Abstract
Objective
We hypothesized that fetal folate serum concentrations are lower and placental folate transport is impaired in pregnancies of obese women.
Study Design
Umbilical vein serum and placental tissue were collected from normal weight and obese pregnant women at term. Cellular localization (immunohistochemistry) of Folate Receptor-α (FR-α), Proton Coupled Folate Transporter (PCFT), and Reduced Folate Carrier (RFC) was established. Protein expression (western blot) and transporter activity (isotope labeled methyltetrahydrofolate) were determined in syncytiotrophoblast microvillous membranes (MVM).
Results
Fetal folate concentrations were similar in obese women as compared to normal weight women. Protein expression of FR-α in MVM was increased (+173%), RFC decreased (-41%), and PCFT unchanged. However, activity of FR-α, PCFT, and RFC was unaltered in obesity.
Conclusion
Fetal serum folate concentrations and placental folate transport activity are not altered in obesity at term, suggesting that limited availability of folate does not contribute to abnormal gene methylation and developmental programming.
Keywords: Methyl Donors, Micronutrient, Obesity, Pregnancy, Trophoblast
Introduction
Overweight (body mass index [BMI] 25.0-29.9) and obesity (BMI ≥ 30) are becoming increasingly common, now reaching epidemic proportions. In 1999-2004, the prevalence of overweight and obesity was 52 % in American women of reproductive age.1 Maternal obesity is associated with a variety of pregnancy complications, including an increased risk of birth defects.2 In particular, overweight/obesity in pregnancy increases the risk of having a child with a neural tube defect (NTD) 3, a risk that is not modified by folate supplementation 4 and cannot be explained by maternal diabetes.5 Furthermore, women with a high BMI are more likely to give birth to large-for-gestational age babies, 6 which have an increased risk to develop metabolic syndrome in childhood 7 as well as obesity, 8 diabetes 9 and cardiovascular disease, 10 in adulthood. Gene methylation and other forms of epigenetic regulation of key metabolic pathways at critical windows of intrauterine development have been implicated as a mechanism underlying developmental programming of metabolic and cardiovascular disease.11 Limited availability of methyl donors, such as folate, may result in abnormal gene methylation patterns and contribute to developmental programming.12
Folate is crucial to the one-carbon cycle by serving as a single carbon donor for 5-methyl-tetrahydrofolate (MTHF) and 10-formyl-THF. MTHF is used to convert homocysteine into methionine, which can then be used to methylate DNA. In addition, 10-formyl-THF is important for de novo purine synthesis. Because of its critical role in DNA synthesis, sufficient folate supply is particularly important during periods of rapid cell division and growth, such as pregnancy. Folate deficiency during pregnancy has been implicated in NTD; therefore, many developed countries have implemented mandatory folic acid fortification.
Cellular uptake of folate is mediated by specific transport mechanisms, including the Folate Receptor-α (FR-α), Proton Coupled Folate Transporter (PCFT), and Reduced Folate Carrier (RFC).13 FR-α transports folate via receptor-mediated endocytosis/exocytosis and functions at a neutral to mildly acidic pH. PCFT mediates the co-transport of folate and protons, has optimal activity at low pH and, accounts for the low pH folate transport activity in the intestine. RFC is an anionic exchanger, mediating the cellular uptake of folate in exchange for various anions such as organic phosphates. RFC has been proposed to be the major route of delivery of folate to systemic tissues at physiologic pH. FR-α, PCFT, RFC have all been shown to be expressed and active in the human placenta.14, 15, 16
The lack of effect of folate supplementation on the high incidence of NTD in obese pregnant women may be due to reduced placental folate transport resulting in fetal folate deficiency. Furthermore, limited folate availability in the fetus of obese women could contribute to developmental programming of metabolic and cardiovascular disease in these babies.17 However, fetal serum folate concentrations and placental folate transport have not been studied in association with maternal obesity. We tested the hypothesis that fetal folate serum concentrations are lower and placental folate transport is impaired in pregnancies complicated by obesity.
Materials and Methods
Participants
This study was approved by the Institutional Review Board at the University of Texas Health Science Center at San Antonio (approval number HSC20070723H). Women with uncomplicated singleton term (37-40 weeks gestation) pregnancies were recruited with written consent prior to undergoing scheduled cesarean deliveries, which were routinely scheduled at 39 0/7 weeks. These women had one or more prior cesarean delivery. They were either not a candidate for a trial of labor after a previous cesarean delivery or declined a trial of labor. Gestational age was estimated from the date of the last menstrual period and confirmed by ultrasound dating.
Pre-pregnancy weight was determined by obtaining a non-pregnant weight stated in the participant's medical record. If no such data were available in the medical record, we obtained the non-pregnant weight verbally from the participant which is not a standardized method. There were a total of two participants in the normal BMI group and four participants in the obese BMI group who did not have a documented non-pregnant weight stated in the medical record. Participants were grouped based on pre-pregnancy BMI into a normal (BMI 18.5-24.9) and an obese group (BMI ≥30).
Women who smoked cigarettes, used any street drugs, or consumed alcohol were excluded from the study. Additionally, gestational diabetes and any chronic medical illnesses were exclusion criteria. All of the women were of Hispanic ethnicity. According to their medical records, all study participants were taking daily prenatal vitamins containing 1 mg of folic acid at the time of delivery.
