AR) and sorbitol dehydrogenase (SDH), hearts from young (4 months) and old (26 months) Fischer 344 rats were isolated, perfused and freeze-clamped and the activities of these enzymes and their products (sorbitol, fructose) were measured using biochemical assays, and the expression levels assessed using western blots. Figure 1 a,b,c indicate that in the absence of I/R injury, aging hearts displayed higher myocardial AR and SDH activities along with their respective products sorbitol and fructose, and protein expression compared to young hearts. These data indicate for the first time that aging is linked to increases in the AR pathway expression and activity in the Fischer 344 rat heart.
Figure 1.
(A) Aldose reductase (AR) (nmols/min/mg prot) and sorbitol dehydrogenase (SDH) (IU/mg prot) activity; (B) Protein expression of AR and SDH; and (C) concentration of polyol pathway products sorbitol and fructose in perfused hearts from young (n=6) and old (n=6) Fischer 344 rat hearts subjected to normoxic perfusion (4 mos vs. 24 mos of age, respectively).
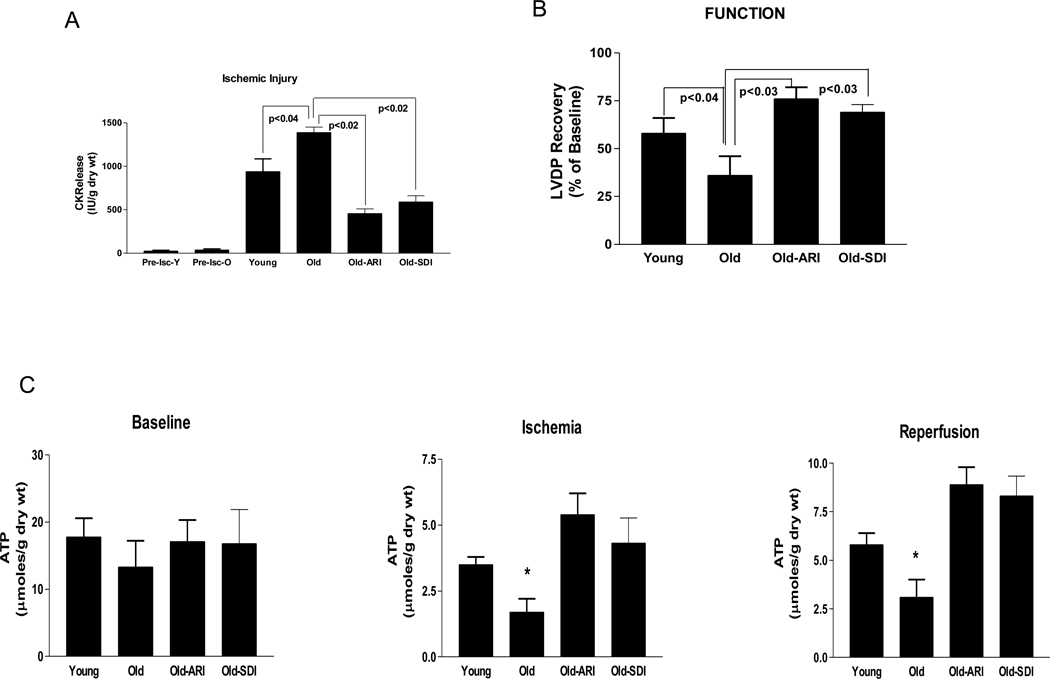
To investigate if increases in the AR pathway impact on the ability of aging rat hearts to recover from I/R injury, isolated perfused hearts were subjected to I/R and the measures of injury and cardiac function monitored. Creatine kinase (CK) release during reperfusion, a marker of ischemic injury, was greater in old hearts than in young rat hearts (Fig 2a). Pharmacological blockade of AR or SDH reduced CK release during reperfusion in old hearts after ischemia. Left ventricular developed pressure recovery was lower in old hearts compared to the young hearts after I/R (Fig 2b), indicating impaired functional recovery in old hearts. ARI or SDI improved cardiac functional recovery on reperfusion in aging hearts. ATP is a critical determinant of the ability of myocardium to recover from ischemic injury. In order to determine if ATP levels are differentially influenced in aging vs. young myocardium, ATP measurements were made during baseline, ischemia, and reperfusion using HPLC methods. Although there were no significant differences in ATP levels in the myocardium at baseline, ATP levels were much less conserved in old hearts vs. young during ischemia and reperfusion (Fig. 2c). Inhibition of AR or SDH improved ATP levels in aging hearts during ischemia and reperfusion. Together, these data indicate that interventions that block AR and SDH protect aging hearts from I/R injury.
