Abstract
Because hyperhomocysteinemia can occur in cholesterol gallstone disease, we hypothesized that this may result from trimethylation of phosphatidylethanolamine (PE), which partakes in biliary phosphatidylcholine (PC) hypersecretion during cholesterol cholelithogenesis. We fed murine strains C57L/J, C57BL/6J, SWR/J, AKR/J, PE N-methyltransferase (PEMT) knockout (KO), PEMT heterozygous (HET), and wild type (WT) mice a cholesterol/cholic acid lithogenic diet (LD) for up to 56 days and documented biliary lipid phase transitions and secretion rates. We quantified plasma total homocysteine (tHcy), folate, and vitamin B12 in plasma and liver, as well as biliary tHcy and cysteine secretion rates. Rate-limiting enzyme activities of PC synthesis, PEMT and cytidine triphosphate: phosphocholine cytidylyltransferase (PCT), S-adenosylmethionine (SAM), and S-adenosylhomocysteine (SAH) were measured in liver homogenates. Other potential sources of plasma tHcy, glycine N-methyltransferase (GNMT) and guanidinoacetate N-methyltransferase (GAMT), were assayed by gene expression. Plasma tHcy and PEMT activities became elevated during cholelithogenesis in gallstone-susceptible C57L, C57BL/6, and SWR mice, but not in the gallstone-resistant AKR mice. Persisting in C57L mice, which exhibit the greatest Lith gene burden, these increases were accompanied by elevated hepatic SAM/SAH ratios and augmented biliary tHcy secretion rates. Counter-regulation included remethylation of Hcy to methionine concurrent with decreased folate and vitamin B12 levels and Hcy transsulfuration to cysteine. Concomitantly, methylenetetrahydrofolate reductase (Mthfr), betaine-homocysteine methyltransferase (Bhmt), and cystathionine-β-synthase (Cbs) were upregulated, but Gnmt and Gamt genes were downregulated. PEMT KO and HET mice displayed biliary lipid secretion rates and high gallstone prevalence rates similar to WT mice without any elevation in plasma tHcy levels.
Conclusion
This work implicates upregulation of PC synthesis by the PEMT pathway as a source of elevated plasma and bile tHcy during cholesterol cholelithogenesis.
Keywords: phosphatidylethanolamine N-methyltransferase, folate, vitamin B12, cysteine, bile
Cholesterol gallstones and atherothrombotic cardiovascular disease are linked epidemiologically (1, 2). Several coronary risk factors, including smoking, abstention from alcohol, obesity, low serum high density lipoprotein-cholesterol, and insulin resistance, are strongly associated with cholesterol gallstone disease (3). Employing a mouse model wherein the liver’s insulin receptor is knocked-out, Biddinger et al (4, 5) demonstrated that hepatic insulin resistance is sufficient to link the metabolic syndrome, accelerated atherogenesis, and increased cholesterol gallstone susceptibility.
Homocysteine (Hcy) is a non-protein, sulfur-containing amino acid that is synthesized in vivo only by demethylation of methionine. Hcy can be remethylated to methionine through two pathways: the methylenetetrahydrofolate reductase (MTHFR) pathway and the betaine-homocysteine methyltransferase (BHMT) pathway. Hcy can also be transsulfurated by cystathionine beta synthase (CBS) to cystathionine, which, in turn, is catabolized to cysteine (6). Homeostasis between Hcy and methionine requires folate and vitamin B12 as cofactors and is coordinated by the tissue S-adenosylmethionine (SAM)/S-adenosylhomocysteine (SAH) ratio. Conversion of Hcy to cysteine via CBS activity is dependent on pyridoxal-6-phosphate (vitamin B6) as an essential cofactor (6).
Over 40 years ago, Carey et al. were the first to uncover subnormal serum folate levels, increased folate clearance, and increased remethylation of Hcy to methionine, especially with pharmacological doses of folic acid, in homocystinuric children (7). In otherwise healthy humans, increased plasma total Hcy (including free Hcy, homocystine, and other conjugated Hcy, tHcy) is an independent risk factor for atherothrombotic disease (8, 9). Relatively small increases may predispose to vascular pathology with its sequelae of stroke as well as coronary artery and peripheral occlusive arterial diseases (8). Hyperhomocysteinemia may also influence the function of other organs and cells such as the brain (10) and bone marrow (11). Recently, on the basis of routine screening tests, hyperhomocysteinemia was reported in middle-aged Japanese (12) and British (13) subjects with gallstones.
In humans and laboratory animals, cholesterol supersaturated (lithogenic) bile is caused principally by hepatic hypersecretion of cholesterol (3, 14, 15). Cholesterol hypersecretion is accompanied invariably by hypersecretion of biliary phosphatidylcholine (PC) (16), the principal (>95%) biliary phospholipid in humans (17) and higher vertebrates (18). In hepatic parenchymal cells, PC is synthesized through two pathways: the cytidine diphosphate-choline (Kennedy) pathway (19) and the phosphatidylethanolamine (PE) methylation pathway (20); the latter is believed to contribute as much as 30% of the total PC in bile (21). We hypothesized that PC hypersecretion in the lithogenic state may be related to the synthesis of PC by hepatic PE N-methyltransferase (PEMT) using methionine as the tri-methyl donor. Here we show in inbred mice that this is the case and that plasma tHcy is increased significantly during cholesterol cholelithogenesis by upregulation of hepatic PEMT, the levels correlating with Lith gene burden. No plasma tHcy elevation occurs in PEMT KO mice even though they are strongly cholesterol cholelithogenic.
Materials and Methods
Animals and Treatments
Male C57L, C57BL/6, SWR, AKR, and PEMT knockout (KO), heterozygous (HET), and wild type (WT) mice, 6–8 wk old, were obtained from The Jackson Laboratory (Bar Harbor, ME) and fed murine chow. A balanced lithogenic diet (LD) containing 1% cholesterol, 0.5% cholic acid, and 17% dairy plus vegetable fat was replete in essential nutrients and vitamins, including folate, vitamin B12, and vitamin B6. Animals were fed the LD continuously for 2–56 days. Official institutional (IACUC) approval was obtained for all studies, and formal ethical standards were maintained.