Sample collection
On the day of the scheduled cesarean delivery and once the participant was consented, maternal blood samples were collected. Following delivery, the cord was double clamped, the placenta was obtained, and blood was collected from the umbilical vein. Because obtaining an umbilical blood sample was unsuccessful in some study participants, the number of umbilical vein blood samples (normal BMI, n = 9; obese BMI, n = 8) were lower than the number of maternal samples (normal BMI, n = 15, obese BMI, n = 13). Serum was prepared and frozen at -80°C until analysis of folate concentrations. The concentrations were measured on an Immulite 1000 (Siemens Healthcare Diagnostics, Deerfield IL), according to the manufacturer's instructions. Intra- and inter-assay coefficients of variation were 6.7 and 7.9% at 700 pg/ml. Villous tissue was dissected free of decidua and fetal membranes. Samples of villous tissue were taken by random sampling and were either fixed for immunostaining or used for isolation of syncytiotrophoblast MVM.
Immunohistochemistry for Folate Transporters
Villous tissue samples were placed in formalin or zinc fixative for 18-24 hours, dehydrated, embedded in paraffin, cut into 4μm thick sections, and subsequently mounted on slides.18, 19 Sections were incubated overnight at +4°C with polyclonal antibodies toward FR-α (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), PCFT (Abcam, Cambridge, MA), or RFC (Alpha Diagnostic International, San Antonio, TX), at 1:250, 1:500, and 1:50 dilution respectively. For negative controls, primary antibodies were incubated in excess antigen peptide. Incubation with appropriate biotinylated secondary antibody was for 1h. Antibody binding was detected by application of avidin-peroxidase for 1 h, followed by incubation with chromogen diaminobenezidine plus nickel to produce a brown-black precipitate.
Preparation of Placental Homogenates and MVMs
Syncytiotrophoblast MVM were prepared as described previously using homogenization, Mg++ precipitation, and differential centrifugation.20, 21, 22 The final pellet was resuspended in an appropriate volume of buffer to give a final protein concentration of 5-10 mg/ml. Vesicles were aliquoted, snap frozen in liquid nitrogen, and stored at -80°C until use. MVM purity was determined as the enrichment of alkaline phosphatase activity compared to homogenates and was assessed using standard activity assays for alkaline phosphatase.23 Enrichment of alkaline phosphatase activity in MVM was 13.6 ± 2.4 fold in placentas from normal (n = 7) weight women, which was not significantly different from a 16.8 ± 3.2 fold enrichment in placentas obtained from obese (n = 7) women (p=0.4). Only MVM preparations with an alkaline phosphatase enrichment >10 were included in the study.
Western Blot Analysis of Folate Transporters
Homogenates and MVMs were diluted 1:1 with sample buffer to a final loading concentration of 35μg with a volume of 20μl, heated to 95°C for 5 minutes, and proteins separated using Biorad 12.5% Tris-HCL, 18 well precast gel. Proteins were transferred electrophoretically onto nitrocellulose membranes at 200 volts for 1.5 hours. The membranes were blocked in Tris-glycine buffered saline containing 0.05% Tween and 5% dry milk overnight at 4°C. After washing in Tris-glycine buffered saline, membranes were incubated in antibodies targeting FR-α (Enzo Life Sciences, Plymouth Meeting, PA) at 1:1000 dilution, PCFT (Abcam, Cambridge, MA) at 1:250 dilution, or RFC (Alpha Diagnostic International, San Antonio, TX) at 1:150 dilution overnight at 4°C. Membranes were incubated in secondary antibodies for 1 hour at room temperature at a dilution of 1:8000 (anti-mouse) for FR-α or at 1:10,000 (anti-rabbit) for PCFT and RFC. Bands were visualized by enhanced chemiluminescence according to the instructions of the manufacturer (Amersham Biosciences). The images were captured using a G:Box with GeneSnap software (Syngene, Frederick, MD).
Methyltetrahydrofolate (MTHF) Uptake by MVMs
The methods to determine MTHF uptake in the MVMs were based on published techniques for amino acid uptake in syncytiotrophoblast plasma membrane vesicles 24 and modified according to methods reported by Yasuda and coworkers.15 In brief, MVM vesicles were preloaded by incubation in 300 mM mannitol, 1 mM ADP and 10 mM Hepes-Tris, pH 7.4 overnight at +4°C. Subsequently, MVM vesicles were pelleted and resuspended in a small volume of the same buffer (final protein concentration ∼5-10 mg/ml). Membrane vesicles were kept on ice until immediately prior to transport measurements when samples were warmed to 37°C. At time zero, 30 μl of vesicles were rapidly mixed (1:3) with the appropriate incubation buffer containing 3H-MTHF (Moravek Biochemicals, Brea CA) to a final concentration of 50 nM. Binding of MTHF to FR-α and transport mediated by RFC were determined using incubation buffers adjusted to pH 7.4. In contrast, PCFT activity was assessed using incubation buffers adjusted to pH 6.0, providing the necessary inwardly directed proton gradient. In initial time course studies, uptake of radio labeled substrate was terminated by addition of 2 ml of ice cold phosphate buffered saline (PBS) after 5-30s. For subsequent uptake studies in MVM isolated from normal and obese placentas, 5s (for pH 7.4) and 20s (for pH 6.0) were used. After stopping the reaction, vesicles were rapidly separated from the substrate medium by filtration on mixed ester filters (0.45 μm pore size, Millipore Corporation, Bedford, MA) and washed with 3 × 2 ml of PBS. In all uptake experiments, each condition was studied in triplicate for each membrane vesicle preparation. Filters were dissolved in 2 ml liquid scintillation fluid, counted, and uptakes were expressed as pmol/mg protein. Non-mediated uptake/non-specific binding was determined in the presence of 1.5 mM unlabelled MTHF. In our uptake experiments all vesicles had 1 mM ADP on the inside, resulting in an outwardly directed gradient of ADP, which is the driving force for exchange with MTHF by RFC. Uptake mediated by RFC was therefore assessed by incubating vesicles in 1 mM ADP present both on the inside and the outside of the vesicle, thereby blocking the uptake mediated by RFC. Finally, the contribution of FR-α to specific binding/uptake was determined in vesicles incubated for 15 minutes in 0.2 units/ml of phosphatidylinositol-specific phospholipase C (PI-PLC), which inhibits the function of glycosylphosphatidylinositol (GPI)-linked cell surface proteins such as FR-α.25
Data Presentation and Statistical Analysis
Data are presented as means ± SEM. Statistical differences between groups were evaluated using the t-test. Statistical significance was set at p≤0.05.
