Abstract
Objective
We examined the effects of an advanced sleep/wake schedule and morning short wavelength (blue) light in 25 adults (mean age±SD = 21.8±3 years; 13 women) with late sleep schedules and subclinical features of delayed sleep phase syndrome (DSPD).
Methods
After a baseline week, participants kept individualized, fixed, advanced 7.5-hour sleep schedules for 6 days. Participants were randomly assigned to groups to receive “blue” (470 nm, ~225 lux, n=12) or “dim” (< 1 lux, n=13) light for one hour after waking each day. Head-worn “Daysimeters” measured light exposure; actigraphs and sleep diaries confirmed schedule compliance. Salivary dim light melatonin onset (DLMO), self-reported sleep, and mood were examined with 2×2 ANOVA.
Results
After 6 days, both groups showed significant circadian phase advances, but morning blue-light was not associated with larger phase shifts than dim-light exposure. The average DLMO advances (mean±SD) were 1.5±1.1 hours in the dim light group and 1.4±0.7 hours in the blue light group.
Conclusions
Adherence to a fixed advanced sleep/wake schedule resulted in significant circadian phase shifts in young adults with subclinical DSPD with or without morning blue light exposure. Light/dark exposures associated with fixed early sleep schedules are sufficient to advance circadian phase in young adults.
Keywords: blue light, human, circadian rhythms, phase shift, sleep, delayed sleep phase syndrome
1. Introduction
Delayed sleep phase disorder (DSPD) is a circadian rhythm sleep disorder characterized by sleep onset and offset times that are delayed relative to conventional sleep patterns, yet with normal sleep architecture and quality (1). To meet criteria for a diagnosis of DPSD, patients must report sleep onset insomnia when they attempt to fall asleep at conventional clock times, extreme difficulty waking in the morning, and daytime sleepiness when forced to rise for school or work without adequate sleep duration and at an inappropriate circadian phase. Earlier editions of the International Classification of Sleep Disorders (2) also specified severity criteria in which patients with an average sleep onset latency of 2 hours and minimal impairment in social/occupational functioning were deemed “mild,” patients with an average sleep onset latency of 3 hours and moderate social/occupational impairment were considered “moderate,” and those with an average sleep latency of 4 hours and significant functional impairment were considered “severe.” On the other hand, certain sleep patterns may represent subclinical forms of DSPD. The subclinical findings are found, for instance, in young adults who experience significant delays in sleep times and circadian phase on weekends, and then have difficulty falling asleep on Sunday nights and waking on Monday mornings (3).
DSPD has an estimated prevalence of about 7% in adolescents and young adults (4, 5) and has been associated with poor school performance and behavioral problems (6), as well as mood dysregulation (7). Many young people have sleep patterns consistent with mild DSPD and exhibit subclinical symptoms without meeting full criteria for the disorder. For instance, a survey of 211 college students (mean age 23 years) performed by Lack found that 16.6% of students met criteria for a mild form of DSPD, including prolonged sleep latency on weeknights (average sleep latency of 42.7 minutes, compared to 17.7 minutes in students without DSPD symptoms), shorter total sleep time on weeknights (average of 43 fewer minutes per night), complaints of insufficient sleep on weeknights, delayed bedtimes and longer sleep times on weekends, and no difficulty maintaining sleep (8). Furthermore, students who exhibited these sleep patterns also had lower grades than students without DSPD symptoms. Thus, misalignment of sleep patterns and school or work schedules is common in young adults and can be associated with mood and performance impairment.
Morning bright light therapy and prescribed sleep-wake schedules are both recommended treatments to produce circadian phase advances and alleviate symptoms of DSPD, though the level of evidence for the clinical efficacy of light therapy (Level 1 and 2 trials) is stronger than the level of evidence for prescribed sleep schedules and chronotherapy (case reports and consensus) (9). Light boxes with conventional broad-spectrum fluorescent lamps are used for bright light treatment (10), yet “white” lights may not be the most effective sources for treatment of circadian phase misalignment because they generate optical radiation that extends beyond the range of wavelengths to which the circadian system is maximally sensitive. Since higher corneal irradiances of “white” light may be required for these sources to be effective, patient compliance may be compromised.
Recent studies comparing different light spectra indicate that nocturnal melatonin suppression is preferentially sensitive to short-wavelength “blue” light (11–13), i.e., showing suppression at relatively low photopic light levels compared to other wavelengths. Multiple photopigments in the retina contribute to the spectral sensitivity of nocturnal melatonin suppression (14), including the intrinsically photosensitive retinal ganglion cells (ipRGC; (15)), yielding a modeled net peak spectral sensitivity at or near 450 nm (16). The available data on circadian phase shifting also show maximum sensitivity to short wavelengths in studies of circadian phase advance (17–20) and circadian phase delay (13, 21, 22). Scheduled blue-light exposures for phase advancing circadian rhythms have not been studied in field settings or in persons with phase misalignment.
The present study examined the effects of a circadian phase advancing sleep schedule together with a morning blue-light intervention or a morning dim-light condition in young adults whose sleep schedules were delayed with respect to school and work commitments, representing a model of subclinical DSPD. Light protocols were imposed in the context of fixed, phase-advanced sleep-wake schedules for one week following an unrestricted baseline monitoring week. We used a novel eye-level light monitor that detects spectrally-weighted radiation, the Daysimeter (Lighting Research Center, Rensselaer Polytechnic Institute, Troy, NY) (23), to ensure compliance to the light intervention and to quantify personal light exposures while participants were awake. We hypothesized that (1) all participants would have a circadian phase advance (as measured by their salivary dim light melatonin onset [DLMO]) due to the earlier sleep-wake (and therefore, light-dark) schedule, and (2) participants who received the morning blue-light intervention would have a larger phase advance than participants in the dim-light condition. We measured stress and mood using self-report questionnaires to investigate psychological effects of the interventions.
