Abstract
When learned in quick succession declarative and motor skill tasks interfere with one another, and subsequent recall is impaired. Depending upon the order of the tasks, we prevented memory interference in humans by applying transcranial magnetic stimulation to either the dorsolateral prefrontal or the primary motor cortex, and neither memory was impaired. Our observations suggest that distinct mechanisms support the communication between different types of memory processing.
Learning facts and motor skills in quick succession can be frustrating; for example, learning a word-list and then a motor skill impairs subsequent word recall, and learning a motor skill and then a word-list impairs subsequent motor skill 1–5. Interference may arise from a direct competition between memory processes, and so it may only be possible to prevent interference by disrupting the interfering memory 2,6,7. Alternatively, a circuit of brain areas bridging between the memory processes may support interference, and so it would not be necessary to directly affect either memory; instead, disrupting the interaction between the tasks could prevent interference. We sought to distinguish between these possibilities by applying Transcranial Magnetic Stimulation (TMS) to either the right dorsolateral prefrontal cortex (DLPFC) or the right primary motor cortex (M1; Supplementary Introduction).
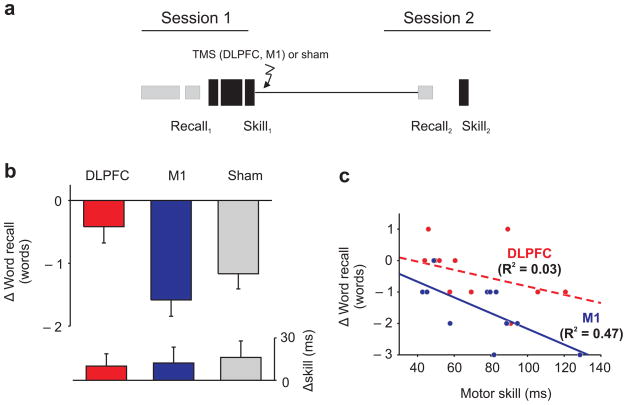
In the first set of experiments, participants learned and recalled a list of words (word list learning task; Recall1) and then learned a motor skill (informed written consent was provided; Supplementary Methods). Immediately after the two learning tasks, participants received sham stimulation or real TMS to the DLPFC or to M1 (session 1; 8am; Fig 1). Initial learning of the word list and motor skill in session one did not differ significantly across the three groups (Recall1; ANOVA, F(2,33) = 1.72, p = 0.2; Skill1; ANOVA, F(2,33) = 0.548, p = 0.583; for word and sequence recall see Supplementary Results). Twelve hours later participants recalled the word-list (Recall2) and had their motor skill retested (session 2; 8pm; Fig 1). The change in word recall between testing and retesting differed significantly across the three groups (ANOVA, F(2,33) = 5.43, p = 0.009). Following sham stimulation, as has been observed in earlier work without TMS 4, declarative word recall decreased significantly between testing and retesting (14.9±0.4 vs. 13.7±0.5 words; mean±sem, paired t-test, t(11) = 4.84, p = 0.001), and the decrease in word recall was correlated with the initial skill acquired in the motor learning task (R = 0.788, F(1,10) = 16.33, p = 0.002). Similarly, despite applying real stimulation over M1 there was also a significant decrease in word recall between testing and retesting (13.5±0.5 vs. 12±0.6 words; mean±sem, paired t-test, t(11) = 6.1, p<0.001), which was also correlated with the initial skill acquired in the motor learning task (R = 0.684, F(1,10) = 8.789, p = 0.014; Fig 1). The decrease in word recall following TMS to M1 was not significantly different from that following sham stimulation (unpaired t-test, t(22) = 1.17, p = 0.252). However, the change in recall following stimulation over the DLPFC was significantly less than following M1 or sham stimulation (DLPFC vs. M1; unpaired t-test, t(22) = 3.17, p = 0.004; DLPFC vs. sham; unpaired t-test, t(22) = 2.2, p = 0.038) because there was no significant decrease in word recall between testing and retesting (14±0.5 vs. 13.7±0.6 words; mean±sem, paired t-test, t(11) = 1.6, p = 0.14; Figs 1 & S3).
Figure 1. Experiment 1, interference between word-list and motor skill learning.
