Abstract
MonoAryloxide-Pyrrolide (MAP) complexes of molybdenum are employed for the selective ethenolysis of 1,2-disubstituted Z olefins in the presence of the corresponding E olefins. Reactions are performed in the presence of 0.02−3.0 mol % catalyst at 22 °C under 20 atm of ethylene. We demonstrate that the Z isomer of an easily accessible E:Z mixture can be destroyed through ethenolysis and the E alkene thereby isolated readily in high yield and exceptional stereoisomeric purity.
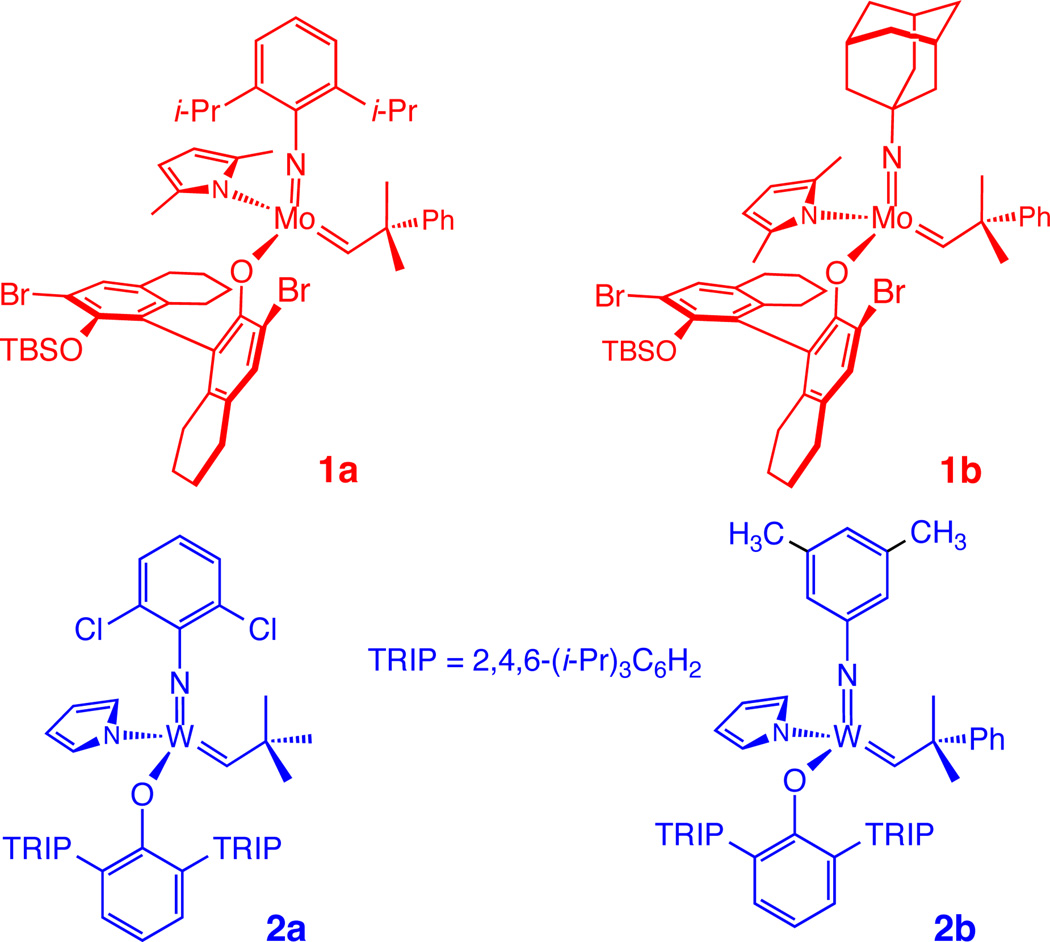
During the last four years, research in these laboratories has led to the discovery and development of MonoAryloxide-Pyrrolide (MAP) complexes 1 and 2 (inter alia; Scheme 1). Variations have proven to be especially efficient catalysts for enantioselective ring-closing,1 enantioselective and Z-selective ring-opening/cross-metathesis,2 and Z-selective homocoupling3 and cross-metathesis reactions.4 We have attributed the origins of Z-selectivity to the presence of a relatively large monoaryloxide, often in combination with a relatively small imido group. An important feature of MAP catalysts is that they contain a stereogenic metal center. Since the aryloxide in 1 is enantiomerically pure, two diastereomers are formed, one of which is more reactive and leads to high enantioselectivity.5 ROMP reactions promoted by Mo complexes analogous to 2 give >95% cis,syndiotactic polymers, with syndiotacticity being a consequence of the directed addition of the monomer trans to the pyrrolide and inversion of the configuration at the metal center with each insertion of monomer.6
Scheme 1.