Figure 2.
(A) Creatine kinase (CK) release during 60 min of reperfusion, a marker of myocardial I/R injury in hearts from young, old, Old-ARI treated (Old-ARI) or Old-SDI treated (Old-SDI) Fischer 344 rats. Pre-ischemic CK release data for young (Pre-Isc-Y) and old (Pre-Isc-O) presented reveal no basal differences in aged vs. young rat hearts. (B) Left ventricular developed pressure (LVDP) recovery in the above hearts. (C) Myocardial ATP content in young, old, old-ARI treated (Old-ARI) or old-SDI treated (old-SDI) Fischer 344 rats. * p<0.05 vs. young in their respective ischemia or reperfusion group. All hearts were subjected to global ischemia followed by reperfusion. ARI = zopolrestat; 1 µM and SDH = CP-470,711, 200 nM. n=6 per group
While most studies have demonstrated that increased flux via AR is detrimental during ischemia-reperfusion in hearts (Ramasamy et al., 1997; Ramasamy et al., 1998; Hwang et al., 2002; Hwang et al., 2004; Tracey et al., 2000; Ananthakrishnan et al., 2009; Iwata et al., 2006; Tang et al., 2008; Li et al., 2008), some studies have shown contrary results (Shinmura et al., 2002; Kaiserova et al., 2008). Though others (Kaiserova et al., 2008) showed increases in AR activity during ischemia consistent with our earlier publication (Hwang et al., 2002), they were unable to demonstrate cardioprotection with ARIs in a glucose perfused isolated rat heart I/R model. Reasons for these contrasting findings in young animals could, in part, be due to model-dependent variations and substrate availability. Our findings here in aging rats indicate that increased flux via AR leads to increased injury during I/R in the heart.
3.2. Metabolic consequences of AR pathway increases in aging
Several studies have shown that increases in flux via the AR pathway lead to increases in cytosolic NADH/NAD+ ratio (Williamson et al. 1993); Trueblood & Ramasamy (1998). Furthermore, it has also been shown that tissue lactate/pyruvate (L/P) ratio reflects the cytosolic ratio of NADH/NAD+ (Williamson et al. 1993); Trueblood & Ramasamy (1998) To determine if increases in AR and SDH seen in aging hearts impact on cytosolic NADH/NAD+, L/P ratios were measured in freeze-clamped heart tissue extracts. As shown in Table 1, the baseline L/P ratios were significantly higher in old hearts than in young hearts. Pharmacological blockade of AR or SDH significantly lowered L/P ratio in both old and young rat hearts. Ischemia results in an increased flux via the AR pathway. Increased flux via this pathway leads to utilization of NADPH by AR and NAD+ by sorbitol dehydrogenase. This flux contributes to increases in cytosolic NADH/NAD+ ratio. As shown previously, increasing flux via the AR pathway increases L/P ratio, impairs glycolysis, and decreases ATP levels in ischemic hearts (Ramasamy et al., 1997; Ramasamy et al., 1998; Hwang et al., 2002; Hwang et al., 2004; Tracey et al., 2000; Ananthakrishnan et al., 2009; Iwata et al., 2006; Tang et al., 2008; Li et al., 2008; Williamson et al., 1993; Trueblood et al., 1998; Hwang et al., 2003). In the present study increases in L/P ratio were attenuated; ATP decline was reversed, and ischemic injury was reduced in aged hearts by treatment with an AR or SDH inhibitor. Inhibition of AR or SDH also resulted in improved functional recovery on reperfusion in aging hearts. These data demonstrate that increased L/P ratio, hence cytosolic NADH/NAD+ ratio, is an important event in AR pathway mediated increases in ischemic injury in aging.
Table 1.
Lactate:pyruvate ratios in young and old Fisher 344 rat hearts
| Group | n | Lactate/Pyruvate ratio |
|---|---|---|
| Young | 12 | 16.8 ± 2.2 |
| Young-ARI | 9 | 6.8 ± 2.8# |
| Old | 6 | 36.2 ± 8.8* |
| Old-ARI | 5 | 7.2 ± 3.1# |
| Old-SDI | 5 | 8.9 ± 4.4# |
p < 0.05 Old vs Young,
p<0.05 Old-ARI, Old-SDI vs Old, young-ARI vs Young. The lactate/pyruvate ratios were measured after perfusion for one hour under normoxic conditions.