2. Methods
2.1 Participants
Participants were recruited through flyers, brochures, radio announcements, and newspaper advertisements targeting “struggling night owls” ages 18–30 years. Interested individuals completed a telephone screening questionnaire. We included participants who reported general good health, were not taking medications known to affect sleep or circadian rhythms, had proficiency in written and spoken English, reported consuming less than 360 mg of caffeine and fewer than 10 cigarettes per day, and had an average reported total sleep time of ≤ 9 hours per night (so that the intervention would not result in sleep deprivation). We excluded those who reported a personal history of a diagnosed sleep disorder, bipolar disorder, psychosis, seizure disorder, or chronic medical condition (e.g., diabetes, asthma, cancer), and those who reported a family history of diagnosed psychosis or bipolar disorder. We excluded participants who reported using recreational drugs and most prescription drugs. Reported medications and over-the-counter supplements among studied participants were multivitamin (n=10), oral contraceptive (n=4), Nuva Ring contraceptive (n=2), calcium supplement (n=2), Vitamin C (n=2), magnesium (n=1), doxycycline for acne (n=1), isoniazid for positive tuberculosis screen (n=1), and ranitidine as needed for gastroesophageal reflux (n=1). Participants were studied in May, October, and November, 2008, January–May, 2009, and October 2009. Participants were not studied during the week after time shifts related to daylight savings time. Assignment of light group was distributed comparably across the study months. Night shift workers and anyone who had traveled beyond two time zones in the month prior to the study were excluded. Female participants were studied at all phases of the menstrual cycle.
We chose to test the phase-advancing effects of blue light in young adults who had misalignment between their actual sleep schedules and daily activities. This situation is common among young adults (6, 8) and may be a useful model of subsyndromal delayed sleep phase, as these individuals meet some criteria for DSPD. Thus, the definitive inclusion criterion was the presence of a misalignment between the participant’s usual sleep-wake times and his or her routine schedule, prospectively defined as the presence of an obligatory morning commitment requiring the participant to wake 1 to 2.5 hours earlier than average reported wake time at least one day per week. For example, if a potential participant reported her average wake time as 10:30 AM, she was included only if she reported a scheduled work or school commitment that required her to be awake between 8:00 and 9:30 am at least 1 day per week (with preparation and travel time factored into the schedule). The early commitment consisted of classes for 64% of participants, work shifts for 20%, and a combination of school and work for 16%. Participants reported a mean (±SD) of 3.0+1.3 “early” days per week.
We chose the specific misalignment range of 1 to 2.5 hours because (1) we anticipated phase shifts of this magnitude could be achieved after 6 days of treatment; (2) we wanted to minimize the possibility of presenting the light stimulus during the phase delay portion of the light phase response curve before the cross-over point to the phase advance portion, which might have been possible for participants if we required very large phase advances; and (3) we wanted to avoid significant sleep deprivation consequent to sleep-onset insomnia more likely if participants had potential shifts greater than 2.5 hours. Unlike for the diagnosis of DSPD, we did not require participants to have significant impairment in their social and/or occupational functioning as a result of their misaligned schedules. The schedule misalignment formed the basis for the imposed phase advance in the participant’s sleep-wake schedule during the intervention week (see below).
2.2 Study Overview
As the sample protocol in Figure 1 illustrates, participants completed one baseline nonscheduled week and one week on a phase-advanced sleep-wake schedule. Participants underwent a laboratory overnight session at the end of each week for saliva collection to measure circadian phase markers. After the informed consent process on day 1, participants began wearing a wrist actigraph to measure activity/rest patterns and a Daysimeter to measure light exposure and activity/rest patterns. They completed the Horne-Östberg morningness-eveningness questionnaire (24) as a measure of circadian phase preference. The study was approved by the Institutional Review Boards at Rhode Island Hospital and at Rensselaer Polytechnic Institute. Data collection and most data analyses took place at the EP Bradley Hospital Sleep and Chronobiology Laboratory. Daysimeter data were analyzed at Rensselaer Polytechnic Institute. Participants were paid for their participation.
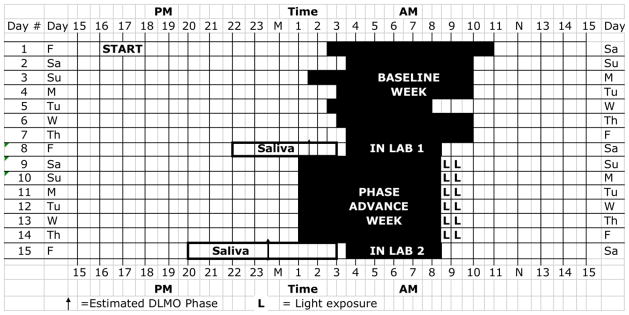
Figure 1. Sample Protocol Diagram.
Participants were on a self-selected schedule during the baseline week. Black bars indicate sleep time during the baseline week and scheduled time in bed during the phase advance week. Note the early wake time on day #5, which illustrates the type of schedule misalignment required for participation in the study.
2.3 Home Monitoring
The Daysimeter (23) is a head-set device worn over the ear that incorporates two calibrated, spectrally-weighted irradiance sensors, one that measures photopic illuminance (lux) and the other that records a specific distribution of short wavelengths. The data from both sensors can be used to calculate “circadian light” (i.e., spectrally weighted corneal irradiance or circadian illuminance, in units of CLA) based upon the spectral sensitivity of human nocturnal melatonin suppression model by Rea and colleagues (16, 25). For this study, we calculated average log CLA and average log lux for the first 90 minutes after waking and across the entire day while the Daysimeter was worn for both the baseline week and the 6 days on the advanced sleep-wake schedule. The Daysimeter also can measure an “activity index” based upon the combined outputs from a pair of orthogonally oriented solid-state accelerometers (26), and a solid-state thermometer in the Daysimeter can be used for sensor calibration and to assess compliance with wearing the device. Participants were instructed to wear the Daysimeter during waking hours throughout the two-week study except during bathing, vigorous exercise, and sleep. While sleeping, they were instructed to place the Daysimeter in a safe place in the room where they slept, exposing the sensor to ambient light. Participants were asked to avoid wearing sunglasses or brimmed hats during the study, so that the Daysimeter data would more accurately reflect corneal light exposures. Participants completed log books to report times the Daysimeter was worn.