Collection of Tissue Samples
After an overnight fast with free access to water, mice were anesthetized with IP injections of ketamine/xylazine (0.09:0.02 mg/g). Hepatic bile was drained acutely through a cannula inserted into the common bile duct (22). Livers were harvested and weighed, then immersed in liquid N2 and kept at −80°C until thawed for further analysis. Blood was collected by cardiac puncture; plasma was separated by centrifugation and stored at −80°C.
Plasma and biliary tHcy, folate, vitamin B12, and cysteine concentrations
Plasma tHcy was determined by HPLC equipped with an electrochemical detector according to the manufacturer’s manual (BAS 200B, Bioanalytical Systems, Mount Vernon, IN). Plasma, liver, and bile folate, and plasma vitamin B12 were determined by radioassay using a BioRad (Hercules, CA) kit. Plasma and bile folate was determined using a 96-well plate microbial assay (23). Plasma cysteine and bile tHcy were assessed by HPLC with fluorometric detection (24). Maximal coefficients of variation were 13% for folate and 8% for cysteine and tHcy.
Gallbladder bile analysis and biliary lipid assays and cholesterol saturation indexes (CSIs)
Gallbladders were weighed and incised. Gallbladder bile was examined by both direct and polarized light microscopy for the presence of liquid crystals, solid crystals, sandy stones, and cholesterol gallstones, as defined elsewhere (25). Mucin gel was scored microscopically on a 0–5 arbitrary scale (26). Biliary bile salt concentrations were assayed using the 3α-hydroxysteroid dehydrogenase method (27). Biliary phospholipid concentrations were determined as inorganic phosphorus (28). Biliary cholesterol was isolated (29) and quantified via HPLC (30). CSIs were calculated using critical tables (31).
S-adenosylmethionine (SAM) and S-adenosylhomocysteine (SAH) concentrations
We measured SAM and SAH levels in fresh liver homogenates by HPLC (Beckman-Coulter, Fullerton, CA) employing UV detection (32), using S-(5′-adenosyl)-L-homocysteine and S-(5′-adenosyl)-L-methionine chloride (Sigma-Aldrich, St. Louis, MO) as standards.
PEMT and cytidine triphosphate:phosphocholine cytidylyltransferase (PCT) enzyme activities
Livers were homogenized with a Potter-Elvehjem tissue homogenizer in five volumes of 20 mM sucrose prepared in 10 mM Tris-HCl and centrifuged at 600×g for 5 min at 4°C; supernatant PEMT activity was assayed (33). One unit of PEMT activity is defined as nmoles SAM converted/mg protein/hour at 37°C. PCT activity was determined as described (34). One unit of PCT activity is defined as nmoles PC converted/mg protein/min at 37°C.
Reverse transcription and quantitative polymerase chain reaction (Q-PCR)
Total hepatic RNA was isolated using an RNeasy Kit (Qiagen, Valencia, CA) and reverse-transcribed using SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA) and oligo-dT primers. Q-PCR was conducted with the Taqman Fast Advanced Master Mix (ABI, Carlsbad, CA). Data for genes Mthfr, Bhmt, Cbs, glycine N-methyltransferase (Gnmt), and guanidinoacetate N-methyltransferase (Gamt) are presented as relative expressions normalized to β-actin levels run simultaneously.
Statistics
Statistical analyses (GraphPad Prism 5.02, GraphPad Software, San Diego, CA) were performed by one-way ANOVA using Dunnett’s post-hoc test.
Results
Plasma tHcy and biliary tHcy secretion rates
Figure 1 shows the effects of LD feeding on plasma tHcy concentrations and biliary tHcy secretion rates in gallstone-susceptible C57L and gallstone-resistant AKR mice. Figure 1A plots plasma tHcy concentrations in gallstone-susceptible C57L mice (n=4–8) that increase from 4.3±0.2 μM to 8.7±0.7 μM (P=0.001) at 4 days, thereafter decreasing ≈50% but remaining elevated for the 56-day duration of LD feeding (P<0.05); Figure 1B shows that bile tHcy secretion rates in C57L mice increase from 1.5±0.1 to 5.3±0.9 pmol/min/g liver (P<0.05) as measured at 42 days of LD feeding. In AKR mice, plasma tHcy concentrations (Figure 1C) as well as bile tHcy secretion rates (Figure 1D) display no changes with LD feeding. Figure 1E illustrates the plasma tHcy elevations at 6 to 16 days in gallstone-susceptible C57BL/6 and SWR mice (P<0.05) that return to normal at 21 and 16 days of LD feeding, respectively.
Figure 1.
Plasma total homocysteine (tHcy) concentrations (μM) (n=4–8) in C57L, AKR, C57BL/6, and SWR mice and bile tHcy secretion rates (pmol/min/g liver) in C57L and AKR mice fed a lithogenic diet (LD) for up to 56 days. A. Plasma tHcy levels are elevated (P<0.05) in C57L mice, the most gallstone-susceptible strain, and remain so during the entire LD feeding period. B. Compared with chow-fed animals, biliary tHcy secretion rates are elevated in C57L mice at 42 days of LD feeding (P<0.05). C. Plasma tHcy concentrations in AKR mice, a gallstone-resistant strain, do not change significantly with LD feeding. D. Biliary tHcy secretion rates are not altered by the LD in AKR mice at 42 days. E. Plasma tHcy levels become elevated in gallstone-susceptible C57BL/6 mice at 6, 9, and 16 days and return to normal at 21 days of LD feeding. In SWR mice, plasma tHcy becomes elevated (P<0.05) at 6 and 9 days of LD feeding, returning to normal at 16 days.
Activities of regulatory enzymes involved in hepatic PC synthesis
PEMT and PCT activities in liver homogenates were assayed during cholelithogenesis-induced hyperhomocysteinemia. Figure 2A shows that PEMT activities in C57L mice are elevated at two days of LD feeding and peak with a 90% elevation at 16 days (P<0.05 at all time points) but do not change in AKR mice (Figure 2B). Figure 2C reveals that PCT activities (Kennedy pathway) remain unchanged in C57L mice during the LD feeding period.
Figure 2.