Representative Mo- and W-Based MAP Complexes
Many MAP catalysts are unusually efficient, perhaps in part as a consequence of highly reactive methylidene species being relatively stable.7 Long-lived, reactive methylidene species and lability of unsubstituted molybdacyclobutane intermediates toward loss of ethylene allow efficient ethenolysis of methyl oleate (eq 1; R = (CH2)7CH3, R1 = (CH2)7CO2CH3) with 0.02 mol % 1a at room temperature and 10 atm of ethylene selectively (>99%) and essentially completely (95%) to 1-decene and methyl-9-decenoate.8
| (1) |
Ethenolysis involving ethylene and Z-R1CH=CHR1 is the reverse of Z-selective metathesis coupling of R1CH=CH2, i.e., the same α,β -disubstituted metallacyclobutane complex must be formed as an intermediate in the forward reaction (homometathesis coupling), shown in eq 2, as in the reverse reaction (ethenolysis). Therefore, ethenolysis of the Z isomer could be significantly more facile than the E isomer under the right circumstances. It should be noted that the olefins involved in the forward and reverse reactions shown in eq 2 coordinate trans to the pyrrolide ligand and the configuration at the metal inverts with each metathesis step.1b,7,9
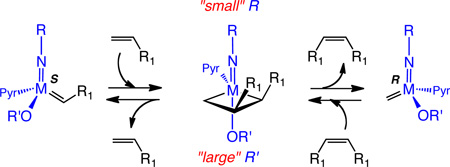 |
(2) |
To explore the proposal that Z olefins can be more prone toward ethenolysis than E olefins, 0.4 mol% of 1a was added to a 1:4 mixture (thermodynamic) of Z-4-octene and E-4-octene (0.6 M in C6D6) and the evacuated vessel was pressurized with four atmospheres of ethylene. After 15 minutes, the solution was exposed to air, filtered through alumina, and analyzed by1H and13C NMR spectroscopy, and gas chromatography. The product mixture contained 1-pentene and >98% E-4-octene (79% yield, Table 1, entry 1). The results of ethenolysis of neat 4:1 E:Z mixtures of 4-octenes, illustrated in entries 2–6 (Table 1), suggest that low catalyst loadings, 20 atm pressure, and neat substrate produce the best results. The most efficient process corresponds to ethenolysis with 0.02 mol% 1a under 20 atm of ethylene (entry 6). The product is readily recovered by passing the mixture through a plug of silica gel and removing the light olefin in vacuo.
Table 1.
Mo-Catalyzed Z-Selective Ethenolysis of a 4:1 E:Z Mixture of 4-Octenesa
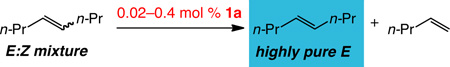 | |||||
|---|---|---|---|---|---|
| entry | mol % 1a | pressure (atm) |
time | yield of Eb | final % Ec |
| 1 | 0.4 | 4 | 15 min | 79 | >98 |
| 2 | 0.1 | 4 | 1 h | 76 | 91 |
| 3 | 0.1 | 20 | 1 h | 77 | 97 |
| 4 | 0.2 | 20 | 1 h | 71 | 89 |
| 5 | 0.05 | 20 | 4 h | 62 | >98 |
| 6 | 0.02 | 20 | 18 h | 77 | >98 |
Performed under an N2 atm; see the Supporting Information for full details.
Yield of pure E isomers after purification (±5%); theory = 80%.
Determined through analysis of 400 MHz 1H NMR spectra (±2%).
