Abstract
Background
Altered cognitive processing following mood challenge is associated with elevated relapse risk in remitted unipolar depressed patients, but little is known about the neural basis of this reactivity and its link to depressive relapse and prophylaxis.
Methods
Remitted unipolar depressed participants (n = 16) and healthy controls (n = 16) underwent functional magnetic resonance imaging (fMRI) while viewing sad and neutral film clips. Correlations were determined between emotional reactivity (neural responses to sad vs. neutral films) in remitted patients and subsequent relapse status over an 18 month follow-up period. An ROC analysis was used to determine signal cutoffs for predicting relapse. Emotional reactivity in relapse prognostic areas was compared between groups.
Results
Within the remitted group, relapse was predicted by medial prefrontal cortical activity (MPFC, BA 32), and contraindicated by visual cortical activity (BA 17). MPFC reactivity predicted rumination, whereas visual cortical reactivity predicted distress tolerance (acceptance). Compared to control participants, remitted depressed patients who sustained remission demonstrated a more pronounced tradeoff between MPFC and visual cortex reactivity. The difference score between MPFC and visual reactivity yielded excellent prediction of depressive relapse.
Conclusions
Medial prefrontal cortical reactivity to mood provocation in remitted unipolar depressed patients serves as a marker of relapse risk rather than successful emotion regulation. Enduring remission is characterized by a normalization of the MPFC to the level of healthy controls. Further, visual cortex reactivity predicts resilience against depressive relapse, indicating a prophylactic role for sensory rather than ruminative cognitive reactivity in the processing of negative emotion.
Keywords: Depression, Imaging, Relapse, Prediction, Emotion, Reactivity
Introduction
Episode remission in recurrent, unipolar depression, while marked by reduced symptom burden, also features increased sensitivity to emotional stress and risk of relapse (1,2). Studies of psychological relapse vulnerability in this population indicate that recovered patients who endorse greater dysfunctional cognitions as mood worsens are at increased risk of relapse up to 18 months later (3,4), yet little is known about the neural regions underlying this reactivity. Extant research implicates two frontal cortical systems in acute unipolar depression and episode remission: first, the ventromedial prefrontal cortex (VMPFC) is hyperactive in depression and reduced reactivity in the VMPFC is associated with successful recovery; second, the dorsolateral prefrontal cortex (DLPFC) is hypoactive in depression, and increased DLPFC activity is associated with successful recovery (5-7). However, high DLPFC reactivity to cognitive or self-referential tasks relative to resting states is maladaptive, predicting lower rates of symptom remission (8). To date, studies have focused on contrasting currently depressed and control participants and their relation to recovery (7-10), suggesting that compromised frontal control of limbic cortex may lead to inefficient over-recruitment of frontal regions. Specifically, while healthy controls activate left-lateralized frontal regions in the regulation of negative emotion, depressed individuals demonstrate bilateral frontal activation, greater effort, and paradoxically increase ventromedial and limbic activity during affect regulation (10). However, this work has not been extended to examine the neural predictors of clinical relapse or sustained remission in recovered patients. Thus, the latent neural vulnerabilities that predispose one to relapse once remitted are unknown.
Reported herein is a prospective functional magnetic imaging (fMRI) analysis method for detecting relapse risk. Remitted unipolar depressed patients and a healthy control group both underwent blood oxygen level-dependent (BOLD) fMRI, during which they watched sad and neutral film clips. Despite the absence of depressive symptoms in the remitted group, we hypothesized that remitted unipolar depressed individuals would demonstrate latent prefrontal vulnerabilities relative to healthy controls, revealed by emotional challenge. To understand the neural vulnerabilities toward relapse, we further examined the association between patterns of neural reactivity and relapse prognosis over an interval of 18 months post-scanning. Improved knowledge of which recovered patients are at elevated risk of relapse could increase referrals to prevention programs and also decrease the considerable personal and social costs of recurrent depression.
Methods and Materials
Participants
Participants were right-handed adults ranging in age from 21 to 61. Sixteen participants fully remitted from unipolar depression (mean age = 44, s.d. = 16; 11 female) and 16 healthy controls (mean age = 39, s.d. = 13; 11 female) participated in the study (Table 1 contains further participant information). All remitted patients had a history of 3 or more past episodes of depression at the time of recruitment (mean # past episodes = 4.6, s.d. = 2.4). All remitted patients were taking prescribed antidepressant medication, without cognitive or behavioral therapy. Full details of patient criteria for study inclusion and assessment of relapse are included in the Supplement. Patients were recruited to the study from the Centre for Addiction and Mental Health in Toronto, Canada, while the control group was recruited from a community sample in the same region.