Activities of phosphatidylethanolamine N-methyltransferase (PEMT), cytidine triphosphate: phosphocholine cytidylyltransferase (PCT), and S-adenosylmethionine/S-adenosylhomocysteine (SAM/SAH) ratios in liver homogenates of C57L and AKR mice fed the LD for 56 days. A. In C57L mice (n=4–8), PEMT activities become elevated (P<0.05) at all time points. B. In AKR mice, PEMT activities are unaffected by LD feeding. C. In C57L mice, PCT activities remain unchanged during LD feeding. D. SAM/SAH ratios in C57L mice are diminished (P<0.05) between days 6 and 16 of LD feeding and then return to normal levels. E. SAM/SAH ratios in AKR mice are not affected by LD feeding.
Liver SAM/SAH ratios
The SAM/SAH ratio is a critical variable influencing tissue Hcy and methionine homeostasis. Figure 2D shows that SAM/SAH ratios in liver homogenates of C57L mice decrease at 6–16 days of LD feeding. This implies that more Hcy is available as a free metabolite for release into plasma (Figure 1A). No significant changes occur in the hepatic SAM/SAH ratios in AKR mice (Figure 2E).
Hcy remethylation, transsulfuration, GNMT, and GAMT pathways
Figure 3 displays schematically that hepatic Hcy is produced by transmethylation reactions, principally utilizing PEMT, GNMT, and GAMT pathways (6). Hcy can be remethylated to methionine through methionine synthase (MS) (with vitamin B12 and folate as cofactors) and BHMT, or transsulfurated to cystathionine by CBS requiring vitamin B6 as cofactor (6). To understand the activities of these pathways during LD feeding, Gnmt, Gamt, Mthfr, Bhmt, and Cbs genes encoding the rate-limiting enzymes, and vitamin B12 and folate cofactors were assayed. In addition, plasma and biliary levels of cysteine, the final product of the transsulfuration pathway, were also assayed.
Figure 3.
Schematic diagram of the principal pathways in methionine/homocysteine homeostasis in hepatocytes. PEMT, glycine N-methyltransferase (GNMT), and guanidinoacetate N-methyltransferase (GAMT) are methyltransferases that utilize SAM as the methyl donor to produce SAH. SAH is then hydrolyzed rapidly to homocysteine. Homocysteine, in turn, can be remethylated to methionine via betaine-homocysteine methyltransferase (BHMT), or by methionine synthase (MS) via the methylenetetrahydrofolate reductase (MTHFR)-dependent pathway. Homocysteine can also be transsulfurated to cysteine via the cystathionine-β-synthase (CBS)-dependent pathway. Gene expression patterns for the major enzymes in these pathways and principal cofactors are quantified in the current work.
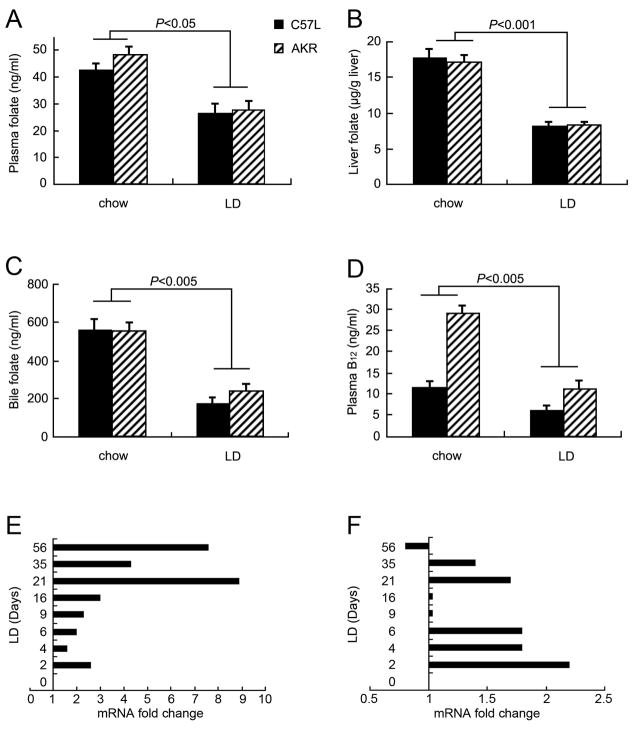
Figure 4(A–D) summarizes the effects of the LD on folate and vitamin B12 levels at 42 days in C57L (n=6–8) and AKR (n=7) mice. In Figure 4A–D, plasma, liver, and bile folate as well as plasma vitamin B12 levels decrease (P<0.05–0.001) in both strains of mice compared with chow-fed animals. Figure 4E illustrates that Mthfr mRNA levels in C57L mouse livers increase over the 56 days of LD feeding. Figure 4F shows that Bhmt mRNA levels increase at most time points of LD feeding. These data indicate that both homocysteine remethylation reactions are upregulated by the LD. Similarly, Bhmt mRNA levels are also elevated by the LD in C57BL/6 and PEMT WT mice (Supplemental Figure 1A and D).
Figure 4.
Effects of LD on vitamin cofactors (folate, vitamin B12) at 42 days and genes (Mthfr, Bhmt) of homocysteine remethylation pathways (viz. Figure 3) over 56 days in C57L and AKR mice. A. Plasma folate levels are decreased in both C57L and AKR mice by the LD. B. Folate levels are reduced in liver homogenates in C57L and AKR mice. C. Biliary folate levels in C57L and AKR mice are also decreased. D. Compared to mice fed chow, plasma vitamin B12 is decreased in C57L and AKR mice. E. Fold changes by Q-PCR demonstrate that hepatic Mthfr mRNA levels in C57L mice are upregulated by the LD after 2 days compared with those fed chow (zero point). F. Bhmt mRNA levels in C57L mice are upregulated at most time points during LD feeding.
Figure 5A illustrates that in response to 42 days of LD feeding, plasma cysteine levels increase in both C57L and AKR mice (P<0.01). Moreover, Figure 5B shows that biliary cysteine secretion rates are elevated in C57L mice (P<0.001), but unchanged in AKR mice, by the LD. Cbs gene expression in C57L mice (Figure 5C) increases during the entire LD feeding course, indicative of increased homocysteine transsulfuration.
Figure 5.
Effects of LD feeding on cysteine, the final product of homocysteine transsulfuration via the CBS pathway, and GNMT and GAMT pathways (viz. Figure 3). A. Plasma cysteine increases in C57L and AKR mice fed the LD for 42 days. B. In C57L mice but not in AKR mice, biliary cysteine secretion rates are increased on the LD. C. The Cbs mRNA level displays 1.3–2-fold elevations at all time points of LD feeding in C57L mice. D. The Gnmt mRNA level is downregulated following 2 days of LD feeding in C57L mice. E. The Gamt mRNA level is also downregulated in C57L mice at all time points during LD feeding.