(a.) Participants learned a word-list and a motor skill in quick succession, TMS (to DLPFC or M1) or sham stimulation was applied and twelve hours later participants word recall and motor skill was retested. (b.) Word recall was impaired by the motor skill-learning task despite sham or real stimulation to M1 (box ± s.e.m.). In contrast, applying TMS to the DLPFC prevented the impairment of word recall by the motor skill learning task. Preventing the interference between the tasks was not dependent on disrupting the interfering memory because motor skill changes were not significantly different across the groups (box ± s.e.m.). (c.) The relationship between the tasks was affected by stimulation. There was a significant correlation between the decrease in word recall and initial motor skill following M1 stimulation; whereas, there was no significant correlation following DLPFC stimulation. The correlation following M1 stimulation was significantly greater than the correlation following DLPFC stimulation (see above R2 values).
Preventing interference between the tasks, which only follows DLPFC stimulation, can not be attributed to TMS disrupting the interfering motor skill because the changes in motor skill between testing and retesting were not significantly different across the three groups (Skill2 − Skill1; ANOVA, F(2,33) = 0.548, p = 0.583), nor was motor skill at retesting significantly different across the three groups (Skill2; ANOVA, F(2,33) = 0.164, p = 0.850). Instead, stimulation may have affected the word-list task; for example, enhancing subsequent recall and so mitigating against the interfering effects of the motor skill task; alternatively, stimulation may have affected the interaction between the tasks. We distinguished between these possibilities by replacing the motor learning task with a motor performance task to create an additional group in which participants learned a word-list, performed a motor performance task, had TMS applied to the right DLPFC, and 12-hrs later recalled the word-list (Supplementary Methods; for performance task analysis; Supplementary Results). When the motor learning task was replaced with a motor performance task, which does not interfere with word recall, there was a significant decrease in word recall following DLPFC stimulation 4 (paired t-test, t(11) = 2.6, p = 0.025; Fig S1). Thus, rather than directly affecting the individual tasks in isolation; for example, by disrupting the interfering motor learning task or by enhancing word recall; stimulation, instead overcomes the interference between the tasks by affecting the interaction between the tasks (Supplementary Discussion 8,9; for state-dependent effects of TMS). We also found that when TMS was applied to the DLPFC the correlation between tasks was significantly reduced (M1 vs. DLPFC, unpaired t-test, t(21) = 2.496, p = 0.021). There was no longer a correlation between the change in word recall, and the initial skill acquired in the motor learning task (R = 0.173, F(1,10) = 0.310, p = 0.590; Fig 1), which again implies that applying TMS to the DLPFC affected the interaction between the tasks.
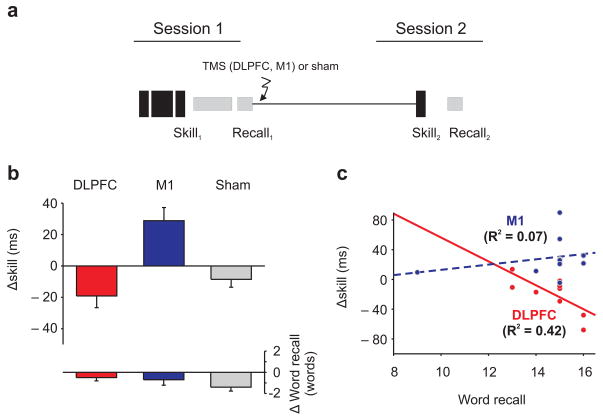
In the second set of experiments, the order of the two memory tasks was reversed; participants learned a motor skill (Skill1) and then a list of words. Immediately after the learning tasks, participants received sham stimulation or real TMS to the DLPFC or to M1 (session 1; 8am; Fig 2). Initial learning of the motor skill and word list in session one did not differ significantly across the groups (Skill1; ANOVA, F(2,27) <1; p = 0.413; recall1; ANOVA, F(2,27) <1, p = 0.945; for motor sequence recall see Supplementary Results). We calculated motor skill as the difference in average response time during the sequential and subsequent random trials (Supplementary Methods 10). Twelve hours later participants performed the motor skill (Skill2) and recalled the word-list (session 2; 8pm; Fig 2). The change in motor skill between testing and retesting differed significantly across the three groups (ANOVA, F(2,27) = 13.7, p<0.001). Following sham stimulation, as has been observed in earlier work without TMS 4, motor skill decreased significantly between testing and subsequent retesting (84±6ms vs. 71±5ms; mean±sem paired t-test, t(9) = 2.526, p =0.032), and the