Table 1. Participant Demographic and Clinical Characteristics.
| Variable | Healthy Controls (n=16) | Remitted MDD (n=16) | P value between groups t-test |
|---|---|---|---|
| Age, mean ± SD | 39.3 ± 15.7 | 43.9 ± 13.3 | 0.370 |
| Female, % | 69 | 69 | 1.000 |
| White, % | 75 | 73 | 0.207 |
| Married/cohabitating, % | 56 | 53 | 0.887 |
| Employed, % | 69 | 47 | 0.711 |
| Education, years | 8.3 ± 1.7 | 7 ± 2.3 | 0.101 |
| HRSD score at study entry, mean ± SD | 0.4 ± 1.0 | 2.0 ± 2.3 | 0.012 |
| Acceptance (AAQR), mean ± SD | 48.1 ± 5.8 | 40.6 ± 9.1 | 0.041 |
| Rumination (RSQ), mean ± SD | 28.8 ± 5.4 | 35.9 ± 5.8 | 0.010 |
| % Relapsing | 62 | ||
| No. of prior episodes, mean ± SD | 4.6 ± 2.4 | ||
| Age of onset, mean ± SD, y | 42.6 ± 13.0 | ||
| Duration of current episode, mean ± D, wk | 61.8 ± 83.4 | ||
| History of prior antidepressant usage, % | 73 | ||
| History of psychiatric hospitalization, % | 7 | ||
| Any Axis I comorbidity, % | 38 | ||
| History of substance abuse or dependence, % | 13 | ||
| Any Axis II comorbidity, % | 19 |
Measures
We employed two behavioral measures to index adaptive and maladaptive cognitive modes in the face of emotional challenge: the Acceptance and Action Questionnaire (AAQ; 11) and the Response Style Questionnaire Rumination subscale (RSQ-R; 12), which measure tendencies to accept or worry about experiences, respectively. We also employed a behavioral measure of depressive severity (HRSD; 13) to provide a more sensitive index of long term depressive affect than the bivariate outcome of relapse status. Further descriptions of these measures are available in the Supplement.
Procedure
Patients were recruited for neuroimaging from a previously published study on depression relapse (14); a summary of the recruitment methods appears in the Supplement. Prior to scanning, all participants met with a licensed psychologist who determined MDD remission status based on SCID interview and HRSD score. At this time acceptance (AAQ) and rumination (RSQ-R) measures were also obtained. Following scanning, patients were contacted monthly by staff psychologists for clinical interviews to determine relapse status, as well as to administer the HRSD as a continuous measure of symptom severity (Figure S1 in the Supplement demonstrates the relationship between maximum HRSD scores and relapse status). Relapse occurred at any point during the 18 month monitoring period and once documented, these patients were re-treated with antidepressant medication within a 48 hour interval.
Sadness Provocation
Participants viewed four sets of film clips, with audio. Each set of clips came from a different source; neutral clips from television programs on gardening and woodworking, and sad clips from the films ‘The Champ’, and ‘Terms of Endearment’. Sets of clips were 3 minutes long and were edited into four 45 second clips. Clips were shown in their original order from each film with an interspersed 30 s reflection period between clips. At the end of each reflection period participants had 6 s to rate their level of sadness on a 5 point Likert scale. The experiment was conducted in two runs, with one set of neutral and sad films (composed of 4 clips each) presented in each run. Further details on the experimental design are available in the Supplement.
Imaging Data
Setup
Imaging data were collected with a Siemens Trio 3.0-Tesla scanner. The block design experiment was designed and implemented using the Visual Basic programming language (version Visual Studio 2005; Redmond, WA, USA; Microsoft). Prior to scanning, participants were provided with instruction and practice on the fMRI task. Additional information on imaging setup and data acquisition is available in the Supplement.
Structural imaging
For each participant, a 3D magnetization prepared rapid gradient echo pulse (MP-RAGE) sequence was employed to obtain a high-resolution T1-weighted structural volume.
Functional imaging
For each subject, a T2*-weighted gradient-echo echo-planar image (EPI) pulse sequence was prescribed and higher order shimmed for the functional trials.
Pre-processing
Functional activation was determined from the blood oxygenation level-dependent (BOLD) signal using the software Statistical Parametric Mapping (SPM8, University College London, UK; http://www.fil.ion.ucl.ac.uk/spm/software/spm8). Following image reconstruction (SPM8 DICOM import utility), the time series data for each participant were motion-corrected and co-registered with their T1-weighted structural image. The T1 image was bias-corrected and segmented using template (ICBM) tissue probability maps for gray/white matter and cerebrospinal fluid. Warping parameters were obtained from the tissue segmentation procedure and subsequently applied to the time-series data (resampling to 3 mm3 voxels). The time-series data were spatially smoothed to a 6 mm3 full-width half maximum Gaussian kernel. Lastly, a voxel level de-trending procedure was applied to remove time-series components correlated with global fluctuations in the BOLD signal (15).
First level statistical models
Single subject time series data were submitted to first-level general linear statistical models examining neural activity during the neutral and sad film viewing periods. Using the SPM8 design specification, the task-specific boxcar stimulus functions were convolved with the canonical hemodynamic response function (HRF) scaled to film clip duration. Each model included high-pass filtering to remove low-frequency signal drift (period = 128 s), and the AR1 method of estimating temporal autocorrelation. At this first level of analysis (within-subject), contrast images were calculated between sad and neutral film conditions; thus for each voxel, Emotional Reactivity = BOLDSad Film Viewing – BOLDNeutral Film Viewing.