The GNMT and GAMT pathways are quantitatively important homocysteine sources in liver (35, 36). However, with LD feeding in C57L mice, liver Gnmt and Gamt genes are down-regulated (Figure 5D and 5E), strongly suggesting that neither is a source of plasma homocysteine during cholelithogenesis.
PEMT KO, HET and WT mice
During LD feeding, plasma tHcy levels do not show any significant changes in PEMT KO (Figure 6A) or HET (Figure 6B) mice. In contrast, in PEMT WT mice (Figure 6C) on the same genetic background, plasma tHcy concentration becomes elevated at days 4 and 6 of LD feeding, and an elevated PEMT activity is detected at 4 days in these WT mice (Figure 6D). In PEMT KO mice, PEMT activities are below the detectable limit and are therefore not displayed.
Figure 6.
Plasma tHcy concentrations (μM) in PEMT KO (n=6–8), HET (n=4), and WT (n=8) mice. A. In PEMT KO mice, and B. in HET mice, plasma tHcy concentrations are not changed significantly by LD feeding. C. Plasma tHcy concentrations increase in WT mice at 4 and 6 days (P<0.05) of LD feeding. D. PEMT activity in liver homogenates of WT mice is elevated at 4 days of LD feeding. PEMT activities in PEMT KO mice are below the detectable limit and are therefore not displayed.
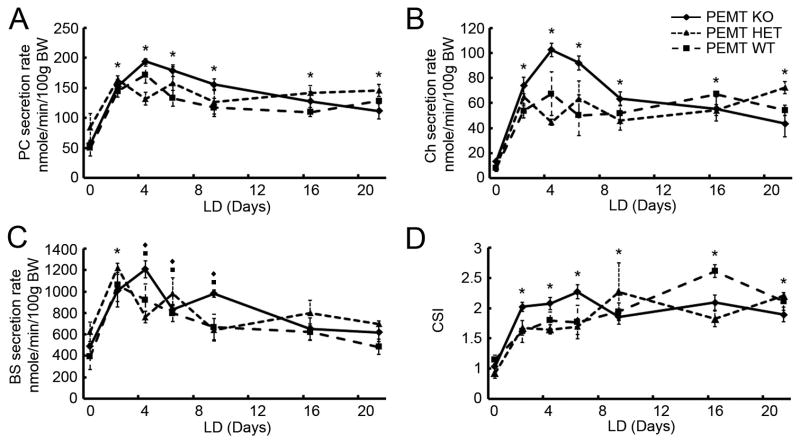
Common biliary lipid secretion rates were measured and CSIs calculated in all three strains of PEMT mice, whose genetic background is C57BL/6J and 129P2/J (Figure 7). PC (Figure 7A) and cholesterol (Ch) (Figure 7B) secretion rates increase at 2 days of LD feeding and remain elevated significantly for the duration of these experiments. Bile salt (BS) secretion rates likewise increase at 2 days of LD feeding but return to normal levels at 16 days. CSIs (Figure 7D) are increased (P<0.05) at all time points. Table 1 summarizes the prevalences of liquid crystals, solid crystals (including cholesterol monohydrate crystals (ChM) and “sandy” stones), and cholesterol gallstones in PEMT KO, HET, and WT mice, which are all similar. Gallbladder mucin gel scores are also comparable in the three mouse strains and consistent with the CSI values. These results indicate that the PEMT KO mice are as strongly gallstone-susceptible as the HET and WT mice as a result of secreting lithogenic amounts of Ch and PC into bile (Figure 7).
Figure 7.
Time dependence of biliary PC, cholesterol (Ch), and bile salt (BS) secretion rates (nmole/min/100g BW) and CSI values in PEMT KO, HET, and WT mice fed the LD. A. In all three strains, PC secretion rates are increased during the entire LD feeding period. B. Ch secretion rates are upregulated markedly over the entire LD feeding period in all three mouse strains. C. BS secretion rates are increased at 2, 4, 6, and 9 days of LD feeding in PEMT KO and WT mice, and increase only at 2 days in PEMT HET mice. D. CSIs are increased significantly by LD feeding in all three mouse strains.
Table 1.
N values, phase transitions, mucin scores and gallstone prevalence rates (%) in PEMT KO, HET, and WT mice fed the LD for 56 days. (See references 24 and 25 for definitions and semi-quantitative mucin scoring system.)
| PEMT KO | Days on LD | 0 | 2 | 4 | 6 | 9 | 16 | 21 | 35 | 56 |
|---|---|---|---|---|---|---|---|---|---|---|
| N value | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 4 | |
| Liquid Crystals * | 0 | 50% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | |
| Solid Crystals † | 0 | 0 | 0 | 25% | 0 | 12.5% | 37.5% | 50% | 25% | |
| Stones ‡ | 0 | 0 | 0 | 0 | 0 | 12.5% | 25% | 25% | 25% | |
| Mucin § | 0 | 0 | 0 | 0.25 | 0.5 | 0.5 | 1.25 | 2.25 | 2 | |
|
| ||||||||||
| PEMT HET | N value | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
|
| ||||||||||
| Liquid Crystals | 0 | 75% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | |
| Solid Crystals | 0 | 0 | 0 | 25% | 0 | 50% | 50% | 25% | 100% | |
| Stones | 0 | 0 | 0 | 0 | 0 | 50% | 25% | 25% | 100% | |
| Mucin | 0 | 0 | 0.4 | 0.25 | 0.25 | 0.25 | 1.75 | 2 | 2 | |
|
| ||||||||||
| PEMT WT | N value | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 4 | 4 |
|
| ||||||||||
| Liquid Crystals | 0 | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | |
| Solid Crystals | 0 | 0 | 0 | 0 | 0 | 50% | 50% | 25% | 0 | |
| Stones | 0 | 0 | 0 | 0 | 0 | 0 | 50% | 25% | 0 | |
| Mucin | 0 | 0.5 | 1 | 0.2 | 1 | 0.25 | 1.25 | 1.5 | 2 | |
Fused liquid crystals with Maltese-cross birefringence and focal conic textures;
Including cholesterol monohydrate crystals (ChM) and/or disintegratable amorphous sandy stones;
Gallstones exhibiting rounded contours and black centers from scattering of transmitted light;
Scoring from 0 (no gel-like material) to 5 (gallbladder full of gelled mucin).