Results
Clinical data
Selected clinical data for the study participants are provided in table 1. By design, BMI in the obese group was higher than in the control group with normal BMI. Furthermore, birth weights were higher in the obese group as compared to women with normal BMI. However, there were no statistical differences between BMI groups with regard to age, parity, gestational age, gestational weight gain, placental weight, or maternal delivery hematocrits.
Table 1. Characteristics of Study Subjects.
| Normal BMI (BMI ≤ 24.9) Mean ± SEM |
Obese (BMI ≥ 30) Mean ± SEM |
p valuesa | |
|---|---|---|---|
| n | 20 | 20 | |
| Age | 27.8 ± 1.8 | 29.8 ± 1.4 | 0.19 |
| Parity (Median/IQR) | 2/1 | 3/2 | |
| Gestational Age (weeks) | 38.9 ±0.1 | 38.8 ± 0.2 | 0.21 |
| Pre-pregnancy BMI | 21.9 ± 0.4 | 35.1 ± 0.8 | <0.01 |
| BMI Range | 18.5-24.8 | 30.6-43.7 | |
| Delivery BMI | 26.4 ± 0.6 | 39.1 ± 0.9 | <0.01 |
| Gestational Weight Gain (lbs) | 24.2 ± 2.3 | 22.0 ± 3.2 | 0.29 |
| Birth Weight (grams) | 3228.6 ± 90.8 | 3537.5 ± 103.6 | 0.02 |
| Placental Weight (grams) | 587.8 ± 38.4 | 646.0 ± 31.7 | 0.13 |
| Infant Gender (M/F) | 8/12 | 10/10 | |
| Maternal Hematocrit at Delivery | 35.3±0.6 | 35.2±0.5 | 0.49 |
BMI, Body Mass Index; SEM, (Standard Error of the Mean); M/F, (Male/Female); IQR, Interquartile Range.
p value for t-test.
Analysis of Type II Error
The Minimal Detectable Difference (MDD, two-tailed) was estimated for a number of key outcome variables using an α of 0.05, 1-β of 0.8 and measures of variability as given in the Result section. The MDD was found to be 34% for fetal serum folate concentrations, 33% for PCFT protein expression in MVM and 79% for pH 6.0 PCFT activity.
Maternal and Umbilical Venous Folate Concentrations
There were no significant differences in maternal (p = 0.27) or fetal (p = 0.15) serum folate concentrations between pregnancies of women with normal weight and obese women (figure 1). The maternal/fetal folate concentration ratios were not significantly different (p=0.22) when comparing the normal group to the obese group. As expected, fetal folate concentrations were significantly higher than maternal (p < 0.01), reflecting the active nature of placental folate transport.
Figure 1. Maternal and fetal serum folate concentrations.
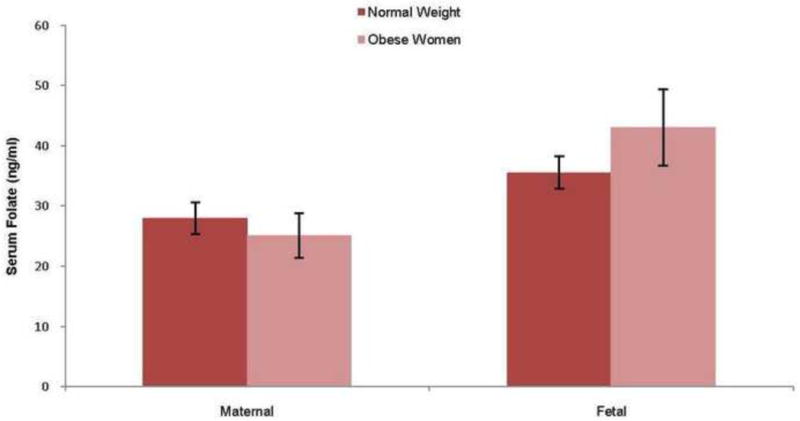
For maternal samples n=15 (Normal BMI) and n=13 (Obese) and for fetal samples n= 9 (Normal BMI) and n=8 (Obese). Data are presented as mean ± SEM, t-test.
