Abstract
The ubiquitous serine/threnine kinase glycogen synthase kinase 3β (Gsk3β) differentially regulates macrophage TLR-triggered pro- and anti-inflammatory cytokine programs. This study was designed to determine in vivo role and therapeutic potential of Gsk3β modulation in tissue inflammation and injury in a murine model of liver partial warm ischemia/reperfusion (IRI). As a constitutively activated liver kinase, Gsk3β became quickly inactivated (phosphorylated) following IR. The active Gsk3β, however, was essential for the development of IRI pathology, as administration of its specific inhibitor SB216763, ameliorated the hepatocellular damage, evidenced by reduced sALT levels and well-preserved liver architecture, compared with controls. The liver protective effect of Gsk3β inhibition was dependent on an immune regulatory mechanism, rather than direct cytoprotection via mitochondria permeability transition pores (MPTP). Indeed: (a) co-administration of SB216763 and atractyloside (MPTP opener) failed to abrogate local cytoprotective Gsk3β inhibition effect; (b) SB216763 selectively inhibited IR-triggered liver pro-inflammatory, but spared IL-10, gene induction program; and (c) IL-10 neutralization restored liver inflammation and IRI in SB216763-treated mice. Gsk3β inactivation by IR was a self-regulatory mechanism in liver homeostasis, critically dependent on phosphoinositide 3 (PI3)-kinase activation, as administration of PI3 kinase inhibitor, wortmannin, reduced Gsk3 phosphorylation and augmented liver damage. In vitro, IL-10 was critical for the suppression of pro-inflammatory gene programs by Gsk3 inhibition in bone marrow-derived macrophages in response to TLR4 stimulation. Our novel findings document the key immune regulatory function of Gsk3β signaling in the pathophysiology of liver IRI, and provide the rationale to target Gsk3β as a refined therapeutic strategy to ameliorate liver IRI.
Introduction
Ischemia and reperfusion injury (IRI) is a common clinical problem associated with liver transplantation, partial hepatic resection or trauma. Although IRI significantly impacts both acute liver failure/graft rejection, as well as chronic liver dysfunction (1–4), no effective therapy is available to prevent or treat the clinical syndrome. The pathogenesis of IRI is a two stage process consisting of the initial cellular damage due to ischemia, followed by reperfusion-initiated inflammation-induced hepatocellular damage. Recently, host innate TLR4 activation by endogenous ligands has been identified as the key trigger in liver immune response against IR (5–9). As cell surface TLR4 ligation activates multiple intracellular signaling pathways (10, 11) leading to the induction of pro- and anti-inflammatory gene programs, our better appreciation of their regulatory mechanisms should identify novel therapeutic targets to selectively alleviate pro-while sparing or promoting anti-inflammatory immune responses and ultimately ameliorate tissue damage. Recent studies have revealed that glycogen synthase kinase 3β (Gsk3β) may represent such a target. Indeed, inhibition of Gsk3β in vitro significantly increased IL-10 production while suppressing release of pro-inflammatory cytokines in macrophages stimulated with TLR ligands. TLR activation leads to Gsk3βphosphorylation by the PI3 kinase-Akt pathway. The resultant increase of cAMP response element-binding (CREB) but decrease of NF-κB activity diminish the expression of pro-inflammatory genes, such as IL-12, TNF-α, and IL-1β, while augmenting the expression of anti-inflammatory IL-10 (12). In vivo, Gsk3βinhibitors effectively protected mice from endotoxin shock (12). These data suggest the therapeutic potential of Gsk3β inhibition and warrant further confirmation in clinically relevant animal inflammatory disease models.
Gsk3, a ubiquitously expressed serine/threonine kinase, was initially found to regulate glycogen synthesis (13, 14). There are two highly homologous Gsk3α and Gsk3β isoforms. Gsk3β, constitutively active (in dephosphorylated form) in resting cells, has the broad range of substrates regulating cell activation, differentiation and survival. In heart IRI models, Gsk3β inactivation represents the convergence point for multiple cytoprotective signaling pathways (15). Gsk3β regulates the induction of the mitochondrial permeability transition pore (MPTP), a key step in triggering mitochondria-mediated cell death, which constitutes the critical IRI effector pathway. Gsk3 inhibition has been tested successfully as a cardioprotection strategy in myocardial infarction (16–18). However, whether Gsk3 inhibition can protect hepatocytes from IR-induced cell death has yet to be determined.
As IR activates TLR4 signaling and triggers pro-inflammatory response in vivo, we need to determine whether IR does indeed activate the PI3 kinase-Akt-Gsk3β pathway. Furthermore, questions of whether Gsk3β may function to regulate liver immune response and IRI are of high significance to further define the disease pathogenesis and explore putative therapeutic strategies. There are potential drawbacks of Gsk3β inhibition in liver IR. It may increase macrophage IFN-β production in response to TLR4 stimulation (19). Since type 1 IFN and its downstream gene product CXCL10 are key pro-inflammatory mediators in liver IRI (20), it becomes questionable as to whether Gsk3β inhibition will truly blunt liver pro-inflammatory program. In addition, Gsk3β genetic deletion results in embryonic lethality due to the severe liver degeneration during development (21), and its inhibition increases hepatocyte apoptosis against TNF-α (22). Thus, Gsk3β inhibition may exert multi-faceted functions in the liver during IR. It remains an open question as to whether or not the Gsk3β inhibition will indeed ameliorate liver IRI cascade.
Materials and Methods
Animals
Male wide-type (WT; C57BL/6) mice (8–12 weeks old) were purchased from the Jackson Laboratory (Bar Harbor, ME). Animals were housed in the UCLA animal facility under specific pathogen-free conditions, and received human care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institute of Health (NIH publication 86–23 revised 1985).