The Octagonal Basic actigraph (Ambulatory Monitoring, Inc., Ardsley, NY) is the size of a large wristwatch and records activity counts using a mass loaded piezoelectric element. Actigraphy data were collected in 1-minute bins in zero crossing mode with a filter setting of 18. Participants wore the actigraph on the nondominant wrist continuously throughout the study, except during bathing. Participants completed a daily sleep diary and phoned the laboratory’s time-stamped voicemail at bedtime and wake time daily. The actigraphy data were downloaded, printed, and compared to the sleep diary and voicemail information at the end of each monitoring week to ensure compliance to the protocol. Diary data were averaged over the 7 days of the baseline “usual schedule” and 6 days of advanced, scheduled sleep to summarize sleep behavior.
2.4 Baseline Sleep Schedule
Participants were instructed to keep their usual reported schedule during the first week of home monitoring, bedtimes and rise times were not fixed, and participants were given no instructions with regard to their exposures to light or dark. For both study weeks, participants were instructed to avoid napping and staying awake all night and were asked to sleep in their own beds.
2.5 In-Laboratory Sessions
Participants came for an overnight laboratory session for saliva collection to assess circadian phase at the end of each home-monitoring week. Laboratory sessions were scheduled based on participants’ predicted baseline dim light melatonin onset (DLMO) time calculated using their habitual sleep schedules with the algorithm of Burgess and Eastman (27). Up to 5 participants in each session sat in a common windowless room and played games, listened to music, watched movies, or worked on their computers. They were aware of clock time during the sessions. Illuminance levels at the cornea were ≤ 40 lux from incandescent lamps; television and computer screens were covered with an amber filter that blocked short wavelengths (Roscolux Filter, #21 Golden Amber, Rosco, Stamford, CT). Snacks were provided ad lib., except during the 5 minutes prior to collection of saliva samples.
Participants used salivettes (Sarstedt, Nümbrecht, Germany) to give a 1 ml saliva sample every 30 minutes from 3.5 hours before the predicted baseline DLMO to 2 hours after predicted baseline DLMO. Saliva samples were frozen at −20° C and were later radioimmunoassayed for melatonin (Alpco, Salem, NH). All samples from an individual participant were assayed with the same kit. The radioimmunoassay has a sensitivity of 0.2 pg/ml, an intra-assay variation of 2.6–20.1%, and an inter-assay variation of 6.6–16.7%. We defined the salivary DLMO threshold as 4 pg/ml, based on the original threshold in plasma of 10 pg/ml (28, 29). DLMO phase was computed by linear interpolation between the times of the saliva samples before and after the participant’s melatonin levels reached 4 pg/ml (30). Phase shifts between the 2 in-lab sessions were determined by subtracting the session 2 DLMO phase from the session 1 DLMO phase. Thus, a positive value indicates a phase advance. Phase angles were calculated by subtracting time of salivary DLMO phase from average diary bedtime for the baseline and intervention weeks.
Participants slept in the laboratory in darkened bedrooms on a shortened sleep schedule during the overnight lab sessions. Bedtime was scheduled to occur immediately following the saliva sample collected 2 hours after predicted DLMO phase, which required staying awake beyond the participant’s usual bedtime. Wake time was scheduled based on each individual participant’s morning schedule requirement. Thus, participants were awakened at the prescribed time that would allow them to sit with a light box for 1 hour every morning before attending to their earliest commitment of the week (factoring in preparation and travel time). Scheduled wake times were 1 to 2.5 hours earlier than each participant’s average wake time during the baseline week, thus representing a phase advance in the sleep schedule. Every participant was scheduled to sleep 7.5 hours during the intervention week. In addition to accommodating saliva collection procedures, a practical rationale for curtailing scheduled sleep time on the laboratory night was that the relative sleep reduction would produce an increased homeostatic drive for sleep and facilitate sleep onset when participants implemented their new earlier bedtime.
2.6 Phase Advanced Schedule Intervention
After the first in-laboratory session, participants continued to wear the Daysimeter and actigraph for the next 6 days and nights at home during which they were required to follow their individualized fixed, advanced sleep-wake schedule that included 7.5 h of time in bed per night. Timing of sleep was dictated by advanced wake up time as described above. Participants were required to be in bed, in the dark, and to try to sleep during their scheduled sleep times. We confirmed sleep schedule compliance with wrist actigraphy, time-stamped phone calls, and sleep diaries. In order to decrease short-wavelength light exposure in the evening and at night during the phase-advanced schedule, participants were provided amber filters (Roscolux #21, Rosco, Stamford, CT) to cover their home computer and television screens when operating these devices after sunset.
2.7 Light Intervention
Participants were provided an Apollo P2 GoLite light box (Phillips Respironics, Amsterdam, The Netherlands) and instructed to sit with it for one hour within 15 minutes after awakening on the six mornings of the advanced sleep-wake schedule. The GoLite P2 is a 6 × 6 inch (15 × 15 cm) device containing an array of 66 blue light emitting diodes (LEDs) with a peak wavelength (λmax) at approximately 470 nm (full width at half maximum, FWHM ≈ 20 nm). We randomly assigned participants to two groups, a “blue-light” group (n=12) that received a high level (~225 lux at the cornea) of the 470 nm light from the GoLite and a “dim-light” group (n = 13) that received very little light directly from the GoLite (< 1 lux). For the blue-light group, the GoLite was set at 50% maximum brightness and participants sat with its light-emitting surface 24 inches (0.61 m) away from the face with illumination directed towards the face. For the dim-light group, the GoLite was set at 10% maximum brightness, and the light-emitting surface was directed perpendicular to the participant’s direction of gaze. Because participants in both groups were instructed to wear the Daysimeter throughout the day, it was in place during the morning light exposure. Neither the participants nor the experimenters were blind to the light group assignments.
