Abstract
Aim
The transient receptor potential vanilloid type 1 (TRPV1) channels have been implicated to play a role in blood pressure regulation. However, contribution of tissue specific TRPV1 to blood pressure regulation is largely unknown. Here we test the hypothesis that TRPV1 expressed in dorsal root ganglia (DRG) of lower thoracic and upper lumbar segments (T8-L3) of the spinal cord and their central and peripheral terminals constitutes a counter regulatory mechanism preventing the increases in blood pressure.
Methods
TRPV1 was knocked down by intrathecal injection of TRPV1 shRNA in rats. Systolic blood pressure and mean arterial pressure (MAP) were recorded. The level of TRPV1 and tyrosine hydroxylase was measured by Western blot.
Results
Intrathecal injection of TRPV1 shRNA (6 μg kg−1 per day) for 3 days increased systolic blood pressure and MAP when compared to rats that received control shRNA (control shRNA: 112±2 vs TRPV1 shRNA: 123±2 mmHg). TRPV1 expression was suppressed in T8-L3 segments of dorsal horn and DRG as well as mesenteric arteries of rats given TRPV1 shRNA. Contents of tyrosine hydroxylase, a marker of sympathetic nerves, were increased in mesenteric arteries of rats treated with TRPV1 shRNA. Pretreatment with the α1-adrenoceptor blocker, prazosin (1 mg/kg/day, p.o.), abolished the TRPV1 shRNA-induced pressor effects.
Conclusion
Our data show that selective knockdown of TRPV1 expressed in DRG of T8-L3 segments of the spinal cord and their central and peripheral terminals increases blood pressure, suggesting that neuronal TRPV1 in these segments possesses a tonic anti-hypertensive effect possibly via suppression of the sympathetic nerve activity.
Keywords: TRPV1, sensory nerves, blood pressure regulation, intrathecal injection, short-hairpin RNA
Introduction
The transient receptor potential vanilloid type 1 (TRPV1) is a non-selective cation channel that can be activated by heat, protons, capsaicin and endovanilloids (Caterina et al. 1997). TRPV1 is abundantly expressed in small-sized primary afferent neurons and their central and peripheral projections including unmyelinated C-fibers and thinly myelinated Aδ-afferent nerve fibers (Caterina et al. 1997, Tominaga et al. 1998). TRPV1-containing peripheral projections of primary afferent neurons distribute widely in peripheral organs and regulate organ/tissue function in specific manners, including sensing thermal pain (Caterina et al. 1997, 2000, Tominaga et al. 1998, Davis et al. 2000), regulating blood pressure (Zygmunt et al. 1999, Zahner et al. 2003), and modulating gastrointestinal motility and excretion (Ward et al. 2003, Barthó et al. 2004).
Indeed, accumulating evidence indicates that systemic impairment of TRPV1-expressing sensory nerves by subcutaneous injection of capsaicin in rats leads to increased blood pressure in the presence of other stressors such as salt loading, suggesting that TRPV1-positive sensory nerves play a counter-regulatory role preventing the increases in blood pressure (Wang et al. 1998, Wang & Li 1999, Wang et al. 2001, Huang & Wang 2001). However, there are a number of limitations with these previous approaches using systemic sensory denervation.
Firstly, TRPV1 colocalizes in the same sensory neurons with other channels, receptors, and neurotransmitters that may play significant functional roles in the regulation of blood pressure. Once TRPV1-expressing sensory nerves are degenerated following neonatal capsaicin administration, not only TRPV1 but also those colocalized components in the same neurons and nerve fibers would be obliterated. As a result, it would be difficult to distinguish the effects of TRPV1 from those of other components on the regulation of cardiovascular function. To solve this problem, specific targeting of TRPV1 for its downregulation or ablation would be needed. Secondly, systemic sensory nerve degeneration following subcutaneous capsaicin administration or systemic ablation of TRPV1 (Pacher et al. 2004) hinders our ability to define the role of specific organ(s)/tissue(s) in blood pressure regulation. An improved approach targeting specific organ(s)/tissue(s) would provide a better tool for dissecting distinctions between organs and tissues.
Here in the present study, we overcame the above limitation by selectively knocking down the TRPV1 expressed in DRGs (the lower thoracic and upper lumbar segments from T8 to L3) and their central and peripheral projections using TRPV1 short-hairpin RNA (shRNA) that was intrathecally administered into T8-L3 segments of the spinal cord. The T8-L3 segments of the spinal cord are mainly for sensory and sympathomotor innervations of mesenteric arteries and kidneys (Ciriello et al. 1983, Donovan et al. 1983, Chevendra et al. 1991, Huang et al. 2002), which are well known for their important roles in the regulation of blood pressure (Kawasaki et al. 1988, Mayer 2008). By using this strategy, we test the hypothesis that TRPV1 expressed in DRGs (T8-L3) and their central and peripheral projections constitutes a counter regulatory mechanism preventing the increases in blood pressure. In response to TRPV1 down regulation after TRPV1 shRNA treatment, several pro-hypertensive and/or anti-hypertensive systems including the sympathetic nervous system, renin-angiotensin-aldosterone system, endothelin system, and nitric oxide system among others might be affected (Wang 2005, Mayer 2008, Kopp et al. 2008). To assess whether the sympathetic nervous system may be involved and may play a role in TRPV1 shRNA-induced blood pressure regulation, the content of tyrosine hydroxylase, a marker of sympathetic nerves, and blood pressure responses to prazosin, a blocker of α1-adrenoceptors, were examined.
Materials and Methods
Animals
Male Wistar rats (201–225 g, Charles River Laboratory; Wilmington, MA) were housed in a 12-hour light/dark cycle facility with free access to food and water. One week after receiving surgery of intrathecal catheter-implantation and removal of the right kidney, rats were injected intrathecally with TRPV1 shRNA or control shRNA once per day for 3 days. Measurement of systolic blood pressure/mean arterial pressure (MAP), water intake, urine excretion, and collection of tissue samples for immunohistochemistry and Western blot analysis were performed on the 3rd day after intrathecal injection of shRNA. All experiments reported here were carried out in accordance with the protocols approved by the Institutional Animals Care and Use Committee of Michigan State University.