Next, we set out to compare the rates of ethenolysis of Z-4-octene and E-4-octene carried out in the presence of 1a. We assumed that the rate would be first order in catalyst, ethylene, and substrate. Reactions were performed without solvent under 20 atm of ethylene; catalyst loadings for the reaction of E-4-octene were 20 or 25 times higher than those employed for Z-4-octene. The points in the ln(C/Co) versus time plot consist of individual runs (7 runs for E-4-octene and 11 runs for Z-4-octene; see the Supporting Information). Comparison of the plot for E-4-octene (R2 = 0.98) with that for Z-4-octene (R2 = 0.86) allows us to deduce that RZ/E = kZ/kE = 25(±5), where kZ and kE are the rate constants for ethenolysis of Z and E olefins. The large error arises from uncertainties in both kZ and kE (see Supporting Information). For example, ~20 % Z-4-octene is present upon workup in runs that involve E-4-octene. Two possible explanations for formation of Z-4-octene are Z-selective metathesis homocoupling of 1-pentene upon release of ethylene pressure before the catalyst is deactivated (a homocoupling "back reaction") or direct isomerization of E to Z via a trisubstituted metallacyclobutane. At this stage it is not known whether catalyst decomposition in a long run with the lowest catalyst loading (entry 6) limits the consumption of E-4-octene and preserves the final % E level.
A value of RZ/E = 25 (= ln([Z]/[Z]o)/ln([E]/[E]o)) is sufficient to produce the findings shown in entry 6 of Table 1. For example, if the 77% yield of product consists of 2% Z-4-octene and 98% E-4-octene, then ln([Z]/[Z]o)/ln([E]/[E]o) = 44. However, this method of determining RZ/E requires accurate measurement of small amounts of remaining Z and small amounts of E consumed and therefore is prone to large errors. A direct measure of the relative rates of consumption of two substrates with the catalyst concentration compensated by the approximate suspected value of RZ/E appears to be a more reliable method of measuring RZ/E at this stage.
Other examples of generating pure E olefins through ethenolysis of a mixture of stereoisomers are shown in Table 2. For example, ethenolysis of a neat 4:1 E:Z mixture of 2-octenes with 0.02 mol% 1a and ethylene (20 atm) led to >98% E-2-octene (20%) after removal of terminal olefins formed through reaction of the Z isomer (entry 1, Table 2). It is likely that the efficiency of the ethenolysis of functionalized olefins illustrated in entries 2 and 3 is limited by reaction of the catalyst with low levels of debilitating impurities (e.g., alcohol, aldehyde, or acid). In run 4 essentially no C9 olefin was formed, which suggests that there is no significant homocoupling "back reaction" between 1-hexene and 1-pentene under these conditions.
Table 2.
Mo-Catalyzed Z-Selective Ethenolysis of E:Z Olefin Mixturesa
| entry | substrate | Initial E:Z | mol % 1a |
pressure (atm) |
time | yield of Eb |
final % Ec |
|---|---|---|---|---|---|---|---|
| 1 |
|
1:4 | 0.02 | 20 | 18 h | 20 | >98 |
| 2 | 1.1:1 | 2.0 | 4 | 2 h | 48 | >98 | |
| 3 | 5:1 | 1.0 | 4 | 15 min | 75 | >98 | |
| 4 | E-4-octene & Z-4-decene | 1:1 | 2.0 | 4 | 15 min | 45 | 96 |
a–c See Table 1.
The data presented in Tables 1 and 2 indicate that it should be possible to access stereoisomerically pure symmetric E olefins from terminal olefins in a two-step process. First, Mo(NAr)(CHCMe2Ph)[OC(CF3)2Me]2 (MoF12; Ar = 2,6-i-Pr2C6H3) or 1a (cat 1; eq 3) was employed to homocouple R(CH2)nCH=CH2 to give an E:Z mixture. This product mixture was then passed through a short silica plug to remove any active Mo catalyst. The eluant was then subjected to Z-selective ethenolysis employing 1a to leave E-R(CH2)nCH=CH(CH2)nR in >98% stereoisomeric purity and high yield (relative to theory) in all cases (Table 3).
Table 3.