2.8 Mood, Stress, and Feedback Questionnaires
Participants completed the Center for Epidemiologic Studies Depression questionnaire (CES-D; (31)), the Positive and Negative Affect Scale (PANAS, (32)), the State-Trait Anxiety Inventory (STAI; (33)), and the Perceived Stress Scale (PSS-14, (34)) at the start of the study and on the evenings of each laboratory session. Participants completed the questionnaires in their study bedrooms, and questionnaires were presented in the same order at each session. A subset of 14 participants also completed a subjective feedback questionnaire about their experiences keeping the sleep schedule.
2.9 Statistical Analyses
Data were analyzed using SPSS software version 18 (SPSS, Chicago, IL, USA). For inferential statistics, we used a mixed two-factor (two weeks by two groups) repeated measure analysis of variance (ANOVA) to test the effects of the schedule and light interventions. Two-tailed Student t-tests and Chi-square were used to compare group means and distributions on demographic variables.
3. Results
3.1 Participant Characteristics
Participants ranged in age from 18 to 30 years (mean ± SD = 21.8 ± 3.0) and included 12 men and 13 women. Fourteen participants were non-Hispanic Caucasian, 5 were Asian, 4 were Hispanic, 1 was African American, and 1 was multiracial. Horne-Östberg morningness-eveningness scores ranged from 28–54; 2 participants were definite evening types, 10 were moderate evening types, and 13 were neither types (24). The two experimental groups did not differ in age, sex, morningness-eveningness score, habitual bedtime and rise times, or baseline mood scores (see Table 1). Horne-Östberg evening types (definite evening types + moderate evening types, n=12) did not differ from neither types (n=13) in bedtimes, wake times, or total sleep times at baseline or during the intervention week (see supplemental table).
Table 1. Participant characteristics assessed at baseline.
| Dim-light Group (n = 13) | Blue-light Group (n = 12) | Entire Sample (N=25) | Between Groups t (p = ) | ||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | ||
| Age (years) | 21.0 (2.2) | 18–26 | 22.7 (3.6) | 18–30 | 21.8 (3.0) | 18–30 | −1.469 |
| MEQ | 41 (7) | 28–54 | 42 (5) | 34–48 | 41 (6) | 28–54 | −.643 (ns) |
| Habitual Bedtime | 2:05 (0:54) | 23:26–3:41 | 1:44 (0:54) | 23:15–3:04 | 1:55 (0:54) | 23:15–3:41 | .982 (ns) |
| Habitual Wake time | 9:40 (0:48) | 7:18–10:36 | 10:05 (0:49) | 7:52–11:07 | 9:51 (0:50) | 7:18–11:07 | −1.297 (ns) |
| STAI-T | 32.8 (7.7) | 21–47 | 33.8 (8.7) | 23–54 | 33.2 (8.0) | 21–54 | −.299 (ns) |
| STAI-S | 29.2 (7.0) | 20–46 | 30.8 (9.8) | 20–52 | 30.0 (8.3) | 20–52 | −.475 (ns) |
| PSS-14 | 16.5 (4.4) | 8–23 | 17.9 (7.3) | 6–31 | 17.2 (5.9) | 6–31 | −.593 (ns) |
| PANAS–positive | 37.0 (6.2) | 23–45 | 33.9 (4.6) | 25–41 | 35.5 (5.6) | 23–45 | 1.405 (ns) |
| PANAS negative | 15.8 (3.5) | 12–22 | 14.6 (4.2) | 10–22 | 15.2 (3.8) | 10–22 | .774 (ns) |
| CES-D | 5.9 (5.4) | 0–19 | 6.9 (5.3) | 0–19 | 6.4 (5.3) | 0–19 | −.499 (ns) |
|
|
|||||||
| χ2 (p = ) | |||||||
|
|
|||||||
| Female, n | 7 | 6 | 13 | .037 (ns) | |||
|
|
|||||||
| Race, n | |||||||
| - Caucasian | 6 | 8 | 14 | 3.451 (ns) | |||
| - Asian | 3 | 2 | 5 | ||||
| - Hispanic | 3 | 1 | 4 | ||||
| - African- American | 1 | 0 | 1 | ||||
| - Other | 0 | 1 | 1 | ||||
MEQ = Horne-Ostberg Morningness-Eveningness Score; STAI-T= State-Trait Anxiety Inventory Trait Score; STAI-S= State-Trait Anxiety Inventory State Score
3.2 Circadian Phase
We found no effect of Group (F1,23=1.27, p =.271) and no Group x Week interaction (F1,23=0.05, p=.817) for salivary DLMO. A significant effect of Week showed that the advanced sleep-wake schedule during the intervention week resulted in significantly earlier times of salivary DLMO for both groups (F1,23=61.4, p <.001). Average DLMO time was 23:24 ± 1:16 at the end of the baseline “usual schedule” week and 21:59 ± 1:03 at the end of 6 days on the earlier, fixed schedule. The average phase advance of DLMO was 1.4 ± 0.9 hours (see Table 2). Figure 2 illustrates phase shifts ≥ 1 hour in 9 of 13 participants in the dim-light group and 9 of 12 participants in blue-light group.
Table 2. Sleep Times, Salivary DLMO, and Phase Angles.