3.3. Aldose reductase in aging mice
To determine if the AR pathway plays a critical role in increasing the vulnerability of aging hearts to I/R injury, we investigated the impact of I/R stress and changes in AR activity and content in young and aging C57BL6 mice hearts and compared them with those in young and old rat hearts. Data in the C57BL6 mice hearts reveal that wild-type mice of this strain do not fully recapitulate the enhanced vulnerability to I/R stress noted in rats (Figure 3). In the isolated perfused heart, aged vs. young C57BL/6 mice did not display significant increases in release of CK nor LVDP recovery – in contrast to data in the rat, as shown above in figure 2. Similar findings in the C57BL/6 strain in aged mice (release of LDH upon I/R for example in aged vs. young C57BL/6 mice) have been demonstrated (Willems et al. 2005). Despite lack of differences in markers of injury in aged mice hearts, Willems et al (2005) did observe poor functional recovery. In our studies we did not observe differences in functional recovery after I/R in aged vs young mice. As reviewed by Boengler et al (2009), the precise manner of induced injury in the model may impact on the changes in injury and functional recovery after I/R.
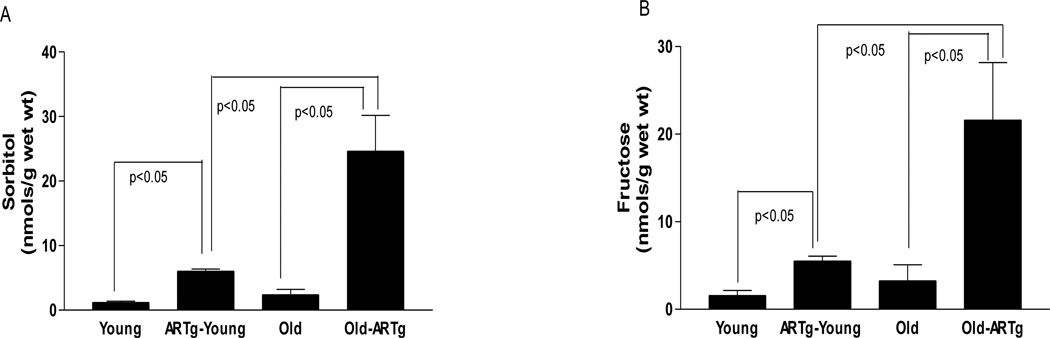
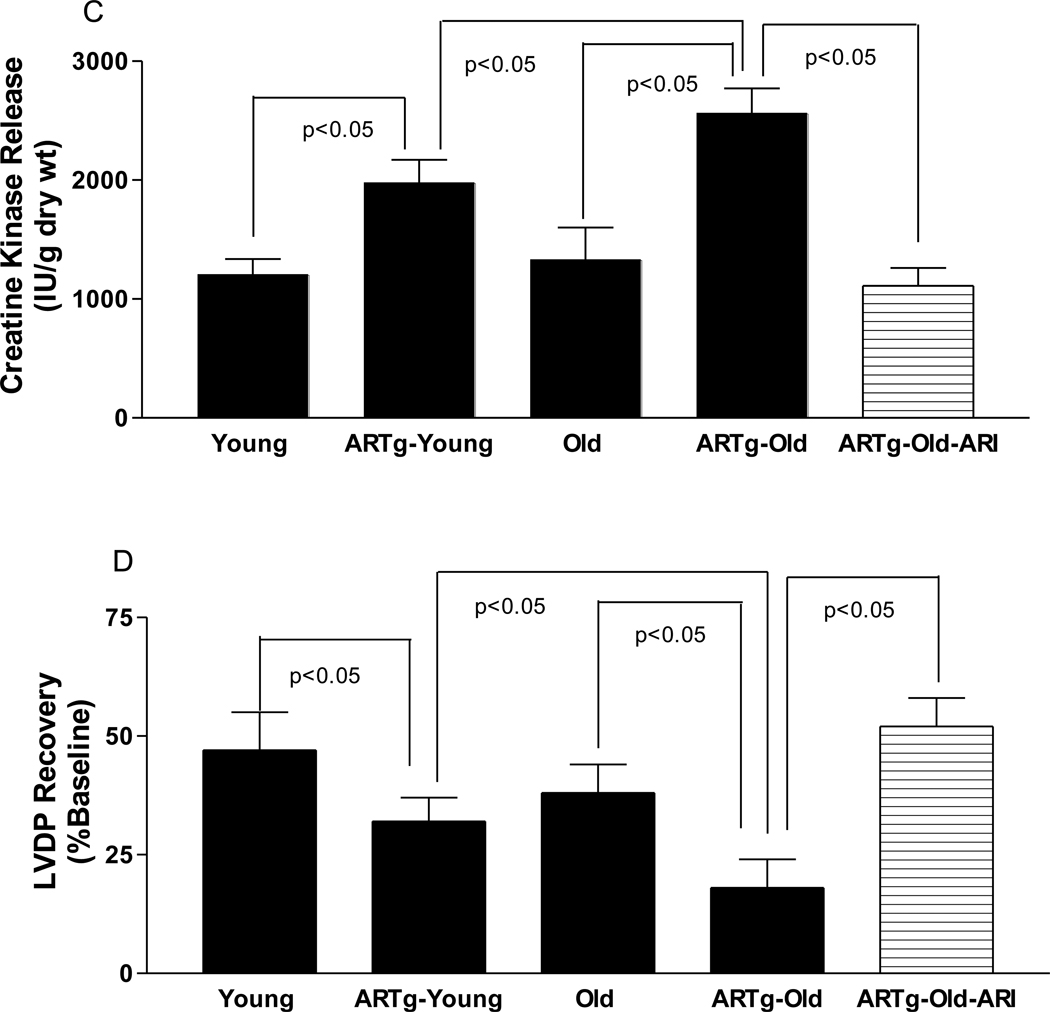
Figure 3.