Expression FR-α, RFC, and PCFT Using Immunohistochemistry
FR-α was predominantly localized in MVM, with little or no staining in the syncytitrophoblast basal plasma membrane (BM) or in other villous cells. Staining for FR-α in MVM was uneven in formalin fixed tissue. PCFT appeared to be highly expressed in the syncytiotrophoblast MVM, BM and cytoplasm as well as in endothelial cells, whereas PCFT staining was weak or absent in cytotrophoblast cells and in villous core connective tissue cells. Syncytiotrophoblast RFC staining was predominantly localized in MVM with weaker staining in cytoplasm and in BM. RFC staining was also present in villous core connective cells and in endothelial cells. The negative controls, using blocking peptides, showed no or negligible staining, indicating specificity of antibodies (figure 2).
Figure 2. Cellular localization of placental folate transporters using immunohistochemistry.
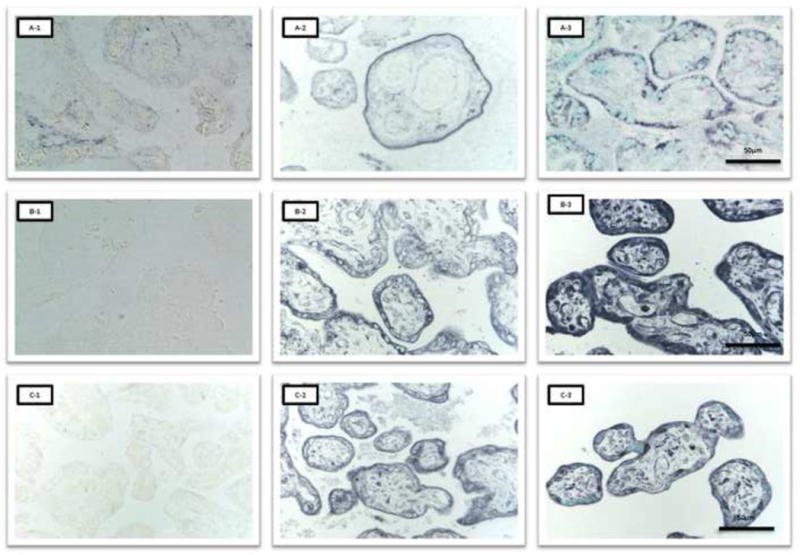
A-1, FR-α negative control; A-2, FR-α zinc fixative; A-3, FR-α formalin fixative, stained with methyl-green. B-1, PCFT negative control; B-2, PCFT zinc fixative; B-3, PCFT formalin fixative, stained with methyl-green. C-1, RFC negative control; C-2, RFC zinc fixative; C-3, RFC formalin fixative, stained with methyl-green.
Quantification of MVM Folate Transporter Protein Expression
Folate transporter expression was studied in both placental homogenates and MVM. This allows for evaluation of changes in total expression (homogenate) and cellular distribution (MVM). FR-α, RFC, and PCFT were all expressed in homogenates and isolated MVM (figure 3). MVM protein expression of FR-α was significantly increased (+173%) in placentas of obese women compared to normal weight women (p <0.01). MVM RFC protein expression was significantly decreased (-41%) in association to obesity (p = 0.03), whereas PCFT expression was unaltered (p = 0.06). There was no significant difference between groups in protein expression of FR-α (p= 0.39), PCFT (p = 0.09), or RFC (p = 1.0) in placental homogenates (figures 3 & 4).
Figure 3. MVM protein expression of folate transporters using Western blot.
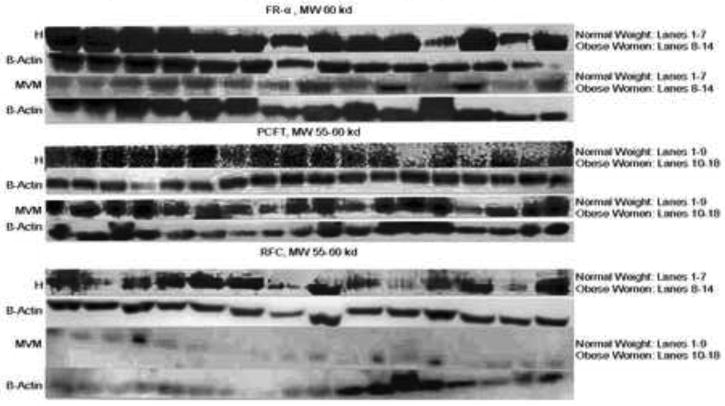
H, Homogenates; MVM, Microvillous Plasma Membranes, FR-α, Folate Receptor-α; PCFT, Proton Coupled Folate Carrier; RFC, Reduced Folate Carrier.
Figure 4. Quantification of Homogenates and MVM protein expression of folate transporters.

n=7-9. A, Homogenates. B, MVM. * p ≤ 0.05, t-test.