Surgery of intrathecal catheter-implantation and uni-nephrectomy
Intrathecal catheters were implanted in rats according to Yaksh & Rudy (1977) with minor modifications. Briefly, rats were anesthetized with ketamine/xylazine (85/5 mg kg−1, i.p.). A small incision was made in the nape skin and the muscles were separated to expose the atlanto-occipital membrane. A small incision was made in the membrane to allow a polyethylene-10 catheter (outer diameter: 0.61 mm) filled with 0.9 % sterile saline to be inserted into the subarachnoid space. The catheter was threaded through the space to as far as the T8 segment of the spinal cord (the length of the catheter from the inside end to the atlanto-occipital membrane was about 7.5 cm) and sutured in place, and the incision closed. About 5 cm of the catheter was left externally for injection of shRNA, and sealed with parafilm film to stop the outflow of cerebrospinal fluid when not used for injection. To accelerate the course of hypertension development in response to shRNA treatment, uni-nephrectomy was performed by making a right flank incision and removal of the right kidney given the well-known role of the kidney in blood pressure regulation (Mayer 2008). To prevent infection, 10 mg kg−1 of kanamycin, 10,000 unit kg−1 of penicillin, and 10 mg kg−1 of streptomycin were injected subcutaneously everyday for 3 days after surgery. At the end of the experiments, the location of the intrathecal catheter tip was verified and rats with misplaced catheter tips were excluded.
Preparation of TRPV1 shRNA or control shRNA and intrathecal injection
TRPV1 shRNA and control shRNA (SureSilencing shRNA plasmid for rat TRPV1 with neomycin, KR42959N) were purchased from SuperArray Bioscience (Frederick, MD). i-Fect (Neuromics, NI35150) was used as a transfection reagent. The shRNA/i-Fect complexes in 1:5 ratio (w/v) were used for intrathecal injection. One week after catheter implantation surgery, rats received bolus intrathecal injection of TRPV1 shRNA or control shRNA at a dose of 6 μg kg−1 in 10 μl, one time per day for 3 consecutive days. The catheter was flushed immediately every time with 10 μl of saline to ensure all drugs getting into the subarachnoid space.
Measurement of systolic blood pressure, water intake, and urine excretion
Systolic blood pressure of conscious rats was measured by the indirect tail cuff method with a sphygmomanometer (Hatteras Instruments SC1000 Blood Pressure Analysis System). Rats were accustomed to the testing environment for 3 days prior to the measurement, and measurements were made on the same day right before intrathecal injection and three days after intrathecal injection. The blood pressure value for each rat was calculated as the average of 9 separate measurements. 24 hour- water intake and urine excretion were determined by the use of metabolic cages one day before intrathecal injection and three days after intrathecal injection. All measurements were performed by the same investigator who was blinded to the protocol of the individual rat.
Measurement of MAP and its response to intravenous injection of capsaicin
The measurement of baseline MAP and its response to intravenous injection of capsaicin were performed in rats under anesthesia with sodium pentobarbital (50 mg kg−1, i.p.). The left jugular vein and right carotid artery were cannulated for administration of capsaicin (30 μg kg−1) and for monitoring of MAP with a Statham 231D pressure transducer coupled to a Gould 2400s recorder (Gould Instruments), respectively. MAP was recorded 30 minutes after the cannulation and recorded every 3 seconds. The MAP value for each rat was calculated as the average of continuing measurements for 10 minutes.
Blockade of α1-adrenoceptor by prazosin
Prazosin, an α1-adrenoceptor blocker, was administrated via a gastric gavage. Prazosin at 0.5 mg kg−1 of each administration dose was given twice per day for 7 days starting 3 days before shRNA injection.
Immunohistochemistry
For TRPV1 staining---Rats were anesthetized with ketamine/xylazine (85/5 mg kg−1, i.p.) and perfused transcardially with freshly made fixative (4 % paraformaldehyde in PBS). The samples of the spinal cord and dorsal root ganglia (DRG) of T8-L3 segments, mesenteric arteries, and kidneys were harvested and kept in the same fixative for 3 hours, followed by incubation with 15 % and then 30 % sucrose for 12 hours each. Samples were embedded in tissue freezing medium (Triangle Biomedical Sciences, Durham, NC) and quickly frozen with liquid nitrogen. Samples were cut into sections of 20 μm by a cryostat (Leica CM 1850). After permeabilizing with 0.1 % Triton X-100 for 20 minutes and being blocked with 10 % donkey serum for 30 minutes, the sections were incubated with guinea pig anti-TRPV1 polyclonal antibody (Millipore, AB5566, 1:500) for 1 hour at room temperature, then incubated with Rhodamine Red (TM)-X donkey anti-guinea pig IgG (Jackson 711-295-152, 1: 100) for 1 hour at room temperature. After mounting, pictures were taken with a fluorescent microscope (Olympus BX41 model; Olympus MicroSuite™-Basic software).
For tyrosine hydroxylase (TH) staining---The whole-mount mesenteric second order arteries (MA) were collected and incubated in 70 % acetone (in saline) for 3 hours. After thorough washing with saline, mesenteric arteries received the same staining treatment mentioned above with the exception that the primary antibody was mouse anti-TH monoclonal antibody (Calbiochem, 657010, 1:1000) and the secondary antibody was Rhodamine red (TM)-X donkey anti-mouse IgG (Jackson 715-295-151, 1: 100).