Discussion
The present work embodies a new paradigm with respect to the cholelithogenic state in inbred mice. We show that hyperproduction of homocysteine occurs in response to augmented hepatic PC synthesis via the PEMT pathway, and a proportion of this tHcy spills into plasma and bile. Fundamental observations on hepatic PC synthesis in mice were made principally by Vance and colleagues (37). They reported reduced plasma tHcy in PEMT KO mice (38), a finding not confirmed here, but that secretion rate of biliary phospholipids remained unaltered (39), as we found (Figure 7A), since the Kennedy pathway of PC synthesis fully compensated (40). We extended these results to show that cholesterol cholelithogenesis in PEMT KO mice is identical to that in WT mice. In the basal state, PEMT contributes approximately 20–40% PC in hepatocytes (21). We show here that PC synthesis is upregulated by exposure to a LD, and tri-methylation of PE is augmented, which results in three SAH molecules accumulating for every PC molecule synthesized. As evidenced by hypersecretion of biliary PC (Supplemental Figure 2A), the upregulated hepatic PEMT pathway contributed to biliary PC during cholelithogenesis (Figure 2A and Figure 6D) since the PCT pathway remained unaltered (Figure 2C). Simultaneously, tHcy elevations in plasma and bile were observed principally during the initial stages of cholelithogenesis because counter-regulatory mechanisms were activated, i.e., remethylation of Hcy to methionine and catabolism of Hcy to cysteine. Plasma elevation of tHcy with cholelithogenesis was ablated in PEMT KO mice (Figure 6A) despite biliary PC secretion similar to WT mice (Figure 7A), and the stages of cholelithogenesis were identical to those of controls (Table 1).
Hyperhomocysteinemia resulted from increased production (Figure 1A and 2A) and remained permanent in C57L mice, the most cholesterol gallstone-susceptible strain (41), but was not observed in gallstone-resistant AKR mice (Figure 1C). Because of muricholate conjugates in murine bile (42), the LD’s 0.5% cholic acid is necessary to induce hyperabsorption of cholesterol; moreover, cholic acid per se is capable of stimulating biliary PC secretion (42). As a result, it was notable that plasma tHcy levels in gallstone-susceptible C57L mice increased in response to 0.5% cholic acid alone, but not with either 1% cholesterol or 11% fat alone (Supplemental Figure 3). It is well known that the LD can induce chronic liver damage in mouse models and that, in humans, plasma tHcy is elevated in hepatic cirrhosis but not in other chronic liver diseases (43). However, in our experiments, the elevated plasma tHcy response is immediate, returning to normal after several days in the less lithogenic mouse strains. Furthermore, there is no evidence in the literature that homocysteine can be an acute phase reactant. In these studies, therefore, the elevated plasma tHcy is the result of a rapid metabolic response and not a response to injury.
The most persuasive evidence that plasma tHcy arose from an upregulated PEMT pathway is its ablation in PEMT KO mice but positive response in PEMT WT mice on the LD, despite nearly identical biliary lipid secretion rates and gallstone susceptibilities in both strains (Figure 7, Table 1). Moreover, hepatic cytosolic SAM/SAH ratios decreased significantly in C57L mice but not in AKR mice, also indicative of overwhelming hepatic hyperproduction of Hcy in the C57L strain. Diminutions with respect to Gnmt and Gamt genes (Figure 5) rule out these pathways (Figure 3) as likely contributors to plasma tHcy in the lithogenic state.
The successful upregulation of homocysteine remethylation and transsulfuration pathways (Figures 4, 5 and Supplemental Figure 1) are likely to be the reason why hyperhomocysteinemia is transient in PEMT WT, C57BL/6, and SWR mice. Clearly, these counter-regulatory mechanisms were overcome in C57L mice by the magnitude of PEMT activity that maintained massive biliary PC hypersecretion (Supplemental Figure 2) accompanying cholesterol hypersecretion (22). We believe that this is a reflection of the complement of 6 Lith genes in C57L mice (41) compared to a single Lith gene in AKR mice (41) and further exemplified by the variable gallstone prevalence rates among strains (90–100% for C57L, 69% for C57BL/6, 25% for SWR, and 15% for AKR, http://phenome.jax.org/).
In healthy mice PE methylation via the PEMT pathway is an appreciable source of plasma tHcy (37, 44). Evidence of this is that plasma tHcy becomes elevated 20–40% in Kennedy pathway-KO mice from increased activity of the PEMT pathway to meet hepatic demands for PC (44). With respect to the possible contribution of the PEMT pathway to tHcy production in humans, Vance et al (37, 38) have argued that human PEMT also plays a major role in SAM consumption and tHcy production in addition to its 30% role in PC synthesis (21, 45, 46).
The LD induced altered homeostasis of vitamin B12, folate, and cysteine in our animal models, but there is currently no simple explanation for the observations that Mthfr gene expression is increased and both plasma and biliary B12 and folate levels are decreased by the LD. The kinetics of the remethylation cycle are influenced by folate, B12, MTHFR activity (not simply gene expression), and NADPH levels in the hepatocyte, as well as by homocysteine and the SAM/SAH ratio. Mathematical modeling of these interactions by Reed and colleagues (47) highlights some of the complexities among these interactions, making it difficult to draw a simple conclusion based on measured concentrations alone. It has been observed that decreased plasma folate and vitamin B6 (the cofactor in the CBS pathway, Figure 3) levels occur in human gallstone subjects (13). This consistency with our current results in mice suggests that the findings here may be relevant to cholelithogenic humans and may indicate that the same mechanisms, i.e., PEMT activation, elevated plasma tHcy (12), and counter-regulatory remethylation and transsulfuration of Hcy, are involved. In addition to atherothrombotic risk from plasma tHcy, it would be important in the future to test whether increased tHcy levels in bile influence gallbladder function, gene expression, or the metastability of supersaturated bile.