Mouse warm hepatic IRI model
Liver partial warm IR was performed, as described (23). In brief, an atraumatic clip was used to interrupt the arterial and portal venous blood supply to the cephalad liver lobes for 90 min (or 60 min in PI3 kinase inhibition experiments). Sham WT controls underwent the same procedure, but without vascular occlusion. To inhibit Gsk3β or PI3 kinase activity, mice were treated with a single dose of SB216763 (25μg/g, Sigma, St. Louis, MO) or wortmannin (1μg/g, Sigma) dissolved in DMSO/PBS, or atractyloside (5μg/g, Enzo Life Sciences, Plymouth Meeting, PA) dissolved in PBS, i.p. 2h or 1h prior to the onset of liver ischemia, respectively. Anti-IL-10 Ab (0.5mg/mouse, Clone JES5–2A5, Bio-Express, West Lebanon, NH) was administered, i.p., 1h prior to the liver ischemia. Mice were sacrificed at various time-points of reperfusion; liver and serum samples were collected for future analysis.
Hepatocellular damage assay
Serum alanine aminotransferase (sALT) levels, an indicator of hepatocellular injury, were measured with an autoanalyzer (ANTECH Diagnostics, Los Angeles, CA).
Liver histology
Liver sections were cut into 5-μm sections, and stained with hematoxylin and eosin (H&E). Histological severity of IRI was graded using Suzuki’s criteria on a scale from 0–4 (24). No necrosis, congestion/centrilobular ballooning is given a score of 0, while severe congestion/degeneration and >60% lobular necrosis is given a value of 4.
MPO assay
To detect neutrophil activity, myeloperoxidase (MPO), an enzyme specific for PMNs, was measured in the liver. Briefly, the frozen tissue was thawed and placed in iced 0.5% hexadecyltrimethyl-ammonium bromide and 50 mmol potassium phosphate buffer solution (pH = 5.0). Each sample was homogenized and centrifuged at 15,000 rpm for 15 min at 4°C. Supernatants were mixed with hydrogen peroxide-sodium acetate and tetramethyl-benzidine solutions. The change in absorbance was measured by spectrophotometry at 655 nm. One unit of MPO activity was defined as the quantity of enzyme degrading 1μmol peroxide/min at 25°C/g of tissue.
Cell cultures
Mouse macrophage RAW264.7 (ATCC, Manassas, VA) cells were maintained in DMEM medium supplemented with 10% heat inactivated fetal bovine serum (FBS). LPS (10ng/ml, Invivogen, San Diego, CA) was used to activate cells. Antimycin A (10μg/ml, Sigma) was used to inhibit cell oxidation and ATP synthesis. SB 216763 (10μM/ml) was used to inhibit Gsk3β.
Murine bone marrow-derived macrophages (BMM) were differentiated from bone marrow from 6–10-week old C57B/6 mice by culturing in 1xDMEM, 10% fetal bovine serum, 1% penicillin/streptomycin, and 20% L929 conditioned medium for 6 days. The cell purity was 94–99% CD11b+.
Quantitative RT-PCR
Two and a half μg of RNA was reverse-transcribed into cDNA using SuperScriptTM III First-Strand Synthesis System (Invitrogen, Carlsbad, CA). Quantitative-PCR was performed using the DNA Engine with Chromo 4 Detector (MJ Research, Waltham, MA). In a final reaction volume of 25 μl, the following were added: 1xSuperMix (Platinum SYBR Green qPCR Kit, Invitrogen), cDNA and 0.5 mM of each primer. Amplification conditions were: 50°C (2 min), 95°C (5 min) followed by 50 cycles of 95 °C (15 s), 60 °C (30 s). Primers used to amplify a specific mouse gene fragments have been described (25).
Western blots
Protein was extracted from liver tissue with ice cold lysis buffer (1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 10% glycerol, 137mM sodium chloride, 20mM Tris, pH 7.4). Proteins (20 μg) were subjected to 12% SDS-PAGE electrophoresis and transferred to PVDF nitrocellulose membrane. Antibodies against phosphorylated and total Gsk3b, extracellular signal-regulated kinase (ERK), Jun N-terminal kinase (JNK), and p38 mitogen-activated protein (MAP) kinase proteins, as well as b-actin (Cell Signaling Technology, Santa Cruz, CA) were used for Western blot analysis. Membranes were probed with primary antibody (1:1000) in 10 ml blocking buffer overnight at 4°C. After washing, membranes were further probed with appropriate HRP-conjugated secondary antibody (1:2000) in 10 ml of blocking buffer for 1 h at room temperature. SuperSignal® West Pico Chemiluminescent Substrates (Thermo Fisher Scientific, Rockford, IL) were used for chemo-luminescence development.
Statistical Analysis
Results are shown as mean±SD. Statistical analyses were performed using unpaired Student’s t test with p < 0.05 (two tailed) considered as significant.
Results
Gsk3β activation/inactivation profile in liver IR
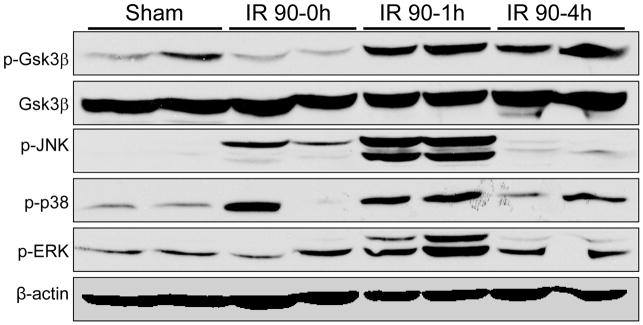
We first determined whether IR stimulation triggers Gsk3β phosphorylation/dephosphorylation in mouse livers subjected to 90 min of warm ischemia, followed by various length of reperfusion. Compared with sham controls, the phosphorylated Gsk3β (at serine 9) level was slightly reduced by ischemia itself (time 0), and then rapidly increased at reperfusion and remained phosphorylated throughout the reperfusion phase (1–6 h) (Fig. 1). As total Gsk3β levels were equal at all time points, these results indicate that the constitutively active Gsk3β in the liver was inactivated by the IR insult and sustained inactive thereafter. Gsk3β activation profile was distinct from that of MAP kinases, which were only transiently activated by IR, but quickly decreased to baseline by 4 h post-reperfusion (Fig. 1). The phosphorylation of tyrosine 216 on Gsk3β was not detected neither was the phosphorylation of Gsk3α in IR livers (data not shown). Thus, liver IR triggers Gsk3β phosphorylation on serine 9, which is sustained throughout the reperfusion period.