Cardiac tissue levels of sorbitol (A) and fructose in young, hAR transgenic young (ARTg-Young), Old, and hAR transgenic old (ARTg-Old) subjected to normoxic perfusion. (n=6 per group). (C) Creatine kinase (CK) release during 60 min of reperfusion, a marker of myocardial I/R injury in hearts from young non-transgenic (n=6 ), hAR transgenic young (n=6), old non-transgenic (n=6), hAR transgenic old (n=6) mice. (D) Left ventricular developed pressure (LVDP) recovery during reperfusion in young and old non-transgenic and hAR transgenic mice.
To address the differences in ischemic injury response in rats (Figure 2) vs. mice (Figure 3), we investigated if differences in AR activity and expression in rats vs. mice might, in part, be responsible, given the therapeutic benefit of ARI in the aged rat heart in I/R. Table 2 illustrates the levels and activities of AR in rats and mice. Rats display significantly higher cardiac AR protein and activity compared to mice of the same age. Aging increased the AR protein level and activity in rat hearts, whereas the activity of cardiac AR and levels in aged C57BL6 mice did not change with age. In humans, the levels of AR protein in heart are 300–800 ng/mg total protein in non-diabetic subjects (Hwang et al. 2004). The AR protein levels in the mouse heart are several fold lower than those observed in humans and rats. These results prompted us to employ transgenic mice expressing human AR to restore human and rat-relevant levels of AR in the mouse; we hypothesized that transgenic expression of AR would better model the human aging heart. Note that transgenic hAR mice (a) display human-relevant levels of AR; and (b) the levels of sorbitol and fructose, and AR activity increase with aging, unlike the C57BL/6 mouse (Figure 3, Table 2). Thus, although mice may display many of the features of aging, their inconsistent enhanced vulnerability to I/R stress in the isolated perfused heart, at least in C57BL/6 strain, suggest that the transgenic hAR mouse model offers distinct advantages to probe our hypotheses in aging.
Table 2.
Aldose reductase levels and activity in rat and mice hearts
| Group | AR protein (ng/mg total prot) |
AR activity (nmols NADPH/min/mg prot) |
|---|---|---|
| Fisher 344 RATS | ||
| Young rats (n=5) | 256 ± 51 | 2.66 ± 0.32 |
| Old rats (n=5) | 540 ± 62* | 5.21 ± 0.61# |
| C57BL6 MICE | ||
| Wild Type mice (Young n=8) | 98 ± 21 | 0.66 ± 0.23 |
| Wild Type mice (Old,n=6) | 122 ± 52 | 1.21 ± 0.33 |
| TghAR mice (Young,n=9) | 456 ± 49@ | 4.12 ± 0.81$ |
| TghAR mice (Old mice, n=5) | 598 ± 25** | 6.89 ± 0.31** |
p<0.03 vs. young rats
p<0.04 vs. young rats
p<0.05 vs. young mice
p<0.05 vs. young and old mice
p<0.05 vs. young TghAR mice
Consistent with the hypothesis that restoration of human relevant levels of AR in the heart will drive increased I/R injury in the mouse, aged transgenic hAR mice (24 months) were subjected to identical degrees of I/R as that induced in the rats as shown in figure 2 above. Aged transgenic hAR mice hearts had similar wet and dry weight, total body weight, and physical appearance as the aged non-transgenic littermates. Data from these aged transgenic hAR mice and aged non-transgenic littermate mice are presented in Figure 3. Transgenic hAR mice exhibited increases in AR activity and polyol content (sorbitol and fructose with aging (fig 3a & b). Thus, increases in AR expression were observed in aging rats and aging hAR mice hearts. Of note, in the transgenic hAR mice, these observed increases in AR expression with aging are likely due, at least in part, to significant increases in human AR transgene transcription. Indeed, studies by others have clearly shown that pathological insults can specifically lead to increases in expression of transgene-driven hAR gene expression in mice (Vikramadithyan et al 2005 and Dan et al 2004). In this context, while the precise mechanisms by which AR expression increases in the aging hAR mice and rats is unclear, it is conceivable that increases in reactive oxygen species and AGEs may play an important role in directly mediating increases in AR expression (Dan et al 2004, Nakamura et al 2000).