A two step synthesis of E-R(CH2)nCH=CH(CH2)nR.a
| entry | R; n | Initial E:Z |
mol % 1a |
pressure (atm) |
time | yield of Eb |
final % Ec |
|---|---|---|---|---|---|---|---|
| 1 | Me; 5 | 4:1 | 0.5 | 20 | 4 h | 67 | >98 |
| 2 | Me; 7 | 4:1 | 2.0 | 4 | 15 min | 77 | >98 |
| 3 | Cy; 1 | 4:1 | 3.0 | 4 | 15 min | 78 | >98 |
| 4 | Ph; 1 | 4:1 | 0.5 | 20 | 5 h | 67 | >98 |
| 5 | CO2Me; 8 | 3:1 | 0.5 | 20 | 20 h | 56 | >98 |
| 6 | CO2Et; 7 | 2.4:1 | 0.5 | 20 | 4 h | 66 | >98 |
| 7 | OBn; 1 | 11:1 | 1.0 | 4 | 30 min | 85 | >98 |
a–c See Table 1. The E:Z mix was prepared with MoF12 in runs 1–3 and 7, and 1a in runs 4–6.
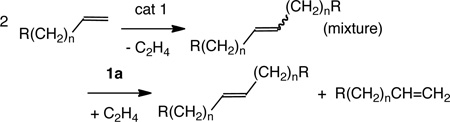 |
(3) |
We were surprised initially to find that a catalyst that is stable towards ethylene and contains the OHIPT (O-2,6-(2,4,6-i-Pr3C6H2)2C6H3) ligand is inferior for Z-selective ethenolysis. For example, only ~1% Z-4-octene (0.2 M in C6D6) is consumed in 45 min when 1 mol% Mo(NAr)(CHCMe2Ph)(Pyr)(OHIPT)3 (Pyr = NC4H4−) is employed. The reason for such a low level of activity might be because the unsubstituted molybdacyclobutane does not readily lose ethylene. Detailed NMR studies have shown that a Mo(CH2CH2CH2) species converts to a Mo(CH2)(CH2CH2) intermediate with kf = 14,500 s−1 and kr = 4900 s−1 in the case of the metallacyclobutane Mo(NAr)(C3H6)(OBr2Bitet)(Me2Pyr) (where OBr2Bitet is the biphenolate in 1a and Me2Pyr = NC4Me2H2−)7. In contrast, for the same transformation in Mo(NAr)(C3H6)(OHIPT)(Pyr) kf was found to be 1.8 s−1 and kr = 9.0 s−1 (20 °C, toluene-d8 in both cases). Differences in the kf values (~8000) and equilibria (3.0 and 0.2, respectively) could account for the inability of OHIPT-bearing catalysts that have been tried so far to promote Z-selective ethenolysis efficiently.
The relatively high stability of tungstacyclobutane complexes toward release of ethylene also limits the effectiveness of tungsten-based catalysts for ethenolysis. The intermediate W(CH2)(CH2CH2) complex has been found to be formed from W(NAr)(C3H6)(OBr2Bitet)(Me2Pyr) with kf = 3.2 s−1 and kr = 69 s−1.3 Therefore, thus far, Mo-OBr2Bitet catalysts appear to be optimal for ethenolysis. The reason why unsubstituted metallacyclobutanes of OHIPT species are so much more stable toward loss of ethylene than OBr2Bitet species might be a consequence of the substantially larger size of an OHIPT relative to a OBr2Bitet ligand. Subtle steric factors also may prove to be critical if the ethylene axis is required to be approximately perpendicular to the M=CH2 axis in the M(CH2)(CH2CH2) intermediate in order for ethylene to be lost readily.10
It should be noted that only catalyst 1a has been successful for Z-selective ethenolysis so far, 1a is a mixture of diastereomers, diastereomers of 1a interconvert readily in the presence of ethylene,1 and 1a is not an especially successful Z-selective homocoupling catalyst.3a,b A detailed mechanistic understanding of Z-selective ethenolysis that includes a discussion of interconversion of and relative rates of reaction of diastereomers of any possible intermediate alkylidene may be necessary to explain all experimental observations eventually, but such an understanding is not at present within reach. It remains to be seen which catalysts, if any, that do not form diastereomers are successful for Z-selective ethenolysis.
Molybdenum-catalyzed Z-selective ethenolysis should allow access to a large variety of E olefins that otherwise would be more difficult to prepare in high stereoisomeric purity through alternative methods. This indirect synthesis of E olefins complements the direct synthesis of Z olefins through Z-selective metathesis reported in earlier papers. The design and development of more efficient catalyst systems and exploration of the scope of Z-selective ethenolysis are in progress.