Figure 1.
Liver ischemia and reperfusion triggers Gsk3β phosphorylation. Liver samples were harvested from B6 mice that were either sham-operated or subjected to 90min of hepatic warm ischemia, followed by various length of reperfusion (0, 1, 4 h). The phosphorylated (serine 9) and total Gsk3β levels, along with phosphorylated MAP kinases including JNK, Erk and p38, and β-actin were measured by Western blots. Representative of two experiments is shown.
Active Gsk3β is critical for the development of liver IRI
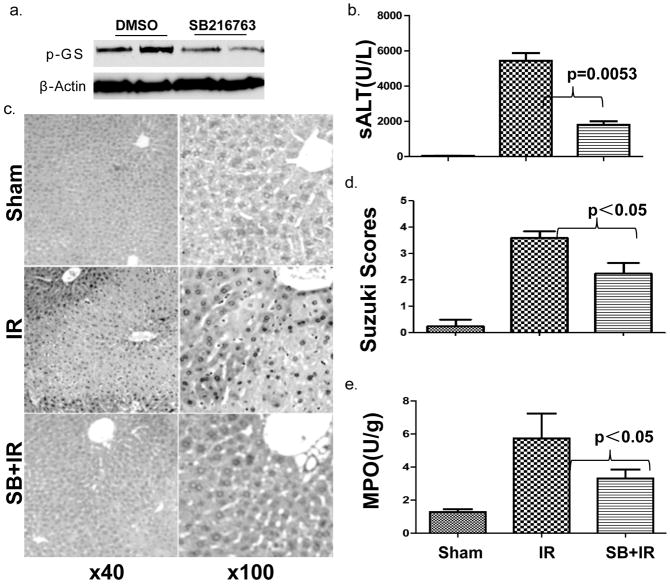
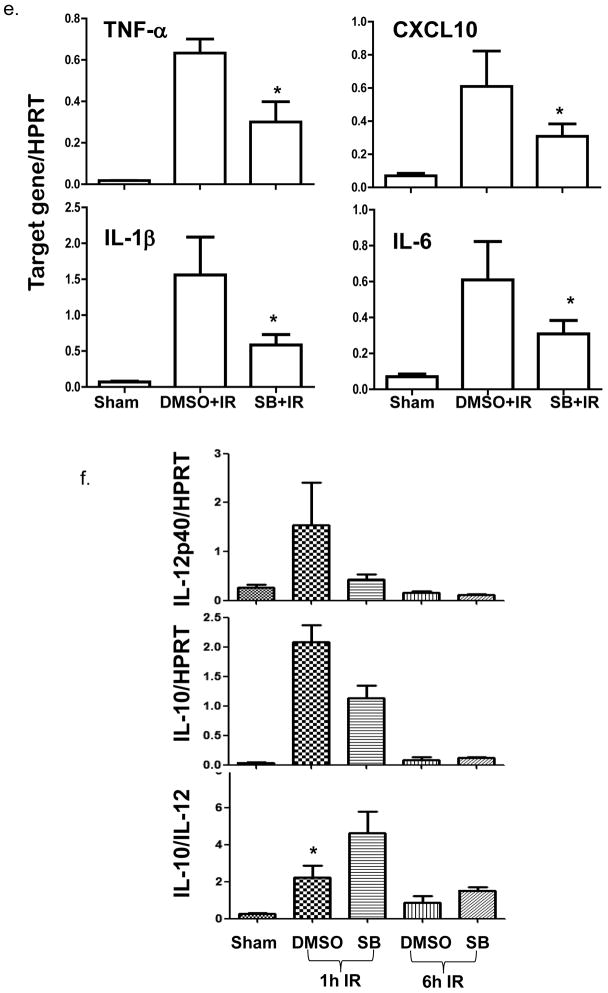
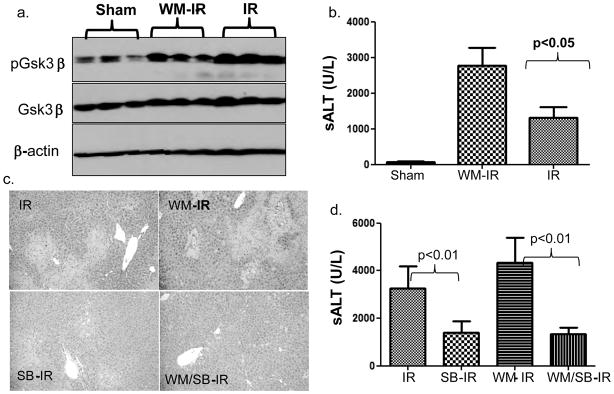
To address the functional significance of the constitutively active Gsk3β, we treated mice with a Gsk3β specific chemical inhibitor, SB216763, prior to the onset of ischemia. The inhibition of liver Gsk3 activity in vivo was confirmed by the reduced phosphorylation level of glycogen synthase, the substrate of Gsk3β (Fig. 2a). As compared with vehicle-treated controls, livers in animals receiving SB216763 suffered less severe IRI, evidenced by significantly lower sALT levels (Fig. 2b, 7709±1689 vs. 2946±513; p=0.0053), better preserved liver architecture by histology (Fig. 2c) and Suzuki grading (Fig. 2d, 3.6±0.24 vs 2.2±0.40 n=5–6/group, p<0.05). In parallel, pretreatment with SB216763 suppressed neutrophil activation, measured by MPO activity (Fig. 2e, 5.73±1.50 vs. 3.30±0.54; p<0.05), and diminished the induction of TNF-α, IL-1β, IL-6 and CXCL10 in IR-livers (Fig. 2f). It has been shown that IR-induced IL-12 and IL-10 are transient and occur early post reperfusion (25). To determine the impact of Gsk3 inhibition on the induction of these two genes by IR, livers were harvested at 1 h post-reperfusion from separate groups of mice. Although Gsk3 inhibition slightly reduced IL-10 expression in IR-livers, its impact on IL-12p40 gene induction was much more pronounced, leading to a significant increase in IL-10/IL-12 ratio in SB216763 treated group, as compared with controls (Fig. 2g). Thus, active Gsk3β was essential for pro-inflammatory immune response, and the development of liver IRI. Its inhibition preferentially suppressed proinflammatory gene program, while IL-10 induction was relatively spared.