Western blot analysis
Western blot analysis was performed as described previously with minor modification (Huang & Wang 2001). Briefly, samples were homogenized in 10 mmol L−1 Tris buffer (pH 7.6) including 0.5 mmol L−1 MgCl2, 50 mmol L−1 NaCl, 10 mg ml−1 leupeptin, 10 mg ml−1 aprotinin, 1 mmol L−1 phenylmethylsulfonyl fluoride, and 1.8 mg ml−1 iodoacetamide. Homogenates were centrifuged at 500 g for 5 min at 4°C. The supernatant was added with EDTA and centrifuged at 50,000 g for 1h at 4°C. The pellet was resuspended with Lysis buffer containing 50 mmol L−1 Tris buffer (pH 7.6), 0.3 mol L−1 NaCl, 0.5% Triton X-100 and protease inhibitors, incubated on ice for 45 min, and centrifuged at 14,000 g for 20 min at 4°C. The supernatant was saved. After determination of protein concentration with a BCA assay kit (Sigma, QPBCA-1KT), sample were electrophoresed on a 10% SDS-PAGE gel for 4 hours at 100 V, semi-dry transferred to PVDF membranes (Bio-Rad) at 15 V. After being blocked with 5% milk in TBS-T buffer for 2 hours, membranes were incubated with rabbit anti-TRPV1 (Affinity BioReagents, PA1-29421, 1:1,000) or mouse anti-TH (Calbiochem, 657010, 1:1,000) at 4 °C overnight, followed by incubation with HRP-donkey anti-rabbit IgG (Jackson, 711-035-152, 1:10,000) or HRP-donkey anti-mouse IgG (Jackson, 711-035-151, 1:10,000) for 2 h at room temperature. The immunoreactive bands were visualized by ECL reagents (Amersham, RPN 2106) and a film (Kodak® X-Omat LS film). The film was scanned by HP Scanjet 8250. The intensity of the bands was determined by using ImageJ (NIH). The protein loading on membrane was normalized to β-actin (mouse anti-β-actin monoclonal antibody, Santa Cruz, sc-69879, 1:1,000).
Drugs
Capsaicin (Sigma, M1022) was dissolved in saline including 0.1 % ethanol for bolus i.v. injection. Prazosin (Sigma, P7791) was dissolved in saline.
Statistical analysis
All data are expressed as mean ± SE. Differences between two groups were analyzed by using the unpaired student’s t-test. Differences among groups were analyzed using one way ANOVA followed by a Bonferroni adjustment for multiple comparisons. Differences were considered statistically significant at P<0.05.
Results
Inthathecal injection of TRPV1 shRNA increased blood pressure
There was no difference in the body weight between rats given control and TRPV1 shRNA (Before treatment: control shRNA: 276±7 vs TRPV1 shRNA: 287±6, p>0.05; after treatment: control shRNA: 284±5 vs TRPV1 shRNA: 300±7 g, p>0.05), indicating that general growth or condition of rats was not affected by intrathecal treatment.
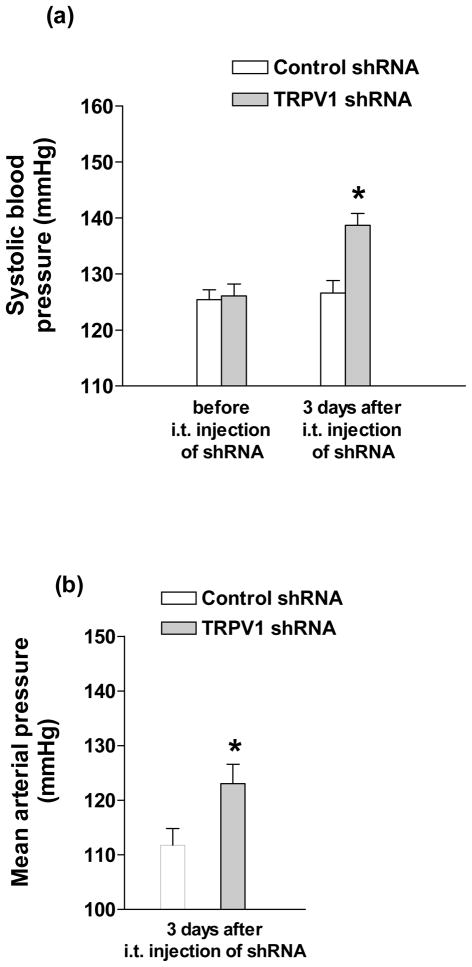
Both systolic blood pressure taken under conscious condition and mean arterial pressure (MAP) by direct measurement were assessed for determining the effect of TRPV1 shRNA intrathecal injection. Systolic blood pressure was elevated 3 days after intrathecal injection of TRPV1 shRNA (Fig. 1a) (control shRNA: 126±2 vs TRPV1 shRNA: 140±2 mmHg, p<0.05), which was confirmed by direct measurement of mean arterial pressure (Fig. 1b) (control shRNA: 112±2 vs TRPV1 shRNA: 123±2 mmHg, p<0.05).
Fig. 1.
Effect of intrathecal (i.t.) injection of TRPV1 shRNA on systolic blood pressure (a) and mean arterial pressure (b) in rats. Values are mean ± SE (n = 12 to 16 for (a); n = 8 to 9 for (b)). *P<0.05 compared with control shRNA-treated group.