decrease in motor skill was correlated with participants’ word recall for the word learning task (R = 0.647, F(1,8) = 5.75, p = 0.043). Similarly, despite applying real stimulation over DLPFC there was also a significant decrease in motor skill between testing and retesting (94±9ms vs. 75 ±8ms; paired t-test, t(9) = 2.552, p = 0.031), which again was correlated with the number of words recalled from the word learning task (R = 0.719, F(1,8) = 8.56, p = 0.019; Fig 2). The decrease in motor skill following TMS to DLPFC was not significantly different from that following sham stimulation (unpaired t-test, t(18) = 0.993, p = 0.334). However, there was a significant increase in motor skill following stimulation over M1 (76±11ms vs. 105±10ms, paired t-test, mean±sem, t(9) = 3.444, p = 0.007), which was significantly greater than the motor skill changes following DLPFC or sham stimulation (M1 vs. DLPFC, unpaired t-test, t(18) = 4.26, p<0.001; M1 vs. sham, unpaired t-test, t(18) = 4.21, p = 0.001). Improvements in motor skill occur between sessions when there is no interference from word-list learning (Supplementary Discussion 4,7,11–13).
Figure 2. Experiment 2, interference between motor skill and word-list learning.
(a.) Participants learned a motor skill and then a word-list in quick succession, TMS (to DLPFC or M1) or sham stimulation was applied and twelve hours later participants motor skill and word recall was retested. (b.) Motor skill was impaired by the word-list learning task after sham or real stimulation to DLPFC (box ± s.e.m.). In contrast, applying TMS to M1 prevented the impairment of motor skill by the word-list learning task. Preventing interference between the tasks was not dependent on disrupting the interfering memory because word recall changes were not significantly different across the groups (box ± s.e.m.). (c.) The relationship between the tasks was affected by stimulation. There was a significant correlation between the decrease in motor skill and initial word recall following DLPFC stimulation; whereas, there was no significant correlation following M1 stimulation. The correlation following DLPFC stimulation was significantly greater than the correlation following M1 stimulation (see above R2 values).
Preventing interference between the tasks, which only occurs following M1 stimulation can not be attributed to TMS disrupting the interfering word-list because changes in word recall between testing and retesting were not significantly different across the groups (Recall2 − Recall1; ANOVA, F(2,27) = 1.34, p = 0.279), nor was word recall significantly different at retesting across the three groups (Recall2 ;ANOVA, F(2,27) = 1.1 , p = 0.338). Instead, stimulation may have affected the motor skill task; for example, by enhancing subsequent motor skill and so mitigating against the interfering effects of the word-list task (Supplementary Discussion); alternatively, stimulation may have affected the interaction between the tasks. We distinguished between these possibilities by replacing the word-list task with a vowel-counting task to create an additional group in which participants learned the motor skill, performed a vowel counting task, had TMS applied to the right M1, and 12-hrs later were retested (Fig S2). When the word-list learning task was replaced with a vowel-counting task, which does not interfere with motor skill, there was a trend to a decrease in motor skill following M1 stimulation 4 (87±13ms vs. 73±12ms, mean±sem; paired t-test, t(9) = 1.98, p = 0.079, Fig S2). Thus, rather than directly affecting the individual tasks in isolation; for example, by disrupting the interfering word recall task or by enhancing motor skill; stimulation, instead overcomes the interference between the tasks by affecting the interaction between the tasks (Supplementary Discussion 8,9; for state-dependent effects of TMS). We also found that when TMS was applied to M1 the correlation between tasks was significantly reduced (DLPFC vs. M1, unpaired t-test, t(17) = 2.42, p = 0.028). There was no longer a correlation between the change in motor skill, and the initial word recall (R = 0.269, F(1,8) = 0.624, p = 0.452; Fig 2; Supplementary Results), which again implies that applying TMS to M1 affected the interaction between the tasks (for a comparison between the experiments see Fig S3).