Aged trangenic hAR mice hearts exhibited increased ischemic injury (CK release, fig. 3c) and poorer LVDP recovery (fig. 3d) after I/R than young transgenic hAR mice hearts. Inhibition of AR with ARI in aged hAR mice reduced ischemic injury (fig 3c) and improved functional recovery (fig 3d) after I/R. It is evident from the present study that in aging hearts, basal increases in AR expression and activity may exert an adverse impact on the ability of myocardium to recover from an added stress such as ischemia. Inhibition of such increases in AR activity reduced ischemic injury and improved functional recovery. Clearly, increased expression and activity of AR was associated with increased markers of injury due to I/R. Furthermore, mice expressing human AR recapitulated the increased vulnerability to I/R stress that were observed in aging Fischer 344 rat hearts. Consistent with earlier findings, we showed that AR pathway inhibition lowered L/P ratio and improved ATP levels in aging hearts (Ramasamy et al., 1997; Ramasamy et al., 1998; Hwang et al., 2002; Hwang et al., 2004; Tracey et al., 2000; Ananthakrishnan et al., 2009; Iwata et al., 2006; Tang et al., 2008; Li et al., 2008; Williamson et al., 1993; Trueblood et al., 1998; Hwang et al., 2003) However, the influence of the AR pathway in impacting intracellular sodium and calcium homeostasis (Ramasamy et al., 1999), oxidative stress (Ananthakrishnan et al., 2009; Tang et al., 2008; Ho et al., 2006) and PKC mediated signaling in aging (Hwang et al., 2005; Ramana et al., 2006) events needs to be investigated. The current studies indicate that AR plays a central role in mediating myocardial ischemic injury and provides a foundation for evaluating AR inhibitors as potential therapeutic adjuncts in treating myocardial infarction in aging.
Research Highlights.
Aging increases flux via PP enzymes AR and SDH;
Increases in PP activity are linked to increased injury and poorer functional recovery after I/R in aging;
Increases in PP flux linked to reduced levels of ATP during I/R
ACKNOWLEDGMENTS
This work was supported by grants from the U.S.P.H.S. AG026467, HL61783, and HL60901. We thank Ms. Latoya Woods for her assistance in manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ananthakrishnan R, Kaneko M, Hwang YC, Quadri N, Gomez T, Li Q, Caspersen C, Ramasamy R. Aldose reductase mediates myocardial ischemia-reperfusion injury in part by opening mitochondrial permeability transition pore. American journal of physiology. 2009;296:H333–H341. doi: 10.1152/ajpheart.01012.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azhar G, Gao W, Liu L, Wei JY. Ischemia-reperfusion in the adult mouse heart influence of age. Experimental gerontology. 1999a;34:699–714. doi: 10.1016/s0531-5565(99)00031-5. [DOI] [PubMed] [Google Scholar]
- Azhar G, Liu L, Zhang X, Wei JY. Influence of age on hypoxia/reoxygenation-induced DNA fragmentation and bcl-2, bcl-xl, bax and fas in the rat heart and brain. Mechanisms of ageing and development. 1999b;112:5–25. doi: 10.1016/s0047-6374(99)00048-2. [DOI] [PubMed] [Google Scholar]
- Boengler K, Schultz R, Heusch G. Loss of cardioprotection with ageing. Cardiovasc Res. 2009;83:247–261. doi: 10.1093/cvr/cvp033. [DOI] [PubMed] [Google Scholar]
- Camici P, Ferrannini E, Opie LH. Myocardial metabolism in ischemic heart disease: basic principles and application to imaging by positron emission tomography. Progress in cardiovascular diseases. 1989;32:217–238. doi: 10.1016/0033-0620(89)90027-3. [DOI] [PubMed] [Google Scholar]
- Cartee GD. Myocardial GLUT-4 glucose transporter protein levels of rats decline with advancing age. Journal of gerontology. 1993;48:B168–B170. doi: 10.1093/geronj/48.4.b168. [DOI] [PubMed] [Google Scholar]