(mean (SD) and range)
| Dim-light Group n=13 | Blue-light Group n=12 | Entire Sample N=25 | ||||
|---|---|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | |
| Bedtimes | ||||||
| Baseline bedtime | 1:51 (1:13) | 23:26–3:41 | 1:41 (1:15) | 23:15–3:04 | 1:46 (1:13) | 23:15-3:41 |
| Intervention bedtime | 23:52 (0:39) | 23:00–1:17 | 00:09 (0:40) | 23:15–1:16 | 00:00 (0:40) | 23:00-1:17 |
| Wake times | ||||||
| Baseline wake time | 9:24 (1:00) | 7:18–10:36 | 9:33 (1:00) | 7:52–11:07 | 9:28 (0:59) | 7:18–11:07 |
| Intervention wake time | 7:18 (0:36) | 6:20–8:30 | 7:29 (0:50) | 5:52–8:44 | 7:24 (0:43) | 5:52–8:44 |
| Reported sleep times | ||||||
| Baseline total sleep time (minutes) | 443 (27) | 401–495 | 467 (43) | 415–511 | 455 (37) | 401–511 |
| Intervention total sleep time (minutes) | 430 (13) | 409–450 | 423 (34) | 326–450 | 427 (25) | 326–450 |
| Difference in total sleep time (minutes) | 13 (27) | –32–60 | 45 (43) | 18–128 | 28 (39) | −32–128 |
| DLMO | ||||||
| Baseline DLMO time | 23:12 (1:25) | 20:56–1:41 | 23:38 (1:07) | 21:37–1:26 | 23:24 (1:16) | 20:56–1:41 |
| Intervention DLMO time | 21:44 (0:46) | 20:16–23:08 | 22:16 (1:16) | 20:22–00:24 | 21:59 (1:03) | 20:16–00:24 |
| DLMO Shift (hrs) | 1.5 (1.1) | –0.3–3.8 | 1.4 (0.7) | 0.4–2.7 | 1.4 (0.9) | −0.3–3.8 |
| Phase Angle Between DLMO and Reported Bedtime | ||||||
| Baseline Phase Angle (hrs) | 2.7 (0.6) | 1.7–3.8 | 2.0 (1.0) | –0.2–3.9 | 2.4 (0.8) | −0.2–3.9 |
| Intervention Phase Angle (hrs) | 2.1 (0.6) | 1.2–3.2 | 1.9 (1.1) | –0.4–3.2 | 2.0 (0.9) | −0.4–3.2 |
Figure 2. Salivary DLMO Phase Shifts.
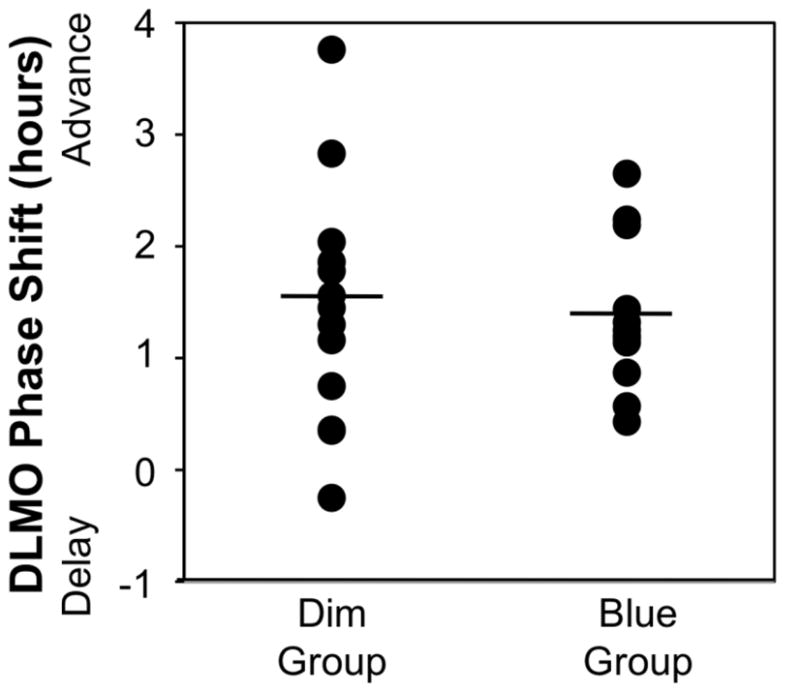
Black discs indicate phase shifts in salivary DLMO for participants in the dim-light (n=13) and blue-light (n=12) groups. Group means are indicated by the horizontal black lines.
3.3 Sleep Timing and Duration
Participants reported an average baseline bedtime of 1:46 AM ± 73 minutes and an average wake time of 9:28 AM± 59 minutes during the “usual schedule” baseline period. Comparison of baseline salivary DLMOs and self-reported bedtimes and wake times indicates that participants’ circadian rhythms were aligned with their usual sleep schedules rather than their earliest school/work commitments. As planned, the fixed 7.5 hour advanced sleep-wake schedule instituted during the second week resulted in significantly earlier reported bedtimes (F1,23=52.45, p<.001) and rise times (F1,23=120.92, p<.001; see Table 2).
Phase angles between time of salivary DLMO and reported bedtime are shown in Table 2. Average phase angle of participants in the dim light group was about 38 minutes wider than the mean phase angle of participants in the blue light group at baseline and 14 minutes wider after the intervention. These differences did not reach statistical significance (F1,23=3.11, p=.09). Phase angles did not differ between Weeks (F1,23=2.25, p=.15) and no roup x Week interaction (F1,23=0.72, p=.40) emerged.
Average self-reported sleep duration decreased significantly from baseline during the 6 days on the advanced sleep-wake schedule, from a mean of 455 ± 37 minutes (range 401–551 minutes) to a mean of 427 ± 25 minutes (range 326–450 minutes) (F1,23= 16.31, p = .001). Nineteen participants (76%) reported obtaining less sleep on the fixed 7.5 hour sleep schedule, with an average decrease in sleep per night of 43 ± 52 minutes (range 9–128 minutes). Among the 6 participants who reported more sleep during the fixed 7.5 hour schedule, the average sleep increase was 17 ± 12 minutes (range 7–32 minutes). Self-reported sleep duration manifested a significant Group x Week interaction (F1,23= 4.70, p=.041), indicating that participants in the blue-light group experienced a greater average decrease in sleep time than participants in the dim light group while adhering to the 7.5 hour advanced sleep-wake schedule, likely due to their higher sleep times during baseline.
3.4 Light Exposure
Daysimeter data were used to confirm participant compliance with the light intervention and to provide patterns of light exposure for participants in both groups across both weeks. Given the skewed distributions of light exposure from daylight during both weeks of the study, logarithmic transforms of the photometric data were performed. Daysimeter data from one participant were lost, so reported here are data from 12 participants in the blue-light group and 12 participants in the dim-light group.
The Daysimeter photometric data confirmed that participants complied with their respective morning light exposure protocols. Table 3 shows the average (log) photopic lux and the (log) CLA exposures for the two groups across both weeks during the first 90 minutes after waking and during the entire waking day while the Daysimeter was worn. Mixed design ANOVAs using the (log) CLA data for the first 90 minutes in the morning showed a significant main effect of Group (F1,22=27.7, p<.0001) and a significant Group x Week interaction (F1,22=31.7, p<.0001); the effect of Week was not statistically significant (F1,22=1.41, ns). Using a two-tail Student’s t-test, post-hoc comparisons between the transformed light exposures recorded by the Daysimeter showed, as expected, a statistically significantly difference in light exposures for the two groups during the mornings of the second week (p<.0001). There was no significant difference between the two groups in their morning circadian light exposures during the first week.
Table 3. Light Exposure (mean ± SD).
Table entries are weekly average light exposures during the first 90 minutes after waking and during the entire wake episode while the Daysimeter was being worn. Periods when participants were in bed or removed the device for more than 30 consecutive minutes were excluded from these analyses. CLA = units of circadian illuminance from the Daysimeter.
| First 90 minutes after waking | Entire wake episode | |||||||
|---|---|---|---|---|---|---|---|---|
| Week 1 | Week 2 | Week 1 | Week 2 | |||||
| Log lux | Log CLA | Log lux | Log CLA | Log lux | Log CLA | Log lux | Log CLA | |
| Group | ||||||||
| Dim-light (n=12) | 1.86±0.26 | 1.93±0.15 | 1.39±0.41 | 1.49±0.25* | 1.61±0.28 | 1.64±0.16 | 1.62±0.30 | 1.66±0.14 |
| Blue-light (n=12) | 1.98±0.29 | 1.96±0.32 | 1.79±0.23 | 2.25±0.21* | 1.70±0.19 | 1.67±0.19 | 1.68±0.31 | 1.66±0.30 |
p<0.0001
Light exposures, (log) CLA and (log) photopic lux for both groups across both weeks during the entire wake episodes are shown in Table 3. Mixed design ANOVAs using the total (log) photopic lux and the total (log) CLA exposures showed no significant main effects for either Group (F1,22=.059, ns) or Week (F1,22=.037, ns), nor was the Group x Week interaction statistically significant (F1,22=.134, ns). Thus, total circadian light exposures for participants in both groups did not differ regardless of the phase advanced sleep-wake schedule or the morning light exposures during the second week.
3.5 Mood, Stress, and Subjective Feedback
Participants’ mood and stress scores at enrollment are shown in Table 1. On average, participants did not report depressive symptoms, negative affect, or stress symptoms in the clinical range at any time. Participants’ scores on the CES-D (Week: F2,22=2.37, p=.12; Group: F1,23=.07, p=.80), STAI (Week: F2,22=2.15, p=.14; Group: F1,23=.02, p=.90), PANAS positive affect (Week: F2,22=.77, p=.48; Group: F1,23=2.44, p=.13), PANAS negative affect (Week: F2,22=.15, p=.86; Group: F1,23=.08, p=.79), or PSS-14 (Week: F2,22=.38, p=.68; Group: F1,23=.02, p=.90) were not affected by the fixed, advanced sleep-wake schedule or the morning light exposure.
Fourteen participants (9 in the dim-light group and 5 in the blue-light group) completed feedback questionnaires at the end of the study about their experiences keeping the intervention sleep schedule. Only 2 (both in the blue-light group) reported that it was easy to awaken in the morning on the earlier intervention schedule. In contrast, only two participants (one from each group) reported difficulty falling asleep on the week 2 schedule, with 9 participants reporting no difficulty and 3 reporting initial difficulty that resolved over the course of the second week. These responses may reflect that the majority of participants (85.7%) reported that they felt they were not getting enough sleep during the intervention week when they were scheduled to be in bed for only 7.5 hours per night. Nine of the participants (5 in the dim light group and 4 in the blue-light group) indicated that keeping the schedule got easier over the course of the week, whereas 4 in the dim-light group and 1 in the blue-light group reported that the schedule became more difficult to maintain as week 2 progressed. Representative comments about the schedule included the observation that the schedule got easier to follow, e.g., “Difficult. It did, however, get progressively easier to wake. Sometimes I would wake before the alarm went off,” and the complaint that the 7.5 hours of scheduled sleep was not adequate, e.g., “The sleep deprivation caught up with me.”
4. Discussion
The principal conclusion of our study is that adherence to a fixed, advanced schedule for 6 consecutive days resulted in significant circadian phase advances in young adults whose usual sleep schedules were delayed with respect to their usual school or work commitments. Although our participants did not meet full criteria for DSPD, they had subclinical features that occur with high frequency in young people in this age cohort and can impact daytime alertness, performance, and mood (5). Adherence to a fixed, earlier sleep schedule advanced circadian phase by an average of 1.4 hours, with eighteen of 25 participants achieving phase shifts > 1 hour. These circadian phase advances resulted in better alignment between participants’ sleep and their school or work obligations. Current recommendations for treating DSPD incorporate sleep schedules as an “option” rather than a “guideline” or a “standard” because the evidence for schedules is based on case reports and committee consensus and because the long-term efficacy and compliance with scheduling as a treatment for DSPD are unknown (9). Our data indicate that adherence to a fixed sleep schedule can advance circadian phase and sleep times with or without a morning dose of blue-light treatment in the short term. We did not study patients with a diagnosis of DSPD, and we did not follow the participants to determine whether they maintained the shifts in their circadian rhythms and sleep patterns long term. Therefore, future work is needed to confirm these findings in a clinical population with longer assessment periods. Nevertheless, these findings provide initial support for the use of behavioral treatment with sleep scheduling to correct misalignment between sleep behavior and school and work schedules, particularly for individuals with symptoms such as difficulty awakening in the morning to attend school or work.
The results did not support our hypothesis that scheduled blue-light exposure in the morning would enhance circadian phase advances compared to morning dim-light exposure. We were able to quantify light exposure using the Daysimeter, and although the blue-light group had more short wavelength corneal irradiance, or “circadian illuminance” (CLA), in the first 90 minutes after waking during the intervention week, phase shifting was not enhanced. We found no difference in circadian light exposures (log CLA) or photopic light exposures (log lux) across the entire wake episodes between our dim-light and blue-light groups. Thus, we speculate that the overall pattern of light and dark exposure produced by the fixed earlier sleep-wake schedule likely resulted in the same phase-advancing effects for the two groups. We speculate that based on the full-spectrum light phase response curve (35) our participants would have received increased exposure to light during the phase-advance portion of the light phase response curve (PRC) by rising earlier and also would have had lower levels of light exposure during the phase delay portion of the light PRC (35) through going to bed earlier.
An alternate explanation for the failure of blue light to enhance circadian phase advances is that the timing of blue light exposure in our study may not have been optimal for producing phase advances. A preliminary blue light PRC published in abstract form (36) indicates that the phase-advance portion of the PRC to short-wavelength light peaks several hours after usual waketime–later than we administered blue light in our participants. In any case, our study indicates that despite the well-documented spectral sensitivity of the circadian system to short wavelength light (11–13, 18–20), parameters for its use need to be established and its utility as a clinical intervention requires further study.
A secondary aim of this project was to investigate whether the advanced schedule and morning light intervention changed reports of symptoms of stress, depression, or anxiety in this group of young adults with misalignment between their sleep and school/work schedules. The participants did not express symptoms of stress, depression, or anxiety by scores in the clinical range, and they did not show a significant increase or decrease in symptoms coincident with the changes in the sleep schedule or the light exposure. Further work is needed to establish whether phase advancing circadian rhythms through scheduling and light exposure results in mood improvements in DSPD patients who are more likely to be experiencing significantly greater emotional distress than the population studied here.
One methodologic concern with this study is that it was conducted in a field setting and participants were not directly observed sleeping or receiving their light intervention. On the other hand, noncompliance with the prescribed protocol appears unlikely based upon the data obtained from the wrist actigraphs, Daysimeter, and laboratory voice mail recordings at bedtime and wake time to confirm schedule compliance. Another limitation is that we recruited participants with subclinical symptoms of DSPD, rather than diagnosed DSPD patients. Still, we believe this group is clinically relevant and may represent a larger portion of the general population who have misalignment between their school or work schedules.
In conclusion, this study demonstrated that significant phase advances in salivary DLMO can be achieved with strict adherence to a fixed, earlier sleep-wake schedule in young adults with misalignment between their usual sleep schedule and their school/work obligations, with or without morning blue-light treatment. Morning blue-light exposure did not produce larger phase advances than the change in the schedule alone. We conclude that the overall pattern of light/dark consequent to the earlier sleep schedule was sufficient to advance circadian rhythms in this study population.
Supplementary Material
Acknowledgments
This work was funded by NIH U01DA023822 to Dr. Rea. The authors thank Andrew Baum,Jena Burgner, David Bushnell, William Coon, Danni Dunlap, Marcy D’Uva, Ellyn Ferriter,Margaret Gordon-Fogelson, Clayton Kim, Jennifer Norton King, Nischal Nadig, Ellen Sweeney, and Celso Teixeira from the EP Bradley Hospital Sleep and Chronobiology Laboratory for technical assistance. We are especially grateful to the late Denise Maceroni, our cherished colleague who performed the melatonin assays for this study. We also thank Andrew Bierman, Brian Donlan, Jim Dunshee, Nicolas Meyer at the Lighting Research Center for technical support. Finally, we are grateful to Stephanie Crowley, PhD, and Harold W. Gordon, PhD, for input on study design.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Academy of Sleep Medicine. Diagnosis and coding manual. 2. Westchester, IL: American Academy of Sleep Medicine; 2005. International classification of sleep disorders. [Google Scholar]
- 2.American Academy of Sleep Medicine. International classification of sleep disorders, revised: Diagnostic and coding manual. Chicago, IL: American Academy of Sleep Medicine; 2001. [Google Scholar]
- 3.Yang CM, Spielman AJ, D’Ambrosio P, Serizawa S, Nunes J, Birnbaum J. A single dose of melatonin prevents the phase delay associated with a delayed weekend sleep pattern. Sleep. 2001 May 1;24(3):272–81. doi: 10.1093/sleep/24.3.272. [DOI] [PubMed] [Google Scholar]
- 4.Weitzman ED, Czeisler CA, Coleman RM, Spielman AJ, Zimmerman JC, Dement W, et al. Delayed sleep phase syndrome. A chronobiological disorder with sleep-onset insomnia. Arch Gen Psychiatry. 1981 Jul;38(7):737–46. doi: 10.1001/archpsyc.1981.01780320017001. [DOI] [PubMed] [Google Scholar]
- 5.Pelayo R, Thorpy M, Glovinsky P. Prevalence of delayed sleep phase syndrome among adolescents. Sleep Research. 1988;17:391. doi: 10.1016/0197-0070(88)90014-9. [DOI] [PubMed] [Google Scholar]
- 6.Thorpy MJ, Korman E, Spielman AJ, Glovinsky PB. Delayed sleep phase syndrome in adolescents. J Adolesc Health Care. 1988 Jan;9(1):22–7. doi: 10.1016/0197-0070(88)90014-9. [DOI] [PubMed] [Google Scholar]
- 7.Shirayama M, Shirayama Y, Iida H, Kato M, Kajimura N, Watanabe T, et al. The psychological aspects of patients with delayed sleep phase syndrome (DSPS) Sleep Med. 2003 Sep;4(5):427–33. doi: 10.1016/s1389-9457(03)00101-1. [DOI] [PubMed] [Google Scholar]
- 8.Lack LC. Delayed sleep and sleep loss in university students. J Am Coll Health. 1986 Nov;35(3):105–10. doi: 10.1080/07448481.1986.9938970. [DOI] [PubMed] [Google Scholar]
- 9.Morgenthaler TI, Lee-Chiong T, Alessi C, Friedman L, Aurora RN, Boehlecke B, et al. Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. An American Academy of Sleep Medicine report. Sleep. 2007 Nov 1;30(11):1445–59. doi: 10.1093/sleep/30.11.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terman M. Evolving applications of light therapy. Sleep Med Rev. 2007 Dec;11(6):497–507. doi: 10.1016/j.smrv.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, et al. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001 Aug 15;21(16):6405–12. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001 Aug 15;535(Pt 1):261–7. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright HR, Lack LC. Effect of light wavelength on suppression and phase delay of the melatonin rhythm. Chronobiol Int. 2001 Sep;18(5):801–8. doi: 10.1081/cbi-100107515. [DOI] [PubMed] [Google Scholar]
- 14.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002 Feb 8;295(5557):1065–70. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002 Feb 8;295(5557):1070–3. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 16.Rea MS, Figueiro MG, Bullough JD, Bierman A. A model of phototransduction by the human circadian system. Brain Res Brain Res Rev. 2005 Dec 15;50(2):213–28. doi: 10.1016/j.brainresrev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Wright HR, Lack LC, Kennaway DJ. Differential effects of light wavelength in phase advancing the melatonin rhythm. J Pineal Res. 2004 Mar;36(2):140–4. doi: 10.1046/j.1600-079x.2003.00108.x. [DOI] [PubMed] [Google Scholar]
- 18.Revell VL, Arendt J, Terman M, Skene DJ. Short-wavelength sensitivity of the human circadian system to phase-advancing light. J Biol Rhythms. 2005 Jun;20(3):270–2. doi: 10.1177/0748730405275655. [DOI] [PubMed] [Google Scholar]
- 19.Smith MR, Revell VL, Eastman CI. Phase advancing the human circadian clock with blue-enriched polychromatic light. Sleep Med. 2009 Mar;10(3):287–94. doi: 10.1016/j.sleep.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warman VL, Dijk DJ, Warman GR, Arendt J, Skene DJ. Phase advancing human circadian rhythms with short wavelength light. Neurosci Lett. 2003 May 15;342(1–2):37–40. doi: 10.1016/s0304-3940(03)00223-4. [DOI] [PubMed] [Google Scholar]
- 21.Lockley SW, Brainard GC, Czeisler CA. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Metab. 2003 Sep;88(9):4502–5. doi: 10.1210/jc.2003-030570. [DOI] [PubMed] [Google Scholar]
- 22.Cajochen C, Munch M, Kobialka S, Krauchi K, Steiner R, Oelhafen P, et al. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J Clin Endocrinol Metab. 2005 Mar;90(3):1311–6. doi: 10.1210/jc.2004-0957. [DOI] [PubMed] [Google Scholar]
- 23.Bierman A, Klein T, Rea MS. The Daysimeter: a device for measuring optical radiation as a stimulus for the human circadian system. Measurement Science and Technology. 2005;16(11):2292. [Google Scholar]
- 24.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4(2):97–110. [PubMed] [Google Scholar]
- 25.Rea MS, Figueiro MG, Bierman A, Bullough JD. Circadian light. J Circadian Rhythms. 2010;8(1):2. doi: 10.1186/1740-3391-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller D, Figueiro MG, Bierman A, Schernhammer E, Rea MS. Ecological measurements of light exposure, activity, and circadian disruption. Lighting Research and Technology. 2010;42:271–84. doi: 10.1177/1477153510367977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burgess HJ, Eastman CI. The dim light melatonin onset following fixed and free sleep schedules. J Sleep Res. 2005 Sep;14(3):229–37. doi: 10.1111/j.1365-2869.2005.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewy AJ, Bauer VK, Ahmed S, Thomas KH, Cutler NL, Singer CM, et al. The human phase response curve (PRC) to melatonin is about 12 hours out of phase with the PRC to light. Chronobiol Int. 1998 Jan;15(1):71–83. doi: 10.3109/07420529808998671. [DOI] [PubMed] [Google Scholar]
- 29.Lewy AJ, Cutler NL, Sack RL. The endogenous melatonin profile as a marker for circadian phase position. J Biol Rhythms. 1999 Jun;14(3):227–36. doi: 10.1177/074873099129000641. [DOI] [PubMed] [Google Scholar]
- 30.Carskadon MA, Wolfson AR, Acebo C, Tzischinsky O, Seifer R. Adolescent sleep patterns, circadian timing, and sleepiness at a transition to early school days. Sleep. 1998 Dec 15;21(8):871–81. doi: 10.1093/sleep/21.8.871. [DOI] [PubMed] [Google Scholar]
- 31.Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurements. 1977;1:385–401. [Google Scholar]
- 32.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988 Jun;54(6):1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 33.Spielberger C, Gorsuch R, Lushene R, Vagg P, Jacobs G. State-Trait Anxiety Inventory for Adults. Mind Garden, Inc; 1977. [Google Scholar]
- 34.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983 Dec;24(4):385–96. [PubMed] [Google Scholar]
- 35.Khalsa SB, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003 Jun 15;549(Pt 3):945–52. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eastman CI, Molina TA, Burgess HJ, Revell VL. Ongoing Go-Lite (blue light) phase response curve in humans. Sleep. 2010;33(Abstract Supplement):A64. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.