These current results taken together allow us to propose a reasonable scenario for an elevation of plasma and bile tHcy in the cholelithogenic state. To supply the increased demand for biliary PC (see Supplemental Figure 2 and reference 20), the PEMT pathway (but not the Kennedy pathway) is upregulated by the lithogenic challenge. Each molecule of PC synthesized from PE via the donation of 3 methyl groups from methionine contributes three molecules of homocysteine to hepatocytes, and ultimately to plasma and bile, as a function of the tissue SAM/SAH ratio. In general, counter-regulatory mechanisms are highly effective in normalizing tHcy in plasma but apparently can be overwhelmed by the magnitude of PEMT activity in regulating PC plus cholesterol hypersecretion, a function of total Lith gene burden, such as in C57L mice with their great proclivity to cholesterol gallstones.
Clearly, prospective studies in human populations with high prevalences of gallstones, especially those in which both males and females develop supersaturated bile during puberty, such as American Indians and some Caucasians (48, 49), are needed to make pathophysiological correlations between this work and the human disease. Parallel research in inbred mouse strains to explore the health of the arterial system, as well as gallbladder wall function, bile metastability, and gene expression, would provide much needed corollary information to illuminate such clinically relevant studies.
Supplementary Material
Figure (Supplemental) 1. Q-PCR results of Bhmt, Mthfr, and Cbs genes in C57BL/6 and PEMT WT mice as functions of days on the LD. In C57BL/6 mice, only the Bhmt gene becomes upregulated during LD feeding (A). Mthfr (B) and Cbs (C) genes do not change with LD feeding. In PEMT WT mice, the Bhmt gene (D) displays an elevation only at 2 days on the LD, but no change is observed in the case of Mthfr (E) and Cbs (F) genes.
Figure (Supplemental) 2. Biliary lipid secretion rates into hepatic bile of C57L mice fed the LD for 56 days. The panels show (A) phosphatidylcholine (PC) secretion rates, (B) cholesterol (Ch) secretion rates, (C) bile salt (BS) secretion rates, and (D) CSI values. In C57L mice, biliary lipid secretion rates and CSI values are elevated significantly at and after 2 days of LD feeding. These data are similar to earlier work from this laboratory (19).
Figure (Supplemental) 3. Effects of dietary cholic acid, cholesterol, and total fat on plasma tHcy concentrations in AKR and C57L mice (n=4). A. In C57L mice, plasma tHcy levels are elevated by 0.5% cholic acid (P<0.05) but not by 1% cholesterol. B. Plasma tHcy levels in AKR mice fed diets containing either 0.5% cholic acid or 1% cholesterol remain unaltered. C. Plasma tHcy levels remain unaltered whether feeding chow (5% fat) or a high (11%) fat diet to C57L mice for 5 wk.
Acknowledgments
Financial Support: Supported in part by NIH (USPHS) grants: DK36588, HL61795, HL81587, HL70819, HL48743, HL89734; and USDA agreements: 1950-51520-008-00D and 58-1950-9-001.
Our thanks to Ms. Monika Leonard for assistance with all aspects of the study. Thanks to Vijay Kotecha and Shou-an Liu for excellent technical support.
Abbreviations used in this paper
- PE
phosphatidylethanolamine
- PC
phosphatidylcholine
- PEMT
phosphatidylethanolamine N-methyltransferase
- LD
lithogenic diet
- tHcy
total homocysteine
- PCT
cytidine triphosphate: phosphocholine cytidylyltransferase
- SAM
S-adenosylmethionine
- SAH
S-adenosylhomocysteine
- GNMT
glycine N-methyltransferase
- GAMT
guanidinoacetate N-methyltransferase
- MTHFR
methylenetetrahydrofolate reductase
- BHMT
betaine-homocysteine methyltransferase
- CBS
cystathionine-β-synthase
Footnotes
Disclosures: The authors have declared that no conflict of interest exists.
References
- 1.Bortnichak EA, Freeman DH, Ostfeld AM, Castelli WP, Kannel WB, Feinleib M, McNamara PM. The association between cholesterol cholelithiasis and coronary heart disease in Framingham, Massachusetts. Am J Epidemiol. 1985;121:19–30. doi: 10.1093/oxfordjournals.aje.a113978. [DOI] [PubMed] [Google Scholar]
- 2.Ruhl CE, Everhart JE. Gallstone Disease Is Associated With Increased Mortality in the United States. Gastroenterology. 2011;140:508–516. doi: 10.1053/j.gastro.2010.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paigen B, Carey MC. Gallstones. In: King RA, Rotter JI, Motulsky AG, editors. The genetic basis of common disease. 2. London: Oxford University Press; 2002. pp. 298–335. [Google Scholar]
- 4.Biddinger SB, Haas JT, Yu BB, Bezy O, Jing E, Zhang W, Unterman TG, et al. Hepatic insulin resistance directly promotes formation of cholesterol gallstones. Nat Med. 2008;14:778–782. doi: 10.1038/nm1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biddinger SB, Hernandez-Ono A, Rask-Madsen C, Haas JT, Alemán JO, Suzuki R, Scapa EF, et al. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab. 2008;7:125–134. doi: 10.1016/j.cmet.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selhub J. Homocysteine metabolism. Annu Rev Nutr. 1999;19:217–246. doi: 10.1146/annurev.nutr.19.1.217. [DOI] [PubMed] [Google Scholar]
- 7.Carey MC, Fennelly JJ, FitzGerald O. Homocystinuria: II. Subnormal serum folate levels, increased folate clearance and effects of folic acid therapy. Am J Med. 1968;45:26–31. doi: 10.1016/0002-9343(68)90004-1. [DOI] [PubMed] [Google Scholar]