Figure 2. Gsk3 inhibition by SB216763 ameliorates liver IRI in B6 mice.
(a) Mice were treated with vehicle (DMSO) or SB216763. Liver samples, harvested 24h later, were subjected to Western blot analysis of phosphorylated glycogen synthases.
(b) Mice were sham-operated, or subjected to 90min warm ischemia followed by 1h or 6h reperfusion. Recipients were treated with vehicle (DMSO) or SB216763. Group averages of sALT levels at 6h were measured and plotted (n=5–12/group).
(c) Representative liver histology (H/E staining at 6h).
(d) Group averages of liver Suzuki scores (6h).
(e) Liver MPO levels at 6h.
(f) Expression levels of inflammatory TNF-α, CXCL10, IL-1β and IL-6 at 6h.
(g) IL-12p40 and IL-10 at 1h and 6h of reperfusion (n=4–5/group). * indicates p<0.05.
IL-10 dependent mechanism, but not direct cytoprotection, mediates Gsk3 inhibition-mediated liver protection
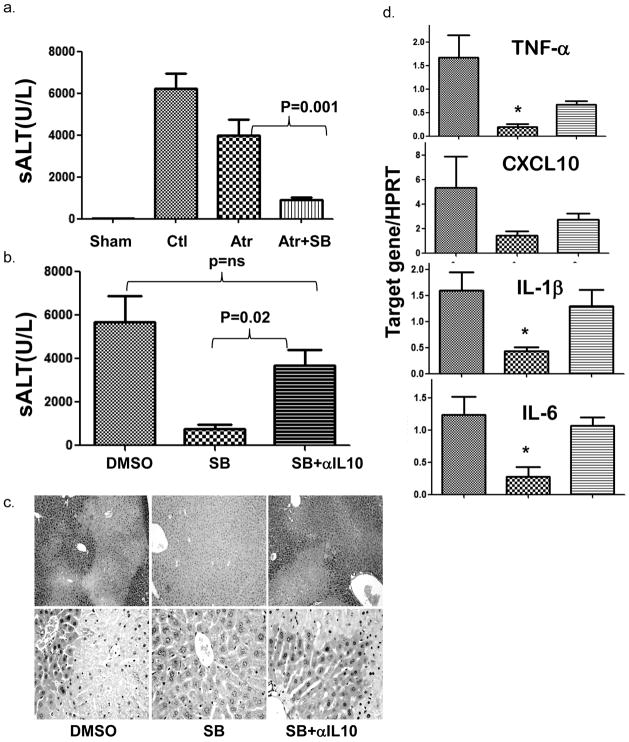
To analyze liver protection mechanisms of Gsk3 inhibition, we differentiated between direct cytoprotection (via inhibition of MPTP) and immune regulation. Mice underwent adjunctive treatment with astractyloside (Atr), a MTPT opener, or anti-IL-10 Ab together with Gsk3 inhibitor (SB216763), followed by liver IR insult. In contrast to its protective effects in the heart (26, 27), treatment with Atr did not affect the beneficial effect of Gsk3 inhibition in the liver. However, concomitant neutralization of IL-10 readily recreated liver damage. Hence, sALT levels in the Atr plus SB216763 treatment group were significantly lower as compared to those in Atr-treated or vehicle-treated groups (Fig. 3a, Atr/SB: 917±104 vs. Atr: 3994±739, p=0.001; vs. Ctl:6239±725, p<0.001). In marked contrast, sALT levels in SB plus anti-IL-10 Ab treatment group were significantly higher than in SB216763 monotherapy group, and became comparable with those in controls (Fig. 3b, SB/anti-IL-10: 3683±720.3 vs. SB: 769.0±203.7; p=0.02; vs. Ctl: 5691±1205, p=ns). The histology evaluation showed the hepatocellular damage, corresponding with sALT levels in the respective animal groups (Fig. 3c). Consequently, IL-10 neutralization restored liver pro-inflammatory immune response against IR in SB-treated mice, as evidenced by increased TNF-α, IL-1β, IL-6 and CXCL10 expression (Fig. 3d). Thus, the liver protective mechanism of Gsk3 inhibition depends on IL-10-mediated immune regulation rather than the direct cytoprotection via mitochondria.
Figure 3. Liver protection against IRI following Gsk3 inhibition is independent of MPTP, but dependent on an IL-10 mediated immune regulation. Groups of B6 mice were treated with vehicle, or astractyloside (Atr) or SB216763 (SB), or SB + Atr or SB +anti-IL-10 Ab, and then subjected to 90min of ischemia, followed by 6h reperfusion, as described in Materials and Methods.
(a, b) Group averages of sALT levels were measured and plotted
(c) Representative liver histology (H/E staining)
(d) Group averages of liver pro-inflammatory gene (TNF-α, CXCL10, IL-1β and IL-6) expression levels. (c), n=3–6/group, * indicates p<0.05.