TRPV1 was knocked down in the spinal cord, DRG, mesenteric arteries, and kidney after TRPV1 shRNA treatment
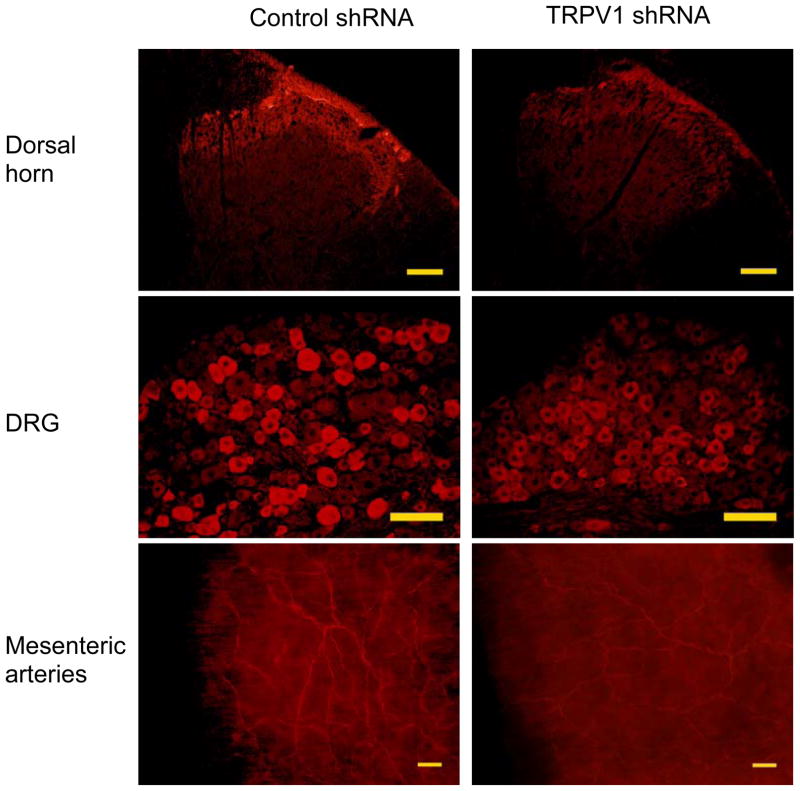
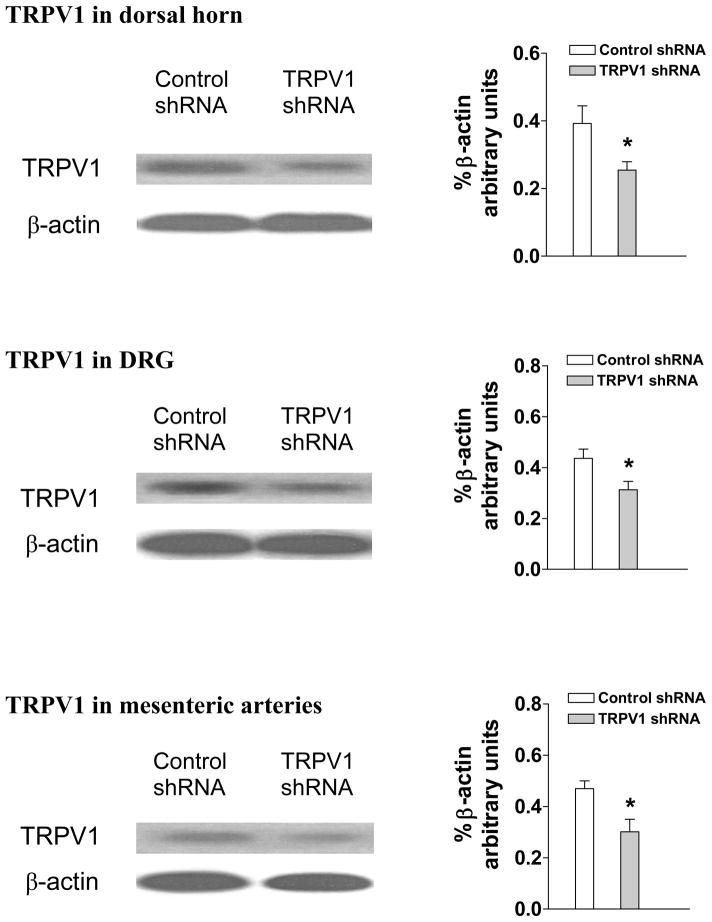
Immunohistochemistry studies revealed that TRPV1 staining in the dorsal horn of the spinal cord (T8-L3 segments), DRG (T8-L3 segments), and mesenteric arteries was attenuated in TRPV1 shRNA-treated rats compared to control shRNA-treated rats (Fig. 2). Negative controls in which TRPV1 antibody was omitted showed no staining (Data not shown). Consistently, quantitative measurement of TRPV1 content by Western blot analysis revealed that TRPV1 expression was knocked down by TRPV1 shRNA treatment in the dorsal horn (control shRNA: 0.38±0.03 vs TRPV1 shRNA: 0.25±0.01, p<0.05), DRG (control shRNA: 0.43±0.03 vs TRPV1 shRNA: 0.28±0.02, p<0.05), mesenteric arteries (control shRNA: 0.24±0.03 vs TRPV1 shRNA: 0.10±0.02, p<0.05) (Fig. 3), as well as in the kidney (control shRNA: 0.38±0.02 vs TRPV1 shRNA: 0.26±0.01, p<0.05). In contrast, there was no significant change in TRPV1 levels in the cervical spinal cord of rats after TRPV1 shRNA treatment (control shRNA: 0.37±0.02 vs TRPV1 shRNA: 0.35±0.03, p>0.05), confirming that shRNA was injected into the targeted segments of the spinal cord.
Fig. 2.
Immunohistochemistry staining showing TRPV1 expression in dorsal horn of spinal cord (T8-L3), dorsal root ganglia (DRG) and mesenteric arteries of rats 3 days after control or TRPV1 shRNA treatment. Scale bars, 100 μm.
Fig. 3.
Western blot analysis showing TRPV1 expression in dorsal horn of the spinal cord (T8-L3), dorsal root ganglia (DRG) and mesenteric arteries of rats 3 days after control or TRPV1 shRNA treatment. The left and right panels indicate representative Western blots and quantification results (% β-actin arbitrary units), respectively. Values are mean ± SE (n = 5). *P<0.05 compared with control shRNA-treated group.