- 8.Clarke R, Daly L, Robinson K, Naughten E, Cahalane S, Fowler B, Graham I. Hyperhomocysteinemia: An Independent Risk Factor for Vascular Disease. N Engl J Med. 1991;324:1149–1155. doi: 10.1056/NEJM199104253241701. [DOI] [PubMed] [Google Scholar]
- 9.Welch GN, Loscalzo J. Homocysteine and atherothrombosis. N Engl J Med. 1998;338:1042–1050. doi: 10.1056/NEJM199804093381507. [DOI] [PubMed] [Google Scholar]
- 10.Mattson MP, Kruman II, Duan W. Folic acid and homocysteine in age-related disease. Ageing Res Rev. 2002;1:95–111. doi: 10.1016/s0047-6374(01)00365-7. [DOI] [PubMed] [Google Scholar]
- 11.Kim DJ, Koh J-M, Lee O, Kim NJ, Lee Y-S, Kim YS, Park J-Y, et al. Homocysteine enhances apoptosis in human bone marrow stromal cells. Bone. 2006;39:582–590. doi: 10.1016/j.bone.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Sakuta H, Suzuki T. Plasma total homocysteine and gallstone in middle-aged Japanese men. J Gastroenterol. 2005;40:1061–1064. doi: 10.1007/s00535-005-1691-z. [DOI] [PubMed] [Google Scholar]
- 13.Worthington HV, Hunt LP, McCloy RF, Ubbink JB, Braganza JM. Dietary antioxidant lack, impaired hepatic glutathione reserve, and cholesterol gallstones. Clin Chim Acta. 2004;349:157–165. doi: 10.1016/j.cccn.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 14.LaMont JT, Carey MC. Cholesterol gallstone formation. 2. Pathobiology and pathomechanics. Prog Liver Dis. 1992;10:165–191. [PubMed] [Google Scholar]
- 15.Shaffer EA, Small DM. Biliary lipid secretion in cholesterol gallstone disease. The effect of cholecystectomy and obesity. J Clin Invest. 1977;59:828–840. doi: 10.1172/JCI108705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carey MC, LaMont JT. Cholesterol gallstone formation. 1. Physical-chemistry of bile and biliary lipid secretion. Prog Liver Dis. 1992;10:139–163. [PubMed] [Google Scholar]
- 17.Hay DW, Cahalane MJ, Timofeyeva N, Carey MC. Molecular species of lecithins in human gallbladder bile. J Lipid Res. 1993;34:759–768. [PubMed] [Google Scholar]
- 18.Moschetta A, Xu F, Hagey LR, van Berge-Henegouwen GP, van Erpecum KJ, Brouwers JF, Cohen JC, et al. A phylogenetic survey of biliary lipids in vertebrates. J Lipid Res. 2005;46:2221–2232. doi: 10.1194/jlr.M500178-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy EP, Weiss SB. The function of cytidine coenzymes in the biosynthesis of phospholipides. J Biol Chem. 1956;222:193–214. [PubMed] [Google Scholar]
- 20.Vance DE, Walkey CJ, Cui Z. Phosphatidylethanolamine N-methyltransferase from liver. Biochim Biophys Acta. 1997;1348:142–150. doi: 10.1016/s0005-2760(97)00108-2. [DOI] [PubMed] [Google Scholar]
- 21.Sundler R, Akesson B. Regulation of phospholipid biosynthesis in isolated rat hepatocytes. Effect of different substrates. J Biol Chem. 1975;250:3359–3367. [PubMed] [Google Scholar]
- 22.Wang DQ, Lammert F, Paigen B, Carey MC. Phenotypic characterization of Lith genes that determine susceptibility to cholesterol cholelithiasis in inbred mice. Pathophysiology of biliary lipid secretion. J Lipid Res. 1999;40:2066–2079. [PubMed] [Google Scholar]
- 23.Horne DW, Patterson D. Lactobacillus casei microbiological assay of folic acid derivatives in 96-well microtiter plates. Clin Chem. 1988;34:2357–2359. [PubMed] [Google Scholar]
- 24.Araki A, Sako Y. Determination of free and total homocysteine in human plasma by high-performance liquid chromatography with fluorescence detection. J Chromatogr. 1987;422:43–52. doi: 10.1016/0378-4347(87)80438-3. [DOI] [PubMed] [Google Scholar]
- 25.Wang DQ, Paigen B, Carey MC. Phenotypic characterization of Lith genes that determine susceptibility to cholesterol cholelithiasis in inbred mice: physical-chemistry of gallbladder bile. J Lipid Res. 1997;38:1395–1411. [PubMed] [Google Scholar]
- 26.Maurer KJ, Ihrig MM, Rogers AB, Ng V, Bouchard G, Leonard MR, Carey MC, et al. Identification of cholelithogenic enterohepatic helicobacter species and their role in murine cholesterol gallstone formation. Gastroenterology. 2005;128:1023–1033. doi: 10.1053/j.gastro.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Turley SD, Dietschy JM. Re-evaluation of the 3α-hydroxysteroid dehydrogenase assay for total bile acids in bile. J Lipid Res. 1978;19:924–928. [PubMed] [Google Scholar]
- 28.Bartlett GR. Phosphorus assay in column chromatography. J Biol Chem. 1959;234:466–468. [PubMed] [Google Scholar]
- 29.Jeske DJ, Dietschy JM. Regulation of rates of cholesterol synthesis in vivo in the liver and carcass of the rat measured using [3H]water. J Lipid Res. 1980;21:364–376. [PubMed] [Google Scholar]
- 30.Vercaemst R, Union A, Rosseneu M. Separation and quantitation of free cholesterol and cholesteryl esters in a macrophage cell line by high-performance liquid chromatography. J Chromatogr. 1989;494:43–52. doi: 10.1016/s0378-4347(00)82655-9. [DOI] [PubMed] [Google Scholar]
- 31.Carey MC. Critical tables for calculating the cholesterol saturation of native bile. J Lipid Res. 1978;19:945–955. [PubMed] [Google Scholar]
- 32.Bottiglieri T. Isocratic high performance liquid chromatographic analysis of S-adenosylmethionine and S-adenosylhomocysteine in animal tissues: the effect of exposure to nitrous oxide. Biomed Chromatogr. 1990;4:239–241. doi: 10.1002/bmc.1130040606. [DOI] [PubMed] [Google Scholar]
- 33.Verma A, Ahmed HA, Davis T, Jazrawi RP, Northfield TC. Demonstration and partial characterisation of phospholipid methyltransferase activity in bile canalicular membrane from hamster liver. J Hepatol. 1999;31:852–859. doi: 10.1016/s0168-8278(99)80286-4. [DOI] [PubMed] [Google Scholar]