PI3 kinase-dependent Gsk3 phosphorylation regulates liver IRI
As Gsk3β phosphorylation occurs spontaneously during liver IR, we then addressed the functional significance of its inactivation. As PI3 kinase-Akt pathway has been shown in vitro to regulate Gsk3βphosphorylation downstream of TLR4 activation (12), we utilized wortmannin, an irreversible PI3 kinase specific inhibitor, to test as to whether or not Gsk3β phosphorylation is PI3 kinase-dependent, and, if so, what is its pathophysiology role in liver IRI. Indeed, livers in wortmannin-treated mice were characterized by significantly lower levels of phosphorylated Gsk3β after IR (Fig. 4a) and suffered more severe injury at 6 h of reperfusion, as compared with vehicle-treated controls. This was most pronounced in the 60 min liver ischemia setting, with the hepatocellular damage less severe than that recorded after 90 min of ischemia (Fig. 4b, sALT Ctl: 1323±295.7 vs. WM: 2775±492.5, n=5, p<0.05; and Fig. 4c, liver histology). To confirm that Gsk3β inactivation functioned downstream of PI3 kinase activation, SB216763 and wortmannin were administered in concert prior to the ischemia insult. Gsk3 inhibition remained hepatocytoprotective against IRI in the presence of PI3 kinase inhibition (Fig. 4d: sALT: Ctl, 7825±583.9 vs. SB, 3511±809.0; p<0.01; WM, 8863±826.9 vs. SB/WM, 3069±741.7; p<0.01). Thus, PI3 kinase-dependent Gsk3β phosphorylation serves as a self-regulatory mechanism of liver homeostasis to limit the excessive IR-triggered tissue damage.
Figure 4. PI3 kinase activation is responsible for Gsk3β phosphorylation and its inhibition deteriorates liver IRI. Groups of B6 mice were treated with SB216763 (SB), Wortmannin (WM) or both or vehicle at 1–2h prior to the liver ischemia insult, as described in Materials and Methods. Liver samples were harvested at 6h post-reperfusion.
(a) Proteins were analyzed by Western blots with Abs against phosphorylated or total Gsk3β, and β-actin
(b) Sixty min ischemia time was used to show the effect of PI3 kinase inhibition in liver IRI. Average sALT levels in different experimental groups were plotted.
(c) Representative liver histology (H/E staining) is shown.
(d) To establish the functional relationship between PI3 kinase and Gsk3β, SB was administered 2h and WM 1h prior to the ischemia insult. Serum ALT levels were measured at 6h of reperfusion.
Gsk3β regulates macrophageTLR4 response via direct and indirect mechanisms
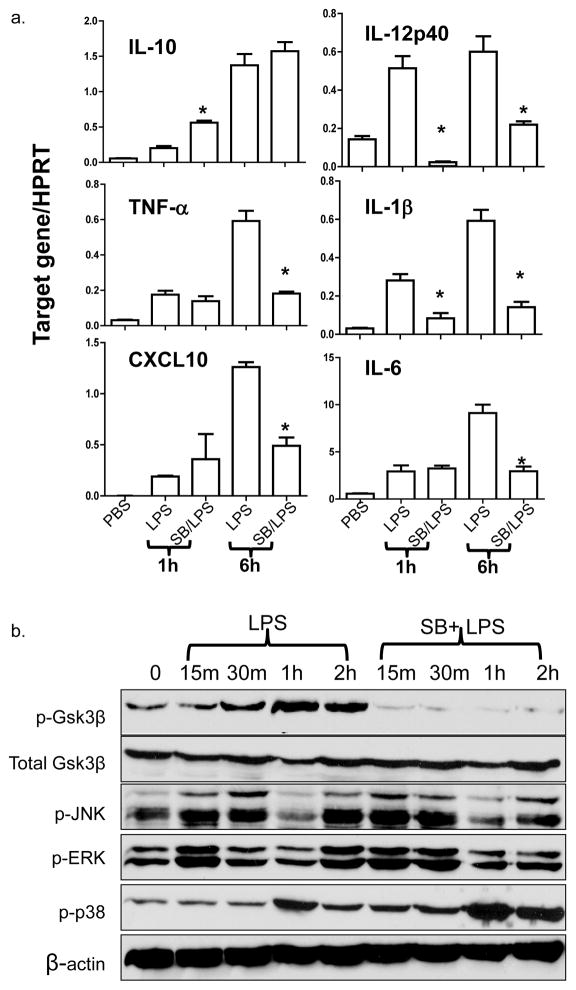
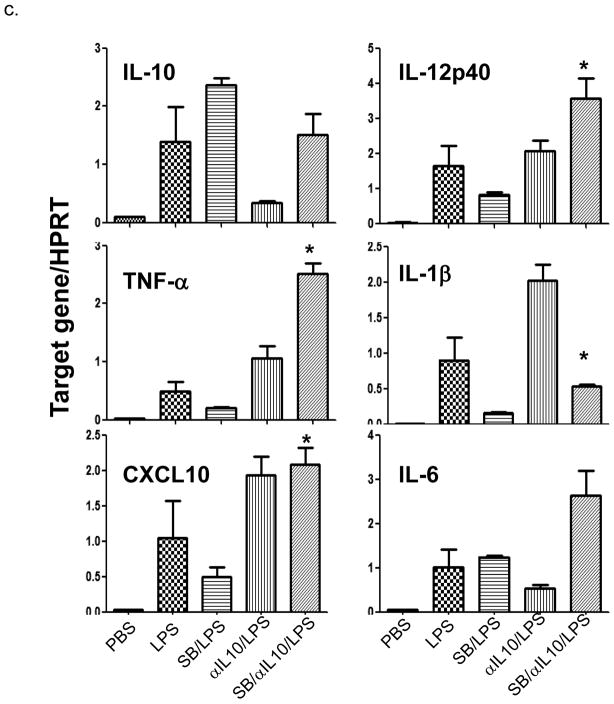
It has been well established that TLR4 activation is the key step in liver inflammatory immune response against IR (5, 9). To investigate the cellular mechanism of our in vivo findings, we analyzed the effects of Gsk3 inhibition in macrophage response to TLR4 stimulation in vitro. Bone marrow-derived macrophages were stimulated with LPS in the absence or presence of SB216763. As shown in Fig. 5a, Gsk3 inhibition significantly reduced IL-12p40 and IL-1β, but increased IL-10 gene induction at 1 h of culture. In contrast, the induction of TNF-α, IL-6, and CXCL10 were unaffected at this early time-point. By 6 h, while the IL-12p40 expression remained lower, levels of TNF-α, IL-6, IL-1β and CXCL10 all became significantly reduced. IL-10 levels were comparable between the two groups. Gsk3 inhibition did not alter LPS-induced MAP kinase activation, as the phosphorylation kinetics of JNK, Erk and p38 were similar in control and SB-treated macrophage cultures (Fig. 5b). The disparities between IL-12/IL-10 and TNF-α/CXCL10 genes at the time points regulated by the Gsk3 inhibition indicated a possible difference in their regulatory mechanisms, i.e., the early regulated genes were the primary targets of Gsk3, whereas the later ones were regulated by the primary gene products. To test whether IL-10 may represent such a primary gene regulating the late inhibition of TNF-α/CXCL10 induction, we added anti-IL-10 Ab in SB216763-treated macrophage cultures. Indeed, LPS-induced TNF-α/CXCL10 levels at 6 h, which remained diminished by Gsk3 inhibitor alone, became restored (or even enhanced) after adjunctive anti-IL-10 Ab and SB216763 (Fig. 5c). Interestingly, anti-IL-10 Ab restored otherwise suppressed IL-12p40 gene induction by SB216763.