Our observations suggest that distinct mechanisms support the communication between different types of memory processing. When this communication is disrupted; for example, by applying TMS to specific brain areas the interference between the motor skill and word-list tasks is reduced, and so both memories are unimpaired. The communication between memory processes may also be disrupted physiologically; for example, due to sleep, explaining the absence of memory interference over sleep (Supplementary Discussion, 4,7,14). Our observations challenge us to no longer view memory interference as the inevitable consequence of a direct competition between memories for resources. Instead, memory interference is an actively mediate process, due to a communication between memory processes, which may serve an important function (for mechanism and possible function; Supplementary Discussion).
Supplementary Material
Acknowledgments
We are grateful to Rachel Brown, Lisa Iguichi, Neechi Mosha and Sanjin Tunovic for their assistance with the experiments described within this manuscript and to Albert Galaburda, Alvaro Pascual-Leone and Robert Stickgold for their encouraging and constructive comments and to the National Institutes of Health (U.S.A; R01 NS051446 and NS051446-03S1; EMR) and the National Science Foundation (U.S.A; Division of Behavioral and Cognitive Sciences 0921177; EMR) for their financial support. Our work also benefited from the infrastructure supplied by the Harvard Clinical and Translational Science Center (from the National Center for Research Resources; UL1 RR025758 and M01 RR 01032).
Footnotes
Author contributions
D.A.C. conducted the experiments and assisted with writing the manuscript; E.M.R. designed the study, conducted the experiments, analyzed the data and wrote the manuscript.
References
- 1.Brashers-Krug T, Shadmehr R, Bizzi E. Consolidation in human motor memory. Nature. 1996;382:252. doi: 10.1038/382252a0. [DOI] [PubMed] [Google Scholar]
- 2.Lechner H, Squire L, Byrne J. 100 years of Consolidation- Remembering Muller and Pilzecker. Learning & Memory. 1999;6:77–87. [PubMed] [Google Scholar]
- 3.Walker MP, Brakefield T, Hobson JA, Stickgold R. Dissociable stages of human memory consolidation and reconsolidation. Nature. 2003;425:616–620. doi: 10.1038/nature01930. [DOI] [PubMed] [Google Scholar]
- 4.Brown RM, Robertson EM. Off-Line Processing: Reciprocal Interactions between Declarative and Procedural Memories. J Neurosci. 2007;27:10468–10475. doi: 10.1523/JNEUROSCI.2799-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keisler A, Shadmehr R. A Shared Resource between Declarative Memory and Motor Memory. J Neurosci. 2010;30:14817–14823. doi: 10.1523/JNEUROSCI.4160-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brashers-Krug T, Shadmehr R, Todorov E. Catastrophic Interference in Human Motor Learning. In: Tesauro G, Touretzky DS, Leen TK, editors. Advances in Neural Information Processing Systems. Vol. 7. MIT Press; Cambridge, MA: 1995. pp. 19–26. [Google Scholar]
- 7.Robertson EM. From creation to consolidation: a novel framework for memory processing. PLoS Biol. 2009;7:e19. doi: 10.1371/journal.pbio.1000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silvanto J, Muggleton N, Walsh V. State-dependency in brain stimulation studies of perception and cognition. Trends Cogn Sci. 2008;12:447–454. doi: 10.1016/j.tics.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Silvanto J, Pascual-Leone A. State-dependency of transcranial magnetic stimulation. Brain Topogr. 2008;21:1–10. doi: 10.1007/s10548-008-0067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robertson EM. The Serial Reaction Time Task: Implicit Motor Skill Learning? J Neurosci. 2007;27:10073–10075. doi: 10.1523/JNEUROSCI.2747-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robertson EM, Pascual-Leone A, Press DZ. Awareness modifies the skill-learning benefits of sleep. Current Biology. 2004;14:208–212. doi: 10.1016/j.cub.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 12.Robertson EM, Press DZ, Pascual-Leone A. Off-Line Learning and the Primary Motor Cortex. J Neurosci. 2005;25:6372–6378. doi: 10.1523/JNEUROSCI.1851-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spencer RM, Sunm M, Ivry RB. Sleep-dependent consolidation of contextual learning. Curr Biol. 2006;16:1001–1005. doi: 10.1016/j.cub.2006.03.094. [DOI] [PubMed] [Google Scholar]
- 14.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.