- 34.Yao ZM, Jamil H, Vance DE. Choline deficiency causes translocation of CTP:phosphocholine cytidylyltransferase from cytosol to endoplasmic reticulum in rat liver. J Biol Chem. 1990;265:4326–4331. [PubMed] [Google Scholar]
- 35.Brosnan JT, Jacobs RL, Stead LM, Brosnan ME. Methylation demand: a key determinant of homocysteine metabolism. Acta Biochim Pol. 2004;51:405–413. [PubMed] [Google Scholar]
- 36.Rowling MJ, McMullen MH, Chipman DC, Schalinske KL. Hepatic glycine N-methyltransferase is up-regulated by excess dietary methionine in rats. J Nutr. 2002;132:2545–2550. doi: 10.1093/jn/132.9.2545. [DOI] [PubMed] [Google Scholar]
- 37.Vance DE, Li Z, Jacobs RL. Hepatic phosphatidylethanolamine N-methyltransferase, unexpected roles in animal biochemistry and physiology. J Biol Chem. 2007;282:33237–33241. doi: 10.1074/jbc.R700028200. [DOI] [PubMed] [Google Scholar]
- 38.Noga AA, Stead LM, Zhao Y, Brosnan ME, Brosnan JT, Vance DE. Plasma homocysteine is regulated by phospholipid methylation. J Biol Chem. 2003;278:5952–5955. doi: 10.1074/jbc.M212194200. [DOI] [PubMed] [Google Scholar]
- 39.Verkade HJ, Havinga R, Shields DJ, Wolters H, Bloks VW, Kuipers F, Vance DE, et al. The phosphatidylethanolamine N-methyltransferase pathway is quantitatively not essential for biliary phosphatidylcholine secretion. J Lipid Res. 2007;48:2058–2064. doi: 10.1194/jlr.M700278-JLR200. [DOI] [PubMed] [Google Scholar]
- 40.Walkey CJ, Yu L, Agellon LB, Vance DE. Biochemical and evolutionary significance of phospholipid methylation. J Biol Chem. 1998;273:27043–27046. doi: 10.1074/jbc.273.42.27043. [DOI] [PubMed] [Google Scholar]
- 41.Lyons MA, Wittenburg H. Cholesterol gallstone susceptibility loci: a mouse map, candidate gene evaluation, and guide to human LITH genes. Gastroenterology. 2006;131:1943–1970. doi: 10.1053/j.gastro.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 42.Wang DQ, Lammert F, Cohen DE, Paigen B, Carey MC. Cholic acid aids absorption, biliary secretion, and phase transitions of cholesterol in murine cholelithogenesis. Am J Physiol. 1999;276:G751–G760. doi: 10.1152/ajpgi.1999.276.3.G751. [DOI] [PubMed] [Google Scholar]
- 43.Ventura P, Rosa MC, Abbati G, Marchini S, Grandone E, Vergura P, Tremosini S, et al. Hyperhomocysteinaemia in chronic liver diseases: role of disease stage, vitamin status and methylenetetrahydrofolate reductase genetics. Liver Int. 2005;25:49–56. doi: 10.1111/j.1478-3231.2005.01042.x. [DOI] [PubMed] [Google Scholar]
- 44.Jacobs RL, Stead LM, Devlin C, Tabas I, Brosnan ME, Brosnan JT, Vance DE. Physiological regulation of phospholipid methylation alters plasma homocysteine in mice. J Biol Chem. 2005;280:28299–28305. doi: 10.1074/jbc.M501971200. [DOI] [PubMed] [Google Scholar]
- 45.Vance DE, Ridgway ND. The methylation of phosphatidylethanolamine. Prog Lipid Res. 1988;27:61–79. doi: 10.1016/0163-7827(88)90005-7. [DOI] [PubMed] [Google Scholar]
- 46.Sehayek E, Wang R, Ono JG, Zinchuk VS, Duncan EM, Shefer S, Vance DE, et al. Localization of the PE methylation pathway and SR-BI to the canalicular membrane: evidence for apical PC biosynthesis that may promote biliary excretion of phospholipid and cholesterol. J Lipid Res. 2003;44:1605–1613. doi: 10.1194/jlr.M200488-JLR200. [DOI] [PubMed] [Google Scholar]
- 47.Reed MC, Nijhout HF, Neuhouser ML, Gregory JF, 3rd, Shane B, James SJ, Boynton A, et al. A Mathematical Model Gives Insights into Nutritional and Genetic Aspects of Folate-Mediated One-Carbon Metabolism. J Nutr. 2006;136:2653–2661. doi: 10.1093/jn/136.10.2653. [DOI] [PubMed] [Google Scholar]
- 48.Bennion LJ, Knowler WC, Mott DM, Spagnola AM, Bennett PH. Development of lithogenic bile during puberty in Pima Indians. N Engl J Med. 1979;300:873–876. doi: 10.1056/NEJM197904193001601. [DOI] [PubMed] [Google Scholar]
- 49.Von Bergmann K, Becker M, Leiss O. Biliary cholesterol saturation in non-obese women and non-obese men before and after puberty. Eur J Clin Invest. 1986;16:531–535. doi: 10.1111/j.1365-2362.1986.tb02173.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure (Supplemental) 1. Q-PCR results of Bhmt, Mthfr, and Cbs genes in C57BL/6 and PEMT WT mice as functions of days on the LD. In C57BL/6 mice, only the Bhmt gene becomes upregulated during LD feeding (A). Mthfr (B) and Cbs (C) genes do not change with LD feeding. In PEMT WT mice, the Bhmt gene (D) displays an elevation only at 2 days on the LD, but no change is observed in the case of Mthfr (E) and Cbs (F) genes.
Figure (Supplemental) 2. Biliary lipid secretion rates into hepatic bile of C57L mice fed the LD for 56 days. The panels show (A) phosphatidylcholine (PC) secretion rates, (B) cholesterol (Ch) secretion rates, (C) bile salt (BS) secretion rates, and (D) CSI values. In C57L mice, biliary lipid secretion rates and CSI values are elevated significantly at and after 2 days of LD feeding. These data are similar to earlier work from this laboratory (19).
Figure (Supplemental) 3. Effects of dietary cholic acid, cholesterol, and total fat on plasma tHcy concentrations in AKR and C57L mice (n=4). A. In C57L mice, plasma tHcy levels are elevated by 0.5% cholic acid (P<0.05) but not by 1% cholesterol. B. Plasma tHcy levels in AKR mice fed diets containing either 0.5% cholic acid or 1% cholesterol remain unaltered. C. Plasma tHcy levels remain unaltered whether feeding chow (5% fat) or a high (11%) fat diet to C57L mice for 5 wk.