Abstract
A limitation of animal models of central pain is their inability to recapitulate all clinical characteristics of the human condition. Specifically, many animal models rely on reflexive measures of hypersensitivity and ignore, or cannot assess spontaneous pain, the hallmark characteristic of central pain in humans. Here, we adopt a conditioned place preference paradigm to test if animals with lesions in the anterolateral quadrant of the spinal cord develop signs consistent with spontaneous pain. This paradigm relies on the fact that pain relief is rewarding to animals, and has been used previously to show that animals with peripheral nerve injury develop tonic pain. With the use of two analgesic treatments commonly used to treat patients with central pain (clonidine infusion and motor cortex stimulation), we demonstrate that analgesic treatments are rewarding to animals with spinal cord lesions but not sham operated controls. These findings are consistent with the conclusion that animals with spinal cord injury suffer from tonic pain.
Keywords: Spontaneous pain, Motor cortex stimulation, Posterior thalamus, Rat, Clonidine
Introduction
A common consequence of spinal cord injury is the development of severe, debilitating chronic pain.1, 29, 36 In patients, the pain manifests with a wide range of intensities and locations. It is usually persistent in the absence of an insult (spontaneous pain), and can present as hypersensitivity to painful stimuli (hyperalgesia) and hypersensitivity to normally innocuous stimuli (allodynia).2 The etiology of the pain is unknown and is thought to be caused by maladaptive changes in the central nervous system.
Several animal models have been developed to study central pain, many of which focused on studying pain due to spinal cord injury. In all of these models, the location, the extent and the means to produce injury vary. Some authors use controlled spinal contusions to mimic clinical traumatic injuries.12, 26, 39 Others have used ischemic lesions,9, 10 or neurotoxic chemical injection into the spinal cord,4, 37 whereas some have used cuts to sever the spinal cord (hemisection),5, 6 or localized regions in the spinal cord (cordotomy).31, 32 Most of these models rely on measures of evoked pain and hypersensitivity, such as mechanical and thermal withdrawal thresholds. However, they commonly do not attempt to quantify spontaneous pain, which is the single most common and debilitating complaint from spinal cord injury patients.8, 29 Our aim was to assess whether animals with spinal cord injury suffer from spontaneous pain.
We have demonstrated recently that localized electrolytic lesions in the anterolateral quadrant of the spinal cord result in consistent, long lasting mechanical and thermal hyperalgesia.19 Like other animal models of central pain, we relied on evoked measures of hypersensitivity to assess hyperalgesia and did not test if animals exhibit symptoms of spontaneous pain. Here, we employ a conditioned place preference paradigm described by King et al.,14 to study tonic pain in animals. This approach takes advantage of the fact that pain relief is rewarding and, therefore, analgesic treatments should only be rewarding in the presence of pain.14 We use the conditioned place preference paradigm combined with two treatments known to alleviate neuropathic pain (clonidine infusion or electrical stimulation of the motor cortex) to test if animals develop signs of spontaneous pain following spinal cord lesions. We demonstrate that lesioned animals, but not sham operated controls, develop rapid preference to the analgesic treatment-paired chamber.
MATERIALS AND METHODS
All procedures were approved by the University of Maryland Animal Care and Use Committee. Experiments were conducted according to institutional guidelines, federal regulations, and the guidelines of the International Association for the Study of Pain.
Protocol overview
Twenty eight adult female Sprague-Dawley rats (Harlan, IN) weighing 250–300 g were used in this study, which was conducted over a 10-week period. Two experiments were conducted concurrently: (1: Drug group) To test the effect of analgesic drug administration (clonidine) on the conditioned place preference of animals with spinal cord injury (n=11). (2: Stimulation group) To test the effect of motor cortex stimulation on the conditioned place preference of animals with spinal cord injury (n=17). In weeks 1 and 2, rats were habituated to handling and trained to stand with their forepaws on the experimenter’s hand, allowing access to the hindpaws, as described in Ren.25 During week 3, rats underwent behavioral tests to measure mechanical hindpaw withdrawal thresholds (see below). During week 4, rats underwent spinal lesion surgery to induce central pain or sham lesion surgery as a control and, for animals receiving motor cortex stimulation, to implant insulated platinum electrodes (see below). Weeks 5 and 6 involved further behavioral testing to measure mechanical hindpaw withdrawal thresholds and monitor the development of injury related hyperalgesia. During week 7, rats in the clonidine/saline group underwent surgery to implant cannulae in the lateral ventricle for drug administration. Week 8 involved recovery from surgery and further testing of mechanical hindpaw withdrawal thresholds. The conditioned place preference protocol was conducted during weeks 9 and 10, along with further testing of mechanical hindpaw withdrawal thresholds.