MAP responses to capsaicin were attenuated after TRPV1 shRNA treatment
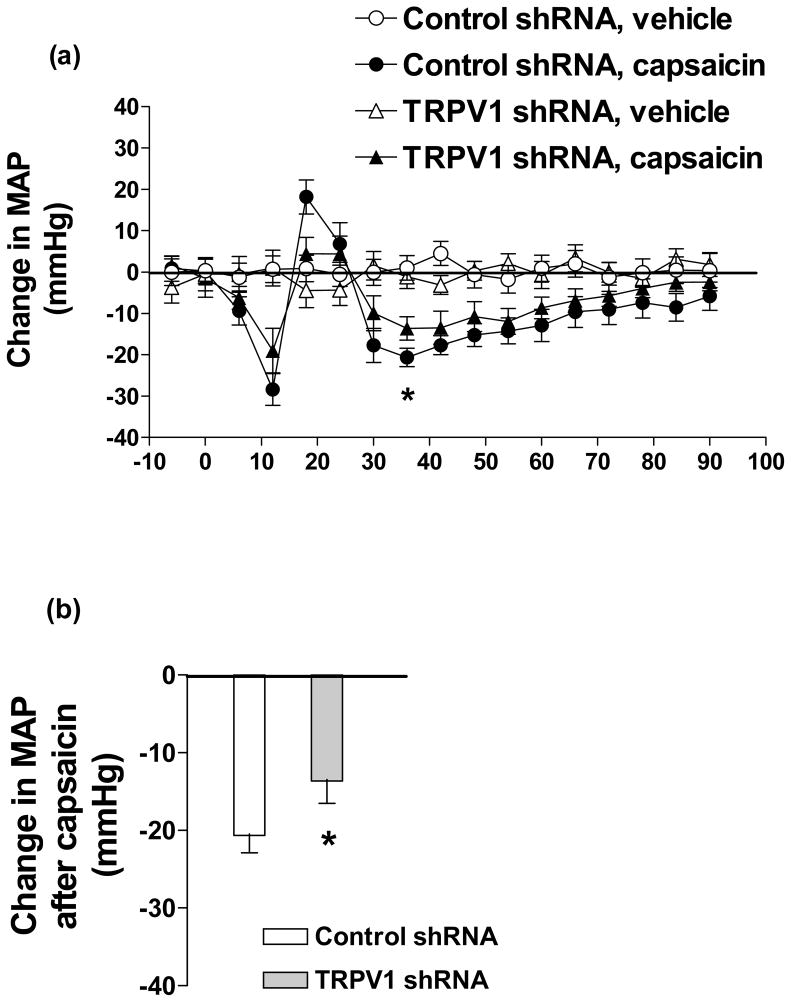
Bolus i.v. injection of capsaicin caused typical tri-phasic MAP responses in rats given control or TRPV1 shRNA. The magnitude of the decreases at the lowest points (36th sec after capsaicin) at the 3rd prolonged phase of the MAP response curve was smaller in rats given TRPV1 shRNA compared to rats given control shRNA (control shRNA: −21±2 vs TRPV1 shRNA: −14±3 mmHg, p<0.05) (Fig. 4).
Fig. 4.
Time course responses of mean arterial pressure (MAP) (a) and the response of MAP at 36th sec (b) to bolus injection of capsaicin (30 μg/kg, i.v.) in rats 3 days after control or TRPV1 shRNA treatment. Values are mean ± SE (n = 6 to 7). * P<0.05 compared with control shRNA-treated group.
Urinary excretion was not altered after TRPV1 shRNA treatment
To explore whether above-mentioned elevation of blood pressure induced by TRPV1 shRNA treatment was due to altered renal function, urine excretion, water intake, and the ratio of urine/water intake were examined. There were no significant differences between control shRNA and TRPV1 shRNA treated rats in 24-hour urine excretion (control shRNA: 29±1 vs TRPV1 shRNA: 27±2 ml, p>0.05), 24-hour water intake (control shRNA: 54±2 vs TRPV1 shRNA: 51±3, p>0.05), or the ratio of 24-hour urine/water intake (control shRNA: 0.54±0.02 vs TRPV1 shRNA: 0.53±0.02, p>0.05).
Blockade of α1-adrenoceptors prevented TRPV1 shRNA-induced elevation of blood pressure
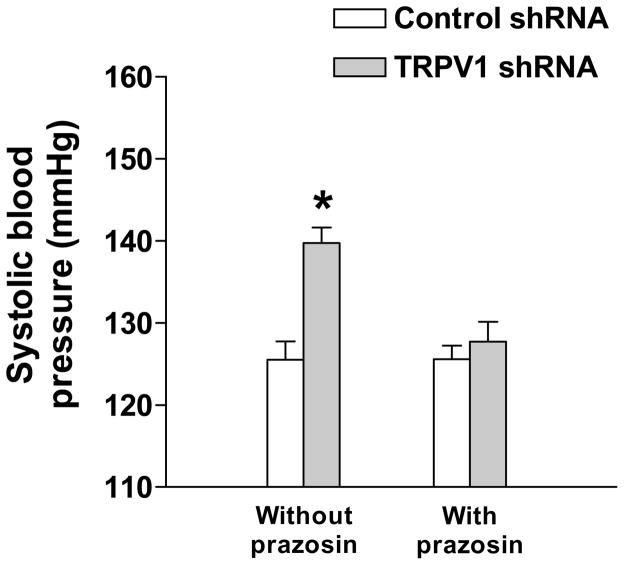
To assess whether enhanced activity of the sympathetic nervous system may contribute to TRPV1 shRNA induced pro-hypertensive effect, the effect of prazosin, an α1-adrenoceptor antagonist, was examined. Fig. 5 showed that blockade of α1-adrenoceptors by prazosin pretreatment prevented TRPV1 shRNA-induced elevation of systolic blood pressure (control shRNA: 126±2 vs TRPV1 shRNA: 128±2 mmHg, p>0.05).
Fig. 5.
Pretreatment with prazosin prevented TRPV1 shRNA-induced elevation of systolic blood pressure (measured by the indirect tail cuff method) in rats. Values are mean ± SE (n = 6 to 7). *P<0.05 compared with control shRNA-treated group.