MTHF Binding by FR-α and MTHF Uptake by RFC and PCFT
Binding/uptake of MTHF increased over time both at pH 7.4 and 6.0 (figure 5). However, at pH 7.4, a plateau was reached at incubation times > 20s. At pH 6.0, there was no plateau noted after 30s. Based on this data, 5 seconds (pH 7.4) and 20 seconds (pH 6.0) were chosen for subsequent studies comparing MTHF binding/uptakes between the two BMI groups. At pH 7.4, specific binding of MTHF to FR-α (54 % of total specific uptake/binding) and mediated uptake by RFC (46% of total specific binding/uptake) were observed whereas no MTHF uptake mediated by PCFT could be demonstrated (figure 6). In contrast, at pH 6.0 PCFT was the only active folate transporter. Obesity was not associated with significant changes in MVM binding of methyltetrahydrofolate to FR-α (p = 0.45) or in the activity of the RFC (p = 0.42) and PCFT (p = 0.39) transporters (figure 6).
Figure 5. Time dependence of MTHF binding/uptake in MVM.
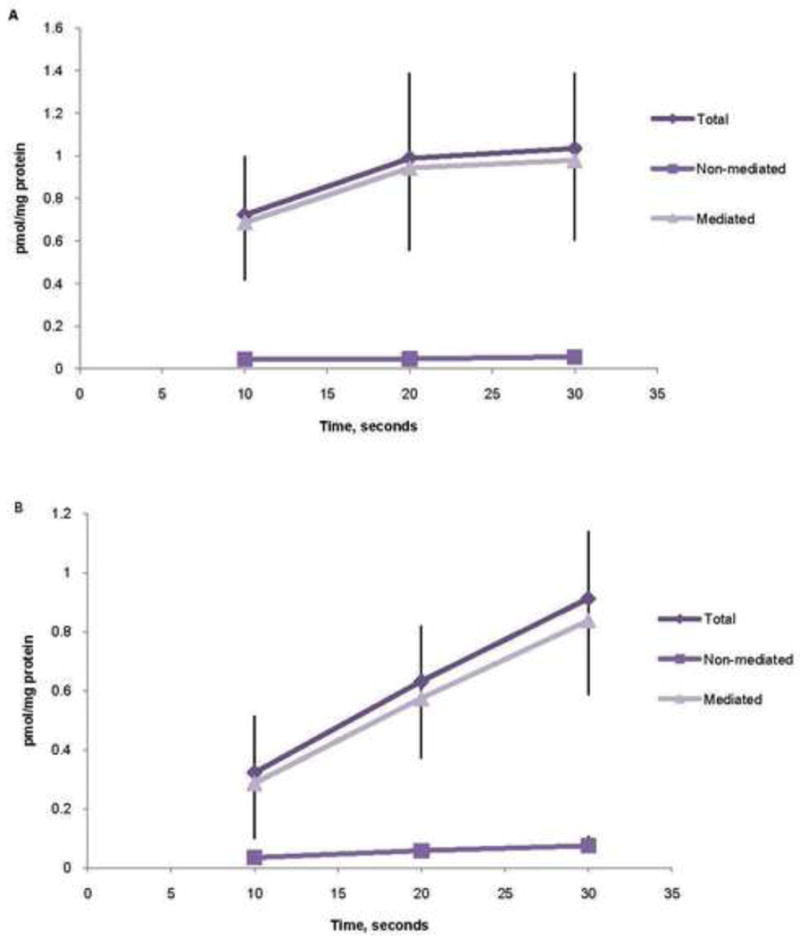
A, MTHF (methyltetrahydrofolate) uptake measured at a pH of 7.4. B, MTHF uptake measured at a pH of 6.0. Each point represents the mean of four determinations.
Figure 6. MVM MTHF binding/uptake.
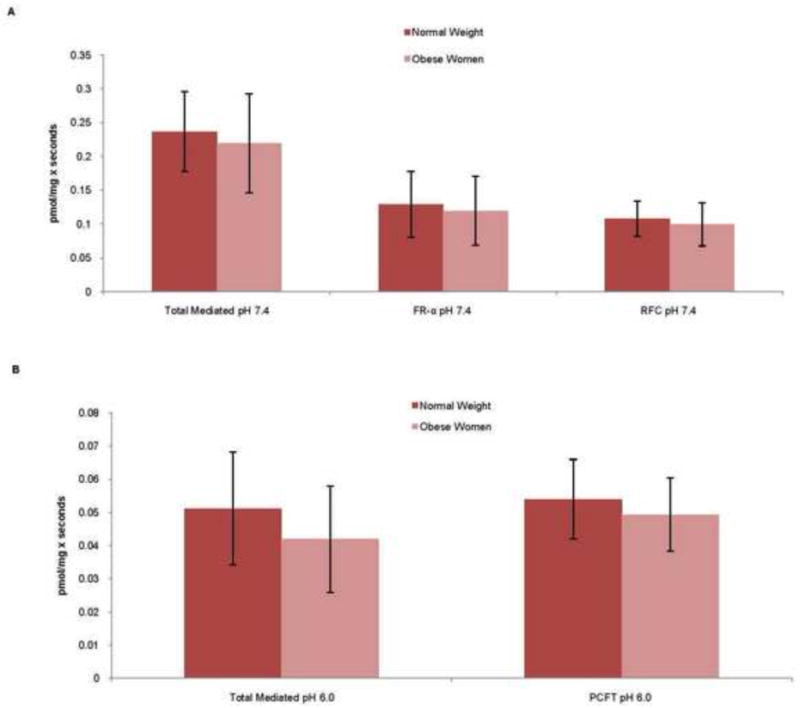
MTHF, Methyltetrahydrofolate; MVM, Microvillus Plasma Membranes; BMI, Body Mass Index. A, MTHF binding/uptake measured at a pH of 7.4. B, MTHF binding/uptake measured at a pH of 6.0. Data is presented as mean ± SEM, t-test.