Mechanical hindpaw withdrawal threshold testing
Mechanical hindpaw withdrawal thresholds were measured bilaterally using calibrated von Frey filaments (Stoelting, IL). Filaments with forces ranging from 10 to 180 g were applied to the dorsal surface of the hindpaw based on studies demonstrating that threshold changes are more reliably and consistently detected at this site.25 Each von Frey filament was applied five times to each hindpaw and the threshold was defined as the force at which the animal withdrew the paw to three or more of the stimuli (>50% response frequency). Animals were not restrained during testing. Rats underwent von Frey testing on three days in week 3 (before spinal or sham lesion surgery) to obtain baseline pre-surgical withdrawal thresholds, and every 7 days post-lesion surgery for the duration of the study. Rats were also tested during the conditioned place preference protocol (week 10) to determine mechanical thresholds in the presence of intraventricular drug treatment or motor cortex stimulation (see below).
Surgical procedures
Spinal lesions
Fifteen adult female Sprague-Dawley rats underwent spinal lesion surgery, and thirteen underwent sham lesion surgery during week 4 of the study. Eleven rats (n=6 lesioned, n=5 sham) underwent surgery to implant a cannula in the right lateral ventricle during week 7 of the study. Surgeries were conducted under strict aseptic conditions. Rats were anesthetized with ketamine/xylazine (100/8 mg/kg, i.p.) and placed on a thermo-regulated heating pad to maintain body temperature. For spinal lesions, a laminectomy was performed to expose the spinal cord between C6 and T2. A quartz-insulated platinum electrode (5 μm tip) was targeted unilaterally to the ventrolateral quadrant of the spinal cord, as described previously.19, 33 Current (10 μA for 10 sec, repeated 4 times) was passed through the electrode to produce an electrolytic lesion (approximately 0.6 mm3; lesion locations: 0.8 mm lateral from midline; depth: 2.1 mm). In some animals (n=9), to produce larger spinal lesions, we modified our approach to produce two lesions, 0.4mm apart (lesion locations: 0.8 mm and 1.2 mm lateral from midline; depth: 2.1 mm). However, the modification in the protocol had no effect on the consistency or features of the resultant hyperalgesia. Sham surgery was performed without laminectomy.
Implantation of motor cortex stimulation electrodes
In 17 animals (“stimulation group”) and, concurrent with spinal lesion surgery, a longitudinal incision was made along the midline of the skull to expose bregma and lambda. The bone overlying the primary motor cortex (MI) was removed contralateral to the spinal lesion site. Custom made epidural bipolar insulated platinum electrodes (diameter: 70 μm, exposed tip: 50 μm, distance between electrodes: 500 μm) were targeted to the MI contralateral to the site of spinal lesion using stereotaxic coordinates (A: 1.8 mm, L: 2 mm). These coordinates were obtained from pilot experiments using electrical microstimulation and from data obtained from our previously published motor cortex mapping work.34 This allowed us to reliably target the hindpaw representation of MI since the location of major subdivisions such as the forelimb or hindlimb areas in the rat motor cortex is somatotopic and consistent from animal to animal.22 Motor cortex stimulation electrodes were attached to amphenol pins to facilitate connection to the isolated pulse stimulator (A-M Systems, WA). Electrodes were fixed in place using four bone screws and acrylic resin. At the end of surgery, the wound edges were approximated and sutured to achieve primary closure.
Cannula implantations
In eleven animals (“drug group”), a craniotomy was performed to expose the brain over the right lateral ventricle in week 7. A guide cannula was advanced to the ventricle and fixed in place using dental resin.
Post operative care
The analgesic buprenorphine (0.05 mg/kg) was administered every 12 hours for 24 hours postoperatively following spinal lesion surgeries and motor cortex electrode implantation, and every 12 hours for 3 days postoperatively following cannula implantations.
Conditioned place preference protocol
Conditioned place preference testing was conducted using a custom built, automated 2-chamber box. The walls of one chamber were white with horizontal black stripes and the walls of the other chamber were white with vertical black stripes. We used chambers with striped walls to ensure that rats would not strongly prefer one chamber over the other, as they would if we had used a more traditional conditioned place preference box with one dark-walled chamber and one light-walled chamber.
Rats were habituated to the conditioned place preference box for 3 days during week 9 of the study. On each habituation day, rats were permitted to move freely between the two chambers for 30 minutes. On day 4 of week 9, a pre-conditioning preference test was conducted in which rats were permitted to move freely between the two chambers for 15 minutes and time spent in each chamber was recorded to determine each rat’s preference.