- Dan Q, Wong RL, Yin S, Chung SK, Chung SS, Lam KS. Interaction between the polyol pathway and non-enzymatic glycation on mesangial cell gene expression. Nephron Exp Nephrol. 2004;98:e89–e99. doi: 10.1159/000080684. [DOI] [PubMed] [Google Scholar]
- Hall JL, Mazzeo RS, Podolin DA, Cartee GD, Stanley WC. Exercise training does not compensate for age-related decrease in myocardial GLUT-4 content. J Appl Physiol. 1994;76:328–332. doi: 10.1152/jappl.1994.76.1.328. [DOI] [PubMed] [Google Scholar]
- Headrick JP. Aging impairs functional, metabolic and ionic recovery from ischemia-reperfusion and hypoxia-reoxygenation. Journal of molecular and cellular cardiology. 1998;30:1415–1430. doi: 10.1006/jmcc.1998.0710. [DOI] [PubMed] [Google Scholar]
- Ho EC, Lam KS, Chen YS, Yip JC, Arvindakshan M, Yamagishi S, Yagihashi S, Oates PJ, Ellery CA, Chung SS, Chung SK. Aldose reductase-deficient mice are protected from delayed motor nerve conduction velocity, increased c-Jun NH2-terminal kinase activation, depletion of reduced glutathione, increased superoxide accumulation, and DNA damage. Diabetes. 2006;55:1946–1953. doi: 10.2337/db05-1497. [DOI] [PubMed] [Google Scholar]
- Hwang YC, Bakr S, Ellery CA, Oates PJ, Ramasamy R. Sorbitol dehydrogenase: a novel target for adjunctive protection of ischemic myocardium. Faseb J. 2003;17:2331–2333. doi: 10.1096/fj.03-0128fje. [DOI] [PubMed] [Google Scholar]
- Hwang YC, Kaneko M, Bakr S, Liao H, Lu Y, Lewis ER, Yan S, Ii S, Itakura M, Rui L, Skopicki H, Homma S, Schmidt AM, Oates PJ, Szabolcs M, Ramasamy R. Central role for aldose reductase pathway in myocardial ischemic injury. Faseb J. 2004;18:1192–1199. doi: 10.1096/fj.03-1400com. [DOI] [PubMed] [Google Scholar]
- Hwang YC, Sato S, Tsai JY, Yan S, Bakr S, Zhang H, Oates PJ, Ramasamy R. Aldose reductase activation is a key component of myocardial response to ischemia. Faseb J. 2002;16:243–245. doi: 10.1096/fj.01-0368fje. [DOI] [PubMed] [Google Scholar]
- Hwang YC, Shaw S, Kaneko M, Redd H, Marrero MB, Ramasamy R. Aldose reductase pathway mediates JAK-STAT signaling: a novel axis in myocardial ischemic injury. Faseb J. 2005;19:795–797. doi: 10.1096/fj.04-2780fje. [DOI] [PubMed] [Google Scholar]
- Isoyama S, Nitta-Komatsubara Y. Acute and chronic adaptation to hemodynamic overload and ischemia in the aged heart. Heart failure reviews. 2002;7:63–69. doi: 10.1023/a:1013701923065. [DOI] [PubMed] [Google Scholar]
- Iwata K, Matsuno K, Nishinaka T, Persson C, Yabe-Nishimura C. Aldose reductase inhibitors improve myocardial reperfusion injury in mice by a dual mechanism. Journal of pharmacological sciences. 2006;102:37–46. doi: 10.1254/jphs.fp0060218. [DOI] [PubMed] [Google Scholar]
- Kaiserova K, Tang XL, Srivastava S, Bhatnagar A. Role of nitric oxide in regulating aldose reductase activation in the ischemic heart. The Journal of biological chemistry. 2008;283:9101–9112. doi: 10.1074/jbc.M709671200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates AM, Herrero P, Dence C, Soto P, Srinivasan M, Delano DG, Ehsani A, Gropler RJ. Impact of aging on substrate metabolism by the human heart. Journal of the American College of Cardiology. 2003;41:293–299. doi: 10.1016/s0735-1097(02)02714-6. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a "set up" for vascular disease. Circulation. 2003a;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003b;107:346–354. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003c;107:490–497. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- Lesnefsky EJ, Gallo DS, Ye J, Whittingham TS, Lust WD. Aging increases ischemia-reperfusion injury in the isolated, buffer-perfused heart. The Journal of laboratory and clinical medicine. 1994;124:843–851. [PubMed] [Google Scholar]