Comment
In this study, we provide new information on specific folate binding and mediated folate transport across the human syncytiotrophoblast microvillous plasma membrane. Furthermore, this study reports maternal and fetal serum folate concentrations in obese women. Our data on folate transporter protein expression and function in obesity is novel. In contrast to our hypothesis, fetal serum folate concentrations and placental folate transport activity are not altered in cases of maternal obesity at term.
The three folate transporters were predominantly expressed in the MVM although PCFT in particular was also expressed in the syncytiotrophoblast cytoplasm and basal plasma membrane; these results are in general agreement with previous reports.26 RFC exchanges folate with anions such as inorganic phosphates, which are present in much higher concentrations inside the cell than extracellularly. RFC will, therefore, promote cellular uptake of folate. Approximately half of the binding/uptake of MTHF at pH 7.4 was mediated by RFC, suggesting that this transporter is a primary mechanism for placental uptake of folate from maternal blood. This is consistent with the view that RFC is the major route of delivery of folate to systemic tissues at physiologic pH. 13 FR-α expressed in the MVM will bind folate in maternal blood and facilitate trophoblast folate uptake via receptor-mediated endocytosis. The endocytotic pathway results in merging of endocytotic vesicles with lysosomes. Since folate binding to FR-α requires neutral pH, folate dissociates from FR-α due to the acidic pH in lysosomes; and, FR-α can recycle to the plasma membrane. Release of folate from the lysosomes to the cytoplasm requires an efficient transport mechanism, such as PCFT, which co-transport protons and folate out of the lysosme. Indeed, the primary function of PCFT in MVM may be to be incorporated in the endocytotic vesicle in order to mediate folate efflux from the lysosome. Thus, placental folate uptake from the maternal circulation is the result of coordinated action of FR-α, PCFT and FRC localized in MVM and conceivably changes in the expression and activity of one of these transporters in conditions such as obesity could affect transplacental folate transport.
We found that the amount of MTHF bound to FR-α was similar to the amount of MTHF uptake mediated by RFC at pH 7.4. The relative importance of FR-α and RFC for folate uptake across MVM in vivo cannot, however, be assessed from these data since the extent of binding of MTHF to FR-α that represents uptake across the plasma membrane is unknown. Determination of the relative contribution of these two uptake mechanisms will require the use of specific inhibitors or gene silencing approaches in intact villous tissue or possibly cultured primary trophoblast cells.
There are only two previous reports investigating MVM binding/uptake of folate by FR-α, RFC, and PCFT. 15, 16 In the study of Yasuda, et al, all three transporters appeared to be active at pH 6.0. At a pH 6.0, folic acid uptake into MVM was 10-fold higher than at pH 7.4. Similarly, in the report of Henriques & Trugo, maximum folate uptake was obtained at pH 6.0 with substantial contributions to total uptake by Fr-α. These findings are in contrast to the results of the present study in which total uptake of MTHF was much higher at pH 7.4 than at pH 6.0 and where PCFT was the only transporter active at pH 6.0. One likely explanation for these discrepant findings is that both previous studies used [3H]-folic acid as the tracer. This is non-physiologic since MTHF and not [3H]-folic acid represents the primary form of folate circulating in blood. 27 At pH 6.0, we found that only PCFT was active, which is consistent with the known pH sensitivity of the three transporters with RFC activity and FR-α binding being inhibited below physiologic pH. Although the main function of PCFT expressed in MVM may be related to release of folate from lysosomes, as discussed above, we cannot excluded the possibility that PCFT mediates MTHF uptake in the MVM in vivo. Wespeculate that proton efflux transporters such as the sodium-proton exchanger, which are highly expressed and active in MVM, 28 create microdomains of the microvillous plasma membrane where the pH on the outside of the membrane is significantly lower than 7.4. Therefore, if PCFT is co-localized with proton efflux transporters, the pH-gradient necessary for PCFT to mediate uptake of MTHF across the MVM may be present.
The protein expression of FR-α was increased and the expression of RFC was decreased in MVM isolated from placentas of obese women; however, the expression of the same transporters was unchanged in placental homogenates. This is consistent with the possibility that obesity affects the cellular distribution of folate transporters rather than total expression. We found a marked discrepancy between the substantial changes in MVM protein expression of FR-α and RFC, and the lack of difference in FR-α and RFC function in cases of maternal obesity. One possible explanation for this difference is that these two folate transporters are subjected to post translational modification.