Whole Brain Analysis
To test for group differences in emotional reactivity, participant emotional reactivity maps from the first level of analysis were subjected to a one-way ANOVA between the Control and Remitted groups. Voxels surviving a whole brain analysis using the False Discovery Rate (16) set at p < 0.05 were deemed statistically significant.
Neural Predictors of Relapse
To determine the neural correlates of relapse risk, we examined whether differences in emotional reactivity within the remitted group predicted relapse status during the follow-up period. We applied a linear regression model using the bivariate outcome variable of relapse status to identify brain voxels predictive of future relapse. Given the small number of participants for this regression and our interest in broad networks predicting relapse risk and prophylaxis, cluster rather than intensity thresholding was applied to correct for multiple comparisons. A minimum critical intensity t-threshold of T = 1.76 was applied with a False Discovery Rate corrected cluster threshold of p < .05 to identify these networks.
Specific Region of Interest (ROI) Analysis
To determine the most reliable specific neural predictors of relapse, functionally defined region-of-interest (ROI) clusters were identified based on a recent cross-run validation technique (17). Relapse status was regressed onto emotional reactivity separately in each of the 2 imaging runs, generating two independent parametric maps for relapse prediction. The conjunction of these maps was then calculated to identify the most reliable neural predictors of relapse. For each run, the minimum critical voxel peak threshold of T= 1.76 was retained, yielding a conjoint uncorrected probability of p < .005 for any voxels surviving the conjunction. A cluster extent threshold of 50 voxels was also applied to this conjunction data, based upon a Monte Carlo simulation predicting that this cluster threshold would approximate a P < .05 corrected threshold. The ROIs with the best cross-run prediction of relapse and sustained remission were retained for further analysis. Mean signal was extracted from a 3mm radius sphere centered on the peak voxels for relapse prediction from the conjunction analysis.
Explanatory Variable Analysis
To relate neural reactivity to patient symptom measures, emotional reactivity signal from both of the conjunction ROIs was tested for correlation with the AAQR and RSQ measures taken at the time of participant scanning. All correlations were computed as both zero-order (raw) and partial correlations, controlling for variability in the number of patient past depression episodes, antidepressant dosage levels, and patient subjective emotional reactivity (sadness ratings) made during the films task.
Anatomical Region of Interest (ROI) Analysis
To explore the robustness of relapse-predicting regions, ROIs were identified using the mean signal from the entire Brodmann area in which the most reliable peak voxels predicting relapse and sustained remission were found. Correlations between emotional reactivity (sad – neutral film clips) in these regions and the HRSD maximum scores were computed as a relatively unbiased prediction of future depressive symptoms.
Mediation Analyses
Mediation analyses were performed to determine the extent to which the prediction of relapse by brain activity could be equally well explained by correlated psychometric variables. These analyses effectively tested whether significant correlations between brain activity and future relapse status were rendered non-significant when examined as a partial correlation between brain and relapse status, controlling for the effects of psychometric variables. In addition to establishing brain-behavior correlations, this allowed an assessment of the added predictive value of fMRI biomarkers beyond that of psychometric variables. Mediation analyses were performed using AMOS 18 (Arbuckle JL, 2008). Maximum likelihood bootstrap estimation was used to determine the optimal model fit for the relationship between brain and behaviour predictors of relapse. This bootstrap method is similar to conventional regression techniques, but uses the observed data to generate a reference distribution rather than assuming characteristics of the data required for conventional regression analyses, leading to great accuracy (18).
Predictive Cutoff ROC analysis
To determine a practical cutoff score for the prediction of relapse on an individual level, an ROC analysis was performed using SPSS 17 (SPSS Inc., 2008), describing the relationship between sensitivity (% of relapsers detected) and specificity (% of non-relapsers rejected) as a function of specific ROI % signal change cutoff scores. The score which maximized both sensitivity and specificity was chosen as the cutoff score.