Figure 5. Gsk3 inhibition regulates TLR4 response in vitro. Bone marrow-derived macrophages (BMMs) were stimulated with LPS for 1h or 6h in the absence or presence of SB216763. Gene induction profiles were measured by qRT-PCR, as described in Materials and Methods.
(a) Average target gene/HPRT ratios for each experimental group were plotted. * indicates p<0.05 between LPS and SB/LPS groups. BMMs were also harvested after 15min, 30min, 1h or 2h stimulation with LPS w/ or w/o SB216763.
(b) Total cell lysates were analyzed for phosphorylated Gsk3β, JNK, Erk and p38 MAP kinases, as well as total Gsk3β and β-actin protein levels by Western blots.
(c) BMMs were stimulated with LPS for 6h w/ or w/o SB216763 in combination with anti-IL-10 Abs. Group averages of target gene/HPRT gene expression ratios were plotted. * indicates p<0.05 between SB/LPS and SB/αIL-10/LPS groups. Representative results of at least 2 separate experiments are shown.
Thus, Gsk3 inhibition regulates macrophage TLR4 response by directly down-regulating the pro-inflammatory IL-12 gene, yet up-regulating the induction of immune regulatory IL-10, which, in turn, further suppresses the pro-inflammatory gene expression programs.
Discussion
Although Gsk3β has been shown to regulate macrophage cytokine production and hepatocyte apoptosis (12, 21), its role in liver IRI cascade, an inflammation-mediated hepatocellular injury process, has not been explored. Our current study demonstrates that liver IR triggered Gsk3β phosphorylation, and the constitutively active Gsk3β was required for the activation of local pro-inflammatory response and the development of hepatocellular damage. Inhibition of Gsk3β protected livers against IRI by down-regulating pro-inflammatory IL-12 and selectively sparing immune regulatory IL-10, resulting in broader suppression of pro-inflammatory gene programs, including TNF-α and CXCL10. Interestingly, IL-10 neutralization recreated liver IRI, stressing the importance of IL-10 immune regulatory mechanism in cytoprotection rendered by Gsk3 inhibition.
Targeting Gsk3 has been proven an effective cardioprotective strategy (16). Unlike pre-conditioning, which triggers Gsk3β phosphorylation, reperfusion with Gsk3 inhibitors, added prior to or even post-ischemia, has reduced cell death (17, 18). However, it was also shown that Gsk3 inactivation was not absolutely required for ischemia pre- and post-conditioning (28). Despite controversial in vivo data, the mechanistic basis of these studies was the finding that inhibition of Gsk3β in cardiomyocytes delayed the opening of the mitochondrial permeability transition pore (MPTP) in the inner membrane, which protects cells from the intrinsic cell death pathway. The MPTP-triggered cell death was closely associated with IRI development (15). Along the same lines of cytoprotection, Gsk3 inhibition was also shown to protect kidneys and brains from IRI pathology (29–31), as well as livers from drug-induced toxicity (32). Our in vitro hepatocyte culture data are consistent with the positive regulatory role of Gsk3 in stress-induced cell death pathway (data not shown). The liver protective effect of Gsk3 inhibition in vivo does not depend on its suppression of MPTP, as atractyloside, a MPTP opener, did not abolish the effect of SB216763 in our liver IR model. Furthermore, Gsk3 inhibition by SB216763 did not sensitize hepatocytes to TNF-α-induced cell death in vitro (data not shown).
Our results show that the immune regulatory function of Gsk3 inhibition is critical for its beneficial effects in vivo, as IL-10 neutralization not only restored liver pro-inflammatory phenotype, but also dictated the severity of hepatocellular damage. In vivo, SB216763-facilitated Gsk3 inhibition protects mice from endotoxin shock (12), in association with the suppression of pro-inflammatory IL-12, IL-6, IFN-γ and the increase of immune regulatory IL-10. Our study provides further evidence that the suppression of pro-inflammatory program by Gsk3 inhibitor both in vitro and in vivo was mediated, at least, partially by an IL-10 autocrine mechanism. In macrophage cultures, Gsk3 inhibition selectively suppressed, as early as at 1 h, LPS-induced IL-12p40 and IL-1β, but not TNF-α, IL-6. A broader suppression of pro-inflammatory cytokines occurred only late (6 h) and was IL-10 dependent. Importantly, IL-10-dependent autocrine mechanism was involved in the regulation of CXCL10 by Gsk3 inhibition in response to TLR4 stimulation. CXCL10 is one of the key components of type I IFN pathway downstream of TLR4, both of which are essential in the pathogenesis of liver IRI (9, 20, 25). It has been shown that TLR4-stimulated IFN-β production, unlike other pro-inflammatory genes, is negatively regulated by Gsk3β (19). We demonstrate that although CXCL10 expression by BMM was not altered by SB216763 at early time points, it was down-regulated later on, as compared with controls. Thus, although CXCL10 induction by TLR4 signaling is not directly downregulated by Gsk3 inhibition, it can be suppressed by IL-10, which can be readily upregulated by SB216763.