- Li Q, Hwang YC, Ananthakrishnan R, Oates PJ, Guberski D, Ramasamy R. Polyol pathway and modulation of ischemia-reperfusion injury in Type 2 diabetic BBZ rat hearts. Cardiovascular Diabetology. 2008;7:33. doi: 10.1186/1475-2840-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Xu B, Cavalieri TA, Hock CE. Age-related difference in myocardial function and inflammation in a rat model of myocardial ischemia-reperfusion. Cardiovascular research. 2002;56:443–453. doi: 10.1016/s0008-6363(02)00603-x. [DOI] [PubMed] [Google Scholar]
- Lopaschuk GD, Stanley WC. Glucose metabolism in the ischemic heart. Circulation. 1997;95:313–315. doi: 10.1161/01.cir.95.2.313. [DOI] [PubMed] [Google Scholar]
- Lopaschuk GD, Wall SR, Olley PM, Davies NJ. Etomoxir, a carnitine palmitoyltransferase I inhibitor, protects hearts from fatty acid-induced ischemic injury independent of changes in long chain acylcarnitine. Circulation Research. 1988;63:1036–1043. doi: 10.1161/01.res.63.6.1036. [DOI] [PubMed] [Google Scholar]
- McMillin JB, Taffet GE, Taegtmeyer H, Hudson EK, Tate CA. Mitochondrial metabolism and substrate competition in the aging Fischer rat heart. Cardiovascular research. 1993;27:2222–2228. doi: 10.1093/cvr/27.12.2222. [DOI] [PubMed] [Google Scholar]
- Misare BD, Krukenkamp IB, Levitsky S. Age-dependent sensitivity to unprotected cardiac ischemia: the senescent myocardium. The Journal of thoracic and cardiovascular surgery. 1992;103:60–64. discussion 64–65. [PubMed] [Google Scholar]
- Mylari BL, Larson ER, Beyer TA, Zembrowski WJ, Aldinger CE, Dee MF, Siegel TW, Singleton DH. Novel, potent aldose reductase inhibitors: 3,4-dihydro-4-oxo-3-[[5-(trifluoromethyl)-2-benzothiazolyl] methyl]-1-phthalazineacetic acid (zopolrestat) and congeners. Journal of medicinal chemistry. 1991;34:108–122. doi: 10.1021/jm00105a018. [DOI] [PubMed] [Google Scholar]
- Mylari BL, Withbroe GJ, Beebe DA, Brackett NS, Conn EL, Coutcher JB, Oates PJ, Zembrowski WJ. Design and synthesis of a novel family of triazine-based inhibitors of sorbitol dehydrogenase with oral activity: 1-[4-[3R,5S-dimethyl-4-(4-methyl-[1,3,5]triazin-2-yl)-piperazin-1-yl]-[1,3 ,5]triazin-2-yl]-(R) ethanol. Bioorganic & medicinal chemistry. 2003;11:4179–4188. doi: 10.1016/s0968-0896(03)00490-5. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Obayashi H, Fujii M, Fukui M, Yoshimori K, Ogata M, Hasegawa G, Shigeta H, Kitagawa Y, Yoshikawa T, Kondo M, Ohta M, Nishimura M, Nishinaka T, Nishimura CY. Induction of aldose reductase in cultured human microvascular endothelial cells by advanced glycation end products. Free Radic Biol Med. 2000;29:17–25. doi: 10.1016/s0891-5849(00)00286-0. [DOI] [PubMed] [Google Scholar]
- Neely JR, Morgan HE. Relationship between carbohydrate and lipid metabolism and the energy balance of heart muscle. Annual review of physiology. 1974;36:413–459. doi: 10.1146/annurev.ph.36.030174.002213. [DOI] [PubMed] [Google Scholar]
- Ramana KV, Willis MS, White MD, Horton JW, DiMaio JM, Srivastava D, Bhatnagar A, Srivastava SK. Endotoxin-induced cardiomyopathy and systemic inflammation in mice is prevented by aldose reductase inhibition. Circulation. 2006;114:1838–1846. doi: 10.1161/CIRCULATIONAHA.106.630830. [DOI] [PubMed] [Google Scholar]
- Ramasamy R, Hwang YC, Whang J, Bergmann SR. Protection of ischemic hearts by high glucose is mediated, in part, by GLUT-4. American journal of physiology. 2001;281:H290–H297. doi: 10.1152/ajpheart.2001.281.1.H290. [DOI] [PubMed] [Google Scholar]
- Ramasamy R, Liu H, Oates PJ, Schaefer S. Attenuation of ischemia induced increases in sodium and calcium by the aldose reductase inhibitor zopolrestat. Cardiovascular research. 1999;42:130–139. doi: 10.1016/s0008-6363(98)00303-4. [DOI] [PubMed] [Google Scholar]