Our study has several potential limitations. First, the findings of the study did not reject the null hypothesis of no differences in the main outcome variables between the two groups. Because of the relatively limited sample size the conclusions based on these findings are subject to type II error. However, the minimal differences that could be detected between the two groups in the current study were estimated to be <35% for many of the key outcome variables, including fetal folate concentrations and MVM transporter expression. This data provide some reassurance with respect to the validity of our conclusions. Second, our study design was dependent on being able to accurately group the participants into a normal BMI or obese BMI group based on the participant's pre-pregnancy BMI. A small subgroup (15%) of our participants had no documented non-pregnant weight in their medical record. These individuals were asked to provide their weight prior to becoming pregnant, which could introduce a recall bias since the values were self-reported. Since most individuals tend to underestimate their weight, the obese participants would have a higher BMI but remain in the obese group. The two normal BMI participants could have had an overweight BMI. However, due to the limited number of individuals who self reported their weight, the likelihood of recall bias significantly impacting our results is small. Lastly, the maternal and fetal folate concentrations in our study are in general higher than reported from in many studies from outside the US29, 30. This may be explained by the folate fortification of food in the USA, which remains uncommon in European and Asian countries. 29, 30
Our data does not support the possibility that the increased risk of fetal programming in obese women is due to limited fetal folate availability at term. The increased risk for fetal malformations in obese mothers is not clearly modifiable by folate supplementation, 4 which led us to postulate that placental folate transport is impaired in association to obesity leading to decreased fetal folate availability. Although the data presented in our report provided no support for this hypothesis, further studies are needed to explore folate metabolism in obese women. Structural malformations develop during the first trimester (the neural tube closes at 4 weeks gestation). Therefore, normal fetal serum folate concentrations in obese women at term do not preclude the possibility that fetal folate availability is limited earlier in pregnancy. These questions are difficult to address in pregnant women and may require studies in animal models that closely resemble the human condition. Moreover, we studied obese women with structurally normal infants, and the participants of the current study may not be representative for the subgroup of obese women who have infants with NTD. Further, folate deficiency many not be the cause of the increased risk of fetal malformations in association with obesity.
Acknowledgments
We would like to thank the patients, nurses, and staff on Labor & Delivery at University Hospital for their assistance with sample collection; Yvette Flahive for her assistance with the microvillous plasma membrane isolation; and Darren Farley, MD for the use of his placental samples which lead to preliminary findings for this study.
This work was supported by funds from the Humana Foundation Distinguished Chair of Obstetrics & Gynecology and Grant HD 21350.
Footnotes
This Study was conducted at the University of Texas Health Science Center at San Antonio and samples were collected from University Hospital in San Antonio, TX.
This information has not been previously presented.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the united states, 1999-2004. JAMA. 2006 Apr 5;295(13):1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Vasudevan C, Renfrew M, McGuire W. Fetal and perinatal consequences of maternal obesity. Arch Dis Child Fetal Neonatal Ed. doi: 10.1136/adc.2009.170928. Published online 2010 Jun 7. [DOI] [PubMed] [Google Scholar]
- 3.Werler MM, Louik C, Shapiro S, Mitchell AA. Pre-pregnant weight in relation to risk of neural tube defects. JAMA. 1996 Apr 10;275(14):1089–92. doi: 10.1001/jama.1996.03530380031027. [DOI] [PubMed] [Google Scholar]
- 4.Ray JG, Wyatt PR, Vermeulen MJ, Meier C, Cole DE. Greater maternal weight and the ongoing risk of neural tube defects after folic acid flour fortification. Obstet Gynecol. 2005 Feb;105(2):261–5. doi: 10.1097/01.AOG.0000151988.84346.3e. [DOI] [PubMed] [Google Scholar]
- 5.Shaw GM, Velie EM, Schaffer D. Risk of neural tube defect-affected pregnancies among obese women. JAMA. 1996 Apr 10;275(14):1093–6. doi: 10.1001/jama.1996.03530380035028. [DOI] [PubMed] [Google Scholar]
- 6.Salihu HM, Mbah AK, Alio AP, Kornosky JL, Bruder K, Belogolovkin V. Success of programming fetal growth phenotypes among obese women. Obstet Gynecol. 2009 Aug;114(2 Pt 1):333–9. doi: 10.1097/AOG.0b013e3181ae9a47. [DOI] [PubMed] [Google Scholar]
- 7.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: Association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005 Mar;115(3):e290–6. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 8.Eriksson J, Forsen T, Osmond C, Barker D. Obesity from cradle to grave. Int J Obes Relat Metab Disord. 2003 Jun;27(6):722–7. doi: 10.1038/sj.ijo.0802278. [DOI] [PubMed] [Google Scholar]