The phosphorylation of Gsk3β downstream of TLR4 is mediated by the PI3 kinase-Akt pathway (12, 33). Indeed, it is known that PI3K/Akt activation protects hearts and brains from IRI pathology (34–37). Our findings imply that PI3 kinase activation was responsible for Gsk3β phosphorylation in IR-livers; and that PI3 kinase-Gsk3β signaling was a self-regulatory mechanism preventing the excessive IR-hepatocellular damage. It is interesting to note that PI3 kinase inhibition by wortmannin exerted the most profound effect when liver IRI was relatively mild, i.e., induced by 60 min rather than by 90 min of warm ischemia. This indicates the functional limit of liver self-protective mechanism that fails after the extended warm ischemia time. Gsk3 inhibition protected livers despite PI3 kinase inhibition, confirming the functional relationship between the two kinases in IRI regulatory pathways. As PI3 kinase is upstream of Gsk3β, targeting the latter may have certain advantages as compared with that of PI3 kinase in terms of both specificity and limited toxicity. Importantly, several potent and specific Gsk3β small molecule inhibitors have been recently tested in pre-clinical diabetic and Alzheimer disease models (13, 33).
In summary, Gsk3β inhibition represents a potent and safe strategy to ameliorate liver IRI pathology. This approach may provide not only the direct cytoprotection means against stress-induced cell death, but also exert immune modulation to reduce local inflammation. Further preclinical studies with Gsk3β chemical inhibitors are warranted to pave the way for the development of a clinically applicable therapeutic strategy against organ IRI.
Acknowledgments
Supported by: NIH Grants RO1 DK083408 (YZ), DK062357 (JWKW), The Dumont Research Foundation.
References
- 1.Fondevila C, Busuttil RW, Kupiec-Weglinski JW. Hepatic ischemia/reperfusion injury--a fresh look. Exp Mol Pathol. 2003;74:86–93. doi: 10.1016/s0014-4800(03)00008-x. [DOI] [PubMed] [Google Scholar]
- 2.Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284:G15–26. doi: 10.1152/ajpgi.00342.2002. [DOI] [PubMed] [Google Scholar]
- 3.Lentsch AB, Kato A, Yoshidome H, McMasters KM, Edwards MJ. Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury. Hepatology. 2000;32:169–173. doi: 10.1053/jhep.2000.9323. [DOI] [PubMed] [Google Scholar]
- 4.Serracino-Inglott F, Habib NA, Mathie RT. Hepatic ischemia-reperfusion injury. Am J Surg. 2001;181:160–166. doi: 10.1016/s0002-9610(00)00573-0. [DOI] [PubMed] [Google Scholar]
- 5.Tsung A, Hoffman RA, Izuishi K, Critchlow ND, Nakao A, Chan MH, Lotze MT, et al. Hepatic ischemia/reperfusion injury involves functional TLR4 signaling in nonparenchymal cells. J Immunol. 2005;175:7661–7668. doi: 10.4049/jimmunol.175.11.7661. [DOI] [PubMed] [Google Scholar]
- 6.Wu HS, Zhang JX, Wang L, Tian Y, Wang H, Rotstein O. Toll-like receptor 4 involvement in hepatic ischemia/reperfusion injury in mice. Hepatobiliary Pancreat Dis Int. 2004;3:250–253. [PubMed] [Google Scholar]
- 7.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhai Y, Qiao B, Shen XD, Gao F, Busuttil RW, Cheng G, Platt JL, et al. Evidence for the pivotal role of endogenous toll-like receptor 4 ligands in liver ischemia and reperfusion injury. Transplantation. 2008;85:1016–1022. doi: 10.1097/TP.0b013e3181684248. [DOI] [PubMed] [Google Scholar]
- 9.Zhai Y, Shen XD, O’Connell R, Gao F, Lassman C, Busuttil RW, Cheng G, et al. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J Immunol. 2004;173:7115–7119. doi: 10.4049/jimmunol.173.12.7115. [DOI] [PubMed] [Google Scholar]
- 10.Hu X, Chakravarty SD, Ivashkiv LB. Regulation of interferon and Toll-like receptor signaling during macrophage activation by opposing feedforward and feedback inhibition mechanisms. Immunol Rev. 2008;226:41–56. doi: 10.1111/j.1600-065X.2008.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 12.Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen P, Goedert M. GSK3 inhibitors: development and therapeutic potential. Nat Rev Drug Discov. 2004;3:479–487. doi: 10.1038/nrd1415. [DOI] [PubMed] [Google Scholar]
- 14.Rayasam GV, Tulasi VK, Sodhi R, Davis JA, Ray A. Glycogen synthase kinase 3: more than a namesake. Br J Pharmacol. 2009;156:885–898. doi: 10.1111/j.1476-5381.2008.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, et al. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113:1535–1549. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy E, Steenbergen C. Does inhibition of glycogen synthase kinase protect in mice? Circ Res. 2008;103:226–228. doi: 10.1161/CIRCRESAHA.108.181602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gross ER, Hsu AK, Gross GJ. Opioid-induced cardioprotection occurs via glycogen synthase kinase beta inhibition during reperfusion in intact rat hearts. Circ Res. 2004;94:960–966. doi: 10.1161/01.RES.0000122392.33172.09. [DOI] [PubMed] [Google Scholar]