After habituation and the pre-conditioning preference test (week 9), rats underwent a 3-day conditioning phase in week 10 of the study. Two sessions were conducted on each day, at least 6 hours apart. (1) In one session the animals were placed in the chamber that they demonstrated preference for during the pre-conditioning test. They spent 30 minutes in that chamber where rats in the drug group received an intraventricular microinjection of vehicle (5 μl saline followed by 10 μl saline flush); rats in the stimulation group received sham motor cortex stimulation (wires attached but no current passed).(2) In the other daily session the animals were placed for 30 min in the chamber that they did not prefer during the pre-conditioning test. Here, rats in the drug group received an intraventricular microinjection of clonidine, an alpha 2-adrenergic agonist (5 μl [2mg/ml] followed by 10 μl saline flush), and those in the stimulation group received motor cortex stimulation (50 μA, 50 Hz, for 30 minutes). We used intraventricular clonidine in these experiments because it has been shown previously to reduce tonic and evoked pain in animals with peripheral neuropathic pain without affecting normal uninjured animals.14
Drug or motor cortex stimulation treatment order was randomized for each rat. That is, some days the rat received vehicle in the first session while other days the rat received drug in the first session. Mechanical hindpaw withdrawal thresholds were measured one hour following saline/clonidine intraventricular injection or immediately after the end of motor cortex stimulation.
One day after the conditioning phase, a post-conditioning place preference test was conducted in which rats received no drug treatment and were permitted to move freely between the two chambers for 15 minutes. Time spent in each chamber was recorded to determine each rat’s chamber preference.
Data analysis
Statistical analyses were performed with SigmaStat (Aspire Software International, Ashburn, VA). To test whether mechanical hindpaw withdrawal thresholds changed over time after surgery, data from spinal-lesioned rats and sham-lesioned rats were analyzed separately with the Friedman test. To test the effects of clonidine or motor cortex stimulation treatment on mechanical hyperalgesia, data from spinal-lesioned rats and sham-lesioned rats were analyzed separately with the Wilcoxon Signed Ranks test. Conditioned place preference test results were analyzed using a two-way analysis of variance (group, conditioning) with repeated measures on one factor (group), followed by a posthoc Fisher least significant difference (LSD) test to compare individual factors. The significance level was set at p<0.05 for all tests.
Results
Animals in the drug group develop mechanical hyperalgesia following spinal lesions
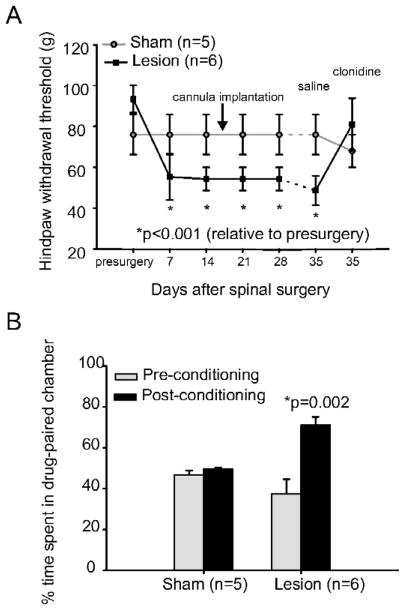
We and others have previously shown that rats with spinal cord lesions develop behavioral signs consistent with central pain, including mechanical and thermal hyperalgesia caudal to the lesion site.19, 27, 32 Consistent with the literature, all spinal-lesioned rats in the clonidine/saline treatment group showed a significant decrease in mechanical hindpaw withdrawal thresholds bilaterally within 7 days of the lesion surgery (Fig. 1A). Mechanical thresholds decreased from 93.3 g (SD 16; median 100; range 60–100; n=6) to 55.3 g (SD 28; median 60; range 26–100; p<0.001, Friedman). Sham surgery (n=5) had no effect on mechanical withdrawal thresholds on either the ipsilateral or contralateral hindpaw (Fig. 1A). Each animal tested had identical withdrawal thresholds on the ipsilateral and contralateral hindpaw at every time point. As a result, Figure 1A shows the behavioral data for the contralateral hindpaw; results for the ipsilateral hindpaw were the same and are therefore not shown.
Figure 1. Intraventricular Administration of Clonidine Reverses Mechanical Hyperalgesia and Unmasks a Tonic Aversive State.