Results
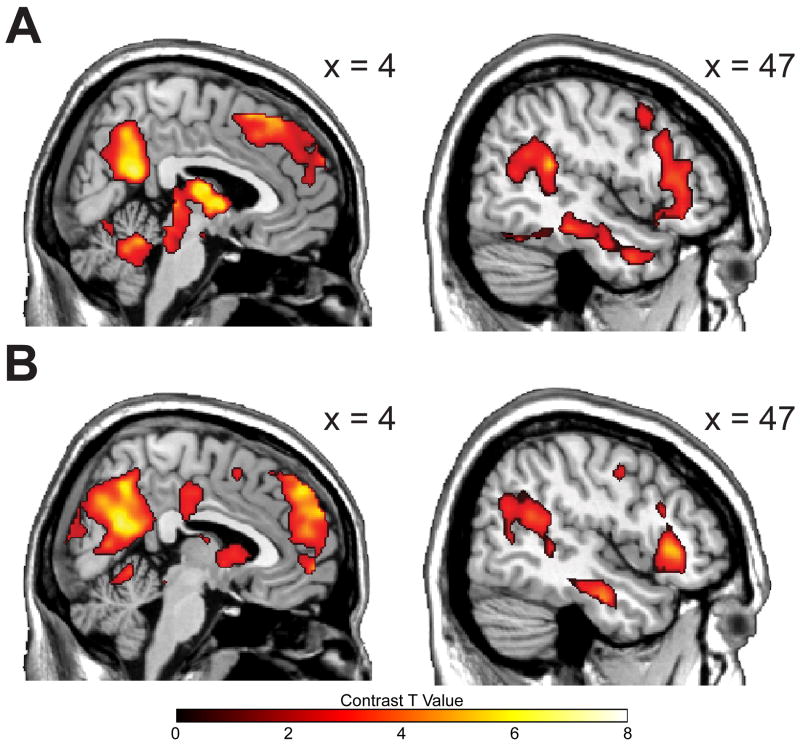
Patient and control groups did not differ on demographic indicators such as gender, age, employment and marital status, or ethnic distribution (Table 1). Patients demonstrated higher depression scores than controls at study intake (t(30) = 2.68, p < .05), although all patients were in the normal range (HRSD < 7). Emotional reactivity (ER) was operationalized through the comparison of sad and neutral film viewing periods. Sad films elicited greater reported sadness than neutral films in both Control (F(1,15) = 19.60, p < .001) and Remitted groups (F(1,15) = 72.36, p < .001), indicating successful emotional challenge. Sadness ratings did not interact with group (F(1,30) = 1.79, p = .191), suggesting equivalent dysphoric mood induction, consistent with the absence of depressive symptoms in both groups at the time of scanning. Mood ratings at the time of scanning did not predict subsequent relapse status (r(14) = -.007, n.s.). Across all groups, ER was associated with widespread lateral and cortical midline BOLD activation (Figure 1A & 1B; see also Table S1 in the Supplement), consistent with a prior study employing this paradigm (18). Refer to the Supplement for a comparison of the neural response associated with emotional reactivity between Control and Patient groups.
Figure 1.
Neural correlates of emotional reactivity (Sad – Neutral film clip viewing). Panel A: control group emotional reactivity in medial and lateral sagittal views. Panel B: a similar pattern is observed in the emotional reactivity for the MDD history group.
Relapse prediction
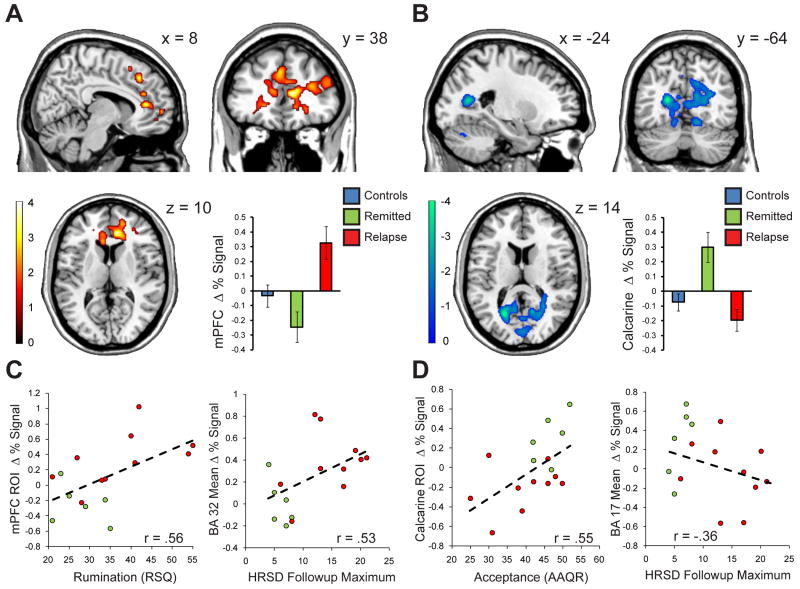
Ten of the sixteen remitted patients relapsed during the 18-month follow-up period. Within the MDD group, relapse was predicted by medial prefrontal cortical reactivity, and contraindicated by calcarine cortical reactivity (Figure 2A & 2B). To improve the reliability of this broad frontal / visual dissociation, we identified specific regions of interest (ROIs) consistently associated with relapse in both of the independent functional imaging runs. In the medial prefrontal region, the most reliable predictor was located on the border of the anterior cingulate and ventral medial prefrontal cortex (r(14)= .68, p < .005) (MPFC; BA 24/32, at peak height: x = 8; y = 38, z = 10), while in the visual cortices, the most reliable predictor was located in the left calcarine sulcus, which negatively predicted relapse, (r(14)= -.73, p < .001) (BA 37; at peak height: x = -24; y = -64; z = 14). The MPFC correlated with peak HRSD during 18 month follow-up, (r(14) = .51, p < .05), and the calcarine trended towards the negative prediction of peak HRSD (r(14)= -.43, p < .10). Relapse prediction also generalized to broad anatomical ROIs: an anatomically more diffuse and conservative follow up analysis revealed the entire MPFC anatomical ROI (BA 32) also predicted relapse status (r(14) = .58, p < .05) and the calcarine anatomical ROI (BA 17) trended towards the negative prediction of relapse (r(14) = -.44, p < .10). Similarly, the MPFC anatomical ROI (BA 32) correlated with peak HRSD scores (r(14) = .51, p < .05) and the calcarine anatomical ROI (BA 17) trended towards the negative prediction of follow-up HRSD scores (r(14) = -.36, p < .2) (Figure 2C & 2D).