- Ramasamy R, Oates PJ, Schaefer S. Aldose reductase inhibition protects diabetic and nondiabetic rat hearts from ischemic injury. Diabetes. 1997;46:292–300. doi: 10.2337/diab.46.2.292. [DOI] [PubMed] [Google Scholar]
- Ramasamy R, Trueblood N, Schaefer S. Metabolic effects of aldose reductase inhibition during low-flow ischemia and reperfusion. The American journal of physiology. 1998;275:H195–H203. doi: 10.1152/ajpheart.1998.275.1.H195. [DOI] [PubMed] [Google Scholar]
- Schaefer S, Carr LJ, Prussel E, Ramasamy R. Effects of glycogen depletion on ischemic injury in isolated rat hearts: insights into preconditioning. The American journal of physiology. 1995;268:H935–H944. doi: 10.1152/ajpheart.1995.268.3.H935. [DOI] [PubMed] [Google Scholar]
- Shinmura K, Bolli R, Liu SQ, Tang XL, Kodani E, Xuan YT, Srivastava S, Bhatnagar A. Aldose reductase is an obligatory mediator of the late phase of ischemic preconditioning. Circulation research. 2002;91:240–246. doi: 10.1161/01.res.0000029970.97247.57. [DOI] [PubMed] [Google Scholar]
- Stanley WC, Lopaschuk GD, Hall JL, McCormack JG. Regulation of myocardial carbohydrate metabolism under normal and ischaemic conditions. Potential for pharmacological interventions. Cardiovascular research. 1997;33:243–257. doi: 10.1016/s0008-6363(96)00245-3. [DOI] [PubMed] [Google Scholar]
- Steenbergen C, Murphy E, Watts JA, London RE. Correlation between cytosolic free calcium, contracture, ATP, and irreversible ischemic injury in perfused rat heart. Circulation research. 1990;66:135–146. doi: 10.1161/01.res.66.1.135. [DOI] [PubMed] [Google Scholar]
- Steenbergen C, Perlman ME, London RE, Murphy E. Mechanism of preconditioning. Ionic alterations. Circulation research. 1993;72:112–125. doi: 10.1161/01.res.72.1.112. [DOI] [PubMed] [Google Scholar]
- Taegtmeyer H, King LM, Jones BE. Energy substrate metabolism, myocardial ischemia, and targets for pharmacotherapy. The American journal of cardiology. 1998;82:54K–60K. doi: 10.1016/s0002-9149(98)00538-4. [DOI] [PubMed] [Google Scholar]
- Tang WH, Wu S, Wong TM, Chung SK, Chung SS. Polyol pathway mediates iron-induced oxidative injury in ischemic-reperfused rat heart. Free radical biology & medicine. 2008;45:602–610. doi: 10.1016/j.freeradbiomed.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Tracey WR, Magee WP, Ellery CA, MacAndrew JT, Smith AH, Knight DR, Oates PJ. Aldose reductase inhibition alone or combined with an adenosine A(3) agonist reduces ischemic myocardial injury. American journal of physiology. 2000;279:H1447–H1452. doi: 10.1152/ajpheart.2000.279.4.H1447. [DOI] [PubMed] [Google Scholar]
- Trueblood N, Ramasamy R. Aldose reductase inhibition improves altered glucose metabolism of isolated diabetic rat hearts. The American journal of physiology. 1998;275:H75–H83. doi: 10.1152/ajpheart.1998.275.1.H75. [DOI] [PubMed] [Google Scholar]
- Vanoverschelde JL, Janier MF, Bergmann SR. The relative importance of myocardial energy metabolism compared with ischemic contracture in the determination of ischemic injury in isolated perfused rabbit hearts. Circulation research. 1994;74:817–828. doi: 10.1161/01.res.74.5.817. [DOI] [PubMed] [Google Scholar]
- Vikramadithyan RK, Hu Y, Noh HL, Liang CP, Hallam K, Tall AR, Ramasamy R, Goldberg IJ. Human aldose reductase expression accelerates diabetic atherosclerosis in transgenic mice. J Clin Invest. 2005;115:2434–2443. doi: 10.1172/JCI24819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei JY. Age and the cardiovascular system. The New England journal of medicine. 1992;327:1735–1739. doi: 10.1056/NEJM199212103272408. [DOI] [PubMed] [Google Scholar]
- Willems L, Zatta A, Holmgren K, Ashton KJ, Headrick JP. Age-related changes in ischemic tolerance in male and female mouse hearts. J Mol Cell Cardiol. 2005;38:245–256. doi: 10.1016/j.yjmcc.2004.09.014. [DOI] [PubMed] [Google Scholar]