- 9.Eriksson JG, Forsen T, Tuomilehto J, Osmond C, Barker DJ. Early adiposity rebound in childhood and risk of type 2 diabetes in adult life. Diabetologia. 2003 Feb;46(2):190–4. doi: 10.1007/s00125-002-1012-5. [DOI] [PubMed] [Google Scholar]
- 10.Forsen T, Eriksson JG, Tuomilehto J, Teramo K, Osmond C, Barker DJ. Mother's weight in pregnancy and coronary heart disease in a cohort of Finnish men: Follow up study. BMJ. 1997 Oct 4;315(7112):837–40. doi: 10.1136/bmj.315.7112.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008 Jul 3;359(1):61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeisel SH. Importance of methyl donors during reproduction. Am J Clin Nutr. 2009 Feb;89(2):673S–7S. doi: 10.3945/ajcn.2008.26811D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao R, Matherly LH, Goldman ID. Membrane transporters and folate homeostasis: Intestinal absorption and transport into systemic compartments and tissues. Expert Rev Mol Med. 2009 Jan 28;11:e4. doi: 10.1017/S1462399409000969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiu A, Jansen M, Sakaris A, Min SH, Chattopadhyay S, Tsai E, et al. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell. 2006 Dec 1;127(5):917–28. doi: 10.1016/j.cell.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 15.Yasuda S, Hasui S, Yamamoto C, Yoshioka C, Kobayashi M, Itagaki S, et al. Placental folate transport during pregnancy. Biosci Biotechnol Biochem. 2008 Sep;72(9):2277–84. doi: 10.1271/bbb.80112. [DOI] [PubMed] [Google Scholar]
- 16.Henriques C, Trugo NM. Partial characterization of folate uptake in microvillous membrane vesicles isolated from human placenta. Braz J Med Biol Res. 1996 Dec;29(12):1583–91. [PubMed] [Google Scholar]
- 17.Van den Veyver IB. Genetic effects of methylation diets. Annu Rev Nutr. 2002;22:255–82. doi: 10.1146/annurev.nutr.22.010402.102932. Epub 2002 Jan 4. [DOI] [PubMed] [Google Scholar]
- 18.Beckstead JH. A simple technique for preservation of fixation-sensitive antigens in paraffin-embedded tissues. J Histochem Cytochem. 1994 Aug;42(8):1127–34. doi: 10.1177/42.8.8027531. [DOI] [PubMed] [Google Scholar]
- 19.Johansson M, Jansson T, Powell TL. Na(+)-K(+)-ATPase is distributed to microvillous and basal membrane of the syncytiotrophoblast in human placenta. Am J Physiol Regul Integr Comp Physiol. 2000 Jul;279(1):R287–94. doi: 10.1152/ajpregu.2000.279.1.R287. [DOI] [PubMed] [Google Scholar]
- 20.Illsley NP, Wang ZQ, Gray A, Sellers MC, Jacobs MM. Simultaneous preparation of paired, syncytial, microvillous and basal membranes from human placenta. Biochim Biophys Acta. 1990 Nov 16;1029(2):218–26. doi: 10.1016/0005-2736(90)90157-j. [DOI] [PubMed] [Google Scholar]
- 21.Johansson M, Glazier JD, Sibley CP, Jansson T, Powell TL. Activity and protein expression of the Na+/H+ exchanger is reduced in syncytiotrophoblast microvillous plasma membranes isolated from preterm intrauterine growth restriction pregnancies. J Clin Endocrinol Metab. 2002 Dec;87(12):5686–94. doi: 10.1210/jc.2002-020214. [DOI] [PubMed] [Google Scholar]
- 22.Magnusson AL, Waterman IJ, Wennergren M, Jansson T, Powell TL. Triglyceride hydrolase activities and expression of fatty acid binding proteins in the human placenta in pregnancies complicated by intrauterine growth restriction and diabetes. J Clin Endocrinol Metab. 2004 Sep;89(9):4607–14. doi: 10.1210/jc.2003-032234. [DOI] [PubMed] [Google Scholar]
- 23.Bowers GN, Jr, McComb RB. A continuous spectrophotometric method for measuring the activity of serum alkaline phosphatase. Clin Chem. 1966 Feb;12(2):70–89. [PubMed] [Google Scholar]
- 24.Jansson T, Ekstrand Y, Bjorn C, Wennergren M, Powell TL. Alterations in the activity of placental amino acid transporters in pregnancies complicated by diabetes. Diabetes. 2002 Jul;51(7):2214–9. doi: 10.2337/diabetes.51.7.2214. [DOI] [PubMed] [Google Scholar]
- 25.Luhrs CA, Slomiany BL. A human membrane-associated folate binding protein is anchored by a glycosyl-phosphatidylinositol tail. J Biol Chem. 1989 Dec 25;264(36):21446–9. [PubMed] [Google Scholar]
- 26.Solanky N, Requena Jimenez A, D'Souza SW, Sibley CP, Glazier JD. Expression of folate transporters in human placenta and implications for homocysteine metabolism. Placenta. 2010 Feb;31(2):134–43. doi: 10.1016/j.placenta.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 27.Pietrzik K, Bailey L, Shane B. Folic acid and L-5-methyltetrahydrofolate: Comparison of clinical pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2010 Aug 1;49(8):535–48. doi: 10.2165/11532990-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 28.Johansson M, Glazier JD, Sibley CP, Jansson T, Powell TL. Activity and protein expression of the Na+/H+ exchanger is reduced in syncytiotrophoblast microvillous plasma membranes isolated from preterm intrauterine growth restriction pregnancies. J Clin Endocrinol Metab. 2002 Dec;87(12):5686–94. doi: 10.1210/jc.2002-020214. [DOI] [PubMed] [Google Scholar]
- 29.Zhang T, Xin R, Gu X, Wang F, Pei L, et al. Maternal serum vitamin B12, folate and homocysteine and the risk of neural tube defects in the offspring in a high-risk area of China. Public Health Nutr. 2009 May;12(5):680–6. doi: 10.1017/S1368980008002735. [DOI] [PubMed] [Google Scholar]
- 30.Han Y, Ha H, Park H, Kim Y, Lee S. Relationships between pregnancy outcomes, biochemical markers and pre-pregnancy body mass index. International Journal of Obesity. 2010 August 31; doi: 10.1038/ijo.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]