Figure 2.
Neural correlates of depressive relapse in the MDD group. Panel A: prefrontal activation during emotional challenge is associated with depressive relapse over the following 18 months, reliably characterized by a region along the right anterior cingulate / MPFC border, whose signal change in each group is portrayed in the bar graph to the bottom right of the panel. Panel B: visual association cortices, as well as the dorsal cerebellum and precuneus activations are associated with sustained MDD remission over the follow-up period. These prophylactic regions are reliably characterized by a region along the left calcarine gyrus, whose signal change in each group is portrayed in the bar graph to the bottom right of the panel. Panel C: The specific (conjunction defined) MPFC relapse-predicting region is positively correlated with trait rumination scores (left); relapse prediction generalizes to an anatomical definition of the MPFC (BA32), whose reactivity predicts peak Hamilton rating scale for depression (HSRD) over the follow-up period (right). Panel D: The specific (conjunction defined) calcarine relapse-predicting region is positively correlated with trait rumination scores (left); relapse prediction generalizes to an anatomical definition of the calcarine (BA17), whose reactivity negatively predicts peak Hamilton rating scale for depression (HSRD) over the follow-up period (right).
Further analyses of the relapsed and sustained remission patients revealed that the two groups did not differ in depressive symptoms at time of scanning (t(14) = .396, n.s.), in number of prior episodes (t(14) = .567, n.s.), or in antidepressant dosage (t(14) = .567, n.s.). As such, the MPFC markers were not merely a reflection of residual depressive symptoms (HRSD scores at scan time), a different history of recurrent depression (number of past episodes), or different dosage levels of psychotropic medication. Further, entering these factors into the relapse prediction analysis only increased the predictive utility of the specific MPFC (partial r(11) = .791, p = .001) and visual cortical (partial r(11) = -.786, p = .001) regions.
Psychometric variables
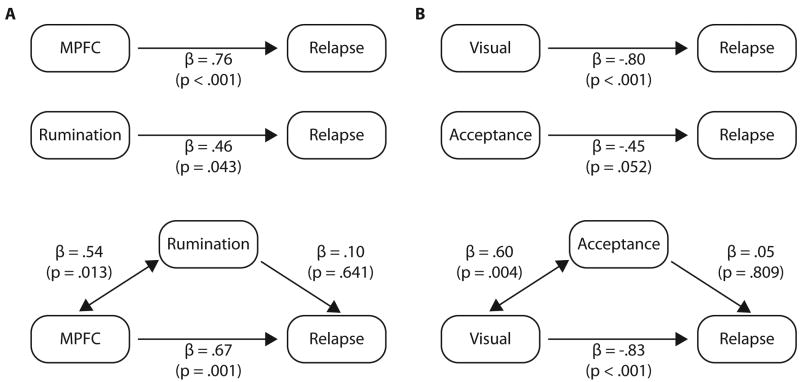
The specific MPFC ROI activity predicted RSQ rumination (r(14) = .56, p < .05), whereas calcarine ROI activity predicted AAQR acceptance (r(14) = .55, p < .05). An inverse correlation was observed between both the medial prefrontal and visual regions of interest, in terms of both specific ROIs (r(14) = -.55, p < .05), and anatomically defined ROIs (r(14) = -.50, p < .05), indicating that the patients with the greatest visual cortical reactivity demonstrated the least medial prefrontal reactivity and vice versa. Relapse was marginally predicted by rumination (r(14) = .46, p = .07) and acceptance r(14) = -.45, p = .08), but these factors did not account for MPFC (partial r(12) = .68, p = .008) or visual cortical (partial r(12) = -.71, p = .004) prediction. Mediation analyses confirmed that rumination and acceptance did not account for the relationship between brain activity and relapse (Figure 3); however, brain activity mediated the relationship between behavioural predictors and relapse. Finally, of all the demographic information, only education predicted protection from relapse (r(14) = -.64, p = .007), but despite this strong association, its mediation effects on brain activity prediction were non-significant, suggesting an independent source of prediction from these two brain regions.
Figure 3.
Mediation analysis of behaviour and brain predictors of relapse. Panel A: prediction of relapse by MPFC % brain signal change to sad – neutral films, and by rumination behavioral RSQ scores. The MPFC ROI mediates relapse prediction by rumination, but rumination does not mediate the MPFC prediction of relapse. Panel B: prediction of sustained remission by calcarine % brain signal change to sad – neutral films, and by acceptance behavioral AAQR scores. The calcarine ROI mediates sustained remission prediction by acceptance, but acceptance does not mediate the calcarine prediction of sustained remission.
Comparison with controls
MPFC and calcarine ROIs were entered into a one-way ANOVA with controls, remitted, and relapsing participants entered as separate groups. A significant main effect of group was found for both the MPFC (F(2,29) = 7.24, p = .003) and the calcarine regions (F(2,29)= 8.32, p = .001). Post-hoc tests for the MPFC revealed significant differences from controls in the relapse (p = .007) but not in the sustained remission groups (p = .164), consistent with the normalization of medial prefrontal reactivity in patients who sustain remission (Figure 2, left bar graph). Post hoc tests for the calcarine regions revealed greater reactivity in the sustained remission group compared to controls (p < .001), but not in the relapse group compared to controls (p = .223), consistent with a compensatory response in the remitted group (Figure 2, right bar graph).
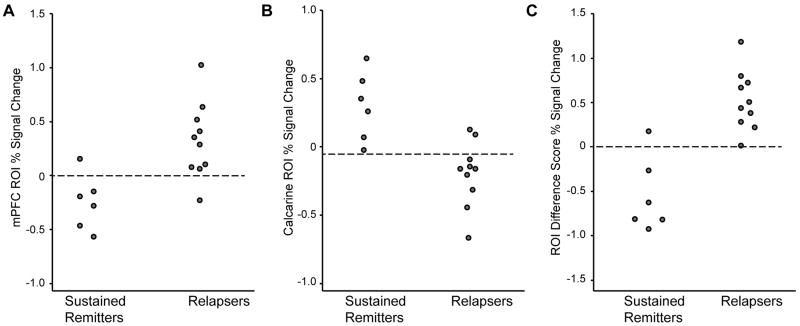
ROC analysis
An ROC analysis was performed to determine the accuracy of relapse prediction using the specific MPFC and calcarine ROIs % signal change during the emotional reactivity paradigm. For the MPFC ROI, a signal change cutoff of 0% or greater resulted in very good sensitivity (90%) and very good specificity (83%) for predicting relapse. For the calcarine ROI, a cutoff score of -0.1% signal change resulted in perfect sensitivity (100%) and good specificity (80%) for predicting sustained remission. Furthermore, using the difference score between the MPFC and calcarine ROIs improved prediction accuracy at a 0% signal change cutoff score, yielding perfect sensitivity (100%) and very good specificity (83%) for predicting relapse. ROC analyses performed using behavioral scores as predictors yielded poorer discrimination than the brain ROIs: the MPFC had a greater area under the ROC curve (AUC) than rumination (.90 versus .75), and the calcarine had a greater AUC than acceptance (.93 versus .75).
Dorsal vs. Ventral MPFC
Both dorsal and ventral aspects of MPFC reactivity predicted relapse, consistent with suggestions that, in depression, dorsal task-oriented processing becomes linked to ventral self-evaluative processing in the MPFC (19). While the optimal prefrontal predictor of relapse was found in the ventral MPFC region described above, a reliable predictor was also found in the left dorsal MPFC (BA 9/32, at peak height: x = -22; y = 34, z = 34). This region demonstrated similar associations with relapse status (r(14) = .67), peak HRSD (r(14) = .35), and rumination (r(14) = .52), and relapsing patients demonstrated heightened reactivity in this dorsal region relative to healthy controls (t(24) = 2.58, p < .05). Between subjects correlations revealed that while the dorsal MPFC demonstrated a positive association with ventral MPFC activity in relapsing patients (r(8) = .49), this correlation was absent in sustained remitters (r(4) = -.02) and these regions were inversely correlated in controls (r(14) = -.53).
Discussion
We employed sad mood provocation to conduct a prospective neuroimaging study of depressive relapse. Expansive medial prefrontal cortex (MPFC) reactivity predicted depressive relapse, encompassing both dorsal and ventral aspects of the MPFC (Figure 2, Panel A). The MPFC prediction of relapse was only partially associated with patient ruminative tendencies, but much of MPFC relapse prediction was not accounted for by rumination (Figure 3, Panel A). It may be that MPFC reactivity represents other maladaptive processes during film viewing, such as the inefficient recruitment of the MPFC in attempts to cognitively regulate negative emotion (10). The failure of MPFC reactivity to protect against relapse in recovered patients is consistent with current research suggesting that frontal connectivity to limbic structures appears to be compromised in depressed populations (9,10).
We also observed an intriguing association between visual cortical activity and relapse prophylaxis (Figure 2). In this case, visual activity was associated with trait acceptance, but just like the MPFC region, this association did not account for the visual area's predictive power (Figure 3, Panel B). The data suggest a distinction between a maladaptive and ruminative form of reactivity in the MPFC, and an adaptive and accepting form of reactivity in the visual cortices. While more research will be needed to determine exactly what the unexplained sources of variance in the MPFC and visual cortex represent, the present data suggests that an attitude of acceptance or observation, rather than interpretation and analysis may help to alter the pattern of cortical reactivity. Both MPFC and visual cortical reactivity contributed to the optimal prediction of relapse (Figure 4), suggesting that both patterns of reactivity are important in the assessment of relapse risk. Currently clinicians do not rely on neuroimaging data to determine a patient's need for maintenance treatment, but the fact that these patterns of neural activation achieved very high sensitivity and high specificity suggest that they would be useful in indexing vulnerability and treatment planning in otherwise non-symptomatic patients.
Figure 4.
Group discrimination between relapsing and sustained remission patients, using peak MPFC and calcarine ROI signal change scores, and the difference score between MPFC and calcarine % signal change. Panel A: The proposed cutoff score as calculated by ROC analysis is 0% signal change for the MPFC, yielding 90% sensitivity and 83% specificity. Panel B: for the calcarine ROI the proposed cutoff score is -.1% signal change, yielding 80% sensitivity and 83% specificity for predicting sustained remission. Panel C: Combining both measures by calculating a difference score between MPFC and calcarine ROI % signal change improves prediction accuracy; at the proposed difference cutoff score of 0% signal change, 100% sensitivity and 83% specificity in predicting relapse was observed.
Further analyses suggested that patients employed one of two opposing modes of reactivity to emotional challenge, as we observed a negative correlation between prefrontal and visual regions in the remitted patients. Such a tradeoff can be viewed as two different types of reactivity, allocating attention either to sensory (i.e. visual) or elaborative (i.e. prefrontal) cortical systems in reaction to an emotionally challenging stimulus. Other investigators have reported a similar tradeoff, in which higher visual or limbic but lower prefrontal reactivity was associated with greater recovery from depression (7, 20), suggesting a rebalancing of perceptual and evaluative networks (21-24), which may be indicative of competing self-empathetic versus other-empathetic responses in the context of viewing another's suffering (25). Easily triggered self-evaluative responses to negative emotion may in turn lead to a rehearsal of dysphoric states, thereby promoting maladaptive cognitive reactivity (23), whereas refraining from engaging these self-evaluative responses may free attention to focus upon less evaluative perceptual representations. What predisposes an individual to react with visual rather than prefrontal processing is still unknown, and will be a critical research question in the development of new preventative therapies.
The expansiveness of relapse-prognostic reactivity within the prefrontal cortices is itself notable, as activity between ventral and dorsal MPFC areas is generally negatively correlated in healthy populations, suggesting dissociated self (ventral) and task (dorsal) oriented processing (19). The co-activation of these regions in relapsing patients suggests that the inability to disengage from self-related processing during external film viewing may be a hallmark relapse risk, an idea supported by the positive coupling of dorsal and ventral MPFC regions in relapsing patients, rather than the more typical anti-correlation observed between these regions in controls. Specifically, recruitment of a ventral prefrontal network may belie maladaptive affective judgment (26-27), an idea supported by our finding of our association between the MPFC ROI and trait rumination. The tendency to react to negative events by engaging in affectively-laden interpretation seems to be a critical determinant of depressive relapse.
In summary, it appears that sustained remission from depression is characterized by a combination of: a) the normalization of MPFC reactivity to levels observed in healthy controls, and b) greater sensory reactivity in visual cortices relative to levels observed in controls. Thus, while sensory reactivity to sadness may be an indicator of depression history (28), it is an indicator of adaptive regulation rather than future pathology. The emergence of prophylactic sensory reactivity is consistent with the use of an alternative coping strategy that avoids relying upon the compromised neural connections between the frontal lobe and limbic system observed in chronic depression (9,10).
This study is limited in its ability to conclusively determine the neural predictors of depressive relapse due to small sample size and a limited number of functional runs for effective cross validation of our regions of interest. However, our findings, while in need of replication, suggest that prevention efforts designed to teach patients how to modify their habitual interpretive biases in reaction to stressors, through a sensory focus or other potential pathways, may enable recruitment of neural networks whose functionality is less impaired.
Supplementary Material
Acknowledgments
This work was generously supported by: NIMH Grant# MH066992 (Segal), CIHR Grant: #MT81164 (Anderson) and an Ontario Mental Health Foundation Postdoctoral Studentship (Farb). The authors would like to thank Assaf Kron for his suggestion to include sensitivity and specificity measures in our analyses, as well as four anonymous reviewers for their helpful comments.
Footnotes
Financial Disclosures: The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. American Journal of Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg PE, Kessler RC, Birnbaum HG, Leong SA, Lowe SW, Berglund PA, et al. The economic burden of depression in the United States: how did it change between 1990 and 2000? Journal of Clinical Psychiatry. 2003;64:1465–1475. doi: 10.4088/jcp.v64n1211. [DOI] [PubMed] [Google Scholar]
- 3.Segal ZV, Kennedy S, Gemar M, Hood K, Pedersen R, Buis T. Cognitive reactivity to sad mood provocation and the prediction of depressive relapse. Archives of General Psychiatry. 2006;63:750–755. doi: 10.1001/archpsyc.63.7.749. [DOI] [PubMed] [Google Scholar]
- 4.Antypa N, Van der Does AJ. Serotonin transporter gene, childhood emotional abuse and cognitive vulnerability to depression. Genes Brain Behav. 2010 May 3; doi: 10.1111/j.1601-183X.2010.00593.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koenigs M, Grafman J. The functional neuroanatomy of depression: Distinct roles for ventromedial and dosrolateral prefrontal cortex. Behavioural Brain Research. 2009;201:239–243. doi: 10.1016/j.bbr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gotlib IH, Hamilton JP. Neuroimaging and Depression: Current Status and Unresolved Issues. Current Directions in Psychological Science. 2008;17(2):159–163. [Google Scholar]
- 7.Siegle GJ, Carter CS, Thase ME. Use of fMRI to Predict Recovery From Unipolar Depression With Cognitive Behavior Therapy. American Journal of Psychiatry. 2006;163:735–738. doi: 10.1176/ajp.2006.163.4.735. [DOI] [PubMed] [Google Scholar]
- 8.Lemogne C, Mayberg H, Bergouignan L, Volle E, Delaveau P, Lehéricy S, Allilaire JF, Fossati P. Self-referential processing and the prefrontal cortex over the course of depression: a pilot study. Journal of Affective Disorders. 2010;124:196–201. doi: 10.1016/j.jad.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Heller AS, Johnstone T, Shackman AJ, Light SN, Peterson MJ, Kolden GG, Kalin NH, Davidson RJ. Reduced capacity to sustain positive emotion in major depression reflect diminished maintenance of fronto-striatal brain activation. Proceedings of the National Academy of Sciences. 2009;106(52):22445–22450. doi: 10.1073/pnas.0910651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: Counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayes SC, Strosahl KD, Wilson KG, Bissett RT, Pistorello J, Toarmino D, Polusny MA, Dykstra TA, Batten SV, Bergan J, Stewart SH, Zvolensky MJ, Eifert GH, Bond FW, Forsyth JP, Karekla M, McCurry SM. Measuring experiential avoidance: A preliminary test of a working model. The Psychological Record. 2004;54:553–578. [Google Scholar]
- 12.Nolen-Hoeksema S, Morrow J. A prospective study of depression and posttraumatic stress symptoms after a natural disaster: The 1989 Loma Prieta earthquake. Journal of Personality and Social Psychology. 1991;61:115–121. doi: 10.1037//0022-3514.61.1.115. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Segal ZV, Bieling P, Young T, MacQueen G, Cooke R, Martin L, Bloch R, Levitan RD. Antidepressant Monotherapy vs Sequential Pharmacotherapy and Mindfulness-Based Cognitive Therapy, or Placebo, for Relapse Prophylaxis in Recurrent Depression. Archives of General Psychiatry. 2010;67(12):1256–1264. doi: 10.1001/archgenpsychiatry.2010.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macey PM, Macey KE, Kumar R, Harper RM. A method for removal of global effects from fMRI time series. Neuroimage. 2004;22:360–366. doi: 10.1016/j.neuroimage.2003.12.042. [DOI] [PubMed] [Google Scholar]
- 16.Genovese CR, Lazar NA, Nichols T. Thresholding of Statistical Maps in Functional Neuroimaging Using the False Discovery Rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 17.Poldrack R, Mumford J. Independence in ROI analysis: where is the voodoo? Social, Cognitive and Affective Neuroscience. 2009;4:208–213. doi: 10.1093/scan/nsp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacKinnon DP, Fairchild AJ, Fritz MS. Mediation Analysis. Annual Review of Psychology. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pizzagalli DA. Frontocingulate Dysfunction in Depression: Toward Biomarkers of Treatment Response. Neuropsychopharmacology Reviews. 2011;36:183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keedwell PA, Drapier D, Surguladze S, Giampietro V, Brammer M, Phillips M. Subgenual cingulate and visual cortex responses to sad faces predict clinical outcome during antidepressant treatment for depression. Journal of Affective Disorders. 2010;120:120–125. doi: 10.1016/j.jad.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 21.Farb NAS, Anderson AK, Mayberg HS, Bean J, McKeon D, Segal ZV. Minding one's emotions: Mindfulness training alters the neural expression of sadness. Emotion. 2010;10(1):25–33. doi: 10.1037/a0017151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fresco D, Segal ZV, Buis T, Kennedy S. Relationship of Posttreatment Decentering and Cognitive Reactivity to Relapse in Major Depression. Journal of Consulting and Clinical Psychology. 2007;75(3):447–455. doi: 10.1037/0022-006X.75.3.447. [DOI] [PubMed] [Google Scholar]
- 23.Lau MA, Segal ZV, Williams JMG. Teasdale's differential activation hypothesis: implications for mechanisms of depressive relapse and suicidal behaviour. Behaviour Research and Therapy. 2004;42(9):1001–1017. doi: 10.1016/j.brat.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Creswell J, Way B, Eisenberger N, Lieberman M. Neural correlates of dispositional mindfulness during affect labeling. Psychosom Med. 2007;69:560–565. doi: 10.1097/PSY.0b013e3180f6171f. [DOI] [PubMed] [Google Scholar]
- 25.Jackson PL, Brunet E, Meltzoff AN, Decety J. Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia. 2006;44:752–761. doi: 10.1016/j.neuropsychologia.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 26.Ochsner KN, Hughes B, Robertson ER, Cooper JC, Gabrieli JDE. Neural Systems Supporting the Control of Affective and Cognitive Conflicts. Journal of Cognitive Neuroscience. 2009;9:1841–1854. doi: 10.1162/jocn.2009.21129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piech RM, Lewis J, Parkinson CH, Owen AR, Roberts AC, Downing PE, Parkinson JA. Neural correlates of affective influence on choice. Brain and Cognition. 2010;72:282–288. doi: 10.1016/j.bandc.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 28.Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, Williams SCR, Phillips ML. A Differential Pattern of Neural Response Toward Sad Versus Happy Facial Expressions in Major Depressive Disorder. Biological Psychiatry. 2005;57:201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.