A. Animals in the clonidine/saline group develop mechanical hyperalgesia following spinal cord lesions (n=6; p<0.001) while sham surgery had no effect on mechanical withdrawal thresholds (n=5). Intraventricular administration of clonidine (5 μl [2mg/ml] followed by 10 μl saline flush) reversed mechanical hyperalgesia in animals with spinal cord lesions. The administration of an equivalent volume of saline had no effect of mechanical withdrawal thresholds. B. The percentage of time spent in the drug paired chamber is shown. Animals with spinal cord injury (n=6) prefer the chamber where they receive clonidine treatment (p=0.002). Clonidine had no effect on chamber preference of sham-operated controls (n=5). Error bar=SEM. *: Statistically significant difference.
Clonidine reverses mechanical hyperalgesia in animals with spinal lesions
Intraventricular injection of clonidine (2 mg/ml) at 35 days after spinal lesion surgery reversed hyperalgesia in lesioned animals (Fig. 1A), returning mechanical withdrawal thresholds to pre-spinal lesion values (pre-surgery: mean 93.3g, SD 16, median 100, range 60–100; clonidine at 35 days: mean 81g, SD 31, median 100, range 26–100; p=1.0, Wilcoxon). The infusion of an equivalent volume of saline at 35 days had no effect on mechanical hyperalgesia in these animals (mean 48.7g, SD 18, median 60, range 26–60; p=0.03, compared with pre-surgical values, Wilcoxon; Fig. 1A). Clonidine and saline treatments had no effect on mechanical hindpaw withdrawal thresholds in sham-lesioned animals (p>0.05). These findings are consistent with previous studies demonstrating that clonidine is effective in reducing mechanical hyperalgesia in animal models of neuropathic pain and that it can be used to examine behaviors related to chronic neuropathic pain.7, 14
Clonidine unmasks the tonic aversive state in animals with spinal lesions
Before conditioning, both sham operated controls and spinal lesioned animals showed a slight but not statistically significant preference for the vertically striped chamber (Fig. 1B). Sham animals spent an average of 46.9% (SD 4, n=5) of the 15 minute test period in the horizontally striped chamber, while spinal lesioned animals spent an average of 37.6% (SD 17, n=6) of the test period in the horizontally striped chamber. During the conditioning phase, all animals received intraventricular infusions of clonidine (2 mg/ml) and were then restricted to the horizontally striped chamber for 30 minutes. Saline injections were paired with restriction in the vertically striped chamber (repeated on 3 days). After this conditioning paradigm, animals with spinal cord lesions, but not sham animals (animals without spinal cord injury), developed a strong and significant preference for the clonidine paired chamber (Fig. 1B), spending an average of 71.1% (SD 10) of the 15 minute test period in the drug-paired horizontally striped chamber (p=0.002, posthoc Fisher LSD; F=6.91, p=0.03 for group × conditioning interaction, two-way ANOVA). The preference of the sham animals remained unchanged, with this group spending an average of 49.6% (SD 2) of the test period in the horizontally striped chamber (p>0.05, posthoc Fisher LSD). The findings that spinal lesioned animals, but not shams, prefer the chamber in which analgesia is provided, suggests that clonidine unmasks a tonic aversive state.
Motor cortex stimulation unmasks the tonic aversive state in animals with spinal lesions
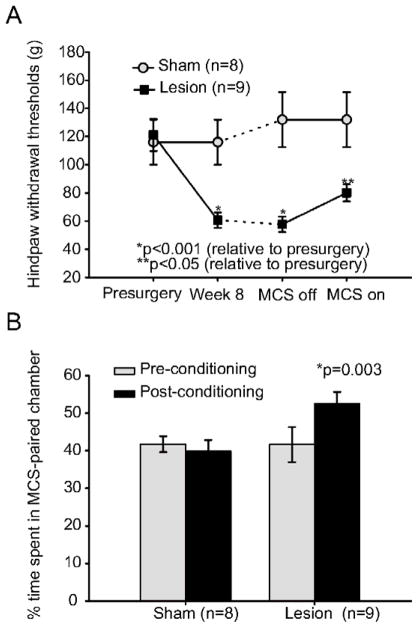
Like the first group of animals in this study, these spinal-lesioned rats developed a significant decrease in mechanical hindpaw withdrawal thresholds bilaterally (Fig. 2A). Mechanical thresholds decreased from 121.2 g (SD 39; median 100; range 60–180; n=9) to 60.0 g at week 8 (SD 17.5; median 60; range 26–100; p<0.001, Friedman). Sham surgery (n=8) had no effect on mechanical withdrawal thresholds on either the ipsilateral (Fig. 2A) or contralateral hindpaw (data not shown).
Figure 2. Motor Cortex Stimulation Reduces Mechanical Hyperalgesia and Unmasks a Tonic Aversive State.