Tyrosine hydroxylase (TH) was up-regulated in mesenteric arteries after TRPV1 shRNA treatment
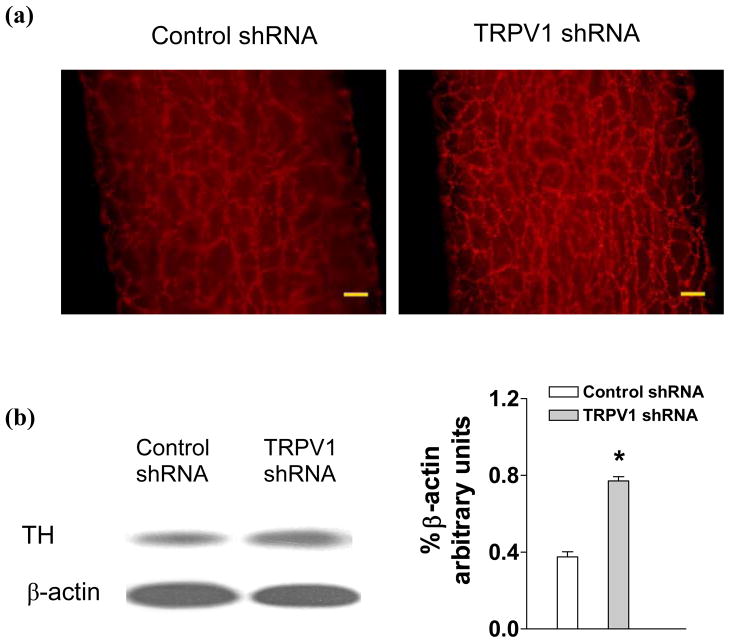
Immunohistochemistry staining revealed that TH expression in mesenteric arteries was enhanced in rats given TRPV1 shRNA compared to control shRNA treatment (Fig. 6a). Negative controls with omitting of TH antibody showed no staining (Data not shown). Quantitative measurement by Western blot analysis revealed that TH levels in mesenteric arteries were markedly enhanced in rats given TRPV1 shRNA compared to control shRNA treatment (Fig. 6b) (control shRNA: 0.33±0.03 vs TRPV1 shRNA: 0.56±0.06, p<0.05). In contrast, there were no significant differences in TH levels in the kidney of rats given TRPV1 shRNA and control shRNA treatment (control shRNA: 0.18±0.02 vs TRPV1 shRNA: 0.23±0.02, p>0.05).
Fig. 6.
Immunohistochemistry staining (a) and Western blot analysis (b) showing tyrosine hydroxylase (TH) expression in mesenteric arteries of rats 3 days after control or TRPV1 shRNA treatment. The left and right panels indicate representative Western blots and quantification results (% β-actin arbitrary units), respectively. Values are mean ± SE (n = 5). *P<0.05 compared with control shRNA-treated group. Scale bars, 100 μm.
Discussion
The goal of the present study was to define the role of TRPV1 expressed in DRGs (T8-L3) and their central and peripheral projections in the regulation of blood pressure by selectively knocking down the TRPV1 using TRPV1 shRNA that was intrathecally administered into the lower thoracic and upper lumbar segments of the spinal cord. We observed that (1) i.t. injection of TRPV1 shRNA suppressed TRPV1 expression in the T8-L3 spinal cord, DRG, mesenteric arteries, and kidneys; (2) suppression of neuronal TRPV1 by i.t. shRNA treatment elevated blood pressure; (3) blockade of the α1 adrenergic receptors with pretreatment of its antagonist abolished TRPV1 shRNA-induced elevation of blood pressure; and (4) TRPV1 shRNA treatment increased TH expression in mesenteric arteries but not in kidneys. These data show that gene silencing of neuronal TRPV1 in T8-L3 DRGs and their central and peripheral projections increases blood pressure, which can be prevented by suppression of the sympathetic nervous system.
One of the advantages of the technique, intrathecal injection, is to limit the action area of drugs to a short length of the dorsal horn of the spinal cord (Yaksh & Rudy 1977). In the present study, TRPV1 shRNA was administered to the lower thoracic and upper lumbar segments of the spinal cord. Indeed, immunohistochemistry results as well as data from Western blot analysis showed that TRPV1 was knocked down within the targeted segments of the spinal cord (Figs 2, 3), with other segments such as the cervical spinal cord unaffected. Thus, the selectivity of knockdown of TRPV1 expressed in targeted segments of the spinal cord justified the use of intrathecal injection of TRPV1 shRNA.
shRNA knocks down the expression of a specific gene by RNA interference (Rao et al. 2009). In the present study, the transfection efficacy of shRNA was further enhanced by the use of a transfection reagent, iFect, which has been particularly designed for facilitating effective transfection for neuronal cells (Luo et al. 2005). No side effects were observed in rats after i.t. injection of shRNA in the present study. Thus, combination of the powerful techniques of i.t injection with shRNA-mediated gene silencing provided us with a viable strategy of conditioned knockout with such characteristic as TRPV1 in only targeted area was knocked down without affecting TRPV1 in other regions or without affecting other components that co-localize with TRPV1 in the same neurons.
After being administrated to the region of the lower thoracic and upper lumbar spinal cord, TRPV1 shRNA would likely encounter the following fate. TRPV1 shRNA would probably be picked up by the central terminals of DRG, transmitted to the DRG soma, suppressed the production of TRPV1 there. As a result, TRPV1 expression not only in DRG but also in DRG central projections, i.e. the dorsal horn of the spinal cord, and DRG peripheral projections, i.e. mesenteric arteries and kidneys, was reduced (Figs. 2, 3). The suppression on TRPV1 production was further confirmed by the functional study in which MAP responses to i.v. injection of capsaicin, a selective TRPV1 agonist, were attenuated after TRPV1 shRNA treatment (Fig. 4). Parallel to decreased expression of TRPV1 in the spinal cord, DRG, mesenteric arteries, and the kidney, blood pressure increased significantly after i.t. injection of TRPV1 shRNA (Fig. 1). These results support the notion that silencing TRPV1 expressed in neurons housed in T8-L3 DRG and their central and peripheral projections conveys a prohypertensive effect.
While TRPV1 expression in the kidney was suppressed, there were no changes in 24-hour urine excretion, water intake, and the ratio of urine/water intake after TRPV1 shRNA treatment. While it was unexpected and the mechanism unknown, several possibilities exist. Partial knockdown of TRPV1 using TRPV1 shRNA or even TRPV1 ablation in the knockout mice under the physiologic condition (Wang 2008) might not be sufficient for causing measurable changes in renal function. Exacerbated impairment of renal function would appear when additional stressors are present with TRPV1 dysfunction (Wang 2008). Alternatively, in response to TRPV1 down regulation in the kidney, several pro-hypertensive and/or anti-hypertensive systems including the renin-angiotensin-aldosterone system, sympathetic nervous system, endothelin system, and nitric oxide system among others might be affected (Wang 2005, Mayer 2008). For example, increased renal sympathetic nerve activity has been shown to contribute to elevated blood pressure induced by afferent renal nerve denervation by dorsal rhizotomy (Kopp et al. 2008). In contrast, renal expression of tyrosine hydroxylase, a sympathetic nerve marker, was unaffected by TRPV1 shRNA treatment in the present study. These results suggest that the prohypertensive effect of TRPV1 shRNA might not depend on altered renal sympathetic nerve density/function leading to altered renal function.
Nonetheless, pretreatment with prazosin, an α1-adrenoceptor antagonist, abolished TRPV1 shRNA-induced prohypertensive effects (Fig. 5), suggesting that heightened sympathetic nerve activity in non-renal vascular beds might occur and contribute to TRPV1 shRNA-induced hypertension. Indeed, expression of tyrosine hydroxylase in mesenteric resistant arteries was markedly up-regulated by TRPV1 shRNA treatment (Fig. 6), indicating that knockdown of TRPV1 in T8-L3 DRG and their central and peripheral projections might trigger an organ-specific reflex via the central nervous system to enhance sympathetic nerve density/function in mesenteric resistant arteries to increase blood pressure. Although underlying mechanisms are unclear, similar organ-specific reflex has been observed in previous studies in which divergent activities of the sympathetic nervous system innervating various tissues were found in response to the same stimulation (Cabassi A et al. 2001, Watson AM et al. 2006).
Indeed, knockdown of TRPV1 expressed in T8-L3 DRG and their central and peripheral projections may cause a decrease in signal input from the afferent nerves to the lower brain stem neurons (Vizzard et al. 1992, Solano-Flores et al. 1997) that project sympatho-excitatory responses to sympathetic pre-ganglionic neurons in the spinal cord (Bard 1960, Barman & Gebber 1981), resulting in enhanced sympathetic nerve density/function peripherally and caused mesenteric vasoconstriction. Furthermore, knockdown of TRPV1 expressed in peripheral terminals in mesenteric arteries would lead to a decrease in the release of vasodilator neuropeptides including calcitonin-gene related peptide (CGRP) (Kawasaki et al. 1988). Indeed, upregulated mesenteric TRPV1 expression led to an increase in N-arachidonoyl-dopamine-induced CGRP release from mesenteric arteries (Wang & Wang 2007). On the other hand, CGRP-mediated vasodilatation in mesenteric arteries was impaired in TRPV1-null mutant mice (Wang et al. 2006).
In conclusion, the present study shows that gene silencing of TRPV1 expressed in T8-L3 DRG and their central and peripheral projections by intrathecal injection of TRPV1 shRNA increases blood pressure. The pressor effect of TRPV1 shRNA may be mediated partly by the enhancement of the sympatho-excitatory response. These results indicate that specific TRPV1-containing nerves play a tonic role in preventing the elevation of blood pressure, and thus modulation of neuronal TRPV1 function may convey preventive as well as therapeutic means for impediment and treatment of hypertension.
Acknowledgments
Source of Funding
This study was supported in part by NIH (grants HL-57853, HL-73287, and DK67620).
Footnotes
Conflict of interest
The authors report no conflicts of interests.
References
- Bard P. Anatomical organization of the central nervous system in relation to control of the heart and blood vessels. Physiol Rev Suppl. 1960;4:3–26. [PubMed] [Google Scholar]
- Barman SM, Gebber GL. Brain stem neuronal types with activity patterns related to sympathetic nerve discharge. Am J Physiol. 1981;240:R335–R347. doi: 10.1152/ajpregu.1981.240.5.R335. [DOI] [PubMed] [Google Scholar]
- Barthó L, Benkó R, Patacchini R, Pethö G, Holzer-Petsche U, Holzer P, Lázár Z, Undi S, Illényi L, Antal A, Horváth OP. Effects of capsaicin on visceral smooth muscle: a valuable tool for sensory neurotransmitter identification. Eur J Pharmacol. 2004;500:143–157. doi: 10.1016/j.ejphar.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Cabassi A, Vinci S, Quartieri F, Moschini L, Borghetti A. Norepinephrine reuptake is impaired in skeletal muscle of hypertensive rats in vivo. Hypertension. 2001;37:698–702. doi: 10.1161/01.hyp.37.2.698. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chevendra V, Weaver LC. Distribution of splenic, mesenteric and renal neurons in sympathetic ganglia in rats. J Auton Nerv Syst. 1991;33:47–53. doi: 10.1016/0165-1838(91)90017-w. [DOI] [PubMed] [Google Scholar]
- Ciriello J, Calaresu FR. Central projections of afferent renal fibers in the rat: an anterograde transport study of horseradish peroxidase. J Auton Nerv Syst. 1983;8:273–285. doi: 10.1016/0165-1838(83)90110-8. [DOI] [PubMed] [Google Scholar]
- Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- Donovan MK, Wyss JM, Winternitz SR. Localization of renal sensory neurons using the fluorescent dye technique. Brain Res. 1983;259:119–122. doi: 10.1016/0006-8993(83)91072-7. [DOI] [PubMed] [Google Scholar]
- Huang J, Chowhdury SI, Weiss ML. Distribution of sympathetic preganglionic neurons innervating the kidney in the rat: PRV transneuronal tracing and serial reconstruction. Auton Neurosci. 2002;95:57–70. doi: 10.1016/s1566-0702(01)00356-3. [DOI] [PubMed] [Google Scholar]
- Huang Y, Wang DH. Role of renin-angiotensin-aldosterone system in salt-sensitive hypertension induced by sensory denervation. Am J Physiol Heart Circ Physiol. 2001;281:H2143–H2149. doi: 10.1152/ajpheart.2001.281.5.H2143. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Takasaki K, Saito A, Goto K. Calcitonin gene-related peptide acts as a novel vasodilator neurotransmitter in mesenteric resistance vessels of the rat. Nature. 1988;335:164–167. doi: 10.1038/335164a0. [DOI] [PubMed] [Google Scholar]
- Kopp UC, Jones SY, DiBona GF. Afferent renal denervation impairs baroreflex control of efferent renal sympathetic nerve activity. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1882–R1890. doi: 10.1152/ajpregu.90529.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo MC, Zhang DQ, Ma SW, Huang YY, Shuster SJ, Porreca F, Lai J. An efficient intrathecal delivery of small interfering RNA to the spinal cord and peripheral neurons. Molecular Pain. 2005;1:29. doi: 10.1186/1744-8069-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer G. An update on the relationship between the kidney, salt and hypertension. Wien Med Wochenschr. 2008;158:365–369. doi: 10.1007/s10354-008-0559-2. [DOI] [PubMed] [Google Scholar]
- Neuhuber WL, Sandoz PA, Fryscak T. The central projections of primary afferent neurons of greater splanchnic and intercostal nerves in the rat. A horseradish peroxidase study. Anat Embryol (Berl) 1986;174:123–144. doi: 10.1007/BF00318344. [DOI] [PubMed] [Google Scholar]
- Pacher P, Bátkai S, Kunos G. Haemodynamic profile and responsiveness to anandamide of TRPV1 receptor knock-out mice. J Physiol. 2004;558:647–657. doi: 10.1113/jphysiol.2004.064824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao DD, Vorhies JS, Senzer N, Nemunaitis J. siRNA vs. shRNA: Similarities and differences. Adv Drug Deliv Rev. 2009;61:746–759. doi: 10.1016/j.addr.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Solano-Flores LP, Rosa-Arellano MP, Ciriello J. Fos induction in central structures after afferent renal stimulation. Brain Res. 1997;753:102–119. doi: 10.1016/s0006-8993(96)01497-7. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The Cloned Capsaicin Receptor Integrates Multiple Pain-Producing Stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Vizzard MA, Standish A, Ammons WS. Renal afferent input to ventrolateral medulla of the cat. Am J Physiol Regul Integr Comp Physiol. 1992;263:R412–R422. doi: 10.1152/ajpregu.1992.263.2.R412. [DOI] [PubMed] [Google Scholar]
- Wang DH, Li J. Antihypertensive mechanisms underlying a novel salt-sensitive hypertensive model induced by sensory denervation. Hypertension. 1999;33:499–503. doi: 10.1161/01.hyp.33.1.499. [DOI] [PubMed] [Google Scholar]
- Wang DH. The vanilloid receptor and hypertension. Acta Pharmacol Sin. 2005;26:286–294. doi: 10.1111/j.1745-7254.2005.00057.x. [DOI] [PubMed] [Google Scholar]
- Wang DH, Li J, Qiu J. Salt-sensitive hypertension induced by sensory denervation: introduction of a new model. Hypertension. 1998;32:649–653. doi: 10.1161/01.hyp.32.4.649. [DOI] [PubMed] [Google Scholar]
- Wang DH, Wu W, Lookingland KJ. Degeneration of capsaicin-sensitive sensory nerves leads to increased salt sensitivity through enhancement of sympathoexcitatory response. Hypertension. 2001;37:440–443. doi: 10.1161/01.hyp.37.2.440. [DOI] [PubMed] [Google Scholar]
- Wang LH, Luo M, Wang Y, Galligan JJ, Wang DH. Impaired vasodilation in response to perivascular nerve stimulation in mesenteric arteries of TRPV1-null mutant mice. J Hypertens. 2006;24:2399–2408. doi: 10.1097/01.hjh.0000251900.78051.56. [DOI] [PubMed] [Google Scholar]
- Wang Y, Babánková D, Huang J, Swain GM, Wang DH. Deletion of TRPV1 channels exaggerates renal damage in DOCA-salt hypertension. Hypertension. 2008;52:264–270. doi: 10.1161/HYPERTENSIONAHA.108.110197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang DH. Increased depressor response to N-arachidonoyl-dopamine during high salt intake: role of the TRPV1 receptor. J Hypertens. 2007;25:2426–2433. doi: 10.1097/HJH.0b013e3282efd1bf. [DOI] [PubMed] [Google Scholar]
- Ward SM, Bayguinov J, Won KJ, Grundy D, Berthoud HR. Distribution of the vanilloid receptor (VR1) in the gastrointestinal tract. J Comp Neurol. 2003;465:121–135. doi: 10.1002/cne.10801. [DOI] [PubMed] [Google Scholar]
- Watson AM, Hood SG, May CN. Mechanisms of sympathetic activation in heart failure. Clin Exp Pharmacol Physiol. 2006;33:1269–1274. doi: 10.1111/j.1440-1681.2006.04523.x. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. Studies on the direct spinal action of narcotics in the production of analgesia in the rat. J Pharmacol Exp Ther. 1997;202:411–428. [PubMed] [Google Scholar]
- Zahner MR, Li DP, Chen SR, Pan HL. Cardiac vanilloid receptor 1-expressing afferent nerves and their role in the cardiogenic sympathetic reflex in rats. J Physiol. 2003;551:515–523. doi: 10.1113/jphysiol.2003.048207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]