Supplementary Material
Acknowledgments
This research was funded by the National Science Foundation (CHE-0841187 to R. R. S.) and by the National Institutes of Health (GM-59426 to R. R. S. and A. H. H.). We thank Materia, Inc., for a gift of 5-decenylacetate.
Footnotes
Supporting Information Available: Experimental procedures and spectral, analytical data for all reaction products (PDF). This material is available on the web: http://www.pubs.acs.org.
References
- 1.(a) Malcolmson SJ, Meek SJ, Sattely ES, Schrock RR, Hoveyda AH. Nature. 2008;456:933. doi: 10.1038/nature07594. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sattely ES, Meek SJ, Malcolmson SJ, Schrock RR, Hoveyda AH. J. Am. Chem. Soc. 2009;131:943. doi: 10.1021/ja8084934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ibrahem I, Yu M, Schrock RR, Hoveyda AH. J. Am. Chem. Soc. 2009;131:3844. doi: 10.1021/ja900097n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jiang AJ, Zhao Y, Schrock RR, Hoveyda AH. J. Am. Chem. Soc. 2009;131:16630. doi: 10.1021/ja908098t. Marinescu SC, Schrock RR, Müller P, Takase MK, Hoveyda AH. Organometallics. 2011;30:1780. doi: 10.1021/om200150c. For recent Ru-catalyzed homocoupling reactions that employ catalyst loadings of 2.0 mol % and give products with 21–95% Z content, see: Keitz BK, Endo K, Herbert MB, Grubbs RH. J. Am. Chem. Soc. 2011;133:9686. doi: 10.1021/ja203488e.
- 4. Meek SJ, O'Brien RV, Llaveria J, Schrock RR, Hoveyda AH. Nature. 2011;471:461. doi: 10.1038/nature09957. For one example of a recent Ru-catalyzed Z-selective cross-metathesis (involving a terminal and a symmetric Z alkene), see: Endo K, Grubbs RH. J. Am. Chem. Soc. 2011;133:8525. doi: 10.1021/ja202818v.
- 5.Meek SJ, Malcolmson SJ, Li B, Schrock RR, Hoveyda AH. J. Am. Chem. Soc. 2009;131:16407. doi: 10.1021/ja907805f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Flook MM, Jiang AJ, Schrock RR, Müller P, Hoveyda AH. J. Am. Chem. Soc. 2009;131:7962. doi: 10.1021/ja902738u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Flook MM, Gerber LCH, Debelouchina GT, Schrock RR. Macromolecules. 2010;43:7515. doi: 10.1021/ma101375v. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Flook MM, Ng VWL, Schrock RR. J. Am. Chem. Soc. 2011;132:1784. doi: 10.1021/ja110949f. [DOI] [PubMed] [Google Scholar]
- 7.Schrock RR, King AJ, Marinescu SC, Simpson JH, Müller P. Organometallics. 2010;29:5241. [Google Scholar]
- 8. Marinescu SC, Schrock RR, Müller P, Hoveyda AH. J. Am. Chem. Soc. 2009;131:10840. doi: 10.1021/ja904786y. For related Ru-catalyzed ethenolyses see: Thomas RM, Keitz BK, Champagne TM, Grubbs RH. J. Am. Chem. Soc. 2011;133:7490. doi: 10.1021/ja200246e.
- 9.Marinescu SC, Schrock RR, Li B, Hoveyda AH. J. Am. Chem. Soc. 2009;131:58. doi: 10.1021/ja808308e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Poater A, Solans-Monfort X, Clot E, Copéret C, Eisenstein O. J. Am. Chem. Soc. 2007;129:8207. doi: 10.1021/ja070625y. [DOI] [PubMed] [Google Scholar]; (b) Leduc A-M, Salameh A, Soulivong D, Chabanas M, Basset J-M, Copéret C, Solans-Monfort X, Clot E, Eisenstein O, Boehm VPW, Roeper M. J. Am. Chem. Soc. 2008;130:6288. doi: 10.1021/ja800189a. [DOI] [PubMed] [Google Scholar]; (c) Solans-Monfort X, Copéret C, Eisenstein O. J. Am. Chem. Soc. 2010;132:7750. doi: 10.1021/ja101597s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.