A. Like the clonidne/saline group, animals in the motor cortex stimulation group developed mechanical hyperalgesia following spinal cord lesions (n=9; p<0.001). Mechanical withdrawal thresholds in animals receiving sham surgery were not significantly different from pre-surgical values. Motor cortex stimulation (50 μA, 50 Hz, for 30 minutes) reduced mechanical hyperalgesia in animals with spinal cord lesions (p=0.04), but had no effect on sham-operated controls. B. Animals with spinal cord lesions (n=9) preferred the chamber where they received motor cortex stimulation (p=0.003). Motor cortex stimulation had no effect on the preference of sham operated controls. Error bar=SEM. *: Statistically significant difference. MCS: Motor cortex stimulation.
Consistent with our previous findings (Lucas, 2010) motor cortex stimulation (50 μA, 50 Hz, 300 μs square pulse, 30 minute duration) 63 days after spinal lesion surgery significantly reduced the hyperalgesia in animals with spinal cord injury (Stimulation on: mean 80 g, SD 21, median 80, range 60–100; Stimulation off: mean 57.7 g, SD 19, median 60, range 26–100; p=0.04, Wilcoxon, n=9). Unlike the clonidine treatment, the reduction in mechanical hyperalgesia after motor cortex stimulation was not complete and mechanical threshold values did not return to pre-surgery levels (p=0.04, Wilcoxon). Motor cortex stimulation in sham lesioned animals (n=8) had no effect on mechanical hindpaw withdrawal thresholds (p>0.05, Fig. 2A).
To test if motor cortex stimulation unmasks the tonic aversive state in animals with anterolateral spinal cord lesions, we used the conditioned place preference test. Before conditioning, both sham and spinal lesioned animals showed a slight but not statistically significant preference for the vertically-striped chamber (Fig. 2B). Sham animals spent an average of 41.7% (SD 5, n=8) of the 15 minute test period in the horizontally striped chamber, while spinal lesioned animals spent an average of 39.9% (SD 10, n=9) of the test period in the horizontally-striped chamber. During the conditioning phase, all animals received motor cortex stimulation (50 μA, 50 Hz, 300 μs square pulse, 30 minute duration) while restricted in the horizontally striped chamber for -striped chamber (repeated on 3 days). After this conditioning paradigm, lesioned animals, but not sham animals, developed a preference to the motor cortex stimulation-paired chamber (Fig. 2B) spending an average of 52% (SD 9) of the 15 minute test period in the motor cortex stimulation-paired chamber (p=0.003, posthoc Fisher LSD; F=4.34, p=0.04 for group × conditioning interaction, two-way ANOVA). The preference of the sham animals remained unchanged, with this group spending an average of 42% (SD 12) of the test period in the horizontally striped chamber (p>0.05, posthoc Fisher LSD). Findings from the conditioned place preference test suggest that motor cortex stimulation reduces tonic pain.
Discussion
This study was designed to test whether animals with anterolateral spinal cord lesions suffer from spontaneous pain. We found that animals with spinal cord injury develop mechanical hyperalgesia that can be attenuated by treatments commonly used for patients with central neuropathic pain: clonidine or motor cortex stimulation. Using the conditioned place preference test we further demonstrate that these treatments unmask a tonic aversive state suggesting that these animals exhibit signs of spontaneous pain.
Signs of spontaneous pain in animals with spinal cord lesions
The conditioned place preference test or modifications of, is commonly used to study the motivational effects of drugs and pain on animals.13, 15 King and colleagues14 adopted the conditioned place preference paradigm to investigate if animals with peripheral neuropathic pain suffer from spontaneous pain. They demonstrated that animals with spinal nerve ligation, but not sham operated controls, rapidly develop a preference to the clonidine-paired chamber. King et al., posited that clonidine administration results in the removal of a tonic state, suggesting that the animals experience pain relief. These findings led King and colleagues to conclude that animals with SNL suffer from tonic pain. 14
In our animal model of central pain, and similar to King et al.,14 intraventricular clonidine administration resulted in negative reinforcement whereby lesioned animals preferred the drug-paired chamber while clonidine was not rewarding in the absence of injury. Therefore, these findings suggest that animals with spinal cord injury suffer from tonic pain. Clonidine not only unmasked the presence of a tonic pain component, but also reversed mechanical hyperalgesia in animals with spinal cord lesions. These findings are consistent with previous reports that clonidine reverses mechanical and thermal hyperalgesia in animal models of neuropathic pain.7, 14 Another treatment for neuropathic pain, motor cortex stimulation, also resulted in negative reinforcement in animals with spinal cord lesions but not in controls. These findings further support the notion that spinal cord lesioned animals suffer from tonic pain.
Spontaneous pain is difficult to assay in animals and especially rodents because they do not display behaviors or postures that reflect the presence of mild to moderate pain.21, 28 Current animal models of central pain are unable to demonstrate that animals with spinal cord injury suffer from spontaneous pain. Previous investigations in animals relied heavily on observations of overgrooming/autotomy, licking, guarding and vocalization.30 These behaviors have been criticized as unreliable and nonspecific.20, 35 The conditioned place preference paradigm offers a suitable supplement to these behaviors especially since it specifically measures cognitive, motivated preference and pain is an emotional cognitive experience.
Neuropathological basis of ongoing pain following spinal cord injury
Spinal cord injury can result in maladaptive plastic changes throughout the neural axis. In the spinal cord, following injury, there is massive reorganization and sprouting in primary afferents.5, 6 Injury causes elevated concentrations of excitatory amino acids18 in the extracellular space and dorsal horn neurons show increased spontaneous activity.10, 11 In the thalamus, spinal cord injury results in increased activity, increased spike bursts and changes in glial activation.33, 40 We have demonstrated that spontaneous activity of PO and SI neurons are dramatically increased (PO: ~30 fold increase; S1: ~3 fold) in animals with spinal lesions when compared to sham operated controls.19, 24 The change in spontaneous activity of thalamic and cortical neurons may contribute to the tonic pain observed in our animal model of spinal cord injury pain.
In humans with spinal cord injury and chronic pain, functional imaging studies reveal significant changes in blood flow in the thalamus during rest3, 23 and electrophysiological recordings reveal abnormal spontaneous discharges in thalamic neurons.16, 17 These maladaptive changes may contribute to the pathogenesis of spontaneous pain in humans. However, the underlying mechanisms remain to be elucidated.
Here, we present findings that suggest that animals with spinal cord injury exhibit signs of spontaneous pain. The presence of tonic pain and hyperalgesia, the small size of spinal damage when compared to other animal models of spinal cord injury,5, 10, 27, 32, 38 the reduced morbidity and the high percentage of animals developing hyperalgesia after lesions (94%)19 make this model ideal to study the neurobiological substrates responsible for the development of central pain.
Acknowledgments
This project was supported by National Institute of Neurological Disorders and Stroke Fellowships F32NS-064775 to R.L.Q and F31NS-070458 to J.M.L, and Research Grants R01-051799 to A.K and R01-NS069568 to R.M. Support was also provided by the Christopher and Dana Reeve Spinal Cord Research Foundation (A.K) and the Department of Defense (SC090126 to R.M).
Footnotes
Disclosures
The authors of this paper have no financial or other conflicts of interest to declare.
Perspective
The hallmark characteristic of central pain in humans is spontaneous pain. Animal models of central pain rely on reflexive measures of hypersensitivity and do not assess spontaneous pain. Demonstrating that animals with spinal cord injury suffer from tonic pain is important to study the etiology of central pain.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Beric A. Post-spinal cord injury pain states. Pain. 1997;72:295–298. [PubMed] [Google Scholar]
- 2.Boivie J. Central Pain. In: McMahon S, Koltzenburg M, editors. Wall and Melzack’s Textbook of Pain. Churchill Livingstone; Oxford: 2005. pp. 1057–1074. [Google Scholar]
- 3.Canavero S, Bonicalzi V. The neurochemistry of central pain: evidence from clinical studies, hypothesis and therapeutic implications. Pain. 1998;74:109–114. doi: 10.1016/s0304-3959(97)00089-4. [DOI] [PubMed] [Google Scholar]
- 4.Caudle RM, Perez FM, King C, Yu CG, Yezierski RP. N-methyl-D-aspartate receptor subunit expression and phosphorylation following excitotoxic spinal cord injury in rats. Neurosci Lett. 2003;349:37–40. doi: 10.1016/s0304-3940(03)00700-6. [DOI] [PubMed] [Google Scholar]
- 5.Christensen MD, Everhart AW, Pickelman JT, Hulsebosch CE. Mechanical and thermal allodynia in chronic central pain following spinal cord injury. Pain. 1996;68:97–107. doi: 10.1016/S0304-3959(96)03224-1. [DOI] [PubMed] [Google Scholar]
- 6.Christensen MD, Hulsebosch CE. Chronic central pain after spinal cord injury. J Neurotrauma. 1997;14:517–537. doi: 10.1089/neu.1997.14.517. [DOI] [PubMed] [Google Scholar]
- 7.Feng X, Zhang F, Dong R, Li W, Liu J, Zhao X, Xue Q, Yu B, Xu J. Intrathecal administration of clonidine attenuates spinal neuroimmune activation in a rat model of neuropathic pain with existing hyperalgesia. Eur J Pharmacol. 2009;614:38–43. doi: 10.1016/j.ejphar.2009.04.044. [DOI] [PubMed] [Google Scholar]
- 8.Finnerup NB, Johannesen IL, Sindrup SH, Bach FW, Jensen TS. Pain and dysesthesia in patients with spinal cord injury: A postal survey. Spinal Cord. 2001;39:256–262. doi: 10.1038/sj.sc.3101161. [DOI] [PubMed] [Google Scholar]
- 9.Hao JX, Xu XJ. Treatment of a chronic allodynia-like response in spinally injured rats: effects of systemically administered excitatory amino acid receptor antagonists. Pain. 1996;66:279–285. [PubMed] [Google Scholar]
- 10.Hao JX, Xu XJ, Aldskogius H, Seiger A, Wiesenfeld-Hallin Z. Allodynia-like effects in rat after ischaemic spinal cord injury photochemically induced by laser irradiation. Pain. 1991;45:175–185. doi: 10.1016/0304-3959(91)90186-2. [DOI] [PubMed] [Google Scholar]
- 11.Hao JX, Xu XJ, Yu YX, Seiger A, Wiesenfeld-Hallin Z. Transient spinal cord ischemia induces temporary hypersensitivity of dorsal horn wide dynamic range neurons to myelinated, but not unmyelinated, fiber input. J Neurophysiol. 1992;68:384–391. doi: 10.1152/jn.1992.68.2.384. [DOI] [PubMed] [Google Scholar]
- 12.Hulsebosch CE, Hains BC, Crown ED, Carlton SM. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res Rev. 2009;60:202–213. doi: 10.1016/j.brainresrev.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johansen JP, Fields HL, Manning BH. The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc Natl Acad Sci U S A. 2001;98:8077–8082. doi: 10.1073/pnas.141218998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Fields HL, Porreca F. Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci. 2009;12:1364–1366. doi: 10.1038/nn.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LaBuda CJ, Fuchs PN. A behavioral test paradigm to measure the aversive quality of inflammatory and neuropathic pain in rats. Exp Neurol. 2000;163:490–494. doi: 10.1006/exnr.2000.7395. [DOI] [PubMed] [Google Scholar]
- 16.Lenz FA, Kwan HC, Dostrovsky JO, Tasker RR. Characteristics of the bursting pattern of action potentials that occurs in the thalamus of patients with central pain. Brain Res. 1989;496:357–360. doi: 10.1016/0006-8993(89)91088-3. [DOI] [PubMed] [Google Scholar]
- 17.Lenz FA, Tasker RR, Dostrovsky JO, Kwan HC, Gorecki J, Hirayama T, Murphy JT. Abnormal single-unit activity recorded in the somatosensory thalamus of a quadriplegic patient with central pain. Pain. 1987;31:225–236. doi: 10.1016/0304-3959(87)90038-8. [DOI] [PubMed] [Google Scholar]
- 18.Liu D, Thangnipon W, McAdoo DJ. Excitatory amino acids rise to toxic levels upon impact injury to the rat spinal cord. Brain Res. 1991;547:344–348. doi: 10.1016/0006-8993(91)90984-4. [DOI] [PubMed] [Google Scholar]
- 19.Masri R, Quiton RL, Lucas JM, Murray PD, Thompson SM, Keller A. Zona Incerta: A Role in Central Pain. J Neurophysiol. 2009;102:181–191. doi: 10.1152/jn.00152.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009;10:283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- 21.Morton DB, Griffiths PH. Guidelines on the recognition of pain, distress and discomfort in experimental animals and an hypothesis for assessment. Vet Rec. 1985;116:431–436. doi: 10.1136/vr.116.16.431. [DOI] [PubMed] [Google Scholar]
- 22.Neafsey EJ, Bold EL, Haas G, Hurley-Gius KM, Quirk G, Sievert CF, Terreberry RR. The organization of the rat motor cortex: a microstimulation mapping study. Brain Res. 1986;396:77–96. doi: 10.1016/s0006-8993(86)80191-3. [DOI] [PubMed] [Google Scholar]
- 23.Peyron R, Garcia-Larrea L, Gregoire MC, Convers P, Richard A, Lavenne F, Barral FG, Mauguiere F, Michel D, Laurent B. Parietal and cingulate processes in central pain. A combined positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) study of an unusual case. Pain. 2000;84:77–87. doi: 10.1016/S0304-3959(99)00190-6. [DOI] [PubMed] [Google Scholar]
- 24.Quiton RL, Masri R, Thompson SM, Keller A. Abnormal Activity of Primary Somatosensory Cortex in Central Pain Syndrome. J Neurophysiol. 2010 doi: 10.1152/jn.00161.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren K. An improved method for assessing mechanical allodynia in the rat. Physiol Behav. 1999;67:711–716. doi: 10.1016/s0031-9384(99)00136-5. [DOI] [PubMed] [Google Scholar]
- 26.Scheff SW, Rabchevsky AG, Fugaccia I, Main JA, Lumpp JEJ. Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J Neurotrauma. 2003;20:179–193. doi: 10.1089/08977150360547099. [DOI] [PubMed] [Google Scholar]
- 27.Siddall P, Xu CL, Cousins M. Allodynia following traumatic spinal cord injury in the rat. Neuroreport. 1995;6:1241–1244. doi: 10.1097/00001756-199506090-00003. [DOI] [PubMed] [Google Scholar]
- 28.Stasiak KL, Maul D, French E, Hellyer PW, VandeWoude S. Species-specific assessment of pain in laboratory animals. Contemp Top Lab Anim Sci. 2003;42:13–20. [PubMed] [Google Scholar]
- 29.Stormer S, Gerner HJ, Gruninger W, Metzmacher K, Follinger S, Wienke C, Aldinger W, Walker N, Zimmermann M, Paeslack V. Chronic pain/dysaesthesiae in spinal cord injury patients: results of a multicentre study. Spinal Cord. 1997;35:446–455. doi: 10.1038/sj.sc.3100411. [DOI] [PubMed] [Google Scholar]
- 30.Vierck CJ, Siddall P, Yezierski RP. Pain following spinal cord injury. animal models and mechanistic studies. Pain. 2000;89:1–5. doi: 10.1016/S0304-3959(00)00463-2. [DOI] [PubMed] [Google Scholar]
- 31.Vierck CJJ, Greenspan JD, Ritz LA. Long-term changes in purposive and reflexive responses to nociceptive stimulation following anterolateral chordotomy. J Neurosci. 1990;10:2077–2095. doi: 10.1523/JNEUROSCI.10-07-02077.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.VierckJr C, Light A. Effects of combined hemotoxic and anterolateral spinal lesions on nociceptive sensitivity. Pain. 1999;83:447–457. doi: 10.1016/S0304-3959(99)00129-3. [DOI] [PubMed] [Google Scholar]
- 33.Wang G, Thompson SM. Maladaptive homeostatic plasticity in a rodent model of central pain syndrome: thalamic hyperexcitability after spinothalamic tract lesions. J Neurosci. 2008;28:11959–11969. doi: 10.1523/JNEUROSCI.3296-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiss DS, Keller A. Specific patterns of intrinsic connections between representation zones in the rat motor cortex. Cerebral Cortex. 1994;4:205–214. doi: 10.1093/cercor/4.2.205. [DOI] [PubMed] [Google Scholar]
- 35.Woolf CJ. Long term alterations in the excitability of the flexion reflex produced by peripheral tissue injury in the chronic decerebrate rat. Pain. 1984;18:325–343. doi: 10.1016/0304-3959(84)90045-9. [DOI] [PubMed] [Google Scholar]
- 36.Yezierski RP. Pain following spinal cord injury: pathophysiology and central mechanisms. Prog Brain Res. 2000;129:429–449. doi: 10.1016/S0079-6123(00)29033-X. [DOI] [PubMed] [Google Scholar]
- 37.Yezierski RP, Liu S, Ruenes GL, Kajander KJ, Brewer KL. Excitotoxic spinal cord injury: behavioral and morphological characteristics of a central pain model. Pain. 1998;75:141–155. doi: 10.1016/S0304-3959(97)00216-9. [DOI] [PubMed] [Google Scholar]
- 38.Yezierski RP, Park SH. The mechanosensitivity of spinal sensory neurons following intraspinal injections of quisqualic acid in the rat. Neurosci Lett. 1993;157:115–119. doi: 10.1016/0304-3940(93)90656-6. [DOI] [PubMed] [Google Scholar]
- 39.Yoon YW, Dong H, Arends JJ, Jacquin MF. Mechanical and cold allodynia in a rat spinal cord contusion model. Somatosens Mot Res. 2004;21:25–31. doi: 10.1080/0899022042000201272. [DOI] [PubMed] [Google Scholar]
- 40.Zhao P, Waxman SG, Hains BC. Modulation of thalamic nociceptive processing after spinal cord injury through remote activation of thalamic microglia by cysteine cysteine chemokine ligand 21. J Neurosci. 2007;27:8893–8902. doi: 10.1523/JNEUROSCI.2209-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]