- 18.Gomez L, Paillard M, Thibault H, Derumeaux G, Ovize M. Inhibition of GSK3beta by postconditioning is required to prevent opening of the mitochondrial permeability transition pore during reperfusion. Circulation. 2008;117:2761–2768. doi: 10.1161/CIRCULATIONAHA.107.755066. [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Garcia CA, Rehani K, Cekic C, Alard P, Kinane DF, Mitchell T, et al. IFN-beta production by TLR4-stimulated innate immune cells is negatively regulated by GSK3-beta. J Immunol. 2008;181:6797–6802. doi: 10.4049/jimmunol.181.10.6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhai Y, Qiao B, Gao F, Shen X, Vardanian A, Busuttil RW, Kupiec-Weglinski JW. Type I, but not type II, interferon is critical in liver injury induced after ischemia and reperfusion. Hepatology. 2008;47:199–206. doi: 10.1002/hep.21970. [DOI] [PubMed] [Google Scholar]
- 21.Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature. 2000;406:86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- 22.Schwabe RF, Brenner DA. Role of glycogen synthase kinase-3 in TNF-alpha-induced NF-kappaB activation and apoptosis in hepatocytes. Am J Physiol Gastrointest Liver Physiol. 2002;283:G204–211. doi: 10.1152/ajpgi.00016.2002. [DOI] [PubMed] [Google Scholar]
- 23.Shen XD, Ke B, Zhai Y, Amersi F, Gao F, Anselmo DM, Busuttil RW, et al. CD154-CD40 T-cell costimulation pathway is required in the mechanism of hepatic ischemia/reperfusion injury, and its blockade facilitates and depends on heme oxygenase-1 mediated cytoprotection. Transplantation. 2002;74:315–319. doi: 10.1097/00007890-200208150-00005. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, Cejalvo D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation. 1993;55:1265–1272. doi: 10.1097/00007890-199306000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Zhai Y, Shen XD, Gao F, Zhao A, Freitas MC, Lassman C, Luster AD, et al. CXCL10 regulates liver innate immune response against ischemia and reperfusion injury. Hepatology. 2008;47:207–214. doi: 10.1002/hep.21986. [DOI] [PubMed] [Google Scholar]
- 26.Gross ER, Hsu AK, Gross GJ. Delayed cardioprotection afforded by the glycogen synthase kinase 3 inhibitor SB-216763 occurs via a KATP- and MPTP-dependent mechanism at reperfusion. Am J Physiol Heart Circ Physiol. 2008;294:H1497–1500. doi: 10.1152/ajpheart.01381.2007. [DOI] [PubMed] [Google Scholar]
- 27.Juhaszova M, Zorov DB, Yaniv Y, Nuss HB, Wang S, Sollott SJ. Role of glycogen synthase kinase-3beta in cardioprotection. Circ Res. 2009;104:1240–1252. doi: 10.1161/CIRCRESAHA.109.197996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishino Y, Webb IG, Davidson SM, Ahmed AI, Clark JE, Jacquet S, Shah AM, et al. Glycogen synthase kinase-3 inactivation is not required for ischemic preconditioning or postconditioning in the mouse. Circ Res. 2008;103:307–314. doi: 10.1161/CIRCRESAHA.107.169953. [DOI] [PubMed] [Google Scholar]
- 29.Wang Z, Havasi A, Gall J, Bonegio R, Li Z, Mao H, Schwartz JH, et al. GSK3beta promotes apoptosis after renal ischemic injury. J Am Soc Nephrol. 2010;21:284–294. doi: 10.1681/ASN.2009080828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koh SH, Yoo AR, Chang DI, Hwang SJ, Kim SH. Inhibition of GSK-3 reduces infarct volume and improves neurobehavioral functions. Biochem Biophys Res Commun. 2008;371:894–899. doi: 10.1016/j.bbrc.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Cowper-Smith CD, Anger GJ, Magal E, Norman MH, Robertson GS. Delayed administration of a potent cyclin dependent kinase and glycogen synthase kinase 3 beta inhibitor produces long-term neuroprotection in a hypoxia-ischemia model of brain injury. Neuroscience. 2008;155:864–875. doi: 10.1016/j.neuroscience.2008.05.051. [DOI] [PubMed] [Google Scholar]
- 32.Shinohara M, Ybanez MD, Win S, Than TA, Jain S, Gaarde WA, Han D, et al. Silencing glycogen synthase kinase-3beta inhibits acetaminophen hepatotoxicity and attenuates JNK activation and loss of glutamate cysteine ligase and myeloid cell leukemia sequence 1. J Biol Chem. 2010;285:8244–8255. doi: 10.1074/jbc.M109.054999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beurel E, Michalek SM, Jope RS. Innate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3) Trends Immunol. 2010;31:24–31. doi: 10.1016/j.it.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li C, Ha T, Kelley J, Gao X, Qiu Y, Kao RL, Browder W, et al. Modulating Toll-like receptor mediated signaling by (1-->3)-beta-D-glucan rapidly induces cardioprotection. Cardiovasc Res. 2004;61:538–547. doi: 10.1016/j.cardiores.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 35.Williams DL, Ozment-Skelton T, Li C. Modulation of the phosphoinositide 3-kinase signaling pathway alters host response to sepsis, inflammation, and ischemia/reperfusion injury. Shock. 2006;25:432–439. doi: 10.1097/01.shk.0000209542.76305.55. [DOI] [PubMed] [Google Scholar]
- 36.Hua F, Ha T, Ma J, Li Y, Kelley J, Gao X, Browder IW, et al. Protection against myocardial ischemia/reperfusion injury in TLR4-deficient mice is mediated through a phosphoinositide 3-kinase-dependent mechanism. J Immunol. 2007;178:7317–7324. doi: 10.4049/jimmunol.178.11.7317. [DOI] [PubMed] [Google Scholar]
- 37.Hua F, Ma J, Ha T, Xia Y, Kelley J, Williams DL, Kao RL, et al. Activation of Toll-like receptor 4 signaling contributes to hippocampal neuronal death following global cerebral ischemia/reperfusion. Journal Of Neuroimmunology. 2007;190:101–111. doi: 10.1016/j.